Synthesis, Conformational Analysis and ctDNA Binding Studies of Flavonoid Analogues Possessing the 3,5-di-tert-butyl-4-hydroxyphenyl Moiety
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis and Characterization
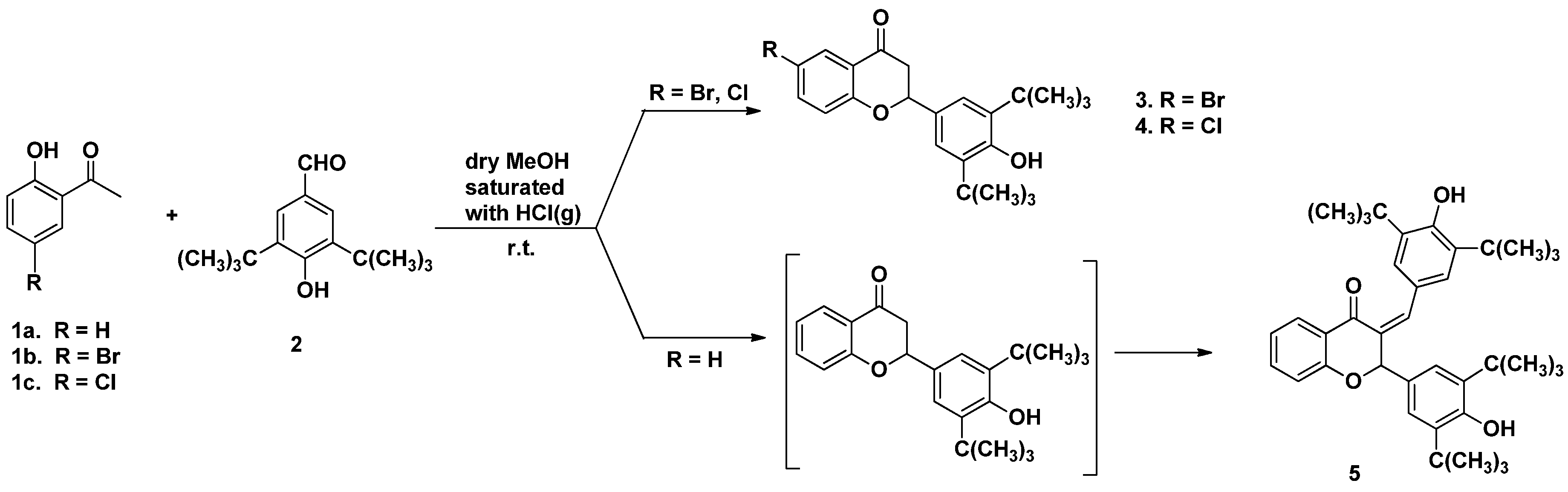
General Procedure for the Synthesis of Flavanones 3–5
- 6-bromo-2-(3,5-di-tert-butyl-4-hydroxyphenyl) chromane-4-one (3)
- 6-chloro-2-(3,5,-di-tert-butyl-4-hydroxyphenyl) chromane-4-one (4)
- 3-(4′-hydroxy-3′,5′-di-tert-butylbenzylidene)-4″-hydroxy-3″,5″-di-tert-butyl-flavanone (5)
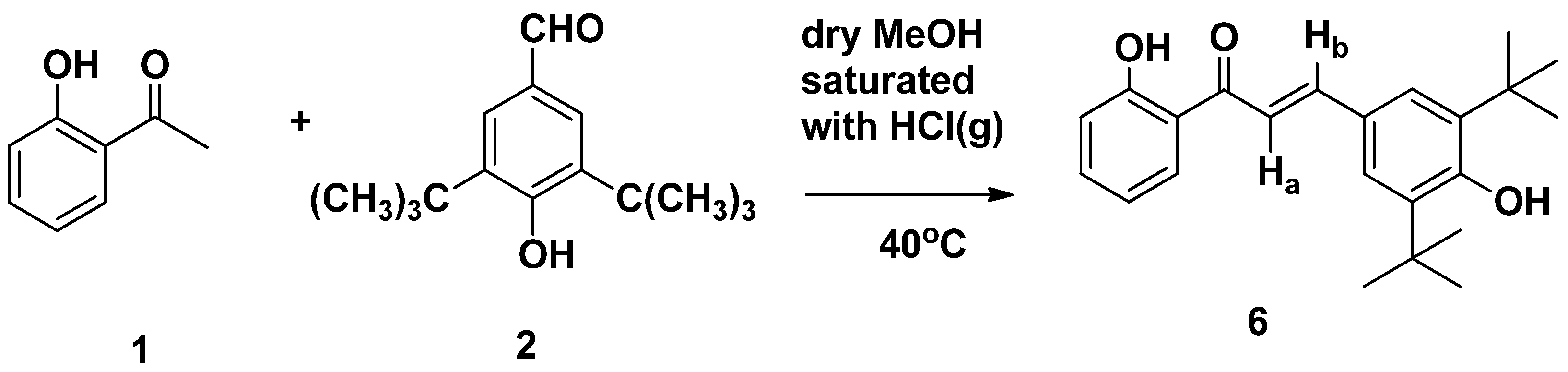
- 2′,4-Dihydroxy-3,5-di-(tert-butyl)-chalcone (6)
2.2. In Vitro Assays
2.2.1. DPPH Radical Scavenging Ability
2.2.2. Inhibition of AAPH Induced Linoleic Acid Oxidation
2.2.3. DNA Binding Studies Using UV-Vis Spectroscopy
2.3. Molecular Docking
2.4. Conformational Analysis
2.5. Calculation of Physicochemical Properties
3. Results
3.1. Chemistry
3.2. Antioxidant Activity
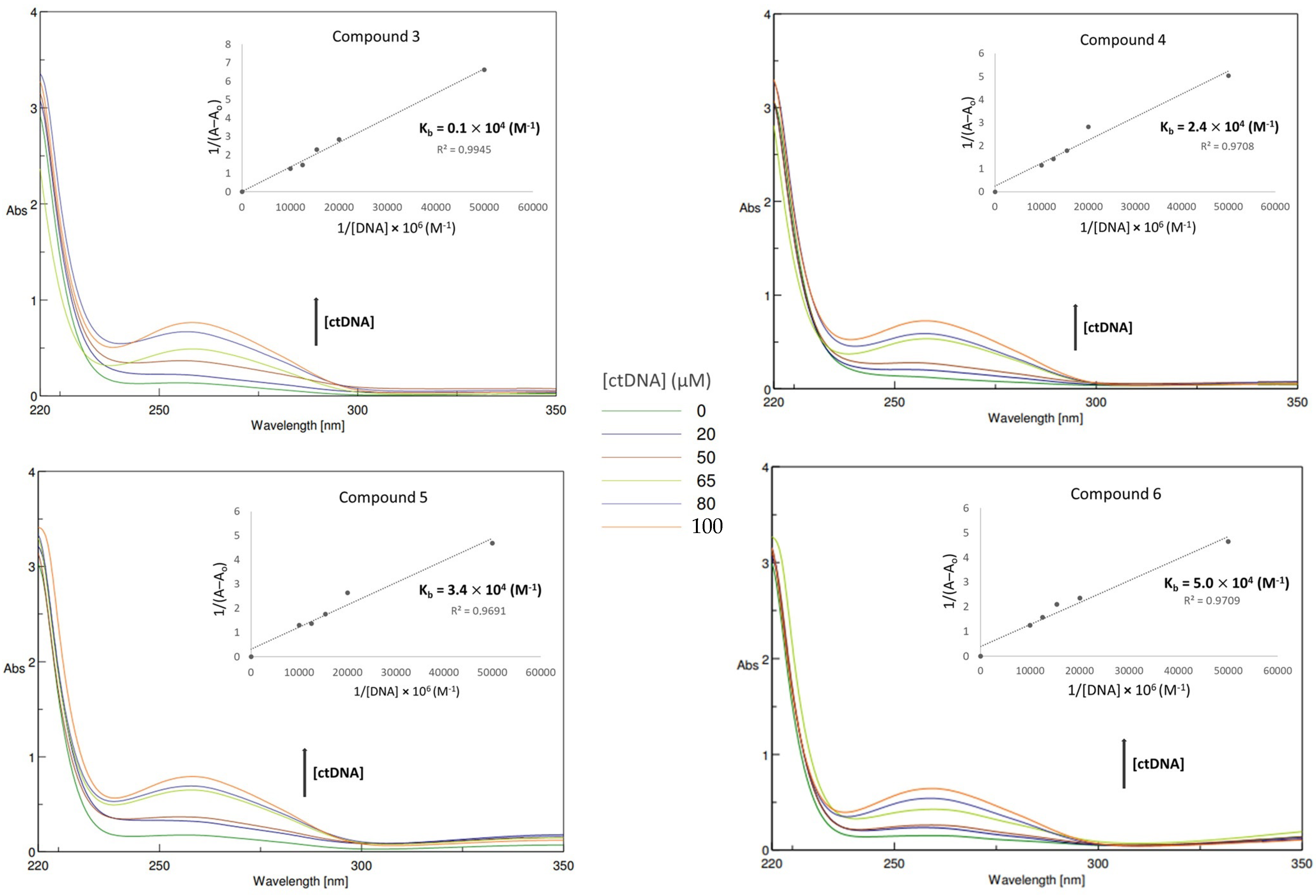
3.3. Binding Studies with ct-DNA Using UV Spectroscopy
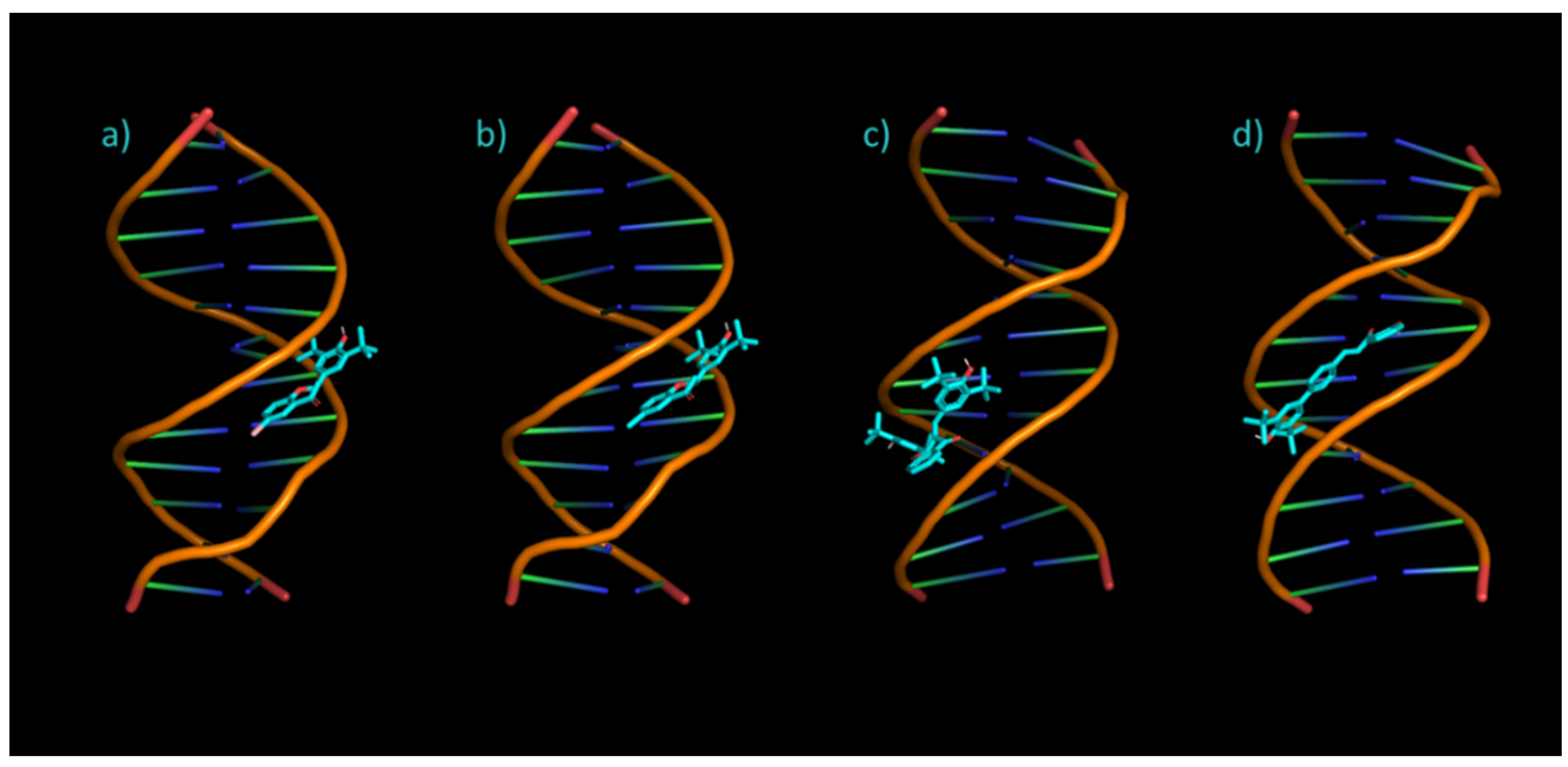
3.4. Molecular Docking
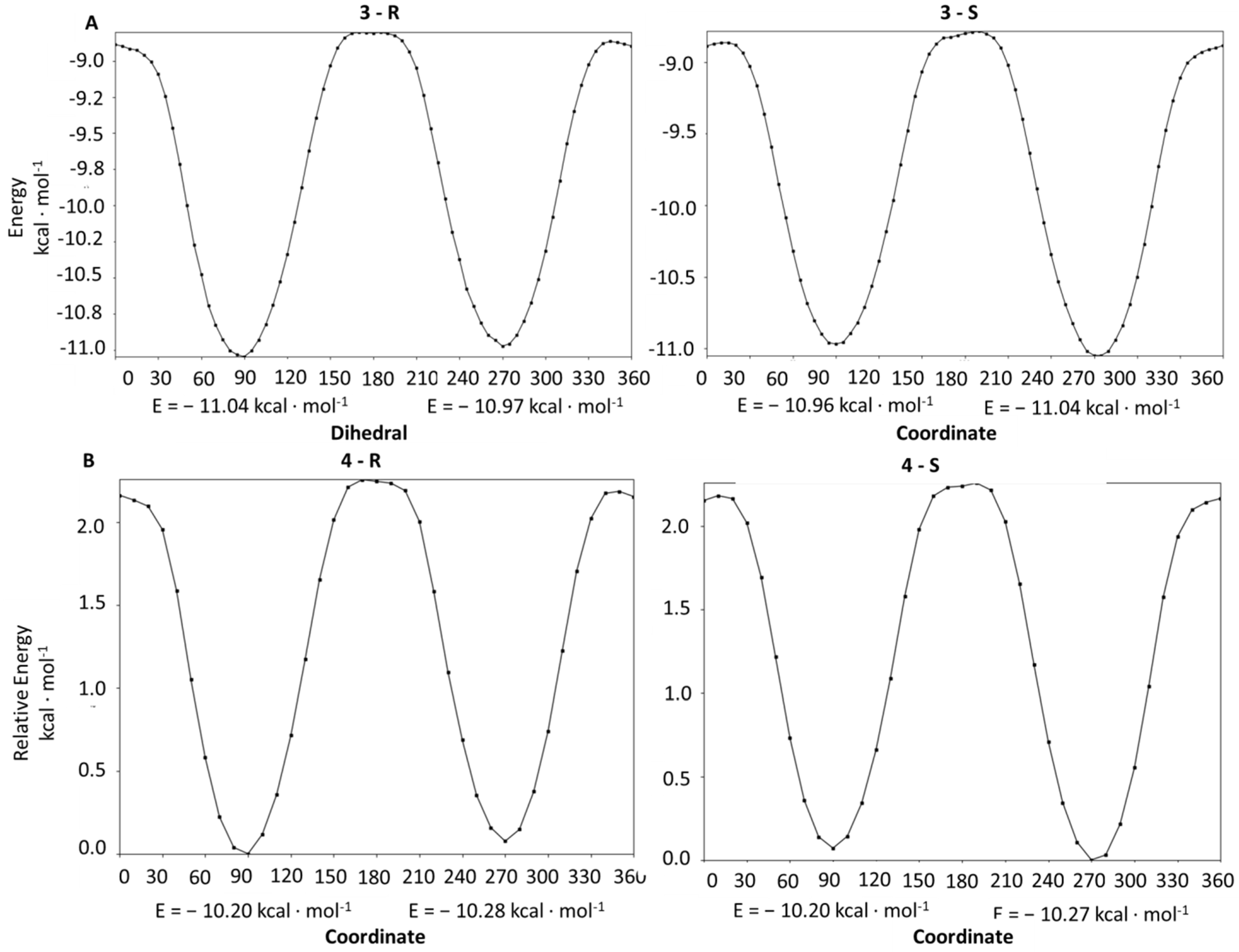
3.5. Conformational Analysis of the Synthesized Compounds
3.6. Calculation of Physicochemical Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
References
- Zhuang, C.; Zhang, W.; Sheng, C.; Zhang, W.; Xing, C.; Miao, Z. Chalcone: A Privileged Structure in Medicinal Chemistry. Chem. Rev. 2017, 117, 7762–7810. [Google Scholar] [CrossRef]
- Kostopoulou, I.; Detsi, A. Recent Developments on Tyrosinase Inhibitors based on the Chalcone and Aurone Scaffolds. Curr. Enzym. Inhib. 2018, 14, 3–17. [Google Scholar] [CrossRef]
- Detsi, A.; Majdalani, M.; Kontogiorgis, C.A.; Hadjipavlou-Litina, D.; Kefalas, P. Natural and synthetic 2′-hydroxy-chalcones and aurones: Synthesis, characterization and evaluation of the antioxidant and soybean lipoxygenase inhibitory activity. Bioorg. Med. Chem. 2009, 17, 8073–8085. [Google Scholar] [CrossRef]
- Kostopoulou, I.; Tzani, A.; Polyzos, N.-I.; Karadendrou, M.-A.; Kritsi, E.; Pontiki, E.; Liargkova, T.; Hadjipavlou-Litina, D.; Zoumpoulakis, P.; Detsi, A. Exploring the 2-Hydroxy-Chalcone Framework for the Development of Dual Antioxidant and Soybean Lipoxygenase Inhibitory Agents. Molecules 2021, 26, 2777. [Google Scholar] [CrossRef]
- Santi, M.D.; Peralta, M.A.; Mendoza, C.S.; Cabrera, J.L.; Ortega, M.G. Chemical and bioactivity of flavanones obtained from roots of Dalea pazensis Rusby. Bioorg. Med. Chem. Lett. 2017, 27, 1789–1794. [Google Scholar] [CrossRef]
- Cheenpracha, S.; Pyne, S.G.; Patrick, B.O.; Andersen, R.J.; Maneerat, W.; Laphookhieo, S. Mallopenins A-E, Antibacterial Phenolic Derivatives from the Fruits of Mallotus philippensis. J. Nat. Prod. 2019, 82, 2174–2180. [Google Scholar] [CrossRef]
- Khan, M.K.; Zill-E-Huma; Dangles, O. A comprehensive review on flavanones, the major citrus polyphenols. J. Food Compos. Anal. 2014, 33, 85–104. [Google Scholar] [CrossRef]
- Wang, S.W.; Sheng, H.; Bai, Y.F.; Weng, Y.Y.; Fan, X.Y.; Zheng, F.; Fu, J.Q.; Zhang, F. Inhibition of histone acetyltransferase by naringenin and hesperetin suppresses Txnip expression and protects pancreatic β cells in diabetic mice: Naringenin and hesperetin protect pancreatic β cells. Phytomedicine 2021, 88, 153454. [Google Scholar] [CrossRef]
- Yang, J.; Liu, L.; Li, M.; Huang, X.; Yang, H.; Li, K. Naringenin inhibits pro-inflammatory cytokine production in macrophages through inducing MT1G to suppress the activation of NF-κB. Mol. Immunol. 2021, 137, 155–162. [Google Scholar] [CrossRef]
- Pontifex, M.G.; Malik, M.M.A.H.; Connell, E.; Müller, M.; Vauzour, D. Citrus Polyphenols in Brain Health and Disease: Current Perspectives. Front. Neurosci. 2021, 15, 640648. [Google Scholar] [CrossRef]
- Justino, G.C.; Rodrigues, M.; Florêncio, M.H.; Mira, L. Structure and antioxidant activity of brominated flavonols and flavanones. J. Mass Spectrom. 2009, 44, 1459–1468. [Google Scholar] [CrossRef]
- Özyürek, M.; Akpınar, D.; Bener, M.; Türkkan, B.; Güçlü, K.; Apak, R. Novel oxime based flavanone, naringin-oxime: Synthesis, characterization and screening for antioxidant activity. Chem. Biol. Interact. 2014, 212, 40–46. [Google Scholar] [CrossRef]
- Di Majo, D.; Giammanco, M.; La Guardia, M.; Tripoli, E.; Giammanco, S.; Finotti, E. Flavanones in Citrus fruit: Structure–antioxidant activity relationships. Int. Food Res. J. 2005, 38, 1161–1166. [Google Scholar] [CrossRef]
- Silva, M.M.; Santos, M.R.; Caroço, G.; Rocha, R.; Justino, G.; Mira, L. Structure-antioxidant activity relationships of flavonoids: A re-examination. Free Radic. Res. 2002, 36, 1219–1227. [Google Scholar] [CrossRef]
- Tajammala, A.; Batoola, M.; Ramzana, A.; Samraa, M.M.; Mahnoora, I.; Verpoortb, F.; Irfancd, A.; Al-Sehemicd, A.G.; Munawara, M.A.; Basra, M.A.R. Synthesis, antihyperglycemic activity and computational studies of antioxidant chalcones and flavanones derived from 2, 5 dihydroxyacetophenone. J. Mol. Struct. 2017, 1148, 512–520. [Google Scholar] [CrossRef]
- Lee, J.H. In-vitro evaluation for antioxidant and anti-inflammatory property of flavanone derivatives. Food Biosci. 2015, 11, 1–7. [Google Scholar] [CrossRef]
- Rice-Evans, C.A.; Miller, N.J.; Paganga, G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic. Biol. Med. 1996, 20, 933–956. [Google Scholar] [CrossRef]
- Ahmed, S.; Shakeel, F. Antioxidant activity coefficient, mechanism, and kinetics of different derivatives of flavones and flavanones towards superoxide radical. Czech J. Food Sci. 2012, 30, 153–163. [Google Scholar] [CrossRef] [Green Version]
- Chuang, S.Y.; Lin, Y.K.; Lin, C.F.; Wang, P.W.; Chen, E.L.; Fang, J.Y. Elucidating the skin delivery of aglycone and glycoside flavonoids: How the structures affect cutaneous absorption. Nutrients 2017, 9, 1304. [Google Scholar] [CrossRef] [Green Version]
- Speisky, H.; Shahidi, F.; Costa de Camargo, A.; Fuentes, J. Revisiting the oxidation of flavonoids: Loss, conservation or enhancement of their antioxidant properties. Antioxidants 2022, 11, 133. [Google Scholar] [CrossRef]
- Mira, L.; Tereza Fernandez, M.; Santos, M.; Rocha, R.; Helena Florêncio, M.; Jennings, K.R. Interactions of flavonoids with iron and copper ions: A mechanism for their antioxidant activity. Free Radic. Res. 2002, 36, 1199–1208. [Google Scholar] [CrossRef]
- Hrelia, S.; Angeloni, C. New mechanisms of action of natural antioxidants in health and disease. Antioxidants 2020, 9, 344. [Google Scholar] [CrossRef] [Green Version]
- Procházková, D.; Boušová, I.; Wilhelmová, N. Antioxidant and prooxidant properties of flavonoids. Fitoterapia 2011, 82, 513–523. [Google Scholar] [CrossRef]
- De Souza Farias, S.A.; da Costa, K.S.; Martins, J.B. Analysis of conformational, structural, magnetic, and electronic properties related to antioxidant activity: Revisiting flavan, anthocyanidin, flavanone, flavonol, isoflavone, flavone, and flavan-3-ol. ACS Omega 2021, 6, 8908–8918. [Google Scholar] [CrossRef]
- Mottaghipisheh, J.; Stuppner, H. A comprehensive review on chemotaxonomic and phytochemical aspects of homoisoflavonoids, as rare flavonoid derivatives. Int. J. Mol. Sci. 2021, 22, 2735. [Google Scholar] [CrossRef]
- Jeong, H.J.; Kim, Y.M.; Kim, J.H.; Kim, J.Y.; Park, J.Y.; Park, S.J.; Ryu, Y.B.; Lee, W.S. Homoisoflavonoids from Caesalpinia sappan displaying viral neuraminidases inhibition. Biol. Pharm. Bull. 2012, 35, 786–790. [Google Scholar] [CrossRef] [Green Version]
- Cuong, T.D.; Hung, T.M.; Kim, J.C.; Kim, E.H.; Woo, M.H.; Choi, J.S.; Lee, J.H.; Min, B.S. Phenolic compounds from caesalpinia sappan heartwood and their anti-inflammatory activity. J. Nat. Prod. 2012, 75, 2069–2075. [Google Scholar] [CrossRef]
- Siddaiah, V.; Rao, C.V.; Venkateswarlu, S.; Krishnaraju, A.V.; Subbaraju, G.V. Synthesis, stereochemical assignments, and biological activities of homoisoflavonoids. Bioorganic Med. Chem. 2006, 14, 2545–2551. [Google Scholar] [CrossRef]
- Zhao, J.; Zhu, A.; Sun, Y.; Zhang, W.; Zhang, T.; Gao, Y.; Shan, D.; Wang, S.; Li, G.; Zeng, K.; et al. Beneficial effects of sappanone A on lifespan and thermotolerance in Caenorhabditis elegans. Eur. J. Pharmacol. 2020, 888, 173558. [Google Scholar] [CrossRef]
- Roy, S.K.; Kumari, N.; Gupta, S.; Pahwa, S.; Nandanwar, H.; Jachak, S.M. 7-Hydroxy-(E)-3-phenylmethylene-chroman-4-one analogues as efflux pump inhibitors against Mycobacterium smegmatis mc2 155. Eur. J. Med. Chem. 2013, 66, 499–507. [Google Scholar] [CrossRef]
- Roy, S.K.; Agrahari, U.C.; Gautam, R.; Srivastava, A.; Jachak, S.M. Isointricatinol, a new antioxidant homoisoflavonoid from the roots of Caesalpinia digyna Rottler. Nat. Prod. Res. 2012, 26, 690–695. [Google Scholar] [CrossRef]
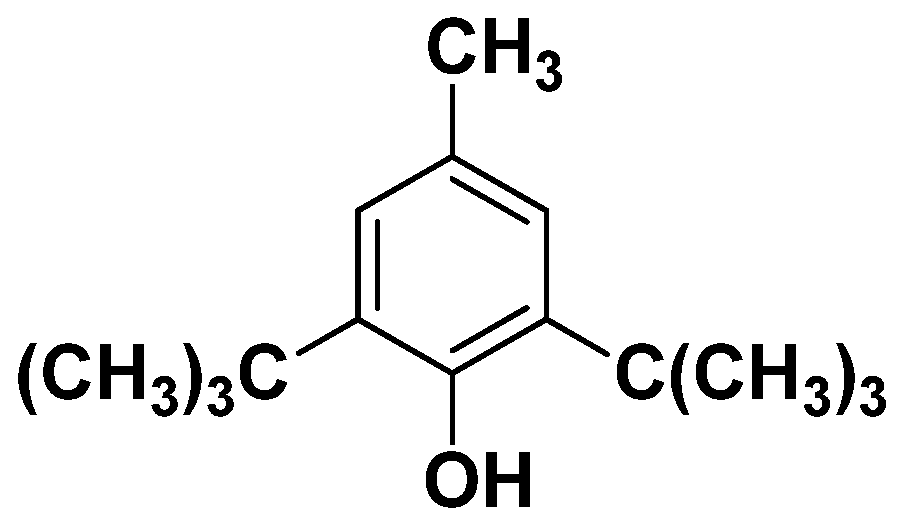
- Yehye, W.A.; Rahman, N.A.; Ariffin, A.; Abd Hamid, S.B.; Alhadi, A.A.; Kadir, F.A.; Yaeghoobi, M. Understanding the chemistry behind the antioxidant activities of butylated hydroxytoluene (BHT): A review. Eur. J. Med. Chem. 2015, 101, 295–312. [Google Scholar] [CrossRef]
- Kostopoulou, I.; Diassakou, A.; Kavetsou, E.; Kritsi, E.; Zoumpoulakis, P.; Pontiki, E.; Hadjipavlou-Litina, D.; Detsi, A. Novel quinolinone–pyrazoline hybrids: Synthesis and evaluation of antioxidant and lipoxygenase inhibitory activity. Mol. Divers. 2020, 25, 723–740. [Google Scholar] [CrossRef]
- Hadjipavlou-Litina, D.; Garnelis, T.; Athanassopoulos, C.M.; Papaioannou, D. Kukoamine A analogs with lipoxygenase inhibitory activity. J. Enzyme Inhib. Med. Chem. 2009, 24, 1188–1193. [Google Scholar] [CrossRef]
- Sirajuddin, M.; Ali, S.; Badshah, A. Drug-DNA interactions and their study by UV-Visible, fluorescence spectroscopies and cyclic voltametry. J. Photochem. Photobiol. B Biol. 2013, 124, 1–19. [Google Scholar] [CrossRef]
- Shahabadi, N.; Fili, S.M.; Kheirdoosh, F. Study on the interaction of the drug mesalamine with calf thymus DNA using molecular docking and spectroscopic techniques. J. Photochem. Photobiol. B Biol. 2013, 128, 20–26. [Google Scholar] [CrossRef]
- Tzani, A.; Kalafateli, S.; Tatsis, G.; Bairaktari, M.; Kostopoulou, I.; Pontillo, A.R.; Detsi, A. Natural Deep Eutectic Solvents (NaDESs) as Alternative Green Extraction Media for Ginger (Zingiber officinale Roscoe). Sustain. Chem. 2021, 2, 576–598. [Google Scholar]
- Pontillo, A.R.N.; Konstanteli, E.; Bairaktari, M.M.; Detsi, A. Encapsulation of the natural product tyrosol in carbohydrate nanosystems and study of their binding with ctdna. Polymers 2021, 13, 87. [Google Scholar] [CrossRef]
- Adams, J.H. Influence of bulky substituents on the syntheses of 4-hydroxy-3, 5-dialkyl flavonoids. J. Org. Chem. 1967, 32, 3992–3998. [Google Scholar]
- Katopodi, A.; Tsotsou, E.; Iliou, T.; Deligiannidou, G.E.; Pontiki, E.; Kontogiorgis, C.; Tsopelas, F.; Detsi, A. Synthesis, bioactivity, pharmacokinetic and biomimetic properties of multi-substituted coumarin derivatives. Molecules 2021, 26, 5999. [Google Scholar] [CrossRef]
- Schrödinger Release 2020-3: MacroModel; Schrödinger, LLC: New York, NY, USA, 2020.
- Jorgensen, W.L.; Maxwell, D.S.; Tirado-Rives, J. Development and testing of the OPLS all-atom force field on conformational energetics and properties of organic liquids. J. Am. Chem. Soc. 1996, 118, 11225–11236. [Google Scholar] [CrossRef]
- Polak, E.; Ribiere, G. Revenue Francaise Informat. Rech. Oper. 1969, 16, 35. [Google Scholar]
- Schrödinger Release 2020-3: Maestro; Schrödinger, LLC: New York, NY, USA, 2020.
- Schrödinger Release 2020-3: QikProp; Schrödinger, LLC: New York, NY, USA, 2020.
- Enes, R.F.; Farinha, A.S.F.; Tomé, A.C.; Cavaleiro, J.A.S.; Amorati, R.; Petrucci, S.; Pedulli, G.F. Synthesis and antioxidant activity of [60] fullerene-flavonoid conjugates. Tetrahedron 2009, 65, 253–262. [Google Scholar] [CrossRef]
- Zhang, Y.L.; Wang, Y.Q. Enantioselective biomimetic cyclization of 2′-hydroxychalcones to flavanones. Tetrahedron Lett. 2014, 55, 3255–3258. [Google Scholar] [CrossRef]
- Desai, J.; Nair, K.B.; Misra, A.N. Synthesis and antimicrobial activities of some new pyrazolines phenyl pyrazolines, flavanones and related compounds. Indian J. Heterocycl. Chem. 2001, 10, 261–266. [Google Scholar]
- Islam, M.M.; Chakraborty, M.; Pandya, P.; Al Masum, A.; Gupta, N.; Mukhopadhyay, S. Binding of DNA with Rhodamine B: Spectroscopic and molecular modeling studies. Dye. Pigment. 2013, 99, 412–422. [Google Scholar] [CrossRef]
- Prieto, D.; Aparicio, G.; Morande, P.E.; Zolessi, F.R. A fast, low cost, and highly efficient fluorescent DNA labeling method using methyl green. Histochem. Cell Biol. 2014, 142, 335–345. [Google Scholar] [CrossRef]
- Ávila, E.P.; Mendes, L.A.O.; De Almeida, W.B.; Santos, H.F.D.; De Almeida, M.V. Conformational analysis and reactivity of naringenin. J. Mol. Struct. 2021, 1245, 131027. [Google Scholar] [CrossRef]

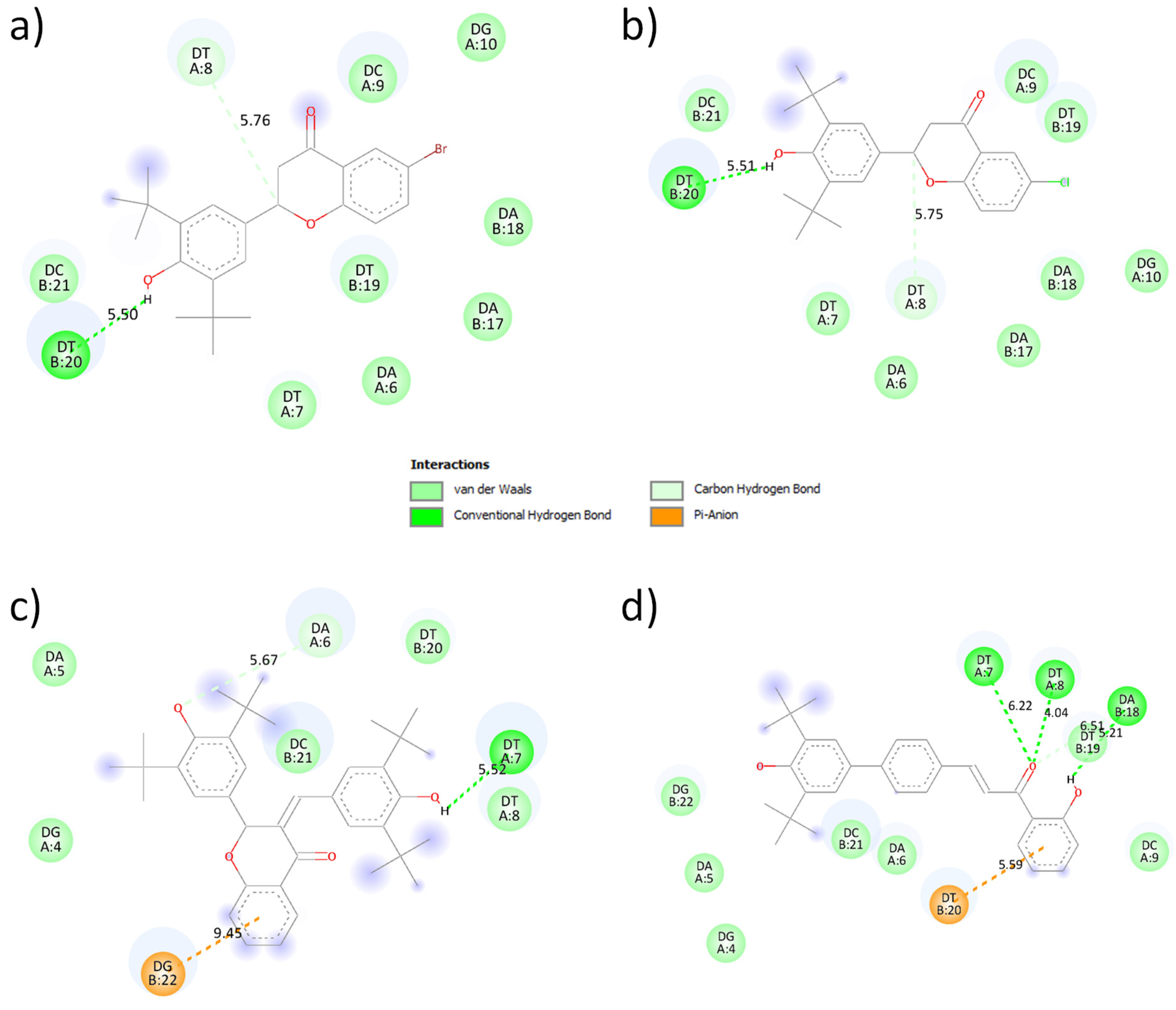


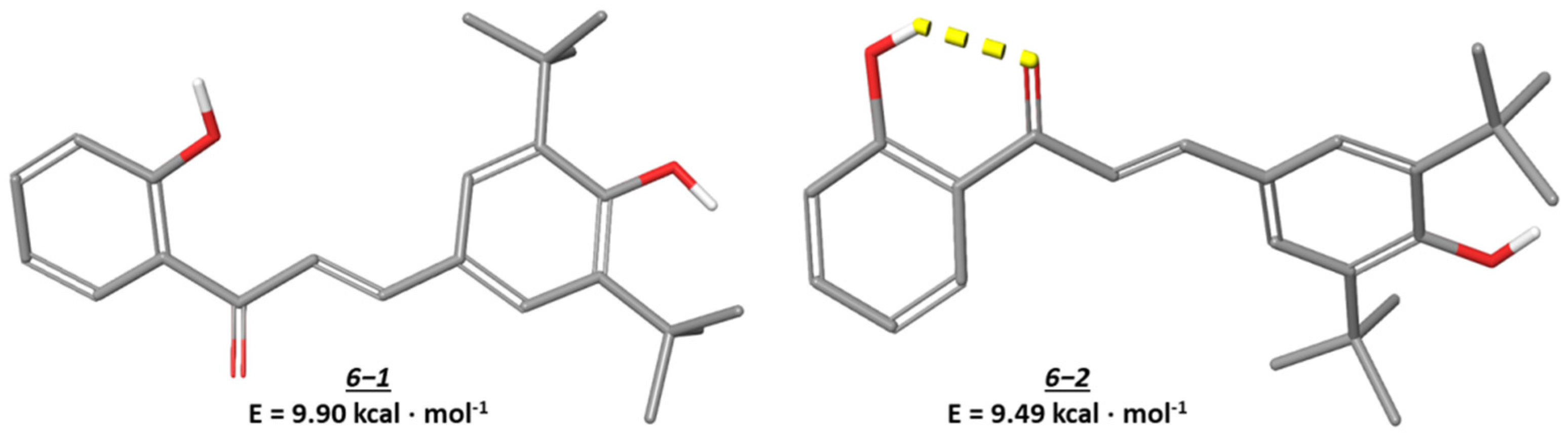
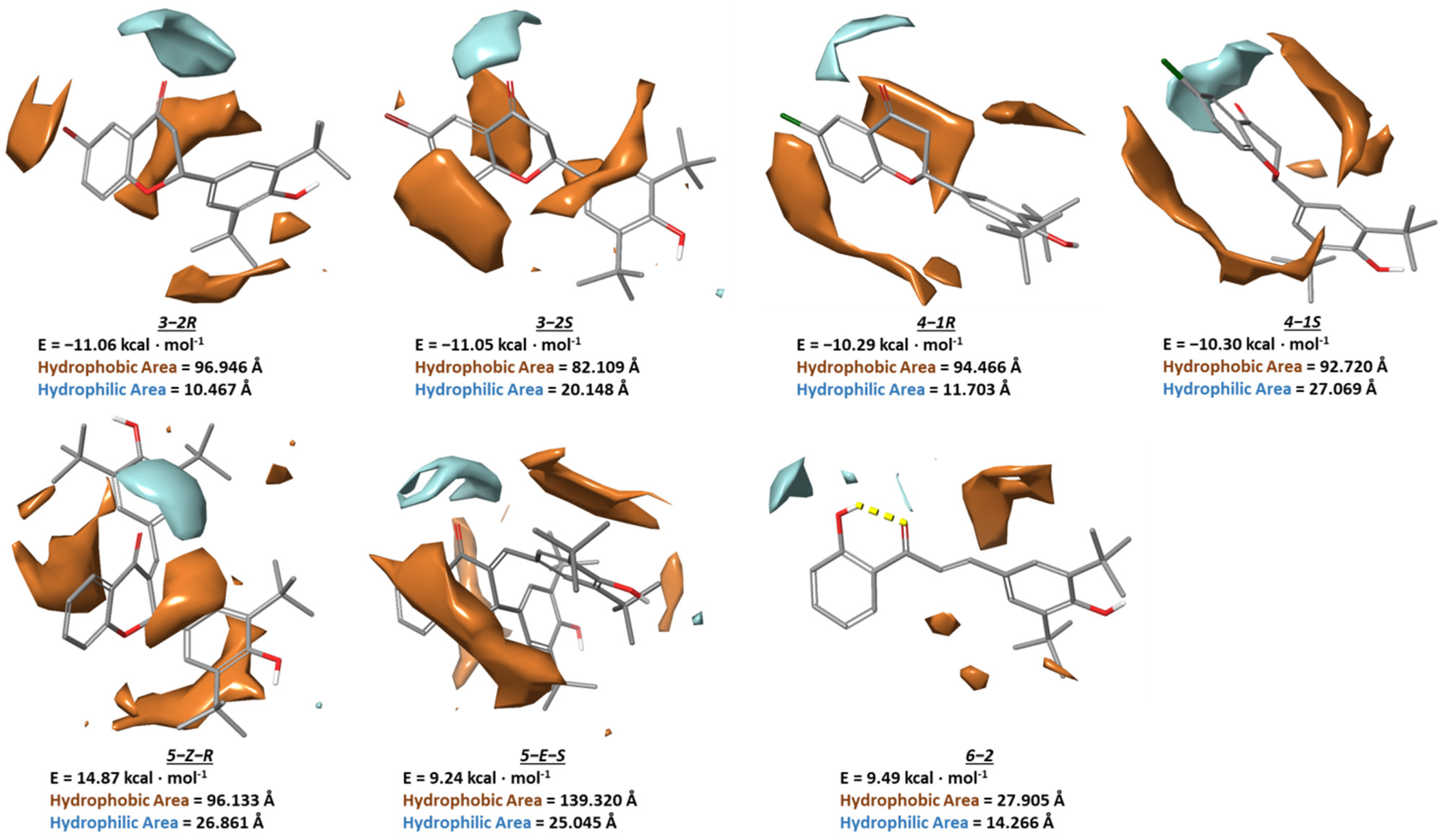
| Compound | Structure | % Inhibition of DPPH Free Radical (100μM) | % Inhibition of Lipid Peroxidation of Linoleic Acid Induced by AAPH Radical (100 μM) | |
|---|---|---|---|---|
| 30 min | 60 min | |||
| 3 | 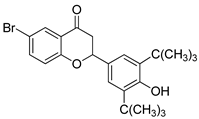 | 29.3 | 28.2 | 21.1 |
| 4 | 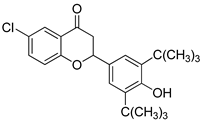 | 32.0 | 39.6 | 59.3 |
| 5 | 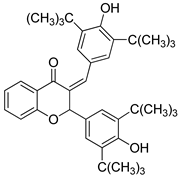 | 70.8 | 60.2 | 77.4 |
| 6 | 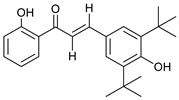 | 61.1 | 39.9 | 54.6 |
| TROLOX | 82.4 | 83.4 | 81.4 | |
| Compound | Structure | Κb (M−1) |
|---|---|---|
| 3 | 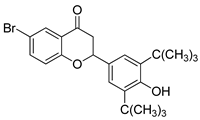 | 0.1 103 |
| 4 |  | 2.4 103 |
| 5 |  | 3.4 103 |
| 6 | 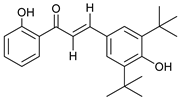 | 5.0 103 |
| Rhodamine B | 2.2 104 | |
| Methyl Green | 2.0 103 | |
| Compound | Binding Energy (kcal/mol) | Inhibition Constant Ki | vdW + Bond + Desolve Energy (kcal/mol) | Electrostatic Energy (kcal/mol) |
|---|---|---|---|---|
| 3 | −9.01 | 250.10 nM | −10.10 | −0.10 |
| 4 | −8.97 | 316.49 nM | −9.97 | −0.09 |
| 5 | −6.35 | 22.11 μM | −8.64 | −0.10 |
| 6 | −9.06 | 229.64 nM | −11.52 | +0.07 |
| Physicochemical Property | Compounds | |||
|---|---|---|---|---|
| 3 | 4 | 5 | 6 | |
| Dipole | 4.97 | 5.26 | 5.87 | 4.81 |
| SASA | 687.00 | 681.97 | 893.34 | 680.30 |
| FOSA | 412.17 | 412.12 | 642.62 | 378.37 |
| FISA | 57.75 | 57.76 | 49.28 | 90.75 |
| PISA | 139.80 | 140.52 | 201.43 | 211.18 |
| WPSA | 77.33 | 71.58 | 0 | 0 |
| volume | 1256.42 | 1247.51 | 1852.64 | 1231.98 |
| donorHB | 1 | 1 | 2 | 1 |
| accptHB | 3.5 | 3.5 | 4.25 | 2.5 |
| dip2/V | 0.019641 | 0.0222095 | 0.0186334 | 0.0187806 |
| QPpolrz | 43.54 | 43.19 | 64.77 | 40.03 |
| QPlogPC16 | 12.06 | 11.94 | 17.30 | 12.02 |
| QPlogPoct | 17.94 | 17.83 | 26.99 | 15.87 |
| QPlogPw | 6.99 | 6.98 | 8.92 | 5.57 |
| QPlogPo/w | 5.77 | 5.69 | 8.90 | 5.60 |
| QPlogS | −7.36 | −7.24 | −9.49 | −6.46 |
| IP(eV) | 9.04 | 9.03 | 8.88 | 8.86 |
| EA(eV) | 0.79 | 0.77 | 0.71 | 0.88 |
| PSA | 47.40 | 47.40 | 57.11 | 58.20 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tzani, A.; Kritsi, E.; Tsamantioti, L.; Kostopoulou, I.; Karadendrou, M.-A.; Zoumpoulakis, P.; Detsi, A. Synthesis, Conformational Analysis and ctDNA Binding Studies of Flavonoid Analogues Possessing the 3,5-di-tert-butyl-4-hydroxyphenyl Moiety. Antioxidants 2022, 11, 2273. https://doi.org/10.3390/antiox11112273
Tzani A, Kritsi E, Tsamantioti L, Kostopoulou I, Karadendrou M-A, Zoumpoulakis P, Detsi A. Synthesis, Conformational Analysis and ctDNA Binding Studies of Flavonoid Analogues Possessing the 3,5-di-tert-butyl-4-hydroxyphenyl Moiety. Antioxidants. 2022; 11(11):2273. https://doi.org/10.3390/antiox11112273
Chicago/Turabian StyleTzani, Andromachi, Eftichia Kritsi, Lamprini Tsamantioti, Ioanna Kostopoulou, Maria-Anna Karadendrou, Panagiotis Zoumpoulakis, and Anastasia Detsi. 2022. "Synthesis, Conformational Analysis and ctDNA Binding Studies of Flavonoid Analogues Possessing the 3,5-di-tert-butyl-4-hydroxyphenyl Moiety" Antioxidants 11, no. 11: 2273. https://doi.org/10.3390/antiox11112273
APA StyleTzani, A., Kritsi, E., Tsamantioti, L., Kostopoulou, I., Karadendrou, M.-A., Zoumpoulakis, P., & Detsi, A. (2022). Synthesis, Conformational Analysis and ctDNA Binding Studies of Flavonoid Analogues Possessing the 3,5-di-tert-butyl-4-hydroxyphenyl Moiety. Antioxidants, 11(11), 2273. https://doi.org/10.3390/antiox11112273










