Maternal Calorie Restriction Induces a Transcriptional Cytoprotective Response in Embryonic Liver Partially Dependent on Nrf2
Abstract
:1. Introduction
2. Materials and Methods
2.1. Mouse Model
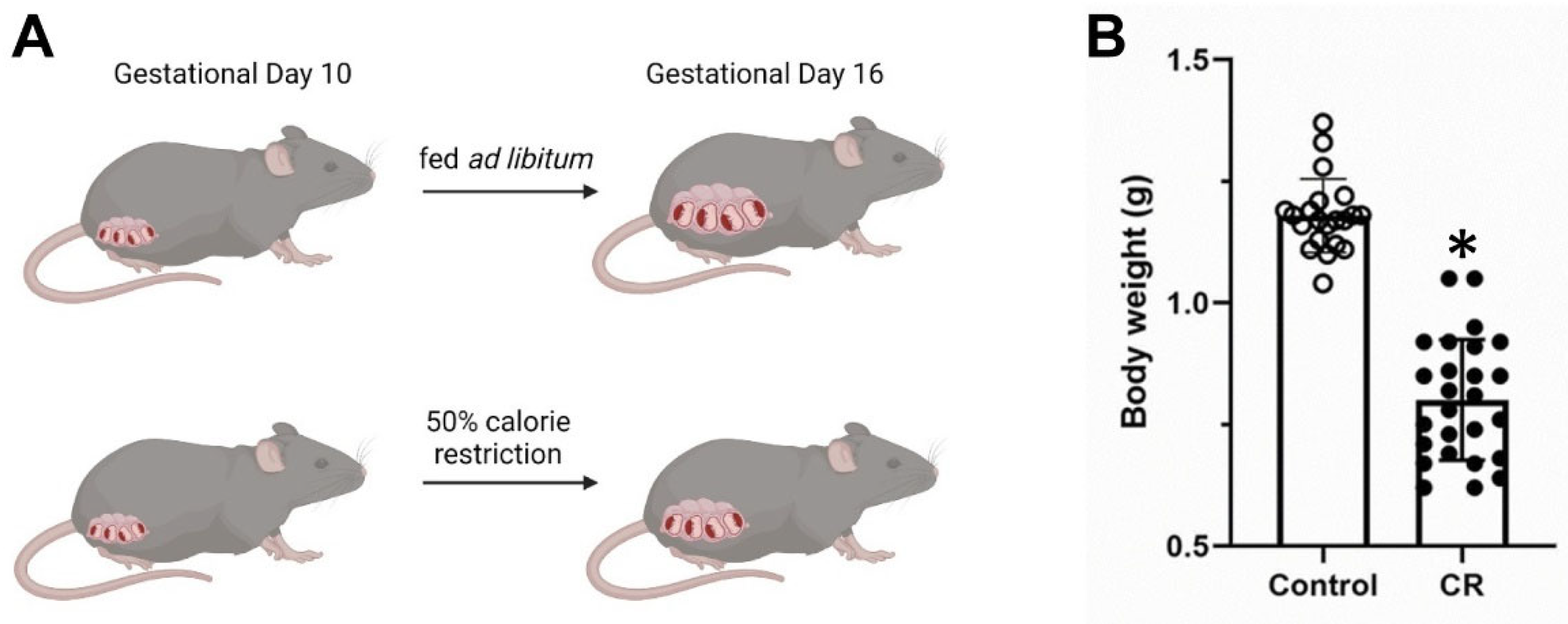
2.2. In Utero Calorie Restriction
2.3. RNA Preparation and Quantitative Real-Time PCR
2.4. OS Assessment
2.5. Statistics
3. Results
3.1. Maternal Calorie Restriction Induces an Antioxidant/Cytoprotective Transcriptional Response in Fetal Liver
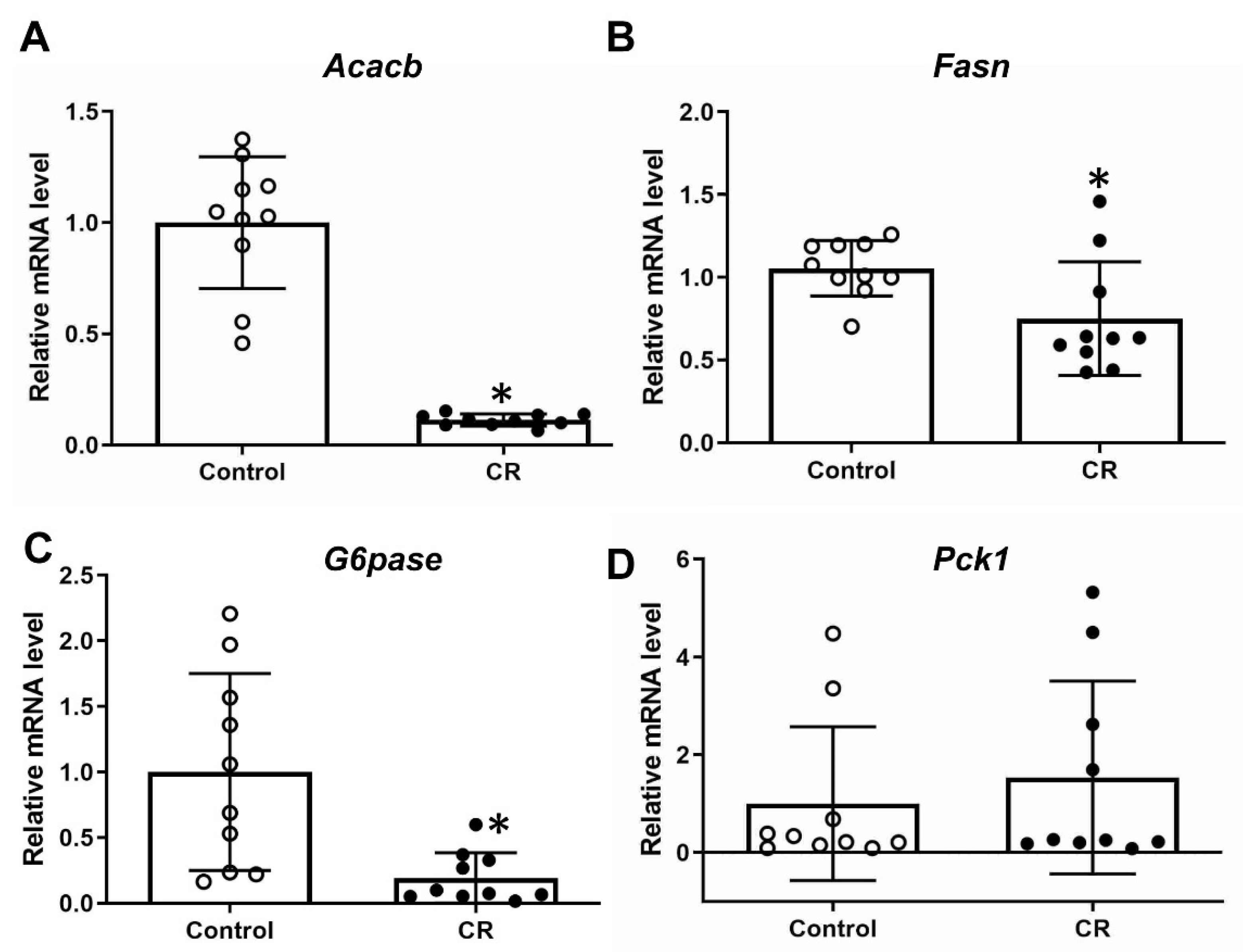
3.2. Calorie Restriction Effects on Gluconeogenic and Lipogenic Gene Expression in Fetal Liver
3.3. No Remarkable Difference in OS Was Found: Neither in the Livers of Embryos, Nor in the Livers of Their Mothers That had been Exposed to Calorie Restriction
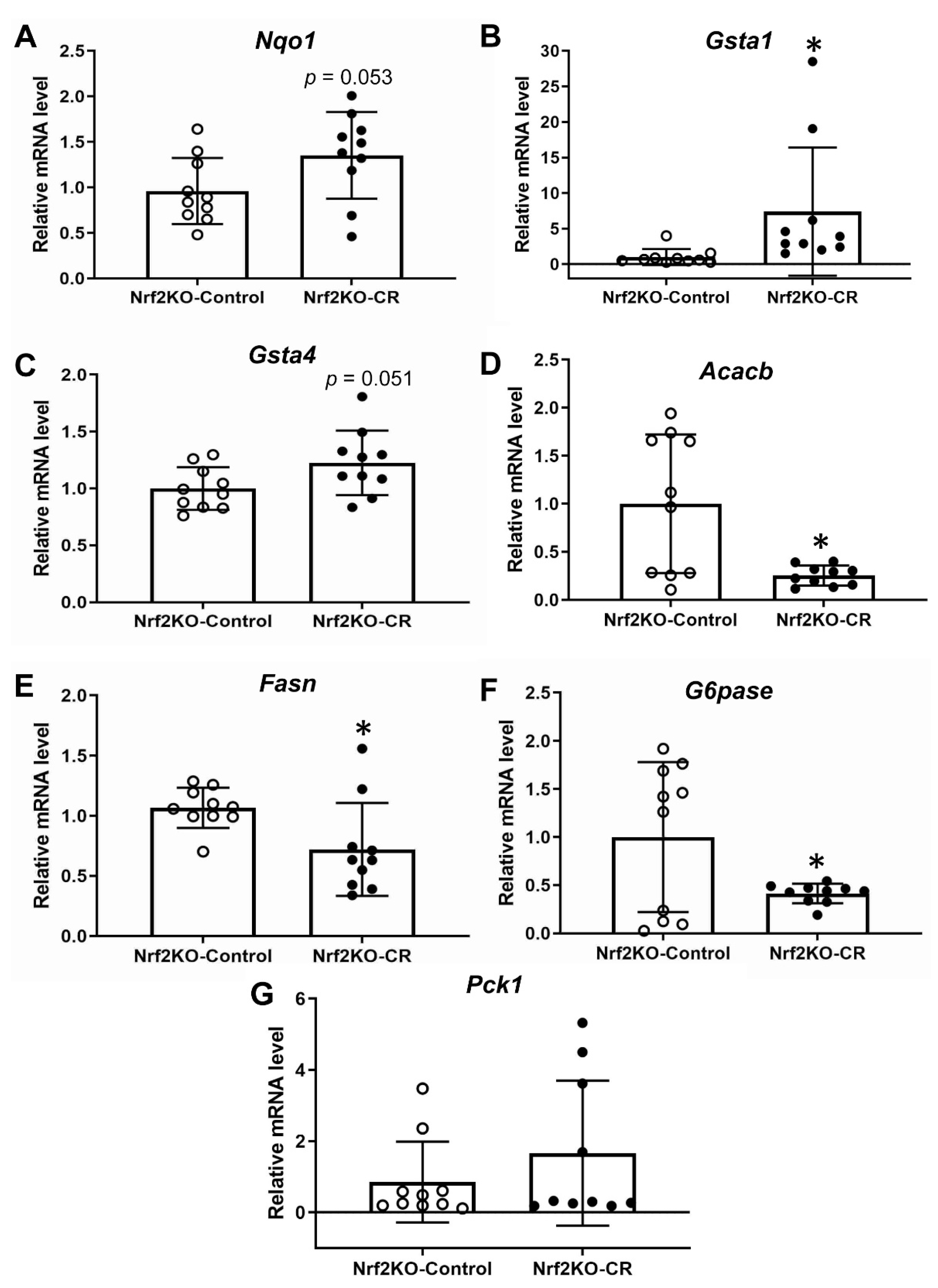
3.4. Lack of Nrf2 Partially Attenuates the Antioxidant Transcriptional Response to Maternal Calorie Restriction in Murine Fetal Livers
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gluckman, P.D.; Hanson, M.A.; Cooper, C.; Thornburg, K.L. Effect of in utero and early-life conditions on adult health and disease. N. Engl. J. Med. 2008, 359, 61–73. [Google Scholar] [CrossRef] [Green Version]
- Godfrey, K.M.; Gluckman, P.D.; Hanson, M.A. Developmental origins of metabolic disease: Life course and intergenerational perspectives. Trends Endocrinol. Metab. 2010, 21, 199–205. [Google Scholar] [CrossRef]
- Lane, M.; Zander-Fox, D.L.; Robker, R.L.; McPherson, N.O. Peri-conception parental obesity, reproductive health, and transgenerational impacts. Trends Endocrinol. Metab. 2015, 26, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Ravelli, A.C.; van der Meulen, J.H.; Michels, R.P.; Osmond, C.; Barker, D.J.; Hales, C.N.; Bleker, O.P. Glucose tolerance in adults after prenatal exposure to famine. Lancet 1998, 351, 173–177. [Google Scholar] [CrossRef]
- Ravelli, A.C.; van Der Meulen, J.H.; Osmond, C.; Barker, D.J.; Bleker, O.P. Obesity at the age of 50 y in men and women exposed to famine prenatally. Am. J. Clin. Nutr. 1999, 70, 811–816. [Google Scholar] [CrossRef] [Green Version]
- Lumey, L.H.; Stein, A.D.; Kahn, H.S.; Romijn, J.A. Lipid profiles in middle-aged men and women after famine exposure during gestation: The Dutch Hunger Winter Families Study. Am. J. Clin. Nutr. 2009, 89, 1737–1743. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lumey, L.H.; Khalangot, M.D.; Vaiserman, A.M. Association between type 2 diabetes and prenatal exposure to the Ukraine famine of 1932-33: A retrospective cohort study. Lancet Diabetes Endocrinol. 2015, 3, 787–794. [Google Scholar] [CrossRef] [PubMed]
- Zimmet, P.; Shi, Z.; El-Osta, A.; Ji, L. Epidemic T2DM, early development and epigenetics: Implications of the Chinese Famine. Nat. Rev. Endocrinol. 2018, 14, 738–746. [Google Scholar] [CrossRef]
- Barker, D.J.; Hales, C.N.; Fall, C.H.; Osmond, C.; Phipps, K.; Clark, P.M. Type 2 (non-insulin-dependent) diabetes mellitus, hypertension and hyperlipidaemia (syndrome X): Relation to reduced fetal growth. Diabetologia 1993, 36, 62–67. [Google Scholar] [CrossRef] [Green Version]
- Barker, D.J.; Osmond, C.; Forsen, T.J.; Kajantie, E.; Eriksson, J.G. Trajectories of growth among children who have coronary events as adults. N. Engl. J. Med. 2005, 353, 1802–1809. [Google Scholar] [CrossRef]
- Tarry-Adkins, J.L.; Ozanne, S.E. Mechanisms of early life programming: Current knowledge and future directions. Am. J. Clin. Nutr. 2011, 94, 1765S–1771S. [Google Scholar] [CrossRef] [Green Version]
- Tobi, E.W.; Slieker, R.C.; Luijk, R.; Dekkers, K.F.; Stein, A.D.; Xu, K.M.; Slagboom, P.E.; van Zwet, E.W.; Lumey, L.H.; Biobank-Based Integrative Omics Studies Consortium; et al. DNA methylation as a mediator of the association between prenatal adversity and risk factors for metabolic disease in adulthood. Sci. Adv. 2018, 4, eaao4364. [Google Scholar] [CrossRef] [Green Version]
- Park, J.H.; Stoffers, D.A.; Nicholls, R.D.; Simmons, R.A. Development of type 2 diabetes following intrauterine growth retardation in rats is associated with progressive epigenetic silencing of Pdx1. J. Clin. Investig. 2008, 118, 2316–2324. [Google Scholar] [CrossRef] [Green Version]
- Raychaudhuri, N.; Raychaudhuri, S.; Thamotharan, M.; Devaskar, S.U. Histone code modifications repress glucose transporter 4 expression in the intrauterine growth-restricted offspring. J. Biol. Chem. 2008, 283, 13611–13626. [Google Scholar] [CrossRef] [Green Version]
- Newsholme, P.; Cruzat, V.F.; Keane, K.N.; Carlessi, R.; de Bittencourt, P.I., Jr. Molecular mechanisms of ROS production and oxidative stress in diabetes. Biochem. J. 2016, 473, 4527–4550. [Google Scholar] [CrossRef]
- Lambert, A.J.; Portero-Otin, M.; Pamplona, R.; Merry, B.J. Effect of ageing and caloric restriction on specific markers of protein oxidative damage and membrane peroxidizability in rat liver mitochondria. Mech. Ageing Dev. 2004, 125, 529–538. [Google Scholar] [CrossRef] [PubMed]
- Sorensen, M.; Sanz, A.; Gomez, J.; Pamplona, R.; Portero-Otin, M.; Gredilla, R.; Barja, G. Effects of fasting on oxidative stress in rat liver mitochondria. Free Radic. Res. 2006, 40, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Ufer, C.; Wang, C.C.; Borchert, A.; Heydeck, D.; Kuhn, H. Redox control in mammalian embryo development. Antioxid Redox Signal. 2010, 13, 833–875. [Google Scholar] [CrossRef] [PubMed]
- Parman, T.; Wiley, M.J.; Wells, P.G. Free radical-mediated oxidative DNA damage in the mechanism of thalidomide teratogenicity. Nat. Med. 1999, 5, 582–585. [Google Scholar] [CrossRef] [PubMed]
- Dennery, P.A. Effects of oxidative stress on embryonic development. Birth Defects Res. C Embryo Today 2007, 81, 155–162. [Google Scholar] [CrossRef]
- Yamamoto, M.; Kensler, T.W.; Motohashi, H. The KEAP1-NRF2 System: A Thiol-Based Sensor-Effector Apparatus for Maintaining Redox Homeostasis. Physiol. Rev. 2018, 98, 1169–1203. [Google Scholar] [CrossRef] [Green Version]
- Chan, K.; Lu, R.; Chang, J.C.; Kan, Y.W. NRF2, a member of the NFE2 family of transcription factors, is not essential for murine erythropoiesis, growth, and development. Proc. Natl. Acad. Sci. USA 1996, 93, 13943–13948. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Itoh, K.; Chiba, T.; Takahashi, S.; Ishii, T.; Igarashi, K.; Katoh, Y.; Oyake, T.; Hayashi, N.; Satoh, K.; Hatayama, I.; et al. An Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem. Biophys. Res. Commun. 1997, 236, 313–322. [Google Scholar] [CrossRef]
- Kweider, N.; Huppertz, B.; Rath, W.; Lambertz, J.; Caspers, R.; ElMoursi, M.; Pecks, U.; Kadyrov, M.; Fragoulis, A.; Pufe, T.; et al. The effects of Nrf2 deletion on placental morphology and exchange capacity in the mouse. J. Matern Fetal. Neonatal Med. 2017, 30, 2068–2073. [Google Scholar] [CrossRef] [PubMed]
- Chapple, S.J.; Puszyk, W.M.; Mann, G.E. Keap1-Nrf2 regulated redox signaling in utero: Priming of disease susceptibility in offspring. Free Radic. Biol. Med. 2015, 88, 212–220. [Google Scholar] [CrossRef] [PubMed]
- Nezu, M.; Souma, T.; Yu, L.; Sekine, H.; Takahashi, N.; Wei, A.Z.; Ito, S.; Fukamizu, A.; Zsengeller, Z.K.; Nakamura, T.; et al. Nrf2 inactivation enhances placental angiogenesis in a preeclampsia mouse model and improves maternal and fetal outcomes. Sci. Signal. 2017, 10, eaam5711. [Google Scholar] [CrossRef]
- Chartoumpekis, D.V.; Yagishita, Y.; Fazzari, M.; Palliyaguru, D.L.; Rao, U.N.; Zaravinos, A.; Khoo, N.K.; Schopfer, F.J.; Weiss, K.R.; Michalopoulos, G.K.; et al. Nrf2 prevents Notch-induced insulin resistance and tumorigenesis in mice. JCI Insight 2018, 3, e97735. [Google Scholar] [CrossRef]
- Slocum, S.L.; Skoko, J.J.; Wakabayashi, N.; Aja, S.; Yamamoto, M.; Kensler, T.W.; Chartoumpekis, D.V. Keap1/Nrf2 pathway activation leads to a repressed hepatic gluconeogenic and lipogenic program in mice on a high-fat diet. Arch. Biochem. Biophys. 2016, 591, 57–65. [Google Scholar] [CrossRef] [Green Version]
- Kulkarni, S.R.; Donepudi, A.C.; Xu, J.; Wei, W.; Cheng, Q.C.; Driscoll, M.V.; Johnson, D.A.; Johnson, J.A.; Li, X.; Slitt, A.L. Fasting induces nuclear factor E2-related factor 2 and ATP-binding Cassette transporters via protein kinase A and Sirtuin-1 in mouse and human. Antioxid Redox Signal. 2014, 20, 15–30. [Google Scholar] [CrossRef] [Green Version]
- Pearson, K.J.; Lewis, K.N.; Price, N.L.; Chang, J.W.; Perez, E.; Cascajo, M.V.; Tamashiro, K.L.; Poosala, S.; Csiszar, A.; Ungvari, Z.; et al. Nrf2 mediates cancer protection but not prolongevity induced by caloric restriction. Proc. Natl. Acad. Sci. USA 2008, 105, 2325–2330. [Google Scholar] [CrossRef]
- Wakabayashi, N.; Shin, S.; Slocum, S.L.; Agoston, E.S.; Wakabayashi, J.; Kwak, M.K.; Misra, V.; Biswal, S.; Yamamoto, M.; Kensler, T.W. Regulation of notch1 signaling by nrf2: Implications for tissue regeneration. Sci. Signal. 2010, 3, ra52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Filippopoulou, F.; Habeos, G.I.; Rinotas, V.; Sophocleous, A.; Sykiotis, G.P.; Douni, E.; Chartoumpekis, D.V. Dexamethasone Administration in Mice Leads to Less Body Weight Gain over Time, Lower Serum Glucose, and Higher Insulin Levels Independently of NRF2. Antioxidants 2021, 11, 4. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Chillaron, J.C.; Isganaitis, E.; Charalambous, M.; Gesta, S.; Pentinat-Pelegrin, T.; Faucette, R.R.; Otis, J.P.; Chow, A.; Diaz, R.; Ferguson-Smith, A.; et al. Intergenerational transmission of glucose intolerance and obesity by in utero undernutrition in mice. Diabetes 2009, 58, 460–468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McFarlane, L.; Truong, V.; Palmer, J.S.; Wilhelm, D. Novel PCR assay for determining the genetic sex of mice. Sex. Dev. 2013, 7, 207–211. [Google Scholar] [CrossRef]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef] [PubMed]
- Vandesompele, J.; De Preter, K.; Pattyn, F.; Poppe, B.; Van Roy, N.; De Paepe, A.; Speleman, F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002, 3, RESEARCH0034. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Spandidos, A.; Wang, H.; Seed, B. PrimerBank: A PCR primer database for quantitative gene expression analysis, 2012 update. Nucleic Acids Res. 2012, 40, D1144–D1149. [Google Scholar] [CrossRef] [Green Version]
- Nioi, P.; McMahon, M.; Itoh, K.; Yamamoto, M.; Hayes, J.D. Identification of a novel Nrf2-regulated antioxidant response element (ARE) in the mouse NAD(P)H:quinone oxidoreductase 1 gene: Reassessment of the ARE consensus sequence. Biochem. J. 2003, 374, 337–348. [Google Scholar] [CrossRef]
- Hayes, J.D.; Chanas, S.A.; Henderson, C.J.; McMahon, M.; Sun, C.; Moffat, G.J.; Wolf, C.R.; Yamamoto, M. The Nrf2 transcription factor contributes both to the basal expression of glutathione S-transferases in mouse liver and to their induction by the chemopreventive synthetic antioxidants, butylated hydroxyanisole and ethoxyquin. Biochem. Soc. Trans. 2000, 28, 33–41. [Google Scholar] [CrossRef]
- van Lieshout, E.M.; Knapen, M.F.; Lange, W.P.; Steegers, E.A.; Peters, W.H. Localization of glutathione S-transferases alpha and pi in human embryonic tissues at 8 weeks gestational age. Hum. Reprod. 1998, 13, 1380–1386. [Google Scholar] [CrossRef]
- Zisimopoulos, D.N.; Kalaitzopoulou, E.; Skipitari, M.; Papadea, P.; Panagopoulos, N.T.; Salahas, G.; Georgiou, C.D. Detection of superoxide radical in all biological systems by Thin Layer Chromatography. Arch. Biochem. Biophys. 2022, 716, 109110. [Google Scholar] [CrossRef]
- Grintzalis, K.; Zisimopoulos, D.; Grune, T.; Weber, D.; Georgiou, C.D. Method for the simultaneous determination of free/protein malondialdehyde and lipid/protein hydroperoxides. Free Radic. Biol. Med. 2013, 59, 27–35. [Google Scholar] [CrossRef]
- Kostopoulou, E.; Kalaitzopoulou, E.; Papadea, P.; Skipitari, M.; Rojas Gil, A.P.; Spiliotis, B.E.; Georgiou, C.D. Oxidized lipid-associated protein damage in children and adolescents with type 1 diabetes mellitus: New diagnostic/prognostic clinical markers. Pediatr. Diabetes 2021, 22, 1135–1142. [Google Scholar] [CrossRef]
- Georgiou, C.D.; Grintzalis, K.; Zervoudakis, G.; Papapostolou, I. Mechanism of Coomassie brilliant blue G-250 binding to proteins: A hydrophobic assay for nanogram quantities of proteins. Anal. Bioanal. Chem. 2008, 391, 391–403. [Google Scholar] [CrossRef]
- Hayes, J.D.; Flanagan, J.U.; Jowsey, I.R. Glutathione transferases. Annu. Rev. Pharmacol. Toxicol. 2005, 45, 51–88. [Google Scholar] [CrossRef] [PubMed]
- Costa-Mattioli, M.; Walter, P. The integrated stress response: From mechanism to disease. Science 2020, 368, 1374–1395. [Google Scholar] [CrossRef] [PubMed]
- Gameiro, P.A.; Struhl, K. Nutrient Deprivation Elicits a Transcriptional and Translational Inflammatory Response Coupled to Decreased Protein Synthesis. Cell Rep. 2018, 24, 1415–1424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chowdhry, S.; Zhang, Y.; McMahon, M.; Sutherland, C.; Cuadrado, A.; Hayes, J.D. Nrf2 is controlled by two distinct beta-TrCP recognition motifs in its Neh6 domain, one of which can be modulated by GSK-3 activity. Oncogene 2013, 32, 3765–3781. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mills, E.L.; Ryan, D.G.; Prag, H.A.; Dikovskaya, D.; Menon, D.; Zaslona, Z.; Jedrychowski, M.P.; Costa, A.S.H.; Higgins, M.; Hams, E.; et al. Itaconate is an anti-inflammatory metabolite that activates Nrf2 via alkylation of KEAP1. Nature 2018, 556, 113–117. [Google Scholar] [CrossRef] [Green Version]
- Boylan, J.M.; Sanders, J.A.; Gruppuso, P.A. Regulation of fetal liver growth in a model of diet restriction in the pregnant rat. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2016, 311, R478–R488. [Google Scholar] [CrossRef]
- Gredilla, R.; Barja, G. Minireview: The role of oxidative stress in relation to caloric restriction and longevity. Endocrinology 2005, 146, 3713–3717. [Google Scholar] [CrossRef] [PubMed]
- Hyun, D.H.; Emerson, S.S.; Jo, D.G.; Mattson, M.P.; de Cabo, R. Calorie restriction up-regulates the plasma membrane redox system in brain cells and suppresses oxidative stress during aging. Proc. Natl. Acad. Sci. USA 2006, 103, 19908–19912. [Google Scholar] [CrossRef] [Green Version]
- McGill, M.R.; Williams, C.D.; Xie, Y.; Ramachandran, A.; Jaeschke, H. Acetaminophen-induced liver injury in rats and mice: Comparison of protein adducts, mitochondrial dysfunction, and oxidative stress in the mechanism of toxicity. Toxicol. Appl. Pharmacol. 2012, 264, 387–394. [Google Scholar] [CrossRef] [Green Version]
- Enomoto, A.; Itoh, K.; Nagayoshi, E.; Haruta, J.; Kimura, T.; O’Connor, T.; Harada, T.; Yamamoto, M. High sensitivity of Nrf2 knockout mice to acetaminophen hepatotoxicity associated with decreased expression of ARE-regulated drug metabolizing enzymes and antioxidant genes. Toxicol. Sci. 2001, 59, 169–177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taguchi, K.; Maher, J.M.; Suzuki, T.; Kawatani, Y.; Motohashi, H.; Yamamoto, M. Genetic analysis of cytoprotective functions supported by graded expression of Keap1. Mol. Cell Biol. 2010, 30, 3016–3026. [Google Scholar] [CrossRef] [Green Version]
- Rada, P.; Rojo, A.I.; Evrard-Todeschi, N.; Innamorato, N.G.; Cotte, A.; Jaworski, T.; Tobon-Velasco, J.C.; Devijver, H.; Garcia-Mayoral, M.F.; Van Leuven, F.; et al. Structural and functional characterization of Nrf2 degradation by the glycogen synthase kinase 3/beta-TrCP axis. Mol. Cell Biol. 2012, 32, 3486–3499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martin, S.A.; DeMuth, T.M.; Miller, K.N.; Pugh, T.D.; Polewski, M.A.; Colman, R.J.; Eliceiri, K.W.; Beasley, T.M.; Johnson, S.C.; Anderson, R.M. Regional metabolic heterogeneity of the hippocampus is nonuniformly impacted by age and caloric restriction. Aging Cell 2016, 15, 100–110. [Google Scholar] [CrossRef] [Green Version]
- Rasmussen, B.B.; Adams, C.M. ATF4 Is a Fundamental Regulator of Nutrient Sensing and Protein Turnover. J. Nutr. 2020, 150, 979–980. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Dai, W.; Kutzler, L.; Lacko, H.A.; Jefferson, L.S.; Dennis, M.D.; Kimball, S.R. ATF4-Mediated Upregulation of REDD1 and Sestrin2 Suppresses mTORC1 Activity during Prolonged Leucine Deprivation. J. Nutr. 2020, 150, 1022–1030. [Google Scholar] [CrossRef]
- Burton, G.J.; Yung, H.W. Endoplasmic reticulum stress in the pathogenesis of early-onset pre-eclampsia. Pregnancy Hypertens. 2011, 1, 72–78. [Google Scholar] [CrossRef]
- Tsai, S.A.; Bendriem, R.M.; Lee, C.D. The cellular basis of fetal endoplasmic reticulum stress and oxidative stress in drug-induced neurodevelopmental deficits. Neurobiol. Stress. 2019, 10, 100145. [Google Scholar] [CrossRef]
- Tsukada, T.; Sakata-Haga, H.; Shimada, H.; Shoji, H.; Hatta, T. Mid-pregnancy maternal immune activation increases Pax6-positive and Tbr2-positive neural progenitor cells and causes integrated stress response in the fetal brain in a mouse model of maternal viral infection. IBRO Neurosci. Rep. 2021, 11, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Kasai, S.; Yamazaki, H.; Tanji, K.; Engler, M.J.; Matsumiya, T.; Itoh, K. Role of the ISR-ATF4 pathway and its cross talk with Nrf2 in mitochondrial quality control. J. Clin. Biochem. Nutr. 2019, 64, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pakos-Zebrucka, K.; Koryga, I.; Mnich, K.; Ljujic, M.; Samali, A.; Gorman, A.M. The integrated stress response. EMBO Rep. 2016, 17, 1374–1395. [Google Scholar] [CrossRef] [Green Version]
- Uruno, A.; Furusawa, Y.; Yagishita, Y.; Fukutomi, T.; Muramatsu, H.; Negishi, T.; Sugawara, A.; Kensler, T.W.; Yamamoto, M. The Keap1-Nrf2 system prevents onset of diabetes mellitus. Mol. Cell Biol. 2013, 33, 2996–3010. [Google Scholar] [CrossRef] [Green Version]
- Pendleton, A.L.; Wesolowski, S.R.; Regnault, T.R.H.; Lynch, R.M.; Limesand, S.W. Dimming the Powerhouse: Mitochondrial Dysfunction in the Liver and Skeletal Muscle of Intrauterine Growth Restricted Fetuses. Front Endocrinol. 2021, 12, 612888. [Google Scholar] [CrossRef]
- Mayeur, S.; Lancel, S.; Theys, N.; Lukaszewski, M.A.; Duban-Deweer, S.; Bastide, B.; Hachani, J.; Cecchelli, R.; Breton, C.; Gabory, A.; et al. Maternal calorie restriction modulates placental mitochondrial biogenesis and bioenergetic efficiency: Putative involvement in fetoplacental growth defects in rats. Am. J. Physiol. Endocrinol. Metab. 2013, 304, E14–E22. [Google Scholar] [CrossRef] [PubMed]



| Gene (NCBI ID) | Forward Primer | Reverse Primer |
|---|---|---|
| Acacb (100705) | CCTTTGGCAACAAGCAAGGTA | AGTCGTACACATAGGTGGTCC |
| Atf4 (11911) | CCTGAACAGCGAAGTGTTGG | TGGAGAACCCATGAGGTTTCAA |
| Chac1 (69065) | CTGTGGATTTTCGGGTACGG | CCCCTATGGAAGGTGTCTCC |
| Ddit3 (13198) | AAGCCTGGTATGAGGATCTGC | TTCCTGGGGATGAGATATAGGTG |
| Fasn (14104) | GGAGGTGGTGATAGCCGGTAT | TGGGTAATCCATAGAGCCCAG |
| Gsta1 (14857) | AAGCCCGTGCTTCACTACTTC | GGGCACTTGGTCAAACATCAAA |
| Gsta4 (14860) | TGATTGCCGTGGCTCCATTTA | CAACGAGAAAAGCCTCTCCGT |
| G6pase (14377) | CTAGCTTTGATCTGGTTGTCAG | GTTGAACCAGTCTCCGACCA |
| Nqo1 (18104) | CATTCTGAAAGGCTGGTTTGA | CTAGCTTTGATCTGGTTGTCAG |
| Pck1 (18534) | CTGCATAACGGTCTGGACTTC | CAGCAACTGCCCGTACTCC |
| Rps29 (20090) | TCTACTGGAGTCACCCACGGAA | GGAAGCACTGGCGGCACA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Habeos, G.I.; Filippopoulou, F.; Habeos, E.E.; Kalaitzopoulou, E.; Skipitari, M.; Papadea, P.; Lagoumintzis, G.; Niarchos, A.; Georgiou, C.D.; Chartoumpekis, D.V. Maternal Calorie Restriction Induces a Transcriptional Cytoprotective Response in Embryonic Liver Partially Dependent on Nrf2. Antioxidants 2022, 11, 2274. https://doi.org/10.3390/antiox11112274
Habeos GI, Filippopoulou F, Habeos EE, Kalaitzopoulou E, Skipitari M, Papadea P, Lagoumintzis G, Niarchos A, Georgiou CD, Chartoumpekis DV. Maternal Calorie Restriction Induces a Transcriptional Cytoprotective Response in Embryonic Liver Partially Dependent on Nrf2. Antioxidants. 2022; 11(11):2274. https://doi.org/10.3390/antiox11112274
Chicago/Turabian StyleHabeos, George I., Fotini Filippopoulou, Evagelia E. Habeos, Electra Kalaitzopoulou, Marianna Skipitari, Polyxeni Papadea, George Lagoumintzis, Athanasios Niarchos, Christos D. Georgiou, and Dionysios V. Chartoumpekis. 2022. "Maternal Calorie Restriction Induces a Transcriptional Cytoprotective Response in Embryonic Liver Partially Dependent on Nrf2" Antioxidants 11, no. 11: 2274. https://doi.org/10.3390/antiox11112274









