Systematic Review of the Therapeutic Role of Apoptotic Inhibitors in Neurodegeneration and Their Potential Use in Schizophrenia
Abstract
:1. Introduction
1.1. Synaptic Pruning in SZ
1.2. Apoptotic Molecular Studies in Neurodegenerative Disorders and Overlap with SZ
1.3. Apoptotic Molecular Studies in SZ
2. Materials and Methods
2.1. Inclusion and exclusion criteria
2.2. Primary and Secondary Outcomes
3. Results
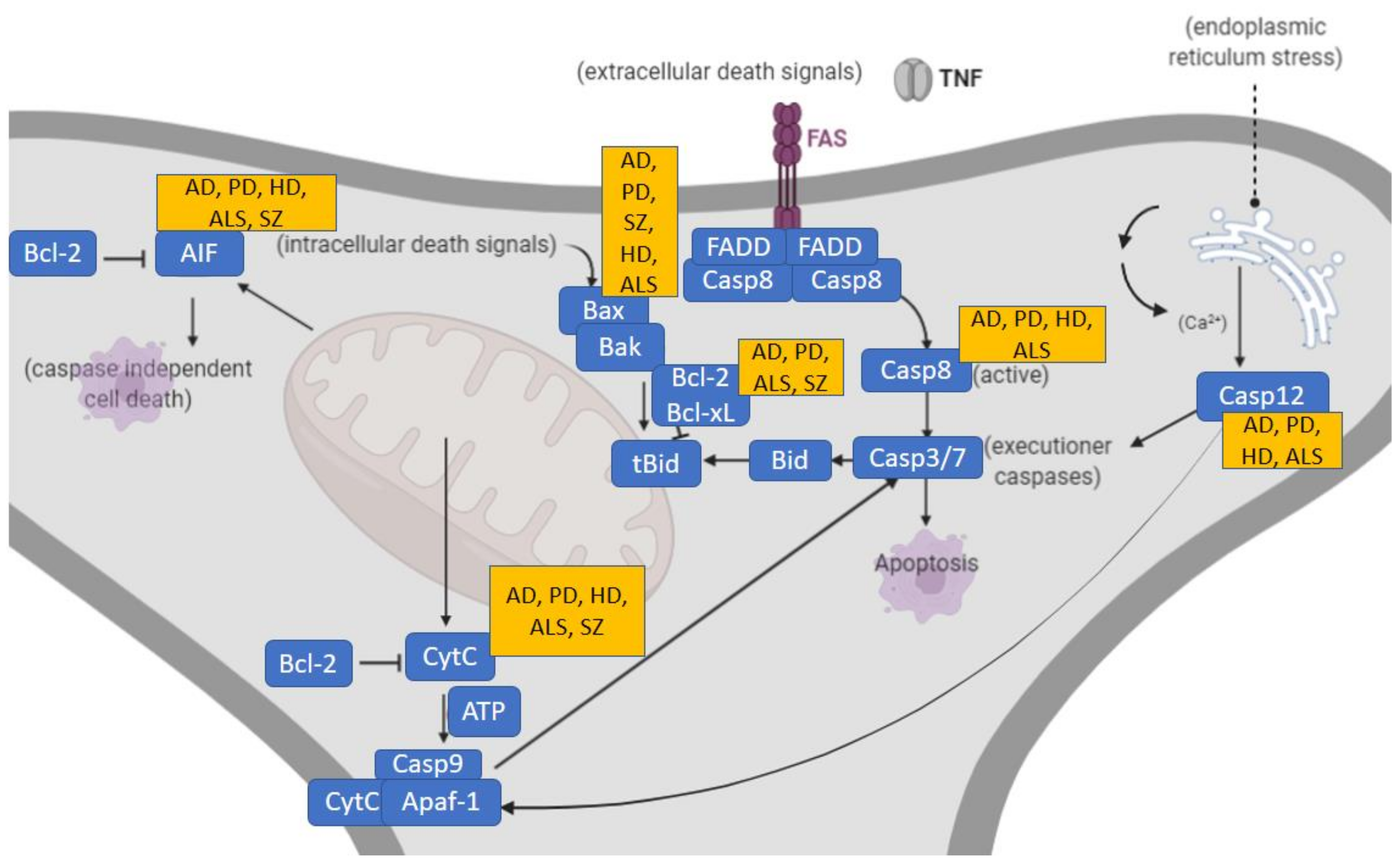
3.1. Apoptotic Alterations in Classic Neurodegenerative Disorders
3.2. Intervention of Apoptotic Molecular Pathways in Neurodegenerative Disorders
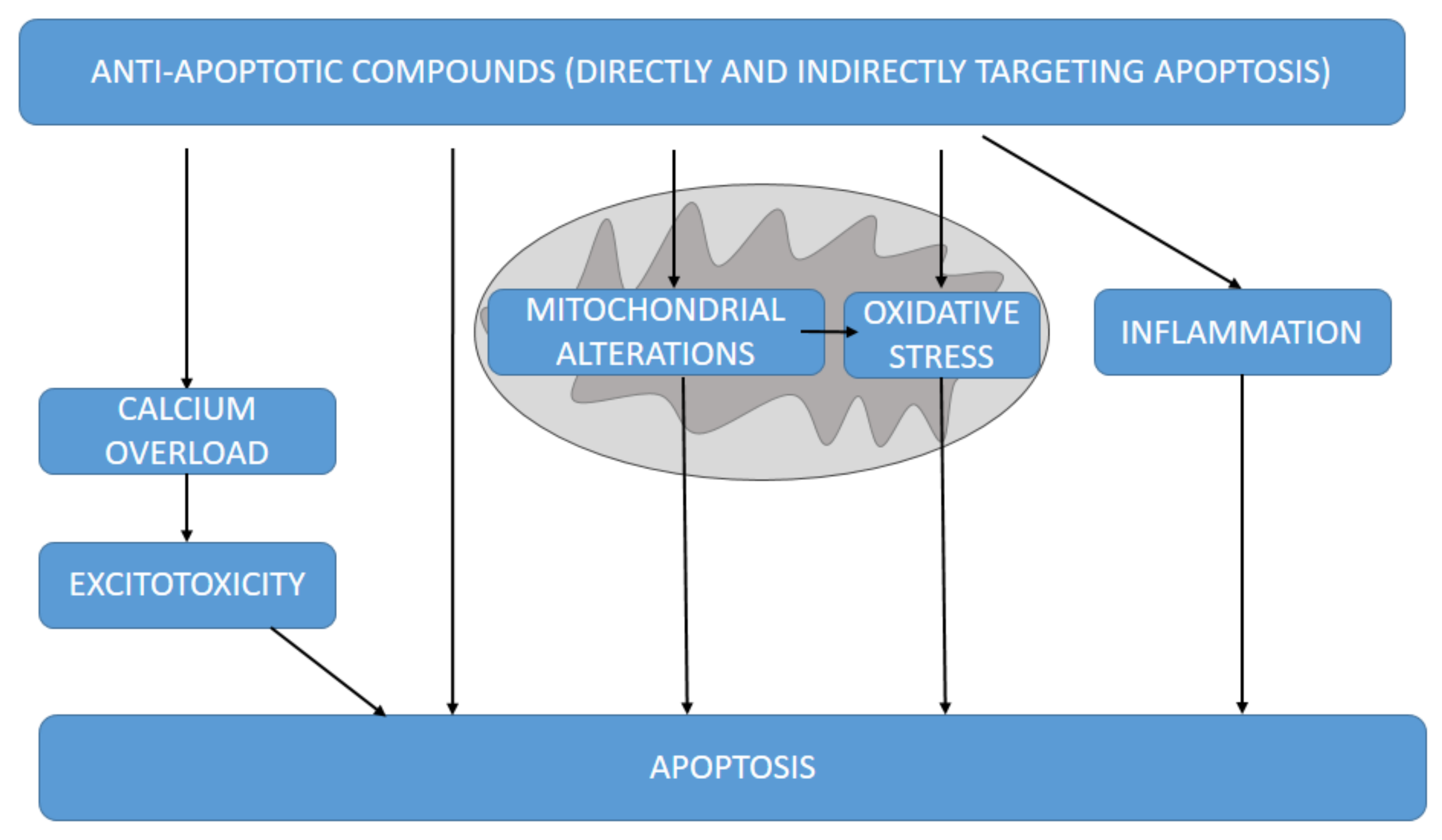
3.3. Mitochondrial Anti-Apoptotic Targets against Neurodegeneration
3.4. Other Anti-Apoptotic-Related Molecular Pathways Associated with Neuroprotection
3.5. Targeting Apoptosis in SZ
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Marder, S.R.; Cannon, T.D. Schizophrenia. N. Engl. J. Med. 2019, 381, 1753–1761. [Google Scholar] [CrossRef]
- Owen, M.J.; O’Donovan, M.; Thapar, A.; Craddock, N. Neurodevelopmental hypothesis of schizophrenia. Br. J. Psychiatry 2011, 198, 173–175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moyer, C.E.; Shelton, M.A.; Sweet, R.A. Dendritic spine alterations in schizophrenia. Neurosci. Lett. 2015, 601, 46–53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cannon, T.D. How Schizophrenia Develops: Cognitive and Brain Mechanisms Underlying Onset of Psychosis. Trends Cogn. Sci. 2015, 19, 744–756. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Talukdar, P.M.; Abdul, F.; Maes, M.; Binu, V.; Venkatasubramanian, G.; Kutty, B.M.; Debnath, M. Maternal Immune Activation Causes Schizophrenia-like Behaviors in the Offspring through Activation of Immune-Inflammatory, Oxidative and Apoptotic Pathways, and Lowered Antioxidant Defenses and Neuroprotection. Mol. Neurobiol. 2020, 57, 4345–4361. [Google Scholar] [CrossRef]
- Vasilescu, A.-N.; Mallien, A.; Pfeiffer, N.; Lang, U.E.; Gass, P.; Inta, D. Rapastinel alleviates the neurotoxic effect induced by NMDA receptor blockade in the early postnatal mouse brain. Eur. Arch. Psychiatry Clin. Neurosci. 2021, 271, 1587–1591. [Google Scholar] [CrossRef]
- Martínez-Pinteño, A.; García-Cerro, S.; Mas, S.; Torres, T.; Boloc, D.; Rodríguez, N.; Lafuente, A.; Gassó, P.; Arnaiz, J.A.; Parellada, E. The positive allosteric modulator of the mGlu2 receptor JNJ-46356479 partially improves neuropathological deficits and schizophrenia-like behaviors in a postnatal ketamine mice model. J. Psychiatr. Res. 2020, 126, 8–18. [Google Scholar] [CrossRef]
- Yuede, C.M.; Wozniak, D.F.; Creeley, C.E.; Taylor, G.T.; Olney, J.W.; Farber, N.B. Behavioral Consequences of NMDA Antagonist-Induced Neuroapoptosis in the Infant Mouse Brain. PLoS ONE 2010, 5, e11374. [Google Scholar] [CrossRef] [Green Version]
- Wesseling, H.; Want, E.J.; Guest, P.C.; Rahmoune, H.; Holmes, E.; Bahn, S. Hippocampal Proteomic and Metabonomic Abnormalities in Neurotransmission, Oxidative Stress, and Apoptotic Pathways in a Chronic Phencyclidine Rat Model. J. Proteome Res. 2015, 14, 3174–3187. [Google Scholar] [CrossRef]
- D’Addario, C.; Micale, V.; Di Bartolomeo, M.; Stark, T.; Pucci, M.; Sulcova, A.; Palazzo, M.; Babinska, Z.; Cremaschi, L.; Drago, F.; et al. A preliminary study of endocannabinoid system regulation in psychosis: Distinct alterations of CNR1 promoter DNA methylation in patients with schizophrenia. Schizophr. Res. 2017, 188, 132–140. [Google Scholar] [CrossRef]
- Ruda-Kucerova, J.; Babinska, Z.; Amchova, P.; Stark, T.; Drago, F.; Sulcova, A.; Micale, V. Reactivity to addictive drugs in the methylazoxymethanol (MAM) model of schizophrenia in male and female rats. World J. Biol. Psychiatry 2017, 18, 129–142. [Google Scholar] [CrossRef] [PubMed]
- Ruda-Kucerova, J.; Babinska, Z.; Stark, T.; Micale, V. Suppression of Methamphetamine Self-Administration by Ketamine Pre-treatment Is Absent in the Methylazoxymethanol (MAM) Rat Model of Schizophrenia. Neurotox. Res. 2017, 32, 121–133. [Google Scholar] [CrossRef] [PubMed]
- Drazanova, E.; Ruda-Kucerova, J.; Krátká, L.; Stark, T.; Kuchar, M.; Maryska, M.; Drago, F.; Starcuk, Z.; Micale, V. Different effects of prenatal MAM vs. perinatal THC exposure on regional cerebral blood perfusion detected by Arterial Spin Labelling MRI in rats. Sci. Rep. 2019, 9, 6062. [Google Scholar] [CrossRef] [Green Version]
- Horska, K.; Kotolova, H.; Karpisek, M.; Babinska, Z.; Hammer, T.; Prochazka, J.; Stark, T.; Micale, V.; Ruda-Kucerova, J. Metabolic profile of methylazoxymethanol model of schizophrenia in rats and effects of three antipsychotics in long-acting formulation. Toxicol. Appl. Pharmacol. 2020, 406, 115214. [Google Scholar] [CrossRef]
- Kucera, J.; Horska, K.; Hruska, P.; Kuruczova, D.; Micale, V.; Ruda-Kucerova, J.; Bienertova-Vasku, J. Interacting effects of the MAM model of schizophrenia and antipsychotic treatment: Untargeted proteomics approach in adipose tissue. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2021, 108, 110165. [Google Scholar] [CrossRef] [PubMed]
- Stark, T.; Iannotti, F.A.; Di Martino, S.; Di Bartolomeo, M.; Ruda-Kucerova, J.; Piscitelli, F.; Wotjak, C.T.; D’Addario, C.; Drago, F.; Di Marzo, V.; et al. Early Blockade of CB1 Receptors Ameliorates Schizophrenia-like Alterations in the Neurodevelopmental MAM Model of Schizophrenia. Biomolecules 2022, 12, 108. [Google Scholar] [CrossRef]
- Micale, V.; Di Bartolomeo, M.; Di Martino, S.; Stark, T.; Dell’Osso, B.; Drago, F.; D’Addario, C. Are the epigenetic changes predictive of therapeutic efficacy for psychiatric disorders? A translational approach towards novel drug targets. Pharmacol. Ther. 2022, 108279. [Google Scholar] [CrossRef]
- Modinos, G.; Allen, P.; Grace, A.A.; McGuire, P. Translating the MAM model of psychosis to humans. Trends Neurosci. 2015, 38, 129–138. [Google Scholar] [CrossRef] [Green Version]
- Glantz, L.A.; Gilmore, J.H.; Lieberman, J.A.; Jarskog, L.F. Apoptotic mechanisms and the synaptic pathology of schizophrenia. Schizophr. Res. 2006, 81, 47–63. [Google Scholar] [CrossRef]
- Parellada, E.; Gassó, P. Glutamate and microglia activation as a driver of dendritic apoptosis: A core pathophysiological mechanism to understand schizophrenia. Transl. Psychiatry 2021, 11, 271. [Google Scholar] [CrossRef]
- Li, W.; Ghose, S.; Gleason, K.; Begovic’, A.; Perez, J.; Bartko, J.; Russo, S.; Wagner, A.D.; Selemon, L.; Tamminga, C.A. Synaptic Proteins in the Hippocampus Indicative of Increased Neuronal Activity in CA3 in Schizophrenia. Am. J. Psychiatry 2015, 172, 373–382. [Google Scholar] [CrossRef] [Green Version]
- Scholz, J.; Broom, D.C.; Youn, D.-H.; Mills, C.D.; Kohno, T.; Suter, M.R.; Moore, K.A.; Decosterd, I.; Coggeshall, R.E.; Woolf, C.J. Blocking Caspase Activity Prevents Transsynaptic Neuronal Apoptosis and the Loss of Inhibition in Lamina II of the Dorsal Horn after Peripheral Nerve Injury. J. Neurosci. 2005, 25, 7317–7323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oppenheim, R.W. Cell Death During Development of the Nervous System. Annu. Rev. Neurosci. 1991, 14, 453–501. [Google Scholar] [CrossRef] [PubMed]
- Fricker, M.; Tolkovsky, A.M.; Borutaite, V.; Coleman, M.; Brown, G.C. Neuronal Cell Death. Physiol. Rev. 2018, 98, 813–880. [Google Scholar] [CrossRef]
- Lin, Y.-C.; Koleske, A.J. Mechanisms of Synapse and Dendrite Maintenance and Their Disruption in Psychiatric and Neurodegenerative Disorders. Annu. Rev. Neurosci. 2010, 33, 349–378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williams, D.W.; Kondo, S.; Krzyzanowska, A.; Hiromi, Y.; Truman, J.W. Local caspase activity directs engulfment of dendrites during pruning. Nat. Neurosci. 2006, 9, 1234–1236. [Google Scholar] [CrossRef]
- Sellgren, C.M.; Gracias, J.; Watmuff, B.; Biag, J.D.; Thanos, J.M.; Whittredge, P.B.; Fu, T.; Worringer, K.; Brown, H.E.; Wang, J.; et al. Increased synapse elimination by microglia in schizophrenia patient-derived models of synaptic pruning. Nat. Neurosci. 2019, 22, 374–385. [Google Scholar] [CrossRef]
- Poon, I.; Lucas, C.; Rossi, A.G.; Ravichandran, K. Apoptotic cell clearance: Basic biology and therapeutic potential. Nat. Rev. Immunol. 2014, 14, 166–180. [Google Scholar] [CrossRef] [Green Version]
- Wyllie, A.; Kerr, J.; Currie, A. Cell Death: The Significance of Apoptosis. Int. Rev. Cytol. 1980, 68, 251–306. [Google Scholar] [CrossRef]
- Oltval, Z.N.; Milliman, C.L.; Korsmeyer, S.J. Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programed cell death. Cell 1993, 74, 609–619. [Google Scholar] [CrossRef]
- Yuan, J.; Yankner, B.A. Apoptosis in the nervous system. Nature 2000, 407, 802–809. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lotocki, G.; Keane, R.W. Inhibitors of Apoptosis Proteins in Injury and Disease. IUBMB Life 2002, 54, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Behl, C. Apoptosis and Alzheimer’s disease. J. Neural Transm. 2000, 107, 1325–1344. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liu, W.; Yang, H.; Liu, W. Balancing Apoptosis and Autophagy for Parkinson’s Disease Therapy: Targeting BCL-2. ACS Chem. Neurosci. 2019, 10, 792–802. [Google Scholar] [CrossRef] [PubMed]
- Hickey, M.A.; Chesselet, M.-F. Apoptosis in Huntington’s disease. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2003, 27, 255–265. [Google Scholar] [CrossRef]
- Sathasivam, S.; Ince, P.; Shaw, P. Apoptosis in amyotrophic lateral sclerosis: A review of the evidence. Neuropathol. Appl. Neurobiol. 2001, 27, 257–274. [Google Scholar] [CrossRef]
- Trubetskoy, V.; Pardiñas, A.F.; Qi, T.; Panagiotaropoulou, G.; Awasthi, S.; Bigdeli, T.B.; Bryois, J.; Chen, C.-Y.; Dennison, C.A.; Hall, L.S.; et al. Mapping genomic loci implicates genes and synaptic biology in schizophrenia. Nature 2022, 604, 502–508. [Google Scholar] [CrossRef]
- Ahmad, S.S.; Sinha, M.; Ahmad, K.; Khalid, M.; Choi, I. Study of Caspase 8 Inhibition for the Management of Alzheimer’s Disease: A Molecular Docking and Dynamics Simulation. Molecules 2020, 25, 2071. [Google Scholar] [CrossRef]
- Vila, M.; Jackson-Lewis, V.; Vukosavic, S.; Djaldetti, R.; Liberatore, G.; Offen, D.; Korsmeyer, S.J.; Przedborski, S. Bax ablation prevents dopaminergic neurodegeneration in the 1-methyl- 4-phenyl-1,2,3,6-tetrahydropyridine mouse model of Parkinson’s disease. Proc. Natl. Acad. Sci. USA 2001, 98, 2837–2842. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Zhu, S.; Pei, Z.; Drozda, M.; Stavrovskaya, I.G.; Del Signore, S.J.; Cormier, K.; Shimony, E.M.; Wang, H.; Ferrante, R.J.; et al. Inhibitors of Cytochrome c Release with Therapeutic Potential for Huntington’s Disease. J. Neurosci. 2008, 28, 9473–9485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kostic, V.; Jackson-Lewis, V.; de Bilbao, F.; Dubois-Dauphin, M.; Przedborski, S. Bcl-2: Prolonging Life in a Transgenic Mouse Model of Familial Amyotrophic Lateral Sclerosis. Science 1997, 277, 559–563. [Google Scholar] [CrossRef]
- Faizi, M.; Salimi, A.; Rasoulzadeh, M.; Naserzadeh, P.; Pourahmad, J. Schizophrenia induces oxidative stress and cytochrome C release in isolated rat brain mitochondria: A possible pathway for induction of apoptosis and neurodegeneration. Iran. J. Pharm. Res. 2014, 13, 93–100. [Google Scholar]
- Roberts, R.C. Mitochondrial dysfunction in schizophrenia: With a focus on postmortem studies. Mitochondrion 2021, 56, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Sastry, P.S.; Rao, K.S. Apoptosis and the nervous system. J. Neurochem. 2000, 74, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Perkins, D.O.; Jeffries, C.D.; Do, K.Q. Potential Roles of Redox Dysregulation in the Development of Schizophrenia. Biol. Psychiatry 2020, 88, 326–336. [Google Scholar] [CrossRef] [PubMed]
- Jarskog, L.F.; Glantz, L.A.; Gilmore, J.H.; Lieberman, J.A. Apoptotic mechanisms in the pathophysiology of schizophrenia. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2005, 29, 846–858. [Google Scholar] [CrossRef]
- Berger, G.E.; Wood, S.; McGorry, P.D. Incipient neurovulnerability and neuroprotection in early psychosis. Psychopharmacol. Bull. 2003, 37, 79–101. [Google Scholar] [PubMed]
- Jarskog, L.F.; Selinger, E.S.; Lieberman, J.A.; Gilmore, J.H. Apoptotic Proteins in the Temporal Cortex in Schizophrenia: High Bax/Bcl-2 Ratio Without Caspase-3 Activation. Am. J. Psychiatry 2004, 161, 109–115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jarskog, L.; Gilmore, J.H.; Selinger, E.S.; Lieberman, J.A. Cortical Bcl-2 protein expression and apoptotic regulation in schizophrenia. Biol. Psychiatry 2000, 48, 641–650. [Google Scholar] [CrossRef]
- Mattson, M.P.; Keller, J.N.; Begley, J.G. Evidence for Synaptic Apoptosis. Exp. Neurol. 1998, 153, 35–48. [Google Scholar] [CrossRef]
- Bennett, M. Schizophrenia: Susceptibility genes, dendritic-spine pathology and gray matter loss. Prog. Neurobiol. 2011, 95, 275–300. [Google Scholar] [CrossRef]
- Ertürk, A.; Wang, Y.; Sheng, M. Local Pruning of Dendrites and Spines by Caspase-3-Dependent and Proteasome-Limited Mechanisms. J. Neurosci. 2014, 34, 1672–1688. [Google Scholar] [CrossRef] [Green Version]
- Gilman, C.P.; Mattson, M.P. Do Apoptotic Mechanisms Regulate Synaptic Plasticity and Growth-Cone Motility? Neuromol. Med. 2002, 2, 197–214. [Google Scholar] [CrossRef]
- Mattson, M.P.; Duan, W. “Apoptotic” biochemical cascades in synaptic compartments: Roles in adaptive plasticity and neurodegenerative disorders. J. Neurosci. Res. 1999, 58, 152–166. [Google Scholar] [CrossRef]
- Gassó, P.; Mas, S.; Molina, O.; Lafuente, A.; Bernardo, M.; Parellada, E. Increased susceptibility to apoptosis in cultured fibroblasts from antipsychotic-naïve first-episode schizophrenia patients. J. Psychiatr. Res. 2014, 48, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Batalla, A.; Bargallo, N.; Gassó, P.; Molina, O.; Pareto, D.; Mas, S.; Roca, J.M.; Bernardo, M.; Lafuente, A.; Parellada, E. Apoptotic markers in cultured fibroblasts correlate with brain metabolites and regional brain volume in antipsychotic-naive first-episode schizophrenia and healthy controls. Transl. Psychiatry 2015, 5, e626. [Google Scholar] [CrossRef] [Green Version]
- Gassó, P.; Mas, S.; Rodríguez, N.; Boloc, D.; García-Cerro, S.; Bernardo, M.; Lafuente, A.; Parellada, E. Microarray gene-expression study in fibroblast and lymphoblastoid cell lines from antipsychotic-naïve first-episode schizophrenia patients. J. Psychiatr. Res. 2017, 95, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Szymona, K.; Dudzińska, E.; Karakuła-Juchnowicz, H.; Gil-Kulik, P.; Chomik, P.; Świstowska, M.; Gałaszkiewicz, J.; Kocki, J. Analysis of the expression of BAX, BCL2, BIRC6, CASP3, CASP9 apoptosis genes during the first episode of schizophrenia. Psychiatr. Polska 2019, 53, 1293–1303. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [Green Version]
- Muka, T.; Glisic, M.; Milic, J.; Verhoog, S.; Bohlius, J.; Bramer, W.; Chowdhury, R.; Franco, O.H. A 24-step guide on how to design, conduct, and successfully publish a systematic review and meta-analysis in medical research. Eur. J. Epidemiol. 2020, 35, 49–60. [Google Scholar] [CrossRef]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan—A web and mobile app for systematic reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef] [PubMed]
- Csernansky, J.G. Neurodegeneration in Schizophrenia: Evidence from In Vivo Neuroimaging Studies. Sci. World J. 2007, 7, 135–143. [Google Scholar] [CrossRef] [Green Version]
- Benn, S.C.; Woolf, C.J. Adult neuron survival strategies—slamming on the brakes. Nat. Rev. Neurosci. 2004, 5, 686–700. [Google Scholar] [CrossRef] [PubMed]
- Giovanni, A.; Keramaris, E.; Morris, E.J.; Hou, S.T.; O’Hare, M.; Dyson, N.; Robertson, G.S.; Slack, R.S.; Park, D.S. E2F1 Mediates Death of B-amyloid-treated Cortical Neurons in a Manner Independent of p53 and Dependent on Bax and Caspase 3. J. Biol. Chem. 2000, 275, 11553–11560. [Google Scholar] [CrossRef] [Green Version]
- Langston, J.W. The MPTP Story. J. Park. Dis. 2017, 7, S11–S19. [Google Scholar] [CrossRef] [Green Version]
- Rosenstock, T.R.; de Brito, O.M.; Lombardi, V.; Louros, S.; Ribeiro, M.; Almeida, S.; Ferreira, I.L.; Oliveira, C.R.; Rego, A.C. FK506 ameliorates cell death features in Huntington’s disease striatal cell models. Neurochem. Int. 2011, 59, 600–609. [Google Scholar] [CrossRef]
- Ishigaki, S.; Liang, Y.; Yamamoto, M.; Niwa, J.-I.; Ando, Y.; Yoshihara, T.; Takeuchi, H.; Doyu, M.; Sobue, G. X-Linked inhibitor of apoptosis protein is involved in mutant SOD1-mediated neuronal degeneration. J. Neurochem. 2002, 82, 576–584. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, C.; Correia, S.; dos Santos, R.X.C.; Cardoso, S.; Moreira, P.; Clark, T.A.; Zhu, X.; Smith, M.A.; Perry, G. Role of mitochondrial-mediated signaling pathways in Alzheimer disease and hypoxia. J. Bioenerg. Biomembr. 2009, 41, 433–440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hartmann, A.; Hunot, S.; Michel, P.P.; Muriel, M.-P.; Vyas, S.; Faucheux, B.A.; Mouatt-Prigent, A.; Turmel, H.; Srinivasan, A.; Ruberg, M.; et al. Caspase-3: A vulnerability factor and final effector in apoptotic death of dopaminergic neurons in Parkinson’s disease. Proc. Natl. Acad. Sci. USA 2000, 97, 2875–2880. [Google Scholar] [CrossRef] [Green Version]
- Hartmann, A.; Michel, P.P.; Troadec, J.-D.; Mouatt-Prigent, A.; Faucheux, B.A.; Ruberg, M.; Agid, Y.; Hirsch, E.C. Is Bax a mitochondrial mediator in apoptotic death of dopaminergic neurons in Parkinson’s disease? J. Neurochem. 2001, 76, 1785–1793. [Google Scholar] [CrossRef]
- Tatton, N.A. Increased Caspase 3 and Bax Immunoreactivity Accompany Nuclear GAPDH Translocation and Neuronal Apoptosis in Parkinson’s Disease. Exp. Neurol. 2000, 166, 29–43. [Google Scholar] [CrossRef]
- Ona, V.O.; Li, M.; Vonsattel, J.P.G.; Andrews, L.J.; Khan, S.Q.; Chung, W.M.; Frey, A.S.; Menon, A.S.; Li, X.-J.; Stieg, P.E.; et al. Inhibition of caspase-1 slows disease progression in a mouse model of Huntington’s disease. Nature 1999, 399, 263–267. [Google Scholar] [CrossRef]
- Farshbaf, M.J.; Ghaedi, K. Huntington’s Disease and Mitochondria. Neurotox. Res. 2017, 32, 518–529. [Google Scholar] [CrossRef]
- Li, M.; Ona, V.O.; Guégan, C.; Chen, M.; Jackson-Lewis, V.; Andrews, L.J.; Olszewski, A.J.; Stieg, P.E.; Lee, J.-P.; Przedborski, S.; et al. Functional Role of Caspase-1 and Caspase-3 in an ALS Transgenic Mouse Model. Science 2000, 288, 335–339. [Google Scholar] [CrossRef]
- Khan, S.; Ahmad, K.; Alshammari, E.M.A.; Adnan, M.; Baig, M.H.; Lohani, M.; Somvanshi, P.; Haque, S. Implication of Caspase-3 as a Common Therapeutic Target for Multineurodegenerative Disorders and Its Inhibition Using Nonpeptidyl Natural Compounds. BioMed Res. Int. 2015, 2015, 379817. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schotte, P.; Declercq, W.; Van Huffel, S.; Vandenabeele, P.; Beyaert, R. Non-specific effects of methyl ketone peptide inhibitors of caspases. FEBS Lett. 1999, 442, 117–121. [Google Scholar] [CrossRef] [Green Version]
- Bilsland, J.; Roy, S.; Xanthoudakis, S.; Nicholson, D.W.; Han, Y.; Grimm, E.; Hefti, F.; Harper, S.J. Caspase Inhibitors Attenuate 1-Methyl-4-Phenylpyridinium Toxicity in Primary Cultures of Mesencephalic Dopaminergic Neurons. J. Neurosci. 2002, 22, 2637–2649. [Google Scholar] [CrossRef] [Green Version]
- Vila, M.; Przedborski, S. Targeting programmed cell death in neurodegenerative diseases. Nat. Rev. Neurosci. 2003, 4, 365–375. [Google Scholar] [CrossRef] [PubMed]
- Wellington, C.L.; Singaraja, R.; Ellerby, L.; Savill, J.; Roy, S.; Leavitt, B.; Cattaneo, E.; Hackam, A.; Sharp, A.; Thornberry, N.; et al. Inhibiting Caspase Cleavage of Huntingtin Reduces Toxicity and Aggregate Formation in Neuronal and Nonneuronal Cells. J. Biol. Chem. 2000, 275, 19831–19838. [Google Scholar] [CrossRef] [Green Version]
- Andreassen, O.A.; Ferrante, R.J.; Hughes, D.B.; Klivenyi, P.; Dedeoglu, A.; Ona, V.O.; Friedlander, R.M.; Beal, M.F. Malonate and 3-Nitropropionic Acid Neurotoxicity Are Reduced in Transgenic Mice Expressing a Caspase-1 Dominant-Negative Mutant. J. Neurochem. 2000, 75, 847–852. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, H.; Gao, Z.; Zheng, L.; Zhang, C.; Liu, Z.; Yang, Y.; Teng, H.; Hou, L.; Yin, Y.; Zou, X. Protective Effects of Fucoidan on Aβ25–35 and d-Gal-Induced Neurotoxicity in PC12 Cells and d-Gal-Induced Cognitive Dysfunction in Mice. Mar. Drugs 2017, 15, 77. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Matthews, R.T.; Schulz, J.B.; Klockgether, T.; Liao, A.W.; Martinou, J.C.; Penney, J.B.; Hyman, B.T.; Beal, M.F. 1-Methyl-4-phenyl-1,2,3,6-tetrahydropyride neurotoxicity is attenuated in mice overexpressing Bcl-2. J. Neurosci. 1998, 18, 8145–8152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mandir, A.S.; Simbulan-Rosenthal, C.M.; Poitras, M.F.; Lumpkin, J.R.; Dawson, V.L.; Smulson, M.E.; Dawson, T.M. A novel in vivo post-translational modification of p53 by PARP-1 in MPTP-induced parkinsonism. J. Neurochem. 2002, 83, 186–192. [Google Scholar] [CrossRef]
- Thornborrow, E.C.; Patel, S.; Mastropietro, A.E.; Schwartzfarb, E.M.; Manfredi, J.J. A conserved intronic response element mediates direct p53-dependent transcriptional activation of both the human and murine bax genes. Oncogene 2002, 21, 990–999. [Google Scholar] [CrossRef] [Green Version]
- Duan, W.; Zhu, X.; Ladenheim, B.; Yu, Q.-S.; Guo, Z.; Oyler, J.; Cutler, R.G.; Cadet, J.L.; Greig, N.H.; Mattson, M.P. p53 inhibitors preserve dopamine neurons and motor function in experimental parkinsonism. Ann. Neurol. 2002, 52, 597–606. [Google Scholar] [CrossRef]
- Picone, P.; Nuzzo, D.; Caruana, L.; Scafidi, V.; Di Carlo, M. Mitochondrial Dysfunction: Different Routes to Alzheimer’s Disease Therapy. Oxidative Med. Cell. Longev. 2014, 2014, 780179. [Google Scholar] [CrossRef] [Green Version]
- Fossati, S.; Giannoni, P.; Solesio, M.E.; Cocklin, S.L.; Cabrera, E.; Ghiso, J.; Rostagno, A. The carbonic anhydrase inhibitor methazolamide prevents amyloid beta-induced mitochondrial dysfunction and caspase activation protecting neuronal and glial cells in vitro and in the mouse brain. Neurobiol. Dis. 2016, 86, 29–40. [Google Scholar] [CrossRef] [Green Version]
- Tsou, Y.-C.; Wang, H.-H.; Hsieh, C.-C.; Sun, K.-H.; Sun, G.-H.; Jhou, R.-S.; Lin, T.-I.; Lu, S.-Y.; Liu, H.-Y.; Tang, S.-J. Down-regulation of BNIP3 by olomoucine, a CDK inhibitor, reduces LPS- and NO-induced cell death in BV2 microglial cells. Neurosci. Lett. 2016, 628, 186–193. [Google Scholar] [CrossRef]
- Park, J.-S.; Davis, R.L.; Sue, C.M. Mitochondrial Dysfunction in Parkinson’s Disease: New Mechanistic Insights and Therapeutic Perspectives. Curr. Neurol. Neurosci. Rep. 2018, 18, 21. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.; Chen, M.; Jiang, J. Mitochondrial dysfunction in neurodegenerative diseases and drug targets via apoptotic signaling. Mitochondrion 2019, 49, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Kumar, V.; Singh, K.; Kumar, S.; Kim, Y.-S.; Lee, Y.-M.; Kim, J.-J. Therapeutic Advances for Huntington’s Disease. Brain Sci. 2020, 10, 43. [Google Scholar] [CrossRef] [PubMed]
- Kuboyama, T.; Tohda, C.; Komatsu, K. Neuritic regeneration and synaptic reconstruction induced by withanolide A. Br. J. Pharmacol. 2005, 144, 961–971. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hannan, A.J. Novel therapeutic targets for Huntington’s disease. Expert Opin. Ther. Targets 2005, 9, 639–650. [Google Scholar] [CrossRef] [PubMed]
- Cialdai, F.; Bolognini, D.; Vignali, L.; Iannotti, N.; Cacchione, S.; Magi, A.; Balsamo, M.; Vukich, M.; Neri, G.; Donati, A.; et al. Effect of space flight on the behavior of human retinal pigment epithelial ARPE-19 cells and evaluation of coenzyme Q10 treatment. Cell. Mol. Life Sci. 2021, 78, 7795–7812. [Google Scholar] [CrossRef] [PubMed]
- Fakhri, S.; Aneva, I.Y.; Farzaei, M.H.; Sobarzo-Sánchez, E. The Neuroprotective Effects of Astaxanthin: Therapeutic Targets and Clinical Perspective. Molecules 2019, 24, 2640. [Google Scholar] [CrossRef] [Green Version]
- Ullah, N.; Ullah, I.; Lee, H.Y.; Naseer, M.I.; Seok, P.M.; Ahmed, J.; Kim, M.O. Protective Function of Nicotinamide Against Ketamine-induced Apoptotic Neurodegeneration in the Infant Rat Brain. J. Mol. Neurosci. 2012, 47, 67–75. [Google Scholar] [CrossRef]
- Wong-Guerra, M.; Jiménez-Martin, J.; Fonseca-Fonseca, L.A.; Ramírez-Sánchez, J.; Montano-Peguero, Y.; Rocha, J.B.; D’avila, F.; De Assis, A.M.; Souza, D.; Pardo-Andreu, G.L.; et al. JM-20 protects memory acquisition and consolidation on scopolamine model of cognitive impairment. Neurol. Res. 2019, 41, 385–398. [Google Scholar] [CrossRef]
- Wang, D.; Wong, H.K.; Feng, Y.-B.; Zhang, Z.-J. Paeoniflorin, a Natural Neuroprotective Agent, Modulates Multiple Anti-Apoptotic and Pro-apoptotic Pathways in Differentiated PC12 Cells. Cell. Mol. Neurobiol. 2013, 33, 521–529. [Google Scholar] [CrossRef] [PubMed]
- Gopinath, K.; Prakash, D.; Sudhandiran, G. Neuroprotective effect of naringin, a dietary flavonoid against 3-Nitropropionic acid-induced neuronal apoptosis. Neurochem. Int. 2011, 59, 1066–1073. [Google Scholar] [CrossRef]
- Rosa, A.I.; Fonseca, I.; Nunes, M.J.; Moreira, S.; Rodrigues, E.; Carvalho, A.N.; Rodrigues, C.M.; Gama, M.J.; Castro-Caldas, M. Novel insights into the antioxidant role of tauroursodeoxycholic acid in experimental models of Parkinson’s disease. Biochim. Biophys. Acta—Mol. Basis Dis. 2017, 1863, 2171–2181. [Google Scholar] [CrossRef]
- Månsson, R.; Hansson, M.J.; Morota, S.; Uchino, H.; Ekdahl, C.T.; Elmér, E. Re-evaluation of mitochondrial permeability transition as a primary neuroprotective target of minocycline. Neurobiol. Dis. 2007, 25, 198–205. [Google Scholar] [CrossRef]
- Thompson, C.K.; Brenowitz, E.A. Caspase Inhibitor Infusion Protects an Avian Song Control Circuit from Seasonal-Like Neurodegeneration. J. Neurosci. 2008, 28, 7130–7136. [Google Scholar] [CrossRef] [Green Version]
- Sharif, R.; Aghsami, M.; Gharghabi, M.; Sanati, M.; Khorshidahmad, T.; Vakilzadeh, G.; Mehdizadeh, H.; Gholizadeh, S.; Taghizadeh, G.; Sharifzadeh, M. Melatonin reverses H-89 induced spatial memory deficit: Involvement of oxidative stress and mitochondrial function. Behav. Brain Res. 2017, 316, 115–124. [Google Scholar] [CrossRef]
- Gilat, M.; Jackson, A.C.; Marshall, N.S.; Rn, D.H.; Mullins, A.E.; Hall, J.M.; Fang, B.A.M.; Yee, B.J.; Wong, K.K.H.; Grunstein, R.R.; et al. Melatonin for Rapid Eye Movement Sleep Behavior Disorder in Parkinson’s disease: A Randomised Controlled Trial. Mov. Disord. 2020, 35, 344–349. [Google Scholar] [CrossRef] [Green Version]
- Kunz, D.; Mahlberg, R. A two-part, double-blind, placebo-controlled trial of exogenous melatonin in REM sleep behaviour disorder. J. Sleep Res. 2010, 19, 591–596. [Google Scholar] [CrossRef]
- Arnold, S.; Beyer, C. Neuroprotection by estrogen in the brain: The mitochondrial compartment as presumed therapeutic target. J. Neurochem. 2009, 110, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wang, B.; Zhu, C.; Feng, Y.; Wang, S.; Shahzad, M.; Hu, C.; Mo, M.; Du, F.; Yu, X. 17β-Estradiol Impedes Bax-Involved Mitochondrial Apoptosis of Retinal Nerve Cells Induced by Oxidative Damage via the Phosphatidylinositol 3-Kinase/Akt Signal Pathway. J. Mol. Neurosci. 2013, 50, 482–493. [Google Scholar] [CrossRef]
- Tai, J.; Liu, W.; Li, Y.; Li, L.; Hölscher, C. Neuroprotective effects of a triple GLP-1/GIP/glucagon receptor agonist in the APP/PS1 transgenic mouse model of Alzheimer’s disease. Brain Res. 2018, 1678, 64–74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, W.; Jalewa, J.; Sharma, M.; Li, G.; Li, L.; Hölscher, C. Neuroprotective effects of lixisenatide and liraglutide in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse model of Parkinson’s disease. Neuroscience 2015, 303, 42–50. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.; Li, Y.; Jalewa, J.; Saunders-Wood, T.; Li, L.; Hölscher, C. Neuroprotective effects of an oxyntomodulin analogue in the MPTP mouse model of Parkinson’s disease. Eur. J. Pharmacol. 2015, 765, 284–290. [Google Scholar] [CrossRef] [PubMed]
- Tewari, D.; Stankiewicz, A.; Mocan, A.; Sah, A.; Tzvetkov, N.T.; Huminiecki, L.; Horbańczuk, J.O.; Atanasov, A.G. Ethnopharmacological Approaches for Dementia Therapy and Significance of Natural Products and Herbal Drugs. Front. Aging Neurosci. 2018, 10, 3. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Flores, J.; Noël, A.; Beauchet, O.; Sjöström, P.J.; Leblanc, A.C. Methylene blue inhibits Caspase-6 activity, and reverses Caspase-6-induced cognitive impairment and neuroinflammation in aged mice. Acta Neuropathol. Commun. 2019, 7, 210. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.-Y.; Zhang, L.-J.; Chen, Z.; Liu, L.-B. The PTEN inhibitor bpV(pic) promotes neuroprotection against amyloid β-peptide (25-35)-induced oxidative stress and neurotoxicity. Neurol. Res. 2017, 39, 758–765. [Google Scholar] [CrossRef]
- Nataraj, J.; Manivasagam, T.; Thenmozhi, A.J.; Essa, M.M. Neuroprotective effect of asiatic acid on rotenone-induced mitochondrial dysfunction and oxidative stress-mediated apoptosis in differentiated SH-SYS5Y cells. Nutr. Neurosci. 2017, 20, 351–359. [Google Scholar] [CrossRef]
- Sagot, Y.; Toni, N.; Perrelet, D.; Lurot, S.; King, B.; Rixner, H.; Mattenberger, L.; Waldmeier, P.C.; Kato, A.C. An orally active anti-apoptotic molecule (CGP 3466B) preserves mitochondria and enhances survival in an animal model of motoneuron disease. Br. J. Pharmacol. 2000, 131, 721–728. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Zhang, Z.; Bao, J.; Yu, Z.; Cai, M.; Li, X.; Wu, T.; Xiang, J.; Cai, D. Jia-Jian-Di-Huang-Yin-Zi decoction reduces apoptosis induced by both mitochondrial and endoplasmic reticulum caspase12 pathways in the mouse model of Parkinson’s disease. J. Ethnopharmacol. 2017, 203, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Rahimian, R.; Fakhfouri, G.; Mehr, S.E.; Ghia, J.-E.; Genazzani, A.A.; Payandemehr, B.; Dehpour, A.R.; Mousavizadeh, K.; Lim, D. Tropisetron attenuates amyloid-beta-induced inflammatory and apoptotic responses in rats. Eur. J. Clin. Investig. 2013, 43, 1039–1051. [Google Scholar] [CrossRef] [PubMed]
- El-Sahar, A.E.; Rastanawi, A.A.; El-Yamany, M.F.; Saad, M.A. Dapagliflozin improves behavioral dysfunction of Huntington’s disease in rats via inhibiting apoptosis-related glycolysis. Life Sci. 2020, 257, 118076. [Google Scholar] [CrossRef]
- Gassó, P.; Mas, S.; Molina, O.; Bernardo, M.; Lafuente, A.; Parellada, E. Neurotoxic/neuroprotective activity of haloperidol, risperidone and paliperidone in neuroblastoma cells. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2012, 36, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.-L.; Ito, H.; Kondo, T.; Uehara, T.; Ikeda, M.; Abe, H.; Saitoh, J.-I.; Noguchi, K.; Suzuki, M.; Kurachi, M. Antipsychotic drugs scavenge radiation-induced hydroxyl radicals and intracellular ROS formation, and protect apoptosis in human lymphoma U937 cells. Free Radic. Res. 2019, 53, 304–312. [Google Scholar] [CrossRef]
- Rajasekaran, A.; Venkatasubramanian, G.; Berk, M.; Debnath, M. Mitochondrial dysfunction in schizophrenia: Pathways, mechanisms and implications. Neurosci. Biobehav. Rev. 2015, 48, 10–21. [Google Scholar] [CrossRef]
- Lundberg, M.; Curbo, S.; Bohman, H.; Agartz, I.; Ögren, S.-O.; Patrone, C.; Mansouri, S. Clozapine protects adult neural stem cells from ketamine-induced cell death in correlation with decreased apoptosis and autophagy. Biosci. Rep. 2020, 40, BSR20193156. [Google Scholar] [CrossRef] [Green Version]
- Chen, A.T.; Chibnall, J.T.; Nasrallah, H.A. Placebo-controlled augmentation trials of the antioxidant NAC in schizophrenia: A review. Ann. Clin. Psychiatry 2016, 28, 190–196. [Google Scholar]
- Morera-Fumero, A.L.; Abreu-Gonzalez, P. Role of Melatonin in Schizophrenia. Int. J. Mol. Sci. 2013, 14, 9037–9050. [Google Scholar] [CrossRef] [Green Version]
- Afonso, A.C.; Pacheco, F.D.; Canever, L.; Wessler, P.G.; Mastella, G.A.; Godoi, A.K.; Hubbe, I.; Bischoff, L.M.; Bialecki, A.V.S.; Zugno, A.I. Schizophrenia-like behavior is not altered by melatonin supplementation in rodents. An. Acad. Bras. Cienc. 2020, 92, e20190981. [Google Scholar] [CrossRef]
- Andrabi, S.S.; Vishnoi, S.; Kaushik, M.; Parveen, K.; Tabassum, H.; Akram, M.; Parvez, S. Reversal of Schizophrenia-like Symptoms and Cholinergic Alterations by Melatonin. Arch. Med. Res. 2019, 50, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Regen, F.; Cosma, N.-C.; Otto, L.R.; Clemens, V.; Saksone, L.; Gellrich, J.; Uesekes, B.; Ta, T.M.T.; Hahn, E.; Dettling, M.; et al. Clozapine modulates retinoid homeostasis in human brain and normalizes serum retinoic acid deficit in patients with schizophrenia. Mol. Psychiatry 2021, 26, 5417–5428. [Google Scholar] [CrossRef]
- Cheng, B.; Martinez, A.A.; Morado, J.; Scofield, V.; Roberts, J.L.; Maffi, S.K. Retinoic acid protects against proteasome inhibition associated cell death in SH-SY5Y cells via the AKT pathway. Neurochem. Int. 2013, 62, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Lerner, V.; Miodownik, C.; Gibel, A.; Kovalyonok, E.; Shleifer, T.; Goodman, A.B.; Ritsner, M.S. Bexarotene as Add-On to Antipsychotic Treatment in Schizophrenia Patients. Clin. Neuropharmacol. 2008, 31, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-B.; Zheng, W.; Ning, Y.-P.; Cai, D.-B.; Yang, X.-H.; Ungvari, G.S.; Ng, C.H.; Wang, C.-Y.; Xiang, Y.-T. Erythropoietin for Cognitive Deficits Associated with Schizophrenia, Bipolar Disorder, and Major Depression: A Systematic Review. Pharmacopsychiatry 2018, 51, 100–104. [Google Scholar] [CrossRef] [Green Version]
- Schwartz, D.L. Quercetin as an Augmentation Agent in Schizophrenia. J. Clin. Psychopharmacol. 2016, 36, 282–283. [Google Scholar] [CrossRef] [PubMed]
- Weiser, M.; Levi, L.; Zamora, D.; Biegon, A.; SanGiovanni, J.P.; Davidson, M.; Burshtein, S.; Gonen, I.; Radu, P.; Pavalache, K.S.; et al. Effect of Adjunctive Estradiol on Schizophrenia Among Women of Childbearing Age: A Randomized Clinical Trial. JAMA Psychiatry 2019, 76, 1009–1017. [Google Scholar] [CrossRef] [PubMed]
- Owen, M.J.; Sawa, A.; Mortensen, P.B. Schizophrenia. Lancet 2016, 388, 86–97. [Google Scholar] [CrossRef] [Green Version]
- Graham, S.H.; Chen, J. Programmed Cell Death in Cerebral Ischemia. J. Cereb. Blood Flow Metab. 2001, 21, 99–109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Inoue, H.; Tsukita, K.; Iwasato, T.; Suzuki, Y.; Tomioka, M.; Tateno, M.; Nagao, M.; Kawata, A.; Saido, T.C.; Miura, M.; et al. The crucial role of caspase-9 in the disease progression of a transgenic ALS mouse model. EMBO J. 2003, 22, 6665–6674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ashton, A.Q. Proteins—Advances in Research and Application: 2012 Edition; ScholarlyEditions: Atlanta, GA, USA, 2012. [Google Scholar]
- Bizat, N.; Galas, M.-C.; Jacquard, C.; Boyer, F.; Hermel, J.-M.; Schiffmann, S.N.; Hantraye, P.; Blum, D.; Brouillet, E. Neuroprotective effect of zVAD against the neurotoxin 3-nitropropionic acid involves inhibition of calpain. Neuropharmacology 2005, 49, 695–702. [Google Scholar] [CrossRef]
- Gray, J.; Haran, M.M.; Schneider, K.; Vesce, S.; Ray, A.M.; Owen, D.; White, I.R.; Cutler, P.; Davis, J.B. Evidence That Inhibition of Cathepsin-B Contributes to the Neuroprotective Properties of Caspase Inhibitor Tyr-Val-Ala-Asp-Chloromethyl Ketone. J. Biol. Chem. 2001, 276, 32750–32755. [Google Scholar] [CrossRef] [Green Version]
- Gallego-Sandín, S.; Novalbos, J.; Rosado, A.; Cano-Abad, M.F.; Arias, E.; Abad-Santos, F.; García, A.G. Albumin prevents mitochondrial depolarization and apoptosis elicited by endoplasmic reticulum calcium depletion of neuroblastoma cells. Eur. J. Pharmacol. 2005, 520, 1–11. [Google Scholar] [CrossRef]
- Eckert, G.P.; Chang, S.; Eckmann, J.; Copanaki, E.; Hagl, S.; Hener, U.; Müller, W.E.; Kögel, D. Liposome-incorporated DHA increases neuronal survival by enhancing non-amyloidogenic APP processing. Biochim. Biophys. Acta —Biomembr. 2011, 1808, 236–243. [Google Scholar] [CrossRef] [Green Version]
- Naguy, A.; Naguy, C. N-acetyl-cysteine in schizophrenia—There is more than meets the eyes! CNS Spectr. 2021, 26, 446–447. [Google Scholar] [CrossRef]
- Martínez-Banaclocha, M. N-acetyl-cysteine in Schizophrenia: Potential Role on the Sensitive Cysteine Proteome. Curr. Med. Chem. 2020, 27, 6424–6439. [Google Scholar] [CrossRef] [PubMed]
- Duarte-Silva, E.; Araújo, S.M.D.R.; Oliveira, W.H.; Lós, D.; de França, M.E.R.; Bonfanti, A.; Peron, G.; Thomaz, L.D.L.; Verinaud, L.; Nunes, A.K.D.S.; et al. Sildenafil ameliorates EAE by decreasing apoptosis in the spinal cord of C57BL/6 mice. J. Neuroimmunol. 2018, 321, 125–137. [Google Scholar] [CrossRef] [PubMed]
- Goff, D.C.; Cather, C.; Freudenreich, O.; Henderson, D.C.; Evins, A.E.; Culhane, M.A.; Walsh, J.P. A placebo-controlled study of sildenafil effects on cognition in schizophrenia. Psychopharmacology 2009, 202, 411–417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Squitieri, F.; Di Pardo, A.; Favellato, M.; Amico, E.; Maglione, V.; Frati, L. Pridopidine, a dopamine stabilizer, improves motor performance and shows neuroprotective effects in Huntington disease R6/2 mouse model. J. Cell. Mol. Med. 2015, 19, 2540–2548. [Google Scholar] [CrossRef]
- Jhou, A.-J.; Chang, H.-C.; Hung, C.-C.; Lin, H.-C.; Lee, Y.-C.; Liu, W.-T.; Han, K.-F.; Lai, Y.-W.; Lin, M.-Y.; Lee, C.-H. Chlorpromazine, an antipsychotic agent, induces G2/M phase arrest and apoptosis via regulation of the PI3K/AKT/mTOR-mediated autophagy pathways in human oral cancer. Biochem. Pharmacol. 2021, 184, 114403. [Google Scholar] [CrossRef]
- Bastianetto, S.; Danik, M.; Mennicken, F.; Williams, S.; Quirion, R. Prototypical antipsychotic drugs protect hippocampal neuronal cultures against cell death induced by growth medium deprivation. BMC Neurosci. 2006, 7, 28. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.-H.; Wang, H.; Wang, X.; Narayanan, M.V.; Stavrovskaya, I.G.; Kristal, B.S.; Friedlander, R.M. Nortriptyline Protects Mitochondria and Reduces Cerebral Ischemia/Hypoxia Injury. Stroke 2008, 39, 455–462. [Google Scholar] [CrossRef] [Green Version]
- Papadopoulos, F.; Isihou, R.; Alexiou, G.A.; Tsalios, T.; Vartholomatos, E.; Markopoulos, G.S.; Sioka, C.; Tsekeris, P.; Kyritsis, A.P.; Galani, V. Haloperidol Induced Cell Cycle Arrest and Apoptosis in Glioblastoma Cells. Biomedicines 2020, 8, 595. [Google Scholar] [CrossRef]
- Pillai, A.; Dhandapani, K.; Pillai, B.A.; Terry, A.V.; Mahadik, S.P. Erythropoietin Prevents Haloperidol Treatment-Induced Neuronal Apoptosis through Regulation of BDNF. Neuropsychopharmacology 2008, 33, 1942–1951. [Google Scholar] [CrossRef]
- Pillai, A.; Veeranan-Karmegam, R.; Dhandapani, K.; Mahadik, S.P. Cystamine prevents haloperidol-induced decrease of BDNF/TrkB signaling in mouse frontal cortex. J. Neurochem. 2008, 107, 941–951. [Google Scholar] [CrossRef]
- Familian, A.; Boshuizen, R.S.; Eikelenboom, P.; Veerhuis, R. Inhibitory effect of minocycline on amyloid β fibril formation and human microglial activation. Glia 2006, 53, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Cankaya, S.; Cankaya, B.; Kilic, U.; Kilic, E.; Yulug, B. The therapeutic role of minocycline in Parkinson’s disease. Drugs Context 2019, 8, 212553. [Google Scholar] [CrossRef] [PubMed]
- Bonelli, R.M.; Hödl, A.K.; Hofmann, P.; Kapfhammer, H.-P. Neuroprotection in Huntington’s disease: A 2-year study on minocycline. Int. Clin. Psychopharmacol. 2004, 19, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Stavrovskaya, I.G.; Drozda, M.; Kim, B.Y.S.; Ona, V.; Li, M.; Sarang, S.; Liu, A.S.; Hartley, D.M.; Wu, D.C.; et al. Minocycline inhibits cytochrome c release and delays progression of amyotrophic lateral sclerosis in mice. Nature 2002, 417, 74–78. [Google Scholar] [CrossRef]
- Du, Y.; Ma, Z.; Lin, S.; Dodel, R.C.; Gao, F.; Bales, K.R.; Triarhou, L.C.; Chernet, E.; Perry, K.W.; Nelson, D.L.G.; et al. Minocycline prevents nigrostriatal dopaminergic neurodegeneration in the MPTP model of Parkinson’s disease. Proc. Natl. Acad. Sci. USA 2001, 98, 14669–14674. [Google Scholar] [CrossRef] [Green Version]
- Fan, R.; Xu, F.; Previti, M.L.; Davis, J.; Grande, A.M.; Robinson, J.K.; Van Nostrand, W.E. Minocycline Reduces Microglial Activation and Improves Behavioral Deficits in a Transgenic Model of Cerebral Microvascular Amyloid. J. Neurosci. 2007, 27, 3057–3063. [Google Scholar] [CrossRef] [Green Version]
- Howard, R.; Zubko, O.; Bradley, R.; Harper, E.; Pank, L.; O’Brien, J.; Fox, C.; Tabet, N.; Livingston, G.; Bentham, P.; et al. Minocycline at 2 Different Dosages vs Placebo for Patients With Mild Alzheimer Disease: A Randomized Clinical Trial. JAMA Neurol. 2020, 77, 164–174. [Google Scholar] [CrossRef]
- Chen, M.; Ona, V.O.; Li, M.; Ferrante, R.J.; Fink, K.B.; Zhu, S.; Bian, J.; Guo, L.; Farrell, L.A.; Hersch, S.M.; et al. Minocycline inhibits caspase-1 and caspase-3 expression and delays mortality in a transgenic mouse model of Huntington disease. Nat. Med. 2000, 6, 797–801. [Google Scholar] [CrossRef]
- Solmi, M.; Veronese, N.; Thapa, N.; Facchini, S.; Stubbs, B.; Fornaro, M.; Carvalho, A.F.; Correll, C.U. Systematic review and meta-analysis of the efficacy and safety of minocycline in schizophrenia. CNS Spectr. 2017, 22, 415–426. [Google Scholar] [CrossRef] [Green Version]
- Deakin, B.; Suckling, J.; Dazzan, P.; Joyce, E.; Lawrie, S.M.; Upthegrove, R.; Husain, N.; Chaudhry, I.B.; Dunn, G.; Jones, P.B.; et al. Minocycline for Negative Symptoms of Schizophrenia and Possible Mechanistic Actions: The BeneMin RCT. Southampton (UK): NIHR Journals Library. 2019. Available online: http://www.ncbi.nlm.nih.gov/books/NBK545707/ (accessed on 17 June 2022).
- Hovens, J.E.; Onderwater, T.A.M. Minocycline for schizophrenia: A brief overview. Tijdschr. Voor Psychiatr. 2014, 56, 402–406. [Google Scholar]
- Zhang, L.; Zheng, H.; Wu, R.; Kosten, T.R.; Zhang, X.-Y.; Zhao, J. The effect of minocycline on amelioration of cognitive deficits and pro-inflammatory cytokines levels in patients with schizophrenia. Schizophr. Res. 2019, 212, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Miyaoka, T.; Yasukawa, R.; Yasuda, H.; Hayashida, M.; Inagaki, T.; Horiguchi, J. Minocycline as Adjunctive Therapy for Schizophrenia. Clin. Neuropharmacol. 2008, 31, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Mouri, A.; Lee, H.; Mamiya, T.; Aoyama, Y.; Matsumoto, Y.; Kubota, H.; Huang, W.; Chiou, L.; Nabeshima, T. Hispidulin attenuates the social withdrawal in isolated disrupted-in-schizophrenia-1 mutant and chronic phencyclidine-treated mice. Br. J. Pharmacol. 2020, 177, 3210–3224. [Google Scholar] [CrossRef] [PubMed]
- Timpe, J.M.; Wang, C.Z.; Kim, J.; Johnson, K.M. α-amino-3-hydroxy-5-methyl-4-isoxazoleproprionic acid receptor activation protects against phencyclidine-induced caspase-3 activity by activating voltage-gated calcium channels. J. Neurosci. Res. 2014, 92, 1785–1791. [Google Scholar] [CrossRef] [PubMed]
- Matsunaga, S.; Kishi, T.; Iwata, N. Memantine Monotherapy for Alzheimer’s Disease: A Systematic Review and Meta-Analysis. PLoS ONE 2015, 10, e0123289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, G.; Li, Y.; Lin, L.; Cao, Y. Anti-autophagic and anti-apoptotic effects of memantine in a SH-SY5Y cell model of Alzheimer’s disease via mammalian target of rapamycin-dependent and -independent pathways. Mol. Med. Rep. 2015, 12, 7615–7622. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Rojas, J.C.; Gonzalez-Lima, F. Methylene blue prevents neurodegeneration caused by rotenone in the retina. Neurotox. Res. 2006, 9, 47–57. [Google Scholar] [CrossRef]
- Gillman, P.K. CNS toxicity involving methylene blue: The exemplar for understanding and predicting drug interactions that precipitate serotonin toxicity. J. Psychopharmacol. 2011, 25, 429–436. [Google Scholar] [CrossRef]
- Ma, H.; Wu, J.; Wang, Y.; Liang, S. Cytoprotective effect of selective small-molecule caspase inhibitors against staurosporine-induced apoptosis. Drug Des. Dev. Ther. 2014, 8, 583–600. [Google Scholar] [CrossRef] [Green Version]
- Zhu, L.-F.; Wu, X.-Y.; Liu, X.; Ma, L.; Zhang, Q.; Yu, Z.; He, Z.-J.; Ying, Y.; Zhang, Z.-T.; Pan, X.-Y.; et al. Absence of BAX differentially affects astrocyte density in the mouse cortex and hippocampus. Sheng Li Xue Bao Acta Physiol. Sin. 2021, 73, 1–9. [Google Scholar]
- D’Orsi, B.; Kilbride, S.M.; Chen, G.; Alvarez, S.P.; Bonner, H.P.; Pfeiffer, S.; Plesnila, N.; Engel, T.; Henshall, D.C.; Düssmann, H.; et al. Bax Regulates Neuronal Ca2+ Homeostasis. J. Neurosci. 2015, 35, 1706–1722. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, M.I.; Deshmukh, M. Endoplasmic reticulum stress-induced apoptosis requires bax for commitment and Apaf-1 for execution in primary neurons. Cell Death Differ. 2007, 14, 1011–1019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wyttenbach, A.; Tolkovsky, A.M. The BH3-only protein Puma is both necessary and sufficient for neuronal apoptosis induced by DNA damage in sympathetic neurons. J. Neurochem. 2006, 96, 1213–1226. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.D.; Xiang, H.; London, S.; Kinoshita, Y.; Knudson, M.; Mayberg, M.; Korsmeyer, S.J.; Morrison, R.S. Evidence for involvement of Bax and p53, but not caspases, in radiation-induced cell death of cultured postnatal hippocampal neurons. J. Neurosci. Res. 1998, 54, 721–733. [Google Scholar] [CrossRef]
- Miller, T.M.; Moulder, K.L.; Knudson, C.M.; Creedon, D.J.; Deshmukh, M.; Korsmeyer, S.J.; Johnson, E.M. Bax Deletion Further Orders the Cell Death Pathway in Cerebellar Granule Cells and Suggests a Caspase-independent Pathway to Cell Death. J. Cell Biol. 1997, 139, 205–217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwon, Y.; Shin, J.; Nam, K.; An, J.S.; Yang, S.; Hong, S.; Bae, M.; Moon, K.; Cho, Y.; Woo, J.; et al. Rhizolutin, a Novel 7/10/6-Tricyclic Dilactone, Dissociates Misfolded Protein Aggregates and Reduces Apoptosis/Inflammation Associated with Alzheimer’s Disease. Angew. Chem. Int. Ed. 2020, 59, 22994–22998. [Google Scholar] [CrossRef]
- Rohn, T.T.; Kokoulina, P.; Eaton, C.R.; Poon, W.W. Caspase activation in transgenic mice with Alzheimer-like pathology: Results from a pilot study utilizing the caspase inhibitor, Q-VD-OPh. Int. J. Clin. Exp. Med. 2009, 2, 300–308. [Google Scholar]
- Teismann, P.; Tieu, K.; Choi, D.-K.; Wu, D.-C.; Naini, A.; Hunot, S.; Vila, M.; Jackson-Lewis, V.; Przedborski, S. Cyclooxygenase-2 is instrumental in Parkinson’s disease neurodegeneration. Proc. Natl. Acad. Sci. USA 2003, 100, 5473–5478. [Google Scholar] [CrossRef] [Green Version]
- Kanthasamy, A.G.; Anantharam, V.; Zhang, D.; Latchoumycandane, C.; Jin, H.; Kaul, S.; Kanthasamy, A. A novel peptide inhibitor targeted to caspase-3 cleavage site of a proapoptotic kinase protein kinase C delta (PKCδ) protects against dopaminergic neuronal degeneration in Parkinson’s disease models. Free Radic. Biol. Med. 2006, 41, 1578–1589. [Google Scholar] [CrossRef]
- Ganjam, G.K.; Bolte, K.; Matschke, L.A.; Neitemeier, S.; Dolga, A.; Höllerhage, M.; Höglinger, G.; Adamczyk, A.; Decher, N.; Oertel, W.H.; et al. Mitochondrial damage by α-synuclein causes cell death in human dopaminergic neurons. Cell Death Dis. 2019, 10, 865. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aharony, I.; Ehrnhoefer, D.E.; Shruster, A.; Qiu, X.; Franciosi, S.; Hayden, M.; Offen, D. A Huntingtin-based peptide inhibitor of caspase-6 provides protection from mutant Huntingtin-induced motor and behavioral deficits. Hum. Mol. Genet. 2015, 24, 2604–2614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahn, S.-W.; Jeon, G.S.; Kim, M.-J.; Shon, J.-H.; Kim, J.-E.; Shin, J.-Y.; Kim, S.-M.; Kim, S.H.; Ye, I.-H.; Lee, K.-W.; et al. Neuroprotective effects of JGK-263 in transgenic SOD1-G93A mice of amyotrophic lateral sclerosis. J. Neurol. Sci. 2014, 340, 112–116. [Google Scholar] [CrossRef] [PubMed]
- Hawking, Z.L. Alzheimer’s disease: The role of mitochondrial dysfunction and potential new therapies: The International Journal of Student Research. Biosci. Horizons 2016, 9. [Google Scholar] [CrossRef] [Green Version]
- Moreira, P.I.; Carvalho, C.; Zhu, X.; Smith, M.A.; Perry, G. Mitochondrial dysfunction is a trigger of Alzheimer’s disease pathophysiology. Biochim. Biophys. Acta Mol. Basis Dis. 2010, 1802, 2–10. [Google Scholar] [CrossRef] [Green Version]
- Sun, X.-Q.; Zhang, R.; Zhang, H.-D.; Yuan, P.; Wang, X.-J.; Zhao, Q.-H.; Wang, L.; Jiang, R.; Bogaard, H.J.; Jing, Z.-C. Reversal of right ventricular remodeling by dichloroacetate is related to inhibition of mitochondria-dependent apoptosis. Hypertens. Res. 2016, 39, 302–311. [Google Scholar] [CrossRef]
- Macdonald, R.; Barnes, K.; Hastings, C.; Mortiboys, H. Mitochondrial abnormalities in Parkinson’s disease and Alzheimer’s disease: Can mitochondria be targeted therapeutically? Biochem. Soc. Trans. 2018, 46, 891–909. [Google Scholar] [CrossRef] [PubMed]
- Flatters, S.J. The Contribution of Mitochondria to Sensory Processing and Pain. Prog. Mol. Biol. Transl. Sci. 2015, 131, 119–146. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.F.; Shaw, P.J.; De Vos, K.J. The role of mitochondria in amyotrophic lateral sclerosis. Neurosci. Lett. 2019, 710, 132933. [Google Scholar] [CrossRef] [PubMed]
- Patergnani, S.; Fossati, V.; Bonora, M.; Giorgi, C.; Marchi, S.; Missiroli, S.; Rusielewicz, T.; Wieckowski, M.; Pinton, P. Mitochondria in Multiple Sclerosis: Molecular Mechanisms of Pathogenesis. Int. Rev. Cell. Mol. Biol. 2017, 328, 49–103. [Google Scholar] [CrossRef]
- Marchionini, D.M.; Collier, T.J.; Pitzer, M.R.; Sortwell, C.E. Reassessment of caspase inhibition to augment grafted dopamine neuron survival. Cell Transplant. 2004, 13, 273–282. [Google Scholar] [CrossRef] [Green Version]
- Sivasangari, K.; Rajan, K.E. Standardized Bacopa monnieri Extract Ameliorates Learning and Memory Impairments through Synaptic Protein, Neurogranin, Pro-and Mature BDNF Signaling, and HPA Axis in Prenatally Stressed Rat Offspring. Antioxidants 2020, 9, 1229. [Google Scholar] [CrossRef]
- Lee, J.-E.; Sim, H.; Yoo, H.M.; Lee, M.; Baek, A.; Jeon, Y.-J.; Seo, K.-S.; Son, M.-Y.; Yoon, J.S.; Kim, J. Neuroprotective Effects of Cryptotanshinone in a Direct Reprogramming Model of Parkinson’s Disease. Molecules 2020, 25, 3602. [Google Scholar] [CrossRef]
- Ravishankar, D.; Corona, G.; Hogan, S.M.; Spencer, J.P.; Greco, F.; Osborn, H.M. Thioflavones as novel neuroprotective agents. Bioorganic Med. Chem. 2016, 24, 5513–5520. [Google Scholar] [CrossRef]
- Nuñez-Figueredo, Y.; Pardo-Andreu, G.L.; Ramírez-Sánchez, J.; Delgado-Hernández, R.; Ochoa-Rodríguez, E.; Verdecia-Reyes, Y.; Naal, Z.; Muller, A.P.; Portela, L.V.; Souza, D.O. Antioxidant effects of JM-20 on rat brain mitochondria and synaptosomes: Mitoprotection against Ca2+-induced mitochondrial impairment. Brain Res. Bull. 2014, 109, 68–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Momeni, H.R.; Kanje, M. Calpain inhibitors delay injury-induced apoptosis in adult mouse spinal cord motor neurons. Neuroreport 2006, 17, 761–765. [Google Scholar] [CrossRef] [PubMed]
- Manigandan, V.; Nataraj, J.; Karthik, R.; Manivasagam, T.; Saravanan, R.; Thenmozhi, A.J.; Essa, M.M.; Guillemin, G.J. Low Molecular Weight Sulfated Chitosan: Neuroprotective Effect on Rotenone-Induced In Vitro Parkinson’s Disease. Neurotox. Res. 2019, 35, 505–515. [Google Scholar] [CrossRef]
- Casarejos, M.J.; Menéndez, J.; Solano, R.M.; Rodríguez-Navarro, J.A.; De Yébenes, J.G.; Mena, M.A. Susceptibility to rotenone is increased in neurons from parkin null mice and is reduced by minocycline. J. Neurochem. 2006, 97, 934–946. [Google Scholar] [CrossRef]
- Sirianni, A.C.; Jiang, J.; Zeng, J.; Mao, L.L.; Zhou, S.; Sugarbaker, P.; Zhang, X.; Li, W.; Friedlander, R.M.; Wang, X. N-acetyl-l -tryptophan, but not N-acetyl-d -tryptophan, rescues neuronal cell death in models of amyotrophic lateral sclerosis. J. Neurochem. 2015, 134, 956–968. [Google Scholar] [CrossRef]
- Mookherjee, P.; Johnson, G.V. Tau phosphorylation during apoptosis of human SH-SY5Y neuroblastoma cells. Brain Res. 2001, 921, 31–43. [Google Scholar] [CrossRef]
- Gu, C.; Zhang, Y.; Hu, Q.; Wu, J.; Ren, H.; Liu, C.-F.; Wang, G. P7C3 inhibits GSK3β activation to protect dopaminergic neurons against neurotoxin-induced cell death in vitro and in vivo. Cell Death Dis. 2017, 8, e2858. [Google Scholar] [CrossRef]
- Hu, X.; Weng, Z.; Chu, C.; Zhang, L.; Cao, G.; Gao, Y.; Signore, A.; Zhu, J.; Hastings, T.; Greenamyre, J.T.; et al. Peroxiredoxin-2 Protects against 6-Hydroxydopamine-Induced Dopaminergic Neurodegeneration via Attenuation of the Apoptosis Signal-Regulating Kinase (ASK1) Signaling Cascade. J. Neurosci. 2011, 31, 247–261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Selvakumar, K.; Bavithra, S.; Suganthi, M.; Benson, C.S.; Elumalai, P.; Arunkumar, R.; Krishnamoorthy, G.; Venkataraman, P.; Arunakaran, J. Protective Role of Quercetin on PCBs-Induced Oxidative Stress and Apoptosis in Hippocampus of Adult Rats. Neurochem. Res. 2012, 37, 708–721. [Google Scholar] [CrossRef]
- Shoshan-Barmatz, V.; Nahon-Crystal, E.; Shteinfer-Kuzmine, A.; Gupta, R. VDAC1, mitochondrial dysfunction, and Alzheimer’s disease. Pharmacol. Res. 2018, 131, 87–101. [Google Scholar] [CrossRef]
- Deleglise, B.; Lassus, B.; Soubeyre, V.; Alleaume-Butaux, A.; Hjorth, J.J.; Vignes, M.; Schneider, B.; Brugg, B.; Viovy, J.-L.; Peyrin, J.-M. Synapto-Protective Drugs Evaluation in Reconstructed Neuronal Network. PLoS ONE 2013, 8, e71103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Wang, W.; Li, Y.; Xiao, Y.; Cheng, J.; Jia, J. The 5-Lipoxygenase Inhibitor Zileuton Confers Neuroprotection against Glutamate Oxidative Damage by Inhibiting Ferroptosis. Biol. Pharm. Bull. 2015, 38, 1234–1239. [Google Scholar] [CrossRef] [PubMed]
- Gómez, C.; Reiriz, J.; Piqué, M.; Gil, J.; Ferrer, I.; Ambrosio, S. Low concentrations of 1-methyl-4-phenylpyridinium ion induce caspase-mediated apoptosis in human SH-SY5Y neuroblastoma cells. J. Neurosci. Res. 2001, 63, 421–428. [Google Scholar] [CrossRef]
- Mukhopadhyay, D.; Hammami, M.; Khalouf, A.; Al Shaikh, Y.; Mohammed, A.K.; Hamad, M.; Salehi, A.; Taneera, J. Dimethyloxalylglycine (DMOG) and the Caspase Inhibitor “Ac-LETD-CHO” Protect Neuronal ND7/23 Cells of Gluocotoxicity. Exp. Clin. Endocrinol. Diabetes 2021, 129, 420–428. [Google Scholar] [CrossRef] [PubMed]
- Ullah, I.; Park, H.Y.; Kim, M.O. Anthocyanins Protect against Kainic Acid-induced Excitotoxicity and Apoptosis via ROS-activated AMPK Pathway in Hippocampal Neurons. CNS Neurosci. Ther. 2014, 20, 327–338. [Google Scholar] [CrossRef]
- Song, D.; Jiang, X.; Liu, Y.; Sun, Y.; Cao, S.; Zhang, Z. Asiaticoside Attenuates Cell Growth Inhibition and Apoptosis Induced by Aβ1-42 via Inhibiting the TLR4/NF-κB Signaling Pathway in Human Brain Microvascular Endothelial Cells. Front. Pharmacol. 2018, 9, 28. [Google Scholar] [CrossRef] [Green Version]
- Werth, J.L.; Deshmukh, M.; Cocabo, J.; Johnson, E.M.; Rothman, S.M. Reversible Physiological Alterations in Sympathetic Neurons Deprived of NGF but Protected from Apoptosis by Caspase Inhibition or Bax Deletion. Exp. Neurol. 2000, 161, 203–211. [Google Scholar] [CrossRef]
- Jung, E.B.; Lee, C.S. Baicalein attenuates proteasome inhibition-induced apoptosis by suppressing the activation of the mitochondrial pathway and the caspase-8- and Bid-dependent pathways. Eur. J. Pharmacol. 2014, 730, 116–124. [Google Scholar] [CrossRef]
- Gliyazova, N.S.; Ibeanu, G.C. The Chemical Molecule B355252 is Neuroprotective in an In Vitro Model of Parkinson’s Disease. Cell. Mol. Neurobiol. 2016, 36, 1109–1122. [Google Scholar] [CrossRef]
- Silva, J.; Alves, C.; Pinteus, S.; Mendes, S.; Pedrosa, R. Seaweeds’ neuroprotective potential set in vitro on a human cellular stress model. Mol. Cell. Biochem. 2020, 473, 229–238. [Google Scholar] [CrossRef]
- Tseng, Y.-T.; Tsai, Y.-H.; Fülöp, F.; Chang, F.-R.; Lo, Y.-C. 2-Iodo-4′-Methoxychalcone Attenuates Methylglyoxal-Induced Neurotoxicity by Activation of GLP-1 Receptor and Enhancement of Neurotrophic Signal, Antioxidant Defense and Glyoxalase Pathway. Molecules 2019, 24, 2249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Boulton, A.A.; Zuo, D.-M.; Yu, P.H. MK-801 induces apoptotic neuronal death in the rat retrosplenial cortex: Prevention by cycloheximide and R(?)-2-Hexyl-N- Methylpropargylamine. J. Neurosci. Res. 1996, 46, 82–89. [Google Scholar] [CrossRef]
- Busquets, O.; Ettcheto, M.; Verdaguer, E.; Castro-Torres, R.D.; Auladell, C.; Beas-Zarate, C.; Folch, J.; Camins, A. JNK1 inhibition by Licochalcone A leads to neuronal protection against excitotoxic insults derived of kainic acid. Neuropharmacology 2018, 131, 440–452. [Google Scholar] [CrossRef]
- Xian, H.; Zhao, J.; Zheng, Y.; Wang, M.; Huang, J.; Wu, B.; Sun, C.; Yang, Y. MADP, a salidroside analog, protects hippocampal neurons from glutamate induced apoptosis. Life Sci. 2014, 103, 34–40. [Google Scholar] [CrossRef] [PubMed]
- El-Mir, M.-Y.; Detaille, D.; R-Villanueva, G.; Esteban, M.D.; Guigas, B.; Attia, S.; Fontaine, E.; Almeida, A.; Leverve, X. Neuroprotective Role of Antidiabetic Drug Metformin Against Apoptotic Cell Death in Primary Cortical Neurons. J. Mol. Neurosci. 2008, 34, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Rojas, J.C.; John, J.M.; Lee, J.; Gonzalez-Lima, F. Methylene Blue Provides Behavioral and Metabolic Neuroprotection Against Optic Neuropathy. Neurotox. Res. 2009, 15, 260–273. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; McInnis, J.; West, J.B.; Bao, J.; Anastasio, N.; Guidry, J.A.; Ye, Y.; Salvemini, D.; Johnson, K.M. Blockade of Phencyclidine-Induced Cortical Apoptosis and Deficits in Prepulse Inhibition by M40403, a Superoxide Dismutase Mimetic. J. Pharmacol. Exp. Ther. 2003, 304, 266–271. [Google Scholar] [CrossRef]
- Kumar, S.N.K.; Deepthy, J.; Saraswathi, U.; Thangarajeswari, M.; Kanna, S.Y.; Ezhil, P.; Kalaiselvi, P. Morinda citrifolia mitigates rotenone-induced striatal neuronal loss in male Sprague-Dawley rats by preventing mitochondrial pathway of intrinsic apoptosis. Redox Rep. 2017, 22, 418–429. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.-B.; Zhou, J.; Qu, Y.; Li, X.; Lu, C.-T.; Xie, K.-L.; Sun, X.-L.; Fei, Z. Neuroprotective effect of osthole on MPP+-induced cytotoxicity in PC12 cells via inhibition of mitochondrial dysfunction and ROS production. Neurochem. Int. 2010, 57, 206–215. [Google Scholar] [CrossRef]
- De Sarno, P.; Shestopal, S.A.; King, T.D.; Zmijewska, A.; Song, L.; Jope, R.S. Muscarinic Receptor Activation Protects Cells from Apoptotic Effects of DNA Damage, Oxidative Stress, and Mitochondrial Inhibition. J. Biol. Chem. 2003, 278, 11086–11093. [Google Scholar] [CrossRef] [Green Version]
- Dong, H.; Li, R.; Yu, C.; Xu, T.; Zhang, X.; Dong, M. Paeoniflorin inhibition of 6-hydroxydopamine-induced apoptosis in PC12 cells via suppressing reactive oxygen species-mediated PKCδ/NF-κB pathway. Neuroscience 2015, 285, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Sanz, E.; Quintana, A.; Battaglia, V.; Toninello, A.; Hidalgo, J.; Ambrosio, S.; Valoti, M.; Marco, J.L.; Tipton, K.F.; Unzeta, M. Anti-apoptotic effect of Mao-B inhibitor PF9601N [N-(2-propynyl)-2-(5-benzyloxy-indolyl) methylamine] is mediated by p53 pathway inhibition in MPP+-treated SH-SY5Y human dopaminergic cells. J. Neurochem. 2008, 105, 2404–2417. [Google Scholar] [CrossRef]
- Hong, S.-Y.; Jeong, W.-S.; Jun, M. Protective Effects of the Key Compounds Isolated from Corni fructus against β-Amyloid-Induced Neurotoxicity in PC12 Cells. Molecules 2012, 17, 10831–10845. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, L.; Sugama, S.; Mischak, R.P.; Kiaei, M.; Bizat, N.; Brouillet, E.; Joh, T.H.; Beal, M.F. A novel systemically active caspase inhibitor attenuates the toxicities of MPTP, malonate, and 3NP in vivo. Neurobiol. Dis. 2004, 17, 250–259. [Google Scholar] [CrossRef] [PubMed]
- Dionne, K.R.; Leser, J.S.; Lorenzen, K.A.; Beckham, J.D.; Tyler, K.L. A brain slice culture model of viral encephalitis reveals an innate CNS cytokine response profile and the therapeutic potential of caspase inhibition. Exp. Neurol. 2011, 228, 222–231. [Google Scholar] [CrossRef] [Green Version]
- Sifringer, M.; Bendix, I.; Borner, C.; Endesfelder, S.; Von Haefen, C.; Kalb, A.; Holifanjaniaina, S.; Prager, S.; Schlager, G.W.; Keller, M.; et al. Prevention of neonatal oxygen-induced brain damage by reduction of intrinsic apoptosis. Cell Death Dis. 2012, 3, e250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, C.S.; Han, E.S.; Han, Y.S.; Bang, H. Differential effect of calmodulin antagonists on MG132-induced mitochondrial dysfunction and cell death in PC12 cells. Brain Res. Bull. 2005, 67, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.S.; Choi, D.-K.; Jung, H.J. Neuroprotective Effects of Vanillyl Alcohol in Gastrodia elata Blume Through Suppression of Oxidative Stress and Anti-Apoptotic Activity in Toxin-Induced Dopaminergic MN9D Cells. Molecules 2011, 16, 5349–5361. [Google Scholar] [CrossRef] [Green Version]
- Perche, O.; Doly, M.; Ranchon-Cole, I. Transient protective effect of caspase inhibitors in RCS rat. Exp. Eye Res. 2008, 86, 519–527. [Google Scholar] [CrossRef]
- Cutillas, B.; Espejo, M.; Gil, J.; Ferrer, I.; Ambrosio, S. Caspase inhibition protects nigral neurons against 6-OHDA-induced retrograde degeneration. Neuroreport 1999, 10, 2605–2608. [Google Scholar] [CrossRef]
- Chen, W.-F.; Chakraborty, C.; Sung, C.-S.; Feng, C.-W.; Jean, Y.-H.; Lin, Y.-Y.; Hung, H.-C.; Huang, T.-Y.; Huang, S.-Y.; Su, T.-M.; et al. Neuroprotection by marine-derived compound, 11-dehydrosinulariolide, in an in vitro Parkinson’s model: A promising candidate for the treatment of Parkinson’s disease. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2012, 385, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Bozzatello, P.; Brignolo, E.; De Grandi, E.; Bellino, S. Supplementation with Omega-3 Fatty Acids in Psychiatric Disorders: A Review of Literature Data. J. Clin. Med. 2016, 5, 67. [Google Scholar] [CrossRef] [PubMed]
- Ben-Azu, B.; Aderibigbe, A.O.; Omogbiya, I.A.; Ajayi, A.M.; Iwalewa, E.O. Morin Pretreatment Attenuates Schizophrenia-Like Behaviors in Experimental Animal Models. Drug Res. 2018, 68, 159–167. [Google Scholar] [CrossRef]
- Morera-Fumero, A.L.; Abreu-Gonzalez, P. Diazepam Discontinuation Through Agomelatine in Schizophrenia With Insomnia and Depression. J. Clin. Psychopharmacol. 2010, 30, 739–741. [Google Scholar] [CrossRef] [PubMed]
- Shamir, E.; Laudon, M.; Barak, Y.; Anis, Y.; Rotenberg, V.; Elizur, A.; Zisapel, N. Melatonin Improves Sleep Quality of Patients With Chronic Schizophrenia. J. Clin. Psychiatry 2000, 61, 373–377. [Google Scholar] [CrossRef] [PubMed]
- Shamir, E.; Barak, Y.; Shalman, I.; Laudon, M.; Zisapel, N.; Tarrasch, R.; Elizur, A.; Weizman, R. Melatonin Treatment for Tardive Dyskinesia: A double-blind, placebo-controlled, crossover study. Arch. Gen. Psychiatry 2001, 58, 1049–1052. [Google Scholar] [CrossRef] [PubMed]
- Hassanpour, F.; Zarghami, M.; Mouodi, S.; Moosazadeh, M.; Barzegar, F.; Bagheri, M.; Hendouei, N. Adjunctive Memantine Treatment of Schizophrenia: A Double-Blind, Randomized Placebo-Controlled Study. J. Clin. Psychopharmacol. 2019, 39, 634–638. [Google Scholar] [CrossRef] [PubMed]
- Sepehrmanesh, Z.; Heidary, M.; Akasheh, N.; Akbari, H.; Heidary, M. Therapeutic effect of adjunctive N-acetyl cysteine (NAC) on symptoms of chronic schizophrenia: A double-blind, randomized clinical trial. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2018, 82, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Kang, S.-S.; Kim, J.Y.; Tchah, H. The Antioxidant N-Acetylcysteine Inhibits Inflammatory and Apoptotic Processes in Human Conjunctival Epithelial Cells in a High-Glucose Environment. Investig. Opthalmology Vis. Sci. 2015, 56, 5614–5621. [Google Scholar] [CrossRef] [Green Version]
- A Wittkopp, T.; Abuzzahab, F.S. Nicotinic acid and nicotinamide adenine dinucleotide (NAD) therapy in schizophrenia: A review. Behav. Neuropsychiatry 1972, 4, 6–12. [Google Scholar] [PubMed]
- Lerner, V.; Miodownik, C.; Gibel, A.; Sirota, P.; Bush, I.; Elliot, H.; Benatov, R.; Ritsner, M.S. The Retinoid X Receptor Agonist Bexarotene Relieves Positive Symptoms of Schizophrenia. J. Clin. Psychiatry 2013, 74, 1224–1232. [Google Scholar] [CrossRef]
- Dakhale, G.N.; Khanzode, S.D.; Saoji, A. Supplementation of vitamin C with atypical antipsychotics reduces oxidative stress and improves the outcome of schizophrenia. Psychopharmacology 2005, 182, 494–498. [Google Scholar] [CrossRef] [PubMed]
- Hoffer, L.J. Vitamin therapy in schizophrenia. Isr. J. Psychiatry Relat. Sci. 2008, 45, 3–10. [Google Scholar] [PubMed]
- Sandyk, R.; Kanofsky, J.D. Vitamin C in the Treatment of Schizophrenia. Int. J. Neurosci. 1993, 68, 67–71. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morén, C.; Treder, N.; Martínez-Pinteño, A.; Rodríguez, N.; Arbelo, N.; Madero, S.; Gómez, M.; Mas, S.; Gassó, P.; Parellada, E. Systematic Review of the Therapeutic Role of Apoptotic Inhibitors in Neurodegeneration and Their Potential Use in Schizophrenia. Antioxidants 2022, 11, 2275. https://doi.org/10.3390/antiox11112275
Morén C, Treder N, Martínez-Pinteño A, Rodríguez N, Arbelo N, Madero S, Gómez M, Mas S, Gassó P, Parellada E. Systematic Review of the Therapeutic Role of Apoptotic Inhibitors in Neurodegeneration and Their Potential Use in Schizophrenia. Antioxidants. 2022; 11(11):2275. https://doi.org/10.3390/antiox11112275
Chicago/Turabian StyleMorén, Constanza, Nina Treder, Albert Martínez-Pinteño, Natàlia Rodríguez, Néstor Arbelo, Santiago Madero, Marta Gómez, Sergi Mas, Patricia Gassó, and Eduard Parellada. 2022. "Systematic Review of the Therapeutic Role of Apoptotic Inhibitors in Neurodegeneration and Their Potential Use in Schizophrenia" Antioxidants 11, no. 11: 2275. https://doi.org/10.3390/antiox11112275
APA StyleMorén, C., Treder, N., Martínez-Pinteño, A., Rodríguez, N., Arbelo, N., Madero, S., Gómez, M., Mas, S., Gassó, P., & Parellada, E. (2022). Systematic Review of the Therapeutic Role of Apoptotic Inhibitors in Neurodegeneration and Their Potential Use in Schizophrenia. Antioxidants, 11(11), 2275. https://doi.org/10.3390/antiox11112275





