Glymphatic Dysfunction Induced Oxidative Stress and Neuro-Inflammation in Major Depression Disorders
Abstract
1. Introduction
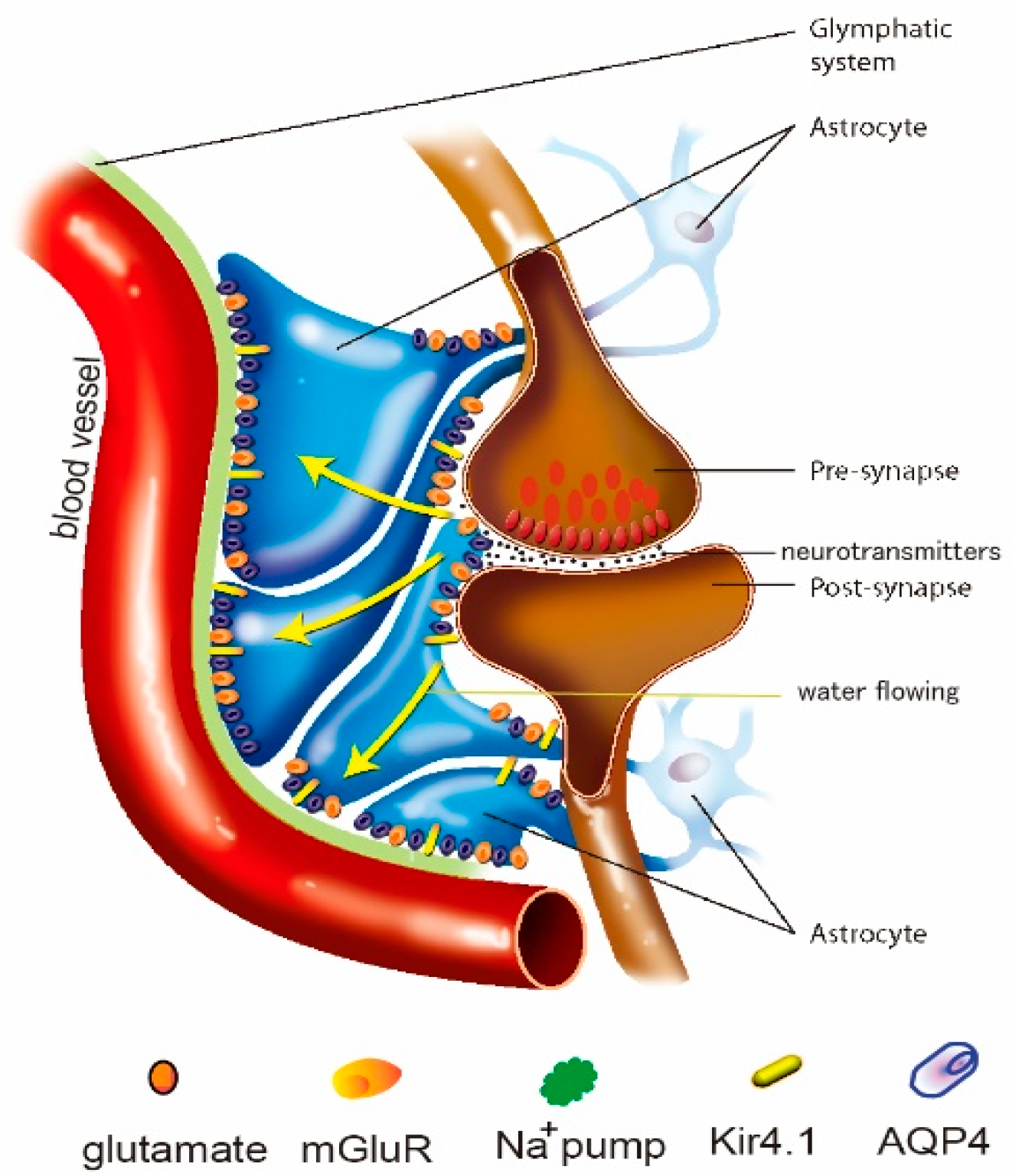
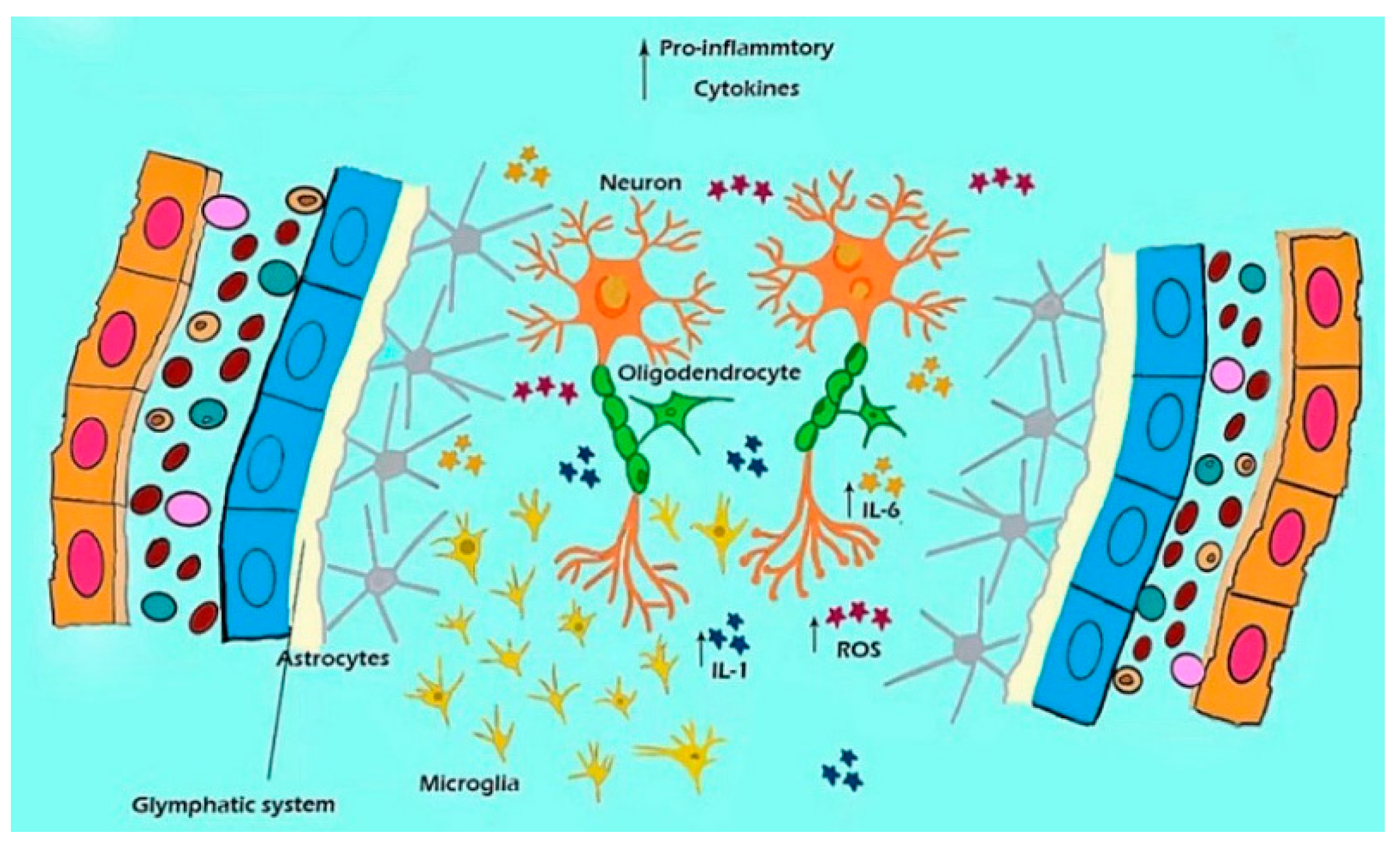
2. The Glymphatic System
2.1. Clearance Function of the Glymphatic System
2.2. Glymphatic System Depends on the Polarization of AQP4
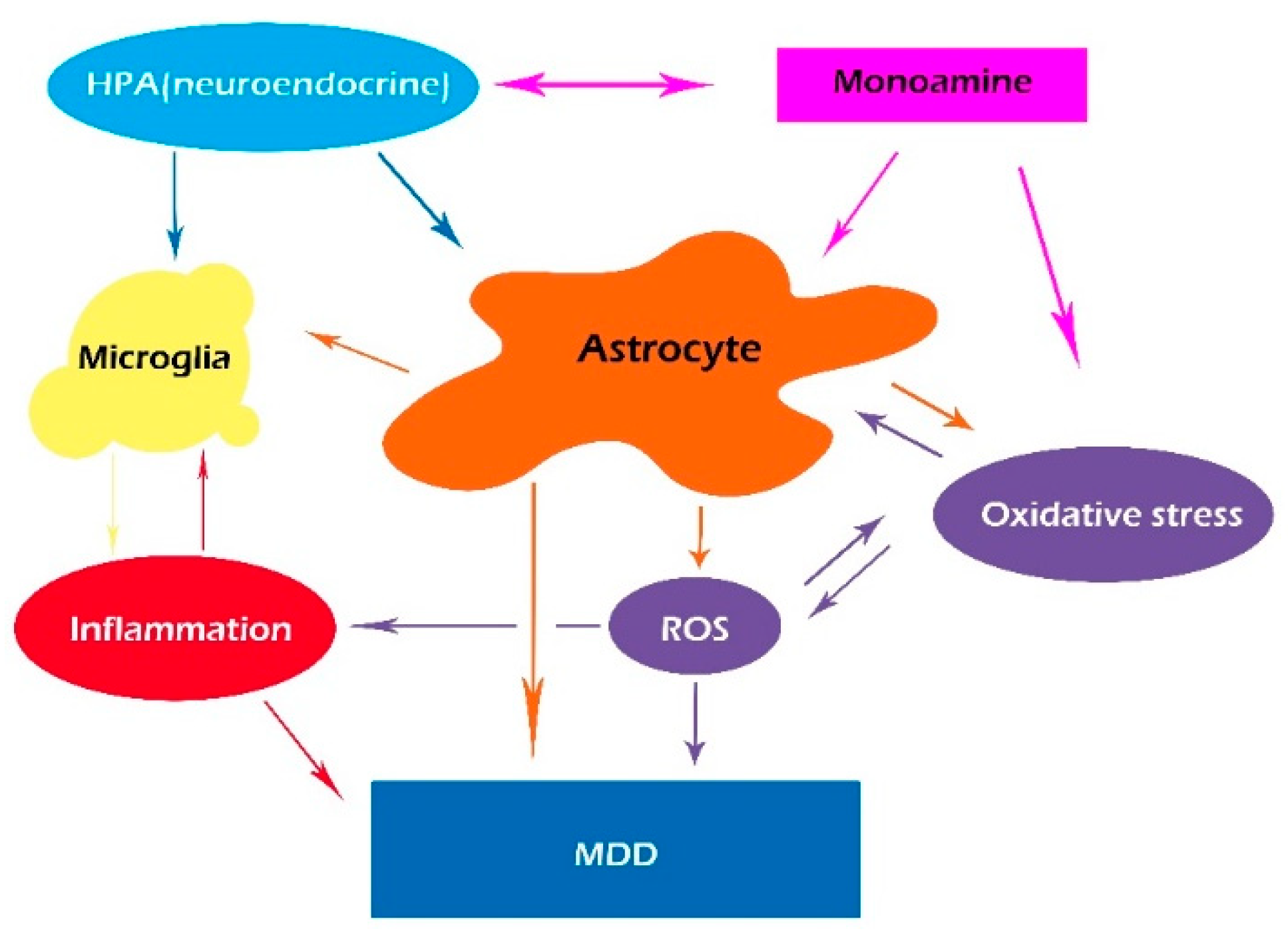
3. ROS and Inflammation
3.1. Reactive Oxygen Species
3.2. Brain-Gut Axis and ROS
3.3. ROS, Glia, and Inflammation
4. Monoamines and the Glymphatic System
5. Astrocytic Ion Channels and the Glymphatic System
6. Glymphatic System and Microglia
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Arioz, B.I.; Tastan, B.; Tarakcioglu, E.; Tufekci, K.U.; Olcum, M.; Ersoy, N.; Bagriyanik, A.; Genc, K.; Genc, S. Melatonin attenuates LPS-induced acute depressive-like behaviors and microglial NLRP3 inflammasome activation through the SIRT1/Nrf2 pathway. Front. Immunol. 2019, 10, 1511. [Google Scholar] [CrossRef] [PubMed]
- Dean, J.; Keshavan, M. The neurobiology of depression: An integrated view. Asian J. Psychiatr. 2017, 27, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Lindqvist, D.; Dhabhar, F.S.; James, S.J.; Hough, C.M.; Jain, F.A.; Bersani, F.S.; Reus, V.I.; Verhoeven, J.E.; Epel, E.S.; Mahan, L.; et al. Oxidative stress, inflammation and treatment response in major depression. Psychoneuroendocrinology 2017, 76, 197–205. [Google Scholar] [CrossRef]
- Rawdin, B.J.; Mellon, S.H.; Dhabhar, F.S.; Epel, E.S.; Puterman, E.; Su, Y.; Burke, H.M.; Reus, V.I.; Rosser, R.; Hamilton, S.P.; et al. Dysregulated relationship of inflammation and oxidative stress in major depression. Brain Behav. Immun. 2013, 31, 143–152. [Google Scholar] [CrossRef]
- Di, L.F.; Kaludercic, N.; Carpi, A.; Menabò, R.; Giorgio, M. Mitochondrial pathways for ROS formation and myocardial injury: The relevance of p66(Shc) and monoamine oxidase. Basic Res. Cardiol. 2009, 104, 131–139. [Google Scholar] [CrossRef]
- Wang, F.; Pan, F.; Tang, Y.Y.; Huang, J.H. Editorial: Uncertainty induced emotional disorders during the COVID-19. Front. Psychol. 2022, 13, 943966. [Google Scholar] [CrossRef] [PubMed]
- Willems, P.H.; Rossignol, R.; Dieteren, C.E.; Murphy, M.P.; Koopman, W.J. Redox homeostasis and mitochondrial dynamics. Cell Metab. 2015, 22, 207–218. [Google Scholar] [CrossRef]
- Bhatt, S.; Nagappa, A.N.; Patil, C.R. Role of oxidative stress in depression. Drug Discov. Today 2020, 25, 1270–1276. [Google Scholar] [CrossRef]
- Yuan, T.F.; Gu, S.; Shan, C.; Marchado, S.; Arias-Carrión, O. Oxidative stress and adult neurogenesis. Stem Cell Rev. Rep. 2015, 11, 706–709. [Google Scholar] [CrossRef]
- Rubinsztein, D.C. The roles of intracellular protein-degradation pathways in neurodegeneration. Nature 2006, 443, 780–786. [Google Scholar] [CrossRef]
- Iliff, J.J.; Goldman, S.A.; Nedergaard, M. Implications of the discovery of brain lymphatic pathways. Lancet Neurol. 2015, 14, 977–979. [Google Scholar] [CrossRef]
- Heo, C.M.; Lee, D.A.; Park, K.M.; Lee, Y.J.; Park, S.; Kim, Y.W.; Ko, J.; Yoo, B.C.; Park, B.S. Glymphatic system dysfunction in patients with early chronic kidney disease. Front. Neurol. 2022, 13, 976089. [Google Scholar] [CrossRef] [PubMed]
- Manouchehrian, O.; Ramos, M.; Bachiller, S.; Lundgaard, I.; Deierborg, T. Acute systemic LPS-exposure impairs perivascular CSF distribution in mice. J. Neuroinflammation 2021, 18, 34. [Google Scholar] [CrossRef] [PubMed]
- Beurel, E.; Toups, M.; Nemeroff, C.B. The bidirectional relationship of depression and inflammation: Double trouble. Neuron 2020, 107, 234–256. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, M.K.; Mestre, H.; Nedergaard, M. The glymphatic pathway in neurological disorders. Lancet Neurol. 2018, 17, 1016–1024. [Google Scholar] [CrossRef]
- Li, X.; Wen, W.; Li, P.; Fu, Y.; Chen, H.; Wang, F.; Dai, Y.; Xu, S. Mitochondrial protection and against glutamate neurotoxicity via Shh/Ptch1 signaling pathway to ameliorate cognitive dysfunction by Kaixin San in multi-infarct dementia rats. Oxidative Med. Cell. Longev. 2021, 2021, 5590745. [Google Scholar] [CrossRef]
- Louveau, A.; Smirnov, I.; Keyes, T.J.; Eccles, J.D.; Rouhani, S.J.; Peske, J.D.; Derecki, N.C.; Castle, D.; Mandell, J.W.; Lee, K.S.; et al. Structural and functional features of central nervous system lymphatic vessels. Nature 2015, 523, 337–341. [Google Scholar] [CrossRef]
- Mogensen, F.L.; Delle, C.; Nedergaard, M. The glymphatic dystem (En) during inflammation. Int. J. Mol. Sci. 2021, 22, 7491. [Google Scholar] [CrossRef]
- Kwon, H.S.; Koh, S.H. Neuroinflammation in neurodegenerative disorders: The roles of microglia and astrocytes. Transl. Neurodegener. 2020, 9, 42. [Google Scholar] [CrossRef]
- Nedergaard, M.; Goldman, S.A. Glymphatic failure as a final common pathway to dementia. Science 2020, 370, 50–56. [Google Scholar] [CrossRef]
- Hablitz, L.M.; Nedergaard, M. The glymphatic system: A novel component of fundamental neurobiology. J. Neurosci. 2021, 41, 7698–7711. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, S.; Thimma Reddygari, J.; Selvaraj, D. G-lymphatic, vascular and immune pathways for Aβ clearance cascade and therapeutic targets for Alzheimer’s disease. Comb. Chem. High Throughput Screen 2021, 24, 1083–1092. [Google Scholar] [CrossRef] [PubMed]
- Harrison, I.F.; Ismail, O.; Machhada, A.; Colgan, N.; Ohene, Y.; Nahavandi, P.; Ahmed, Z.; Fisher, A.; Meftah, S.; Murray, T.K.; et al. Impaired glymphatic function and clearance of tau in an Alzheimer’s disease model. Brain 2020, 143, 2576–2593. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Sun, Y.; Song, M.; Song, Y.; Fang, Y.; Zhang, Q.; Li, X.; Song, N.; Ding, J.; Lu, M.; et al. NLRP3/caspase-1/GSDMD-mediated pyroptosis exerts a crucial role in astrocyte pathological injury in mouse model of depression. JCI Insight 2021, 6, e146852. [Google Scholar] [CrossRef]
- Schneider, A.; Mandelkow, E. Tau-based treatment strategies in neurodegenerative diseases. Neurotherapeutics 2008, 5, 443–457. [Google Scholar] [CrossRef]
- Kumamoto, T.; Tsurugizawa, T. Potential of multiscale astrocyte Iimaging for revealing mechanisms underlying neurodevelopmental disorders. Int. J. Mol. Sci. 2021, 22, 10312. [Google Scholar] [CrossRef]
- Wang, F.; Smith, N.A.; Xu, Q.; Fujita, T.; Baba, A.; Matsuda, T.; Takano, T.; Bekar, L.; Nedergaard, M. Astrocytes modulate neural network activity by Ca²+-dependent uptake of extracellular K+. Sci. Signal. 2012, 5, ra26. [Google Scholar] [CrossRef]
- Lan, Y.L.; Chen, J.J.; Hu, G.; Xu, J.; Xiao, M.; Li, S. Aquaporin 4 in astrocytes is a target for therapy in Alzheimer’s disease. Curr. Pharm. Des. 2017, 23, 4948–4957. [Google Scholar] [CrossRef]
- Vandebroek, A.; Yasui, M. Regulation of AQP4 in the central nervous system. Int. J. Mol. Sci. 2020, 21, 1603. [Google Scholar] [CrossRef]
- Singla, B.; Aithabathula, R.V.; Kiran, S.; Kapil, S.; Kumar, S.; Singh, U.P. Reactive oxygen species in regulating lymphangiogenesis and lymphatic function. Cells 2022, 11, 1750. [Google Scholar] [CrossRef]
- Semwal, M.K.; Jones, N.E.; Griffith, A.V. Metabolic regulation of thymic epithelial cell function. Front. Immunol. 2021, 12, 636072. [Google Scholar] [CrossRef] [PubMed]
- Gallina, P.; Nicoletti, C.; Scollato, A.; Lolli, F. The “glymphatic-lymphatic system pathology” and a new categorization of neurodegenerative disorders. Front. Neurosci. 2021, 15, 669681. [Google Scholar] [CrossRef]
- Liu, X.; Wu, G.; Tang, N.; Li, L.; Liu, C.; Wang, F.; Ke, S. Glymphatic drainage blocking aggravates brain edema, neuroinflammation via modulating TNF-α, IL-10, and AQP4 after intracerebral hemorrhage in rats. Front. Cell Neurosci. 2021, 15, 784154. [Google Scholar] [CrossRef] [PubMed]
- Hsu, S.J.; Zhang, C.; Jeong, J.; Lee, S.I.; McConnell, M.; Utsumi, T.; Iwakiri, Y. Enhanced meningeal lymphatic drainage ameliorates neuroinflammation and hepatic encephalopathy in cirrhotic rats. Gastroenterology 2021, 160, 1315–1329.e13. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Cao, C.; Xu, Q.; Gu, S.; Wang, F.; Huang, X.; Xu, S.; Wu, E.; Huang, J.H. Piperine attenuates TBI-induced seizures via inhibiting Cytokine-activated reactive astrogliosis. Front. Neurol. 2020, 11, 431. [Google Scholar] [CrossRef] [PubMed]
- Salman, M.M.; Kitchen, P.; Halsey, A.; Wang, M.X.; Törnroth-Horsefield, S.; Conner, A.C.; Badaut, J.; Iliff, J.J.; Bill, R.M. Emerging roles for dynamic aquaporin-4 subcellular relocalization in CNS water homeostasis. Brain 2022, 145, 64–75. [Google Scholar] [CrossRef]
- Mestre, H.; Hablitz, L.M.; Xavier, A.L.; Feng, W.; Zou, W.; Pu, T.; Monai, H.; Murlidharan, G.; Castellanos Rivera, R.M.; Simon, M.J.; et al. Aquaporin-4-dependent glymphatic solute transport in the rodent brain. Elife 2018, 7, e40070. [Google Scholar] [CrossRef]
- Wu, J.; Carlock, C.; Shim, J.; Moreno-Gonzalez, I.; Glass, W., 2nd; Ross, A.; Barichello, T.; Quevedo, J.; Lou, Y. Requirement of brain interleukin33 for aquaporin4 expression in astrocytes and glymphatic drainage of abnormal tau. Mol. Psychiatry 2021, 26, 5912–5924. [Google Scholar] [CrossRef]
- Kress, B.T.; Iliff, J.J.; Xia, M.; Wang, M.; Wei, H.S.; Zeppenfeld, D.; Xie, L.; Kang, H.; Xu, Q.; Liew, J.A.; et al. Impairment of paravascular clearance pathways in the aging brain. Ann. Neurol. 2014, 76, 845–861. [Google Scholar] [CrossRef]
- Thrane, A.S.; Rappold, P.M.; Fujita, T.; Torres, A.; Bekar, L.K.; Takano, T.; Peng, W.; Wang, F.; Rangroo Thrane, V.; Enger, R.; et al. Pivotal role of aquaporin-4 (AQP4) in astrocytic Ca2+ signaling events elicited by cerebral edema. Proc. Natl. Acad. Sci. USA 2011, 108, 846–851. [Google Scholar] [CrossRef]
- Fan, Y.; Zhang, J.; Sun, X.L.; Gao, L.; Zeng, X.N.; Ding, J.H.; Cao, C.; Niu, L.; Hu, G. Sex- and region-specific alterations of basal amino acid and monoamine metabolism in the brain of aquaporin-4 knockout mice. J. Neurosci. Res. 2005, 82, 458–464. [Google Scholar] [CrossRef]
- de Melo, L.; Nunes, S.; Anderson, G.; Vargas, H.O.; Barbosa, D.S.; Galecki, P.; Carvalho, A.F.; Maes, M. Shared metabolic and immune-inflammatory, oxidative and nitrosative stress pathways in the metabolic syndrome and mood disorders. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2017, 78, 34–50. [Google Scholar] [CrossRef] [PubMed]
- Roomruangwong, C.; Barbosa, D.S.; Matsumoto, A.K.; Nogueira, A.S.; Kanchanatawan, B.; Sirivichayakul, S.; Carvalho, A.F.; Duleu, S.; Geffard, M.; Moreira, E.G.; et al. Activated neuro-oxidative and neuro-nitrosative pathways at the end of term are associated with inflammation and physio-somatic and depression symptoms: While predicting outcome characteristics in mother and baby. J. Affect. Disord. 2017, 223, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Sanada, K.; Nakajima, S.; Kurokawa, S.; Barceló-Soler, A.; Ikuse, D.; Hirata, A.; Yoshizawa, A.; Tomizawa, Y.; Salas-Valero, M.; Noda, Y.; et al. Gut microbiota and major depressive disorder: A systematic review and meta-analysis. J. Affect. Disord. 2020, 266, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Kepp, O.; Galluzzi, L.; Zitvogel, L.; Kroemer, G. Pyroptosis-A cell death modality of its kind? Eur. J. Immunol. 2010, 40, 627–630. [Google Scholar] [CrossRef] [PubMed]
- Rainville, J.R.; Hodes, G.E. Inflaming sex differences in mood disorders. Neuropsychopharmacology 2019, 44, 184–199. [Google Scholar] [CrossRef]
- Yan, T.; Qiu, Y.; Yu, X.; Yang, L. Glymphatic dysfunction: A bridge between sleep disturbance and mood disorders. Front. Psychiatry 2022, 2, 658340. [Google Scholar] [CrossRef] [PubMed]
- Poljsak, B.; Šuput, D.; Milisav, I. Achieving the balance between ROS and antioxidants: When to use the synthetic antioxidants. Oxidative Med. Cell. Longev. 2013, 2013, 956792. [Google Scholar] [CrossRef]
- Roomruangwong, C.; Anderson, G.; Berk, M.; Stoyanov, D.; Carvalho, A.F.; Maes, M. A neuro-immune, neuro-oxidative and neuro-nitrosative model of prenatal and postpartum depression. Prog. Neuropsychopharmacol. Biol. Psychiatry 2018, 81, 262–274. [Google Scholar] [CrossRef]
- Christensen, J.; Yamakawa, G.R.; Shultz, S.R.; Mychasiuk, R. Is the glymphatic system the missing link between sleep impairments and neurological disorders? Examining the implications and uncertainties. Prog. Neurobiol. 2021, 198, 101917. [Google Scholar] [CrossRef]
- Liu, Y.; Feng, S.; Subedi, K.; Wang, H. Attenuation of ischemic stroke-caused brain injury by a monoamine oxidase inhibitor involves improved proteostasis and reduced neuroinflammation. Mol. Neurobiol. 2020, 57, 937–948. [Google Scholar] [CrossRef] [PubMed]
- Franchi, L.; Muñoz-Planillo, R.; Núñez, G. Sensing and reacting to microbes through the inflammasomes. Nat. Immunol. 2012, 13, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Priyanka, H.P.; Singh, R.V.; Mishra, M.; ThyagaRajan, S. Diverse age-related effects of Bacopa monnieri and donepezil in vitro on cytokine production, antioxidant enzyme activities, and intracellular targets in splenocytes of F344 male rats. Int. Immunopharmacol. 2013, 15, 260–274. [Google Scholar] [CrossRef] [PubMed]
- Maes, M.; Galecki, P.; Chang, Y.S.; Berk, M. A review on the oxidative and nitrosative stress (O&NS) pathways in major depression and their possible contribution to the (neuro)degenerative processes in that illness. Prog. Neuropsychopharmacol. Biol. Psychiatry 2011, 35, 676–692. [Google Scholar] [CrossRef] [PubMed]
- Estes, M.L.; McAllister, A.K. Maternal immune activation: Implications for neuropsychiatric disorders. Science 2016, 353, 772–777. [Google Scholar] [CrossRef]
- Valles-Colomer, M.; Falony, G.; Darzi, Y.; Tigchelaar, E.F.; Wang, J.; Tito, R.Y.; Schiweck, C.; Kurilshikov, A.; Joossens, M.; Wijmenga, C.; et al. The neuroactive potential of the human gut microbiota in quality of life and depression. Nat. Microbiol. 2019, 4, 623–632. [Google Scholar] [CrossRef]
- Riveros, M.E.; Ávila, A.; Schruers, K.; Ezquer, F. antioxidant biomolecules and their potential for the treatment of difficult-to-treat depression and conventional treatment-resistant depression. Antioxidants 2022, 11, 540. [Google Scholar] [CrossRef]
- Konturek, P.C.; Konturek, K.; Brzozowski, T.; Wojcik, D.; Magierowski, M.; Targosz, A.; Krzysiek-Maczka, G.; Sliwowski, Z.; Strzalka, M.; Magierowska, K.; et al. Participation of the intestinal microbiota in the mechanism of beneficial effect of treatment with synbiotic Syngut on experimental colitis under stress conditions. J. Physiol. Pharmacol. 2020, 71, 329–342. [Google Scholar] [CrossRef]
- Ayala, A.; Muñoz, M.F.; Argüelles, S. Lipid peroxidation: Production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxidative Med. Cell. Longev. 2014, 2014, 360438. [Google Scholar] [CrossRef]
- Bastiaanssen, T.F.S.; Cussotto, S.; Claesson, M.J.; Clarke, G.; Dinan, T.G.; Cryan, J.F. Gutted! Unraveling the role of the microbiome in major depressive disorder. Harv. Rev. Psychiatry 2020, 28, 26–39. [Google Scholar] [CrossRef]
- Manosso, L.M.; Lin, J.; Carlessi, A.S.; Recco, K.C.C.; Quevedo, J.; Gonçalves, C.L.; Réus, G.Z. Sex-related patterns of the gut-microbiota-brain axis in the neuropsychiatric conditions. Brain Res. Bull. 2021, 171, 196–208. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Jiang, M.; Gu, S.; Zhang, X.; Feng, G.; Ma, X.; Xu, S.; Wu, E.; Huang, J.H.; Wang, F. Metabolomics changes in brain-gut axis after unpredictable chronic mild stress. Psychopharmacology 2022, 239, 729–743. [Google Scholar] [CrossRef] [PubMed]
- Sanmarco, L.M.; Wheeler, M.A.; Gutiérrez-Vázquez, C.; Polonio, C.M.; Linnerbauer, M.; Pinho-Ribeiro, F.A.; Li, Z.; Giovannoni, F.; Batterman, K.V.; Scalisi, G.; et al. Gut-licensed IFNγ+ NK cells drive LAMP1+TRAIL+ anti-inflammatory astrocytes. Nature 2021, 590, 473–479. [Google Scholar] [CrossRef]
- Simpson, D.; Oliver, P.L. ROS generation in microglia: Understanding oxidative stress and inflammation in neurodegenerative disease. Antioxidants 2020, 9, 743. [Google Scholar] [CrossRef] [PubMed]
- Hinwood, M.; Morandini, J.; Day, T.A.; Walker, F.R. Evidence that microglia mediate the neurobiological effects of chronic psychological stress on the medial prefrontal cortex. Cereb. Cortex 2012, 22, 1442–1454. [Google Scholar] [CrossRef]
- Iwata, M.; Ota, K.T.; Li, X.Y.; Sakaue, F.; Li, N.; Dutheil, S.; Banasr, M.; Duric, V.; Yamanashi, T.; Kaneko, K.; et al. Psychological stress activates the inflammasome via release of adenosine triphosphate and stimulation of the purinergic type 2X7 receptor. Biol. Psychiatry 2016, 80, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Wilms, H.; Sievers, J.; Rickert, U.; Rostami-Yazdi, M.; Mrowietz, U.; Lucius, R. Dimethylfumarate inhibits microglial and astrocytic inflammation by suppressing the synthesis of nitric oxide, IL-1beta, TNF-alpha and IL-6 in an in-vitro model of brain inflammation. J. Neuroinflammation 2010, 7, 30. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.; Sahu, A.; Prabhakar, A.; Chatterjee, T.; Tyagi, T.; Kumari, B.; Khan, N.; Nair, V.; Bajaj, N.; Sharma, M.; et al. Activation of NLRP3 inflammasome complex potentiates venous thrombosis in response to hypoxia. Proc. Natl. Acad. Sci. USA 2017, 114, 4763–4768. [Google Scholar] [CrossRef] [PubMed]
- Yirmiya, R.; Rimmerman, N.; Reshef, R. Depression as a microglial disease. Trends. Neurosci. 2015, 38, 637–658. [Google Scholar] [CrossRef]
- Müller, N.; Schwarz, M.J. The immune-mediated alteration of 5-HT and glutamate: Towards an integrated view of depression. Mol. Psychiatry 2007, 12, 988–1000. [Google Scholar] [CrossRef]
- Sun, X.L.; Ding, J.H.; Fan, Y.; Zhang, J.; Gao, L.; Hu, G. Aquaporin 4 regulates the effects of ovarian hormones on monoamine neurotransmission. Biochem. Biophys. Res. Commun. 2007, 353, 457–462. [Google Scholar] [CrossRef] [PubMed]
- Gu, S.; Wang, W.; Wang, F.; Huang, J.H. Neuromodulator and emotion biomarker for stress induced mental disorders. Neural. Plast. 2016, 2016, 2609128. [Google Scholar] [CrossRef]
- Jiang, Y.; Zou, D.; Li, Y.; Gu, S.; Dong, J.; Ma, X.; Xu, S.; Wang, F.; Huang, J.H. Monoamine Neurotransmitters Control Basic Emotions and Affect Major Depressive Disorders. Pharmaceuticals 2022, 15, 1203. [Google Scholar] [CrossRef]
- Gu, S.; Wang, F.; Patel, N.P.; Bourgeois, J.A.; Huang, J.H. A model for basic emotions using observations of behavior in drosophila. Front. Psychol. 2019, 10, 781. [Google Scholar] [CrossRef] [PubMed]
- Gu, S.; He, Z.; Xu, Q.; Dong, J.; Xiao, T.; Liang, F.; Ma, X.; Wang, F.; Huang, J.H. The relationship between 5-hydroxytryptamine and its metabolite changes with post-stroke depression. Front. Psychiatry 2020, 13, 871754. [Google Scholar] [CrossRef]
- Wang, F.; Smith, N.A.; Xu, Q.; Goldman, S.; Peng, W.; Huang, J.H.; Takano, T.; Nedergaard, M. Photolysis of caged Ca2+ but not receptor-mediated Ca2+ signaling triggers astrocytic glutamate release. J. Neurosci. Off. J. Soc. Neurosci. 2012, 33, 17404–17412. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wotton, C.A.; Quon, E.F.; Palmer, A.C.; Bekar, L.K. Corticosterone and 5-HT similarly influence GABAergic and purinergic pathways to affect cortical inhibitory networks. J. Neuroendocrinol. 2018, 30, e12592. [Google Scholar] [CrossRef] [PubMed]
- Ding, F.; O’Donnell, J.; Thrane, A.S.; Zeppenfeld, D.; Kang, H.; Xie, L.; Wang, F.; Nedergaard, M. α1-Adrenergic receptors mediate coordinated Ca2+ signaling of cortical astrocytes in awake, behaving mice. Cell Calcium 2013, 54, 387–394. [Google Scholar] [CrossRef]
- Ding, F.; O’Donnell, J.; Xu, Q.; Kang, N.; Goldman, N.; Nedergaard, M. Changes in the composition of brain interstitial ions control the sleep-wake cycle. Science 2016, 352, 550–555. [Google Scholar] [CrossRef]
- Bola, R.A.; Kiyatkin, E.A. Inflow of oxygen and glucose in brain tissue induced by intravenous NE: Relationships with central metabolic and peripheral vascular responses. J. Neurophysiol. 2018, 119, 499–508. [Google Scholar] [CrossRef]
- Goldman, N.; Hablitz, L.M.; Mori, Y.; Nedergaard, M. The glymphatic system and pain. Med. Acupunct. 2020, 32, 373–376. [Google Scholar] [CrossRef] [PubMed]
- Sugama, S.; Kakinuma, Y. Noradrenaline as a key neurotransmitter in modulating microglial activation in stress response. Neurochem. Int. 2021, 143, 104943. [Google Scholar] [CrossRef] [PubMed]
- Saller, S.; Merz-Lange, J.; Raffael, S.; Hecht, S.; Pavlik, R.; Thaler, C.; Berg, D.; Berg, U.; Kunz, L.; Mayerhofer, A. Norepinephrine, active norepinephrine transporter, and norepinephrine-metabolism are involved in the generation of reactive oxygen species in human ovarian granulosa cells. Endocrinology 2012, 153, 1472–1483. [Google Scholar] [CrossRef]
- Chen, Y.; Feng, X.; Hu, X.; Sha, J.; Li, B.; Zhang, H.; Fan, H. dexmedetomidine ameliorates acute stress-induced kidney injury by attenuating oxidative stress and apoptosis through inhibition of the ROS/JNK signaling pathway. Oxidative Med. Cell. Longev. 2018, 2018, 4035310. [Google Scholar] [CrossRef]
- Hu, H. Reward and aversion. Annu. Rev. Neurosci. 2016, 39, 297–324. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Cui, Y.; Sang, K.; Dong, Y.; Ni, Z.; Ma, S.; Hu, H. Ketamine blocks bursting in the lateral habenula to rapidly relieve depression. Nature 2018, 554, 317–322. [Google Scholar] [CrossRef]
- Corkrum, M.; Araque, A. Astrocyte-neuron signaling in the mesolimbic DA system: The hidden stars of DA signaling. Neuropsychopharmacology 2021, 46, 1864–1872. [Google Scholar] [CrossRef]
- Fuxe, K.; Agnati, L.F.; Marcoli, M.; Borroto-Escuela, D.O. volume transmission in central dopamine and noradrenaline neurons and its astroglial targets. Neurochem. Res. 2015, 40, 2600–2614. [Google Scholar] [CrossRef]
- Fullana, N.; Gasull-Camós, J.; Tarrés-Gatius, M.; Castañé, A.; Bortolozzi, A.; Artigas, F. Astrocyte control of glutamatergic activity: Downstream effects on serotonergic function and emotional behavior. Neuropharmacology 2020, 166, 107914. [Google Scholar] [CrossRef]
- Schipke, C.G.; Heuser, I.; Peters, O. Antidepressants act on glial cells: SSRIs and serotonin elicit astrocyte calcium signaling in the mouse prefrontal cortex. J. Psychiatr. Res. 2011, 45, 242–248. [Google Scholar] [CrossRef]
- Müller, F.E.; Schade, S.K.; Cherkas, V.; Stopper, L.; Breithausen, B.; Minge, D.; Varbanov, H.; Wahl-Schott, C.; Antoniuk, S.; Domingos, C.; et al. 5-HT receptor 4 regulates hippocampal astrocyte morphology and function. Glia 2021, 69, 872–889. [Google Scholar] [CrossRef] [PubMed]
- Quon, E.F.; Wotton, C.A.; Bekar, L.K. Evidence for astrocyte purinergic signaling in cortical sensory adaptation and 5-HT-mediated neuromodulation. Mol. Cell Neurosci. 2018, 88, 53–61. [Google Scholar] [CrossRef]
- Azizi, H.; Hwang, J.; Suen, V.; Kang, N.; Somvanshi, R.K.; Tadavarty, R.; Kumar, U.; Sastry, B.S. Sleep deprivation induces changes in 5-HT actions and 5-HT1A receptor expression in the rat hippocampus. Neurosci. Lett. 2017, 655, 151–155. [Google Scholar] [CrossRef] [PubMed]
- Barnes, N.M.; Sharp, T. A review of central 5-HT receptors and their function. Neuropharmacology 1999, 38, 1083–1152. [Google Scholar] [CrossRef]
- Gul, S.; Saleem, D.; Haleem, M.A.; Haleem, D.J. Inhibition of hormonal and behavioral effects of stress by tryptophan in rats. Nutr. Neurosci. 2019, 22, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Pattij, T.; Schoffelmeer, A.N. Serotonin and inhibitory response control: Focusing on the role of 5-HT(1A) receptors. Eur. J. Pharmacol. 2015, 753, 140–145. [Google Scholar] [CrossRef] [PubMed]
- Catena-Dell’Osso, M.; Rotella, F.; Dell’Osso, A.; Fagiolini, A.; Marazziti, D. Inflammation, 5-HT and major depression. Curr. Drug. Targets 2013, 14, 571–577. [Google Scholar] [CrossRef]
- Talaei, F.; Bouma, H.R.; Van der Graaf, A.C.; Strijkstra, A.M.; Schmidt, M.; Henning, R.H. Serotonin and dopamine protect from hypothermia/rewarming damage through the CBS/H2S pathway. PLoS ONE 2011, 6, e22568. [Google Scholar] [CrossRef]
- Rajkowska, G.; Hughes, J.; Stockmeier, C.A.; Javier Miguel-Hidalgo, J.; Maciag, D. Coverage of blood vessels by astrocytic endfeet is reduced in major depressive disorder. Biol. Psychiatry 2013, 73, 613–621. [Google Scholar] [CrossRef]
- Di Benedetto, B.; Malik, V.A.; Begum, S.; Jablonowski, L.; Gómez-González, G.B.; Neumann, I.D.; Rupprecht, R. fluoxetine requires the endfeet protein aquaporin-4 to enhance plasticity of astrocyte processes. Front. Cell Neurosci. 2016, 10, 8. [Google Scholar] [CrossRef]
- Wallensten, J.; Nager, A.; Åsberg, M.; Borg, K.; Beser, A.; Wilczek, A.; Mobarrez, F. Leakage of astrocyte-derived extracellular vesicles in stress-induced exhaustion disorder: A cross-sectional study. Sci. Rep. 2021, 11, 2009. [Google Scholar] [CrossRef] [PubMed]
- Wagner, S.; Rokita, A.G.; Anderson, M.E.; Maier, L.S. Redox regulation of sodium and calcium handling. Antioxid. Redox. Signal 2013, 18, 1063–1077. [Google Scholar] [CrossRef] [PubMed]
- Harper, A.G.; Mason, M.J.; Sage, S.O. A key role for dense granule secretion in potentiation of the Ca2+ signal arising from store-operated calcium entry in human platelets. Cell Calcium 2009, 45, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Dada, L.A.; Chandel, N.S.; Ridge, K.M.; Pedemonte, C.; Bertorello, A.M.; Sznajder, J.I. Hypoxia-induced endocytosis of Na, K-ATPase in alveolar epithelial cells is mediated by mitochondrial reactive oxygen species and PKC-zeta. J. Clin. Investig. 2003, 111, 1057–1064. [Google Scholar] [CrossRef]
- Blaustein, M.P.; Hamlyn, J.M. Ouabain, endogenous ouabain and ouabain-like factors: The Na+ pump/ouabain receptor, its linkage to NCX, and its myriad functions. Cell Calcium 2020, 86, 102159. [Google Scholar] [CrossRef] [PubMed]
- Andrikopoulos, P.; Kieswich, J.; Harwood, S.M.; Baba, A.; Matsuda, T.; Barbeau, O.; Jones, K.; Eccles, S.A.; Yaqoob, M.M. endothelial angiogenesis and barrier function in response to thrombin require Ca2+ influx through the Na+/Ca2+ exchanger. J. Biol. Chem. 2015, 290, 18412–18428. [Google Scholar] [CrossRef] [PubMed]
- Larsen, B.R.; Assentoft, M.; Cotrina, M.L.; Hua, S.Z.; Nedergaard, M.; Kaila, K.; Voipio, J.; MacAulay, N. Contributions of the Na⁺/K⁺-ATPase, NKCC1, and Kir4.1 to hippocampal K⁺ clearance and volume responses. Glia 2014, 62, 608–622. [Google Scholar] [CrossRef]
- MacAulay, N. Molecular mechanisms of K+ clearance and extracellular space shrinkage-Glia cells as the stars. Glia 2020, 68, 2192–2211. [Google Scholar] [CrossRef] [PubMed]
- Sampieri, R.; Fuentes, E.; Carrillo, E.D.; Hernández, A.; García, M.C.; Sánchez, J.A. pharmacological preconditioning using diazoxide regulates store-operated Ca2+ Channels in adult rat cardiomyocytes. Front. Physiol. 2020, 10, 1589. [Google Scholar] [CrossRef]
- Fischer, T.; Prey, J.; Eschholz, L.; Rotermund, N.; Lohr, C. NE-Induced calcium signaling and store-operated calcium entry in olfactory bulb astrocytes. Front. Cell Neurosci. 2021, 15, 639754. [Google Scholar] [CrossRef]
- Zhou, X.; Li, Y.; Lenahan, C.; Ou, Y.; Wang, M.; He, Y. glymphatic system in the central nervous system, a novel therapeutic direction against brain edema after stroke. Front. Aging Neurosci. 2021, 13, 698036. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, J.; You, Z. Switching of the microglial activation phenotype is a possible treatment for depression disorder. Front. Cell Neurosci. 2018, 12, 306. [Google Scholar] [CrossRef] [PubMed]
- Corcoran, S.E.; O’Neill, L.A. HIF1α and metabolic reprogramming in inflammation. J. Clin. Investig. 2016, 126, 3699–3707. [Google Scholar] [CrossRef] [PubMed]
- Fava, M.; Hwang, I.; Rush, A.J.; Sampson, N.; Walters, E.E.; Kessler, R.C. The importance of irritability as a symptom of major depressive disorder: Results from the National Comorbidity Survey Replication. Mol. Psychiatry 2010, 15, 856–867. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, S.H. Core symptoms of major depressive disorder: Relevance to diagnosis and treatment. Dialogues Clin. Neurosci. 2008, 10, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Munk, A.S.; Wang, W.; Bèchet, N.B.; Eltanahy, A.M.; Cheng, A.X.; Sigurdsson, B.; Benraiss, A.; Mäe, M.A.; Kress, B.T.; Kelley, D.H.; et al. PDGF-B is required for development of the glymphatic system. Cell Rep. 2019, 26, 2955–2969. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gu, S.; Li, Y.; Jiang, Y.; Huang, J.H.; Wang, F. Glymphatic Dysfunction Induced Oxidative Stress and Neuro-Inflammation in Major Depression Disorders. Antioxidants 2022, 11, 2296. https://doi.org/10.3390/antiox11112296
Gu S, Li Y, Jiang Y, Huang JH, Wang F. Glymphatic Dysfunction Induced Oxidative Stress and Neuro-Inflammation in Major Depression Disorders. Antioxidants. 2022; 11(11):2296. https://doi.org/10.3390/antiox11112296
Chicago/Turabian StyleGu, Simeng, Yumeng Li, Yao Jiang, Jason H. Huang, and Fushun Wang. 2022. "Glymphatic Dysfunction Induced Oxidative Stress and Neuro-Inflammation in Major Depression Disorders" Antioxidants 11, no. 11: 2296. https://doi.org/10.3390/antiox11112296
APA StyleGu, S., Li, Y., Jiang, Y., Huang, J. H., & Wang, F. (2022). Glymphatic Dysfunction Induced Oxidative Stress and Neuro-Inflammation in Major Depression Disorders. Antioxidants, 11(11), 2296. https://doi.org/10.3390/antiox11112296










