Conventional vs. Green Extraction Using Natural Deep Eutectic Solvents—Differences in the Composition of Soluble Unbound Phenolic Compounds and Antioxidant Activity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Reagents
2.2. Preparation of NaDES
2.3. Extraction
2.4. Total Phenolic Content Determination
2.5. Total Flavonoid Content Determination
2.6. HPLC Analysis
2.7. Antioxidant Activity Determination
2.7.1. DPPH Radical Scavenging Activity
- AS was absorbance of test solution treated with DPPH radical solution;
- AC was absorbance of control solution.
2.7.2. Ferric Ion Reducing Antioxidant Power (FRAP Assay)
2.7.3. ABTS Radical Scavenging Activity
- AS was absorbance of solution of the extract treated with ABTS radical solution;
- AC was absorbance of control solution.
2.8. Statistical Analysis
3. Results
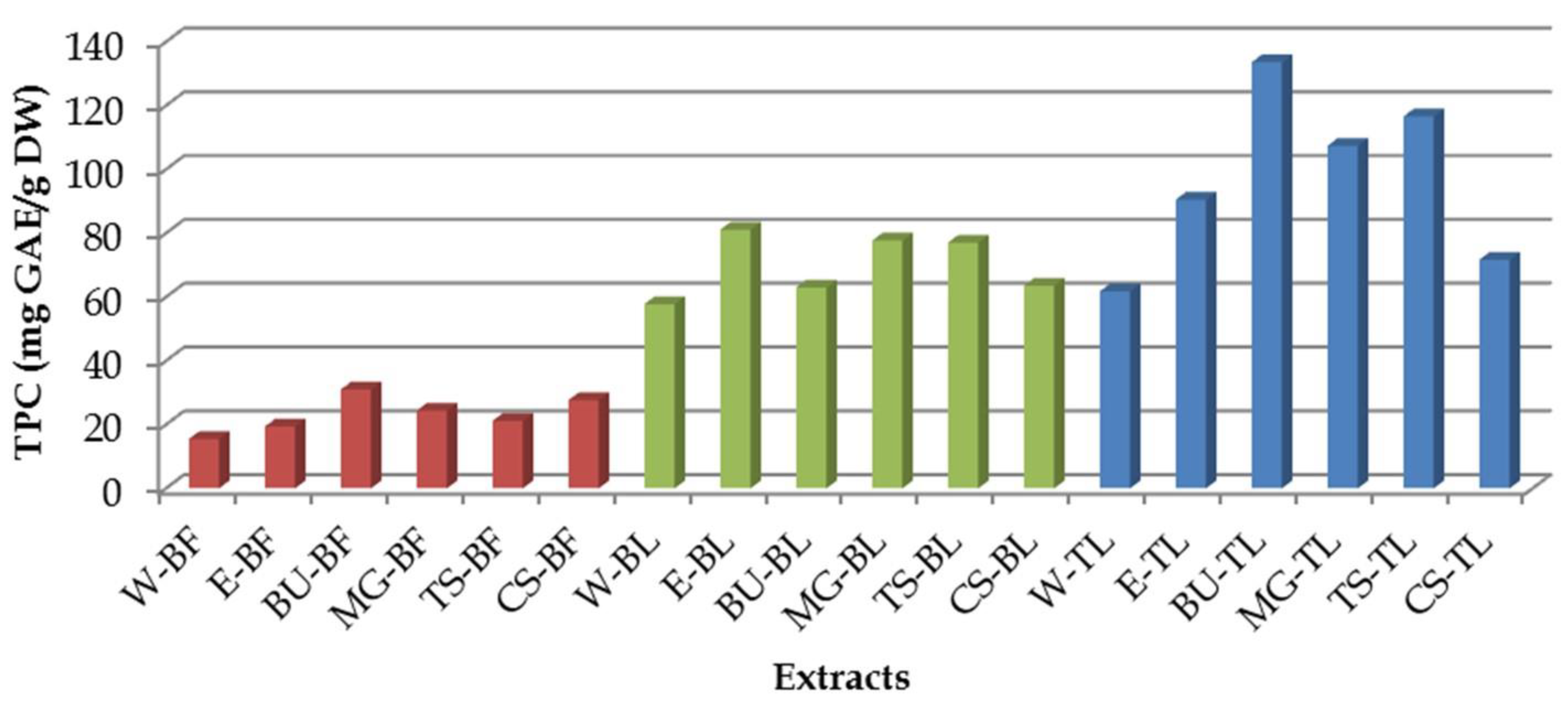
3.1. Total Phenolic Content
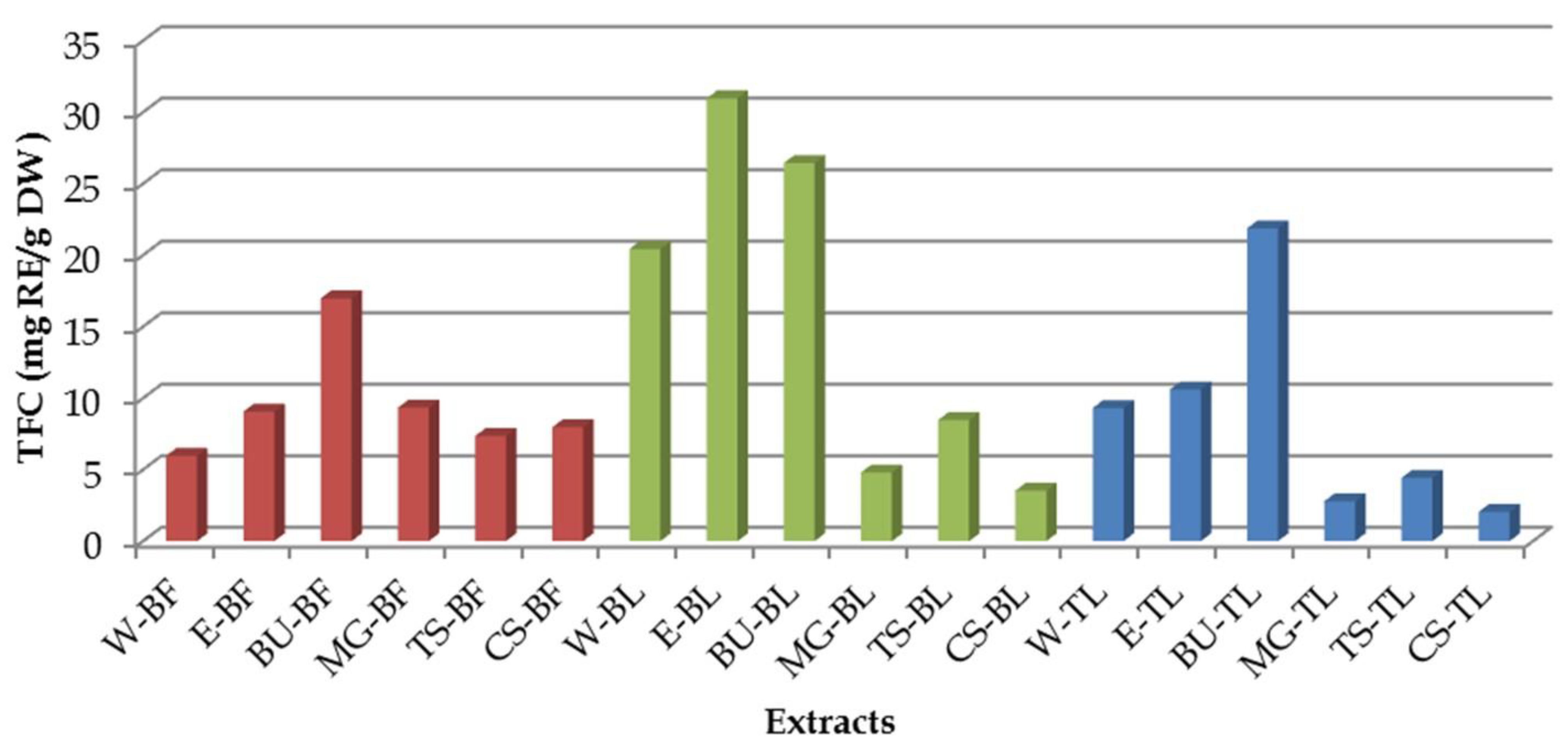
3.2. Total Flavonoid Content
3.3. Chemical (HPLC) Analysis
3.4. Antioxidant Activity
3.5. Correlation Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alara, O.R.; Abdurahman, N.H.; Ukaegbu, C.I. Extraction of phenolic compounds: A review. Curr. Res. Food Sci. 2021, 4, 200–214. [Google Scholar] [CrossRef]
- Balasundram, N.; Sundram, K.; Samman, S. Phenolic compounds in plants and agri-industrial by-products: Antioxidant activity, occurrence, and potential uses. Food Chem. 2006, 99, 191–203. [Google Scholar] [CrossRef]
- Caleja, C.; Ribeiro, A.; Filomena Barreiro, M.; Ferreira, I.C. Phenolic compounds as nutraceuticals or functional food ingredients. Curr. Pharm. Des. 2017, 23, 2787–2806. [Google Scholar] [CrossRef] [PubMed]
- Durazzo, A.; Lucarini, M.; Souto, E.B.; Cicala, C.; Caiazzo, E.; Izzo, A.A.; Novellino, E.; Santini, A. Polyphenols: A concise overview on the chemistry, occurrence, and human health. Phytother. Res. 2019, 33, 2221–2243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soto, M.L.; Falqué, E.; Domínguez, H. Relevance of natural phenolics from grape and derivative products in the formulation of cosmetics. Cosmetics 2015, 2, 259–276. [Google Scholar] [CrossRef] [Green Version]
- Perez, A.; Gonzalez-Manzano, S.; Jimenez, R.; Perez-Abud, R.; Haro, J.M.; Osuna, A.; Santos-Buelga, C.; Duarte, J.; Perez-Vizcaino, F. The flavonoid quercetin induces acute vasodilator effects in healthy volunteers: Correlation with beta-glucuronidase activity. Pharmacol. Res. 2014, 89, 11–18. [Google Scholar] [CrossRef]
- Kent, K.; Charlton, K.; Roodenrys, S.; Batterham, M.; Potter, J.; Traynor, V.; Gilbert, H.; Morgan, O.; Richards, R. Consumption of anthocyanin-rich cherry juice for 12 weeks improves memory and cognition in older adults with mild-to-moderate dementia. Eur. J. Nutr. 2017, 56, 333–341. [Google Scholar] [CrossRef] [Green Version]
- Albuquerque, B.R.; Heleno, S.A.; Oliveira, M.B.; Barros, L.; Ferreira, I.C. Phenolic compounds: Current industrial applications, limitations and future challenges. Food Funct. 2021, 12, 14–29. [Google Scholar] [CrossRef]
- Acosta-Estrada, B.A.; Gutiérrez-Uribe, J.A.; Serna-Saldívar, S.O. Bound phenolics in foods, a review. Food Chem. 2014, 152, 46–55. [Google Scholar] [CrossRef]
- Suo, H.; Peng, Z.; Guo, Z.; Wu, C.; Liu, J.; Wang, L.; Xiao, J.; Li, X. Deep eutectic solvent-based ultrasonic-assisted extraction of phenolic compounds from different potato genotypes: Comparison of free and bound phenolic profiles and antioxidant activity. Food Chem. 2022, 388, 133058. [Google Scholar] [CrossRef]
- Wang, X.; Jia, W.; Lai, G.; Wang, L.; del Mar Contreras, M.; Yang, D. Extraction for profiling free and bound phenolic compounds in tea seed oil by deep eutectic solvents. J. Food Sci. 2020, 85, 1450–1461. [Google Scholar] [CrossRef] [PubMed]
- Alara, O.R.; Abdurahman, N.H.; Ukaegbu, C.I.; Azhari, N.H. Vernonia cinerea leaves as the source of phenolic compounds, antioxidants, and anti-diabetic activity using microwave-assisted extraction technique. Ind. Crop. Prod. 2018, 122, 533–544. [Google Scholar] [CrossRef]
- Kudłak, B.; Owczarek, K.; Namieśnik, J. Selected issues related to the toxicity of ionic liquids and deep eutectic solvents—A review. Environ. Sci. Pollut. Res. 2015, 22, 11975–11992. [Google Scholar] [CrossRef] [PubMed]
- Jeong, K.M.; Ko, J.; Zhao, J.; Jin, Y.; Yoo, D.E.; Han, S.Y.; Lee, J. Multi-functioning deep eutectic solvents as extraction and storage media for bioactive natural products that are readily applicable to cosmetic products. J. Clean. Prod. 2017, 151, 87–95. [Google Scholar] [CrossRef]
- Xing, C.; Cui, W.Q.; Zhang, Y.; Zou, X.S.; Hao, J.Y.; Zheng, S.D.; Wang, T.T.; Wang, X.Z.; Wu, T.; Liu, Y.Y.; et al. Ultrasound-assisted deep eutectic solvents extraction of glabridin and isoliquiritigenin from Glycyrrhiza glabra: Optimization, extraction mechanism and in vitro bioactivities. Ultrason. Sonochem. 2022, 83, 105946. [Google Scholar] [CrossRef]
- Shams, K.A.; Abdel-Azim, N.S.; Saleh, I.A.; Hegazy, M.F.; El-Missiry, M.M.; Hammouda, F.M.; Bohouth, E.; Tahrir, E. Green technology: Economically and environmentally innovative methods for extraction of medicinal & aromatic plants (MAP) in Egypt. J. Chem. Pharm. Res. 2015, 7, 1050–1074. [Google Scholar]
- Shirzad, H.; Niknam, V.; Taheri, M.; Ebrahimzadeh, H. Ultrasound-assisted extraction process of phenolic antioxidants from Olive leaves: A nutraceutical study using RSM and LC–ESI–DAD–MS. J. Food Sci. Technol. 2017, 54, 2361–2371. [Google Scholar] [CrossRef]
- Vinatoru, M. An overview of the ultrasonically assisted extraction of bioactive principles from herbs. Ultrason. Sonochemistry 2001, 8, 303–313. [Google Scholar] [CrossRef]
- Stojiljković, D.; Arsić, I.; Tadić, V. Extracts of Wild apple fruit (Malus sylvestris (L.) Mill., Rosaceae) as a source of antioxidant substances for use in production of nutraceuticals and cosmeceuticals. Ind. Crop. Prod. 2016, 60, 165–176. [Google Scholar] [CrossRef]
- Sultana, B.; Anwar, F.; Ashraf, M. Effect of extraction solvent/technique on the antioxidant activity of selected medicinal plant extracts. Molecules. 2009, 14, 2167–2180. [Google Scholar] [CrossRef] [Green Version]
- Jablonský, M.; Škulcová, A.; Šima, J. Use of deep eutectic solvents in polymer chemistry–a review. Molecules 2019, 24, 3978. [Google Scholar] [CrossRef]
- Abbott, A.P.; Boothby, D.; Capper, G.; Davies, D.L.; Rasheed, R.K. Deep eutectic solvents formed between choline chloride and carboxylic acids: Versatile alternatives to ionic liquids. J. Am. Chem. Soc. 2004, 126, 9142–9147. [Google Scholar] [CrossRef]
- Qi, X.L.; Peng, X.; Huang, Y.Y.; Li, L.; Wei, Z.F.; Zu, Y.G.; Fu, Y.J. Green and efficient extraction of bioactive flavonoids from Equisetum palustre L. by deep eutectic solvents-based negative pressure cavitation method combined with macroporous resin enrichment. Ind. Crops Prod. 2015, 70, 142–148. [Google Scholar] [CrossRef]
- Zhou, P.; Wang, X.; Liu, P.; Huang, J.; Wang, C.; Pan, M.; Kuang, Z. Enhanced phenolic compounds extraction from Morus alba L. leaves by deep eutectic solvents combined with ultrasonic-assisted extraction. Ind. Crop. Prod. 2018, 120, 147–154. [Google Scholar] [CrossRef]
- Silva, D.T.; Pauletto, R.; Cavalheiro, S.D.S.; Bochi, V.C.; Rodrigues, E.; Weber, J.; Silva, C.B.; Morisso, F.D.P.; Barcia, M.T.; Emanuelli, T. Natural deep eutectic solvents as a biocompatible tool for the extraction of bilberry anthocyanins. J. Food Compost. Anal. 2020, 89, 103470. [Google Scholar] [CrossRef]
- Bujor, O.C.; Le Bourvellec, C.; Volf, I.; Popa, V.I.; Dufour, C. Seasonal variations of the phenolic constituents in bilberry (Vaccinium myrtillus L.) leaves, stems and fruits, and their antioxidant activity. Food Chem. 2016, 213, 58–68. [Google Scholar] [CrossRef]
- Tadić, V.M.; Nešić, I.; Martinović, M.; Rój, E.; Brašanac-Vukanović, S.; Maksimović, S.; Žugić, A. Old plant, new possibilities: Wild bilberry (Vaccinium myrtillus L., ericaceae) in topical skin preparation. Antioxidants 2021, 10, 465. [Google Scholar] [CrossRef]
- Salah, N.; Miller, N.J.; Paganga, G.; Tijburg, L.; Bolwell, G.P.; Riceevans, C. Polyphenolic flavanols as scavengers of aqueous phase radicals and as chain-breaking antioxidants. Arch. Biochem. 1995, 322, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Naczk, M.; Amarowicz, R.; Zadernowski, R.; Pegg, R.B.; Shahidi, F. Antioxidant activity of crude phenolic extracts from wild bilberry leaves. Pol. J. Food Nutr. Sci. 2003, 12, 166–169. [Google Scholar]
- Brašanac-Vuksanović, S.; Mutić, J.; Stanković, D.; Arsić, I.; Blagojević, N.; Vuksanović-Pešić, V.; Tadić, V. Wild Bilberry (Vaccinium myrtillus L., Ericaceae) from Montenegro as a Source of Antioxidants for Use in the Production of Nutraceuticals. Molecules 2018, 23, 1864. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amberg, N.; Fogarassy, C. Green consumer behavior in the cosmetics market. Resources 2019, 8, 137. [Google Scholar] [CrossRef] [Green Version]
- Sharma, S.; Das, J.; Braje, W.M.; Dash, A.K.; Handa, S. A glimpse into green chemistry practices in the pharmaceutical industry. ChemSusChem 2020, 13, 2859–2875. [Google Scholar] [CrossRef]
- Chanioti, S.; Tzia, C. Extraction of phenolic compounds from olive pomace by using natural deep eutectic solvents and innovative extraction techniques. Innov. Food Sci. Emerg. Technol. 2018, 48, 228–239. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenols with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Woisky, R.; Salatino, A. Analysis of propolis: Some parameters and procedures for chemical quality control. J. Apic. Res. 1998, 37, 99–105. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT—Food. Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. Ferric reducing ability of plasma (FRAP) as a measure of antioxidant power: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [Green Version]
- Idris, O.A.; Wintola, O.A.; Afolayan, A.J. Phytochemical and antioxidant activities of Rumex crispus L. in treatment of gastrointestinal helminths in Eastern Cape Province, South Africa. Asian Pac. J. Trop. Biomed. 2017, 7, 1071–1078. [Google Scholar] [CrossRef]
- Abdussalam-Mohammed, W.; Ali, A.Q.; Errayes, A.O. Green chemistry: Principles, applications, and disadvantages. Chem. Methodol. 2020, 4, 408–423. [Google Scholar] [CrossRef]
- Vilková, M.; Płotka-Wasylka, J.; Andruch, V. The role of water in deep eutectic solvent-base extraction. J. Mol. Liq. 2020, 304, 112747. [Google Scholar] [CrossRef]
- Vanda, H.; Dai, Y.; Wilson, E.G.; Verpoorte, R.; Choi, Y.H. Green solvents from ionic liquids and deep eutectic solvents to natural deep eutectic solvents. Comptes Rendus Chim. 2018, 21, 628–638. [Google Scholar] [CrossRef]
- Benvenutti, L.; Zielinski, A.A.F.; Ferreira, S.R.S. Which is the best food emerging solvent: IL, DES or NADES? Trends Food Sci. Technol. 2019, 90, 133–146. [Google Scholar] [CrossRef]
- Lachenmeier, D.W. Safety evaluation of topical applications of ethanol on the skin and inside the oral cavity. J. Occup. Med. Toxicol. 2008, 3, 26. [Google Scholar] [CrossRef] [PubMed]
- Celleno, L. Topical urea in skincare: A review. Derm. Ther. 2018, 31, e12690. [Google Scholar] [CrossRef]
- Smith, W.P. Comparative effectiveness of α-hydroxy acids on skin properties. Int. J. Cosmet. Sci. 1996, 18, 75–83. [Google Scholar] [CrossRef]
- Tang, S.C.; Yang, J.H. Dual effects of alpha-hydroxy acids on the skin. Molecules 2018, 23, 863. [Google Scholar] [CrossRef] [Green Version]
- Tang, B.; Zhang, H.; Row, K.H. Application of deep eutectic solvents in the extraction and separation of target compounds from various samples. J. Sep. Sci. 2015, 38, 1053–1064. [Google Scholar] [CrossRef]
- Bi, W.; Tian, M.; Row, K.H. Evaluation of alcohol-based deep eutectic solvent in extraction and determination of flavonoids with response surface methodology optimization. J. Chromatogr. A 2013, 1285, 22–30. [Google Scholar] [CrossRef]
- Park, H.E.; Tang, B.; Row, K.H. Application of deep eutectic solvents as additives in ultrasonic extraction of two phenolic acids from Herba Artemisiae Scopariae. Anal. Lett. 2014, 47, 1476–1484. [Google Scholar] [CrossRef]
- Zannou, O.; Koca, I. Greener extraction of anthocyanins and antioxidant activity from blackberry (Rubus spp) using natural deep eutectic solvents. LWT 2022, 158, 113184. [Google Scholar] [CrossRef]
- Ruesgas-Ramón, M.; Figueroa-Espinoza, M.C.; Durand, E. Application of deep eutectic solvents (DES) for phenolic compounds extraction: Overview, challenges, and opportunities. J. Agric. Food Chem. 2017, 65, 3591–3601. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.H.; Zhang, D.Y.; Duan, M.H.; Cui, Q.; Xu, W.J.; Luo, M.; Li, C.Y.; Zu, Y.G.; Fu, Y.J. Preparation and determination of phenolic compounds from Pyrola incarnata Fisch. with a green polyols based-deep eutectic solvent. Sep. Purif. Technol. 2015, 149, 116–123. [Google Scholar] [CrossRef]
- Cui, Q.; Peng, X.; Yao, X.H.; Wei, Z.F.; Luo, M.; Wang, W.; Zhao, C.J.; Fu, Y.J.; Zu, Y.G. Deep eutectic solvent-based microwave-assisted extraction of genistin, genistein and apigenin from pigeon pea roots. Sep. Purif. Technol. 2015, 150, 63–72. [Google Scholar] [CrossRef]
- Jiang, Z.M.; Wang, L.J.; Gao, Z.; Zhuang, B.; Yin, Q.; Liu, E.H. Green and efficient extraction of different types of bioactive alkaloids using deep eutectic solvents. Microchem. J. 2019, 145, 345–353. [Google Scholar] [CrossRef]
- Bajkacz, S.; Adamek, J. Evaluation of new natural deep eutectic solvents for the extraction of isoflavones from soy products. Talanta 2017, 168, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Han, Z.; Zou, Y.; Yu, B. Efficient extraction of major catechins in Camellia sinensis leaves using green choline chloride-based deep eutectic solvents. RSC Adv. 2015, 5, 93937–93944. [Google Scholar] [CrossRef]
- Silva, D.T.; Smaniotto, F.A.; Costa, I.F.; Baranzelli, J.; Muller, A.; Somacal, S.; Monteiro, C.S.; Vizzotto, M.; Rodrigues, E.; Barcia, M.T.; et al. Natural deep eutectic solvent (NADES): A strategy to improve the bioavailability of bilberry phenolic compounds in a ready-to-use extract. Food Chem. 2021, 364, 130370. [Google Scholar] [CrossRef]
- Grillo, G.; Gunjević, V.; Radošević, K.; Redovniković, I.R.; Cravotto, G. Deep eutectic solvents and nonconventional technologies for bilberry-peel extraction: Kinetics, anthocyanin stability, and antiproliferative activity. Antioxidants 2020, 9, 1069. [Google Scholar] [CrossRef]
- Benlebna, M.; Ruesgas-Ramón, M.; Bonafos, B.; Fouret, G.; Casas, F.; Coudray, C.; Durand, E.; Cruz Figueroa-Espinoza, M.; Feillet-Coudray, C. Toxicity of natural deep eutectic solvent betaine: Glycerol in rats. J. Agri. Food Chem. 2018, 66, 6205–6212. [Google Scholar] [CrossRef]
- Altunay, N.; Elik, A.; Gürkan, R. Preparation and application of alcohol based deep eutectic solvents for extraction of curcumin in food samples prior to its spectrophotometric determination. Food Chem. 2020, 310, 125933. [Google Scholar] [CrossRef]
- Fanali, C.; Della Posta, S.; Dugo, L.; Gentili, A.; Mondello, L.; De Gara, L. Choline-chloride and betaine-based deep eutectic solvents for green extraction of nutraceutical compounds from spent coffee ground. J. Pharm. Biomed. Anal. 2020, 189, 113421. [Google Scholar] [CrossRef] [PubMed]
- Mitar, A.; Panić, M.; PrlićKardum, J.; Halambek, J.; Sander, A.; ZagajskiKučan, K.; RadojčićRedovniković, I.; Radošević, K. Physicochemical properties, cytotoxicity, and antioxidative activity of natural deep eutectic solvents containing organic acid. Chem. Biochem. Eng. Q. 2019, 33, 1–8. [Google Scholar] [CrossRef]
- Shi, J.; Yu, J.; Pohorly, J.; Young, J.; Bryan, M.; Wu, Y. Optimization of the extraction of polyphenols from grape seed meal byaqueous ethanol solution. J. Food Agric. Environ. 2003, 1, 42–47. [Google Scholar]
- Babova, O.; Occhipinti, A.; Capuzzo, A.; Maffei, M.E. Extraction of bilberry (Vaccinium myrtillus) antioxidants using supercritical/subcritical CO2 and ethanol as co-solvent. J. Supercrit. Fluids 2016, 107, 358–363. [Google Scholar] [CrossRef]
- Andrade, T.A.; Hamerski, F.; López Fetzer, D.E.; Roda-Serrat, M.C.; Corazza, M.L.; Norddahl, B.; Errico, M. Ultrasound-assisted pressurized liquid extraction of anthocyanins from Aronia melanocarpa pomace. Sep. Purif. Technol. 2021, 276, 119290. [Google Scholar] [CrossRef]
- Wang, T.; Xu, W.J.; Wang, S.X.; Kou, P.; Wang, P.; Wang, X.Q.; Fu, Y.J. Integrated and sustainable separation of chlorogenic acid from bilberry leaves by deep eutectic solvents coupled with aqueous two-phase system. Food Bioprod. Process. 2017, 105, 205–214. [Google Scholar] [CrossRef]
- Perva-Uzunalić, A.; Škerget, M.; Knez, Ž.; Weinreich, B.; Otto, F.; Grüner, S. Extraction of active ingredients from green tea (Camellia sinensis): Extraction efficiency of major catechins and caffeine. Food Chem. 2006, 96, 597–605. [Google Scholar] [CrossRef]
- Floegel, A.; Kim, D.O.; Chung, S.J.; Koo, S.I.; Chun, O.K. Comparison of ABTS/DPPH assays to measure antioxidant capacity in popular antioxidant-rich US foods. J. Food Compost. Anal. 2011, 24, 1043–1048. [Google Scholar] [CrossRef]
- Xiao, F.; Xu, T.; Lu, B.; Liu, R. Guidelines for antioxidant assays for food components. Food Front. 2020, 1, 60–69. [Google Scholar] [CrossRef] [Green Version]
- Shahidi, F.; Janitha, P.K.; Wanasundara, P.D. Phenolic antioxidants. Crit. Rev. Food Sci. Nutr. 1992, 32, 67–103. [Google Scholar] [CrossRef]

| Abbreviation | Component 1 | Component 2 | Mole Ratio |
|---|---|---|---|
| TS | tartaric acid | sorbitol | 1:2 |
| CS | citric acid | sorbitol | |
| BU | betaine | urea | |
| MG | malic acid | glycerol |
| Extraction Solvent | Bilberry Fruit | Bilberry Leaves | Green Tea Leaves |
|---|---|---|---|
| Water | W − BF | W −BL | W − TL |
| 50% Ethanol | E − BF | E − BL | E − TL |
| Betaine + Urea | BU − BF | BU − BL | BU − TL |
| Malic acid + Glycerol | MG − BF | MG − BL | MG − TL |
| Tartaric acid + Sorbitol | TS − BF | TS − BL | TS − TL |
| Citric acid + Sorbitol | CS − BF | CS − BL | CS − TL |
| Bilberry Fruit | ||||||
|---|---|---|---|---|---|---|
| Water | Ethanol | Betaine + Urea | Malic Acid + Glycerol | Tartaric Acid + Sorbitol | Citric Acid + Sorbitol | |
| Protocatechuic acid (mg/g) | 0.94 ± 0.04 b | 1.03 ± 0.02 ab | 1.60 ± 0.15 a | 1.35 ± 0.15 ab | 1.48 ± 0.39 ab | 1.30 ± 0.28 ab |
| Chlorogenic acid (mg/g) | 0.86 ± 0.06 b | 0.83 ± 0.03 b | 1.40 ± 0.16 a | 1.47 ± 0.37 a | 1.53 ± 0.11 a | 1.51 ± 0.01 a |
| Hyperoside (mg/g) | 0.15 ± 0.03 b | 0.53 ± 0.03 a | 0.59 ± 0.03 a | 0.57 ± 0.06 a | 0.49 ± 0.01 a | 0.50 ± 0.07 a |
| Delphinidin-3-O-glucoside (mg/g) | tr | tr | Tr | 1.01 ± 0.05 a | 1.07 ± 0.02 a | 0.99 ± 0.05 a |
| Cyanidin-3-O-galactoside (mg/g) | tr | tr | Tr | 0.10 ± 0.03 a | 0.10 ± 0.04 a | 0.11 ± 0.03 a |
| Cyanidin-3-O-glucoside (mg/g) | tr | tr | Tr | 0.19 ± 0.02 a | 0.19 ± 0.01 a | 0.18 ± 0.01 a |
| Bilberry Leaves | ||||||
| Water | Ethanol | Betaine + Urea | Malic Acid + Glycerol | Tartaric Acid + Sorbitol | Citric Acid + Sorbitol | |
| Chlorogenic acid (mg/g) | 8.37 ± 0.06 c | 22.51 ± 0.50 a | 17.13 ± 0.21 b | 21.17 ± 1.75 ab | 19.48 ± 0.92 ab | 22.48 ± 1.57 ab |
| Procyanidin B2 (mg/g) | nd | 14.41 ± 0.80 a | 11.63 ± 0.85 | 18.59 ± 1.23 | 7.57 ± 1.16 | 15.77 ± 0.68 a |
| Epicatechin (mg/g) | nd | 1.92 ± 0.13 a | 2.19 ± 0.42 a | 1.61 ± 0.27 a | 0.51 ± 0.09 | 1.74 ± 0.08 a |
| Rutin (mg/g) | 1.93 ± 0.15 | 3.79 ± 0.12 a | 2.54 ± 0.13 | 3.24 ± 0.10 bc | 3.16 ± 0.05 c | 3.60 ± 0.26 ab |
| Hyperoside (mg/g) | 1.32 ± 0.11 | 3.31 ± 0.10 | 1.79 ± 0.09 | 2.57 ± 0.11 | 2.21 ± 0.07 | 2.85 ± 0.09 |
| Quercetin-3-O-glucoside (mg/g) | 4.88 ± 0.20 | 9.58 ± 0.22 | 6.03 ± 0.05 | 8.02 ± 0.08 a | 6.76 ± 0.16 | 8.29 ± 0.12 a |
| Quercitrin (mg/g) | 0.56 ± 0.07 de | 0.99 ± 0.10 a | 0.50 ± 0.02 e | 0.76 ± 0.05 bc | 0.69 ± 0.04 cd | 0.90 ± 0.06 ab |
| Green tea leaves | ||||||
| Water | Ethanol | Betaine + Urea | Malic Acid + Glycerol | Tartaric Acid + Sorbitol | Citric Acid + Sorbitol | |
| Epigallocatechin (mg/g) | 34.65 ± 1.55 b | 36.85 ± 0.59 b | 54.00 ± 1.11 a | 54.56 ± 0.49 a | 32.08 ± 0.84 | 60.88 ± 0.11 |
| Epicatechin (mg/g) | 6.66 ± 0.07 | 4.51 ± 0.07 | 9.02 ± 0.06 | 5.98 ± 0.11 a | 3.57 ± 0.06 | 5.84 ± 0.04 a |
| Epigallocatechin gallate (mg/g) | nd | 36.76 ± 0.67 a | 29.20 ± 1.31 a | 42.33 ± 0.58 | 19.18 ± 0.33 | 34.86 ± 1.58 a |
| Epicatechin gallate (mg/g) | nd | 12.18 ± 0.54 a | 17.79 ± 0.20 | 13.05 ± 0.43 a | 5.63 ± 0.16 | 9.97 ± 0.07 a |
| Kaempferol-3-O-glucoside (mg/g) | nd | 0.25 ± 0.05 ab | 0.76 ± 0.05 | 0.30 ± 0.03 a | 0.13 ± 0.00 b | 0.16 ± 0.01 b |
| Bilberry Fruit | ||||||
|---|---|---|---|---|---|---|
| Water | Ethanol | Betaine + Urea | Malic Acid + Glycerol | Tartaric Acid + Sorbitol | Citric Acid + Sorbitol | |
| FRAP (mmol Fe2+/g DW) | 0.25 | 0.3 | 0.47 | 0.38 | 0.14 | 0.3 |
| DPPH − IC50 (mg/mL) | 4.47 | 3.24 | 1.05 | 1.64 | 2.38 | 2.43 |
| ABTS − IC50 (μg/mL) | 128.17 | 78.55 | 43.27 | 92.72 | 80.68 | 90.47 |
| Bilberry Leaves | ||||||
| Water | Ethanol | Betaine + Urea | Malic Acid + Glycerol | Tartaric Acid + Sorbitol | Citric Acid + Sorbitol | |
| FRAP (mmol Fe2+/g DW) | 0.41 | 0.66 | 0.82 | 0.94 | 0.94 | 0.51 |
| DPPH −IC50 (mg/mL) | 0.78 | 0.48 | 0.41 | 0.59 | 0.64 | 1.2 |
| ABTS − IC50 (μg/mL) | 49.49 | 15.56 | 23.75 | 29.82 | 39.38 | 26.57 |
| Green Tea Leaves | ||||||
| Water | Ethanol | Betaine + Urea | Malic Acid + Glycerol | Tartaric Acid + Sorbitol | Citric Acid + Sorbitol | |
| FRAP (mmol Fe2+/g DW) | 0.87 | 1.49 | 1.91 | 1.45 | 1.66 | 0.96 |
| DPPH − IC50 (mg/mL) | 0.42 | 0.24 | 0.09 | 0.28 | 0.25 | 0.44 |
| ABTS − IC50 (μg/mL) | 16.01 | 8.17 | 7.03 | 12.51 | 9.78 | 20.14 |
| Ascorbic Acid | |
|---|---|
| FRAP (mmol Fe2+/g DW) | 15.94 |
| DPPH − IC50 (μg/mL) | 4.45 |
| ABTS − IC50 (μg/mL) | 2.31 |
| TPC | 1 | ||||
| TFC | 0.0161 | 1 | |||
| FRAP | 0.9773 | 0.0973 | 1 | ||
| DPPH | −0.8460 | −0.1724 | −0.8519 | 1 | |
| ABTS | −0.1965 | −0.2045 | −0.3545 | 0.1003 | 1 |
| TPC | TFC | FRAP | DPPH | ABTS |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martinović, M.; Krgović, N.; Nešić, I.; Žugić, A.; Tadić, V.M. Conventional vs. Green Extraction Using Natural Deep Eutectic Solvents—Differences in the Composition of Soluble Unbound Phenolic Compounds and Antioxidant Activity. Antioxidants 2022, 11, 2295. https://doi.org/10.3390/antiox11112295
Martinović M, Krgović N, Nešić I, Žugić A, Tadić VM. Conventional vs. Green Extraction Using Natural Deep Eutectic Solvents—Differences in the Composition of Soluble Unbound Phenolic Compounds and Antioxidant Activity. Antioxidants. 2022; 11(11):2295. https://doi.org/10.3390/antiox11112295
Chicago/Turabian StyleMartinović, Milica, Nemanja Krgović, Ivana Nešić, Ana Žugić, and Vanja Milija Tadić. 2022. "Conventional vs. Green Extraction Using Natural Deep Eutectic Solvents—Differences in the Composition of Soluble Unbound Phenolic Compounds and Antioxidant Activity" Antioxidants 11, no. 11: 2295. https://doi.org/10.3390/antiox11112295
APA StyleMartinović, M., Krgović, N., Nešić, I., Žugić, A., & Tadić, V. M. (2022). Conventional vs. Green Extraction Using Natural Deep Eutectic Solvents—Differences in the Composition of Soluble Unbound Phenolic Compounds and Antioxidant Activity. Antioxidants, 11(11), 2295. https://doi.org/10.3390/antiox11112295







