Defining the S-Glutathionylation Proteome by Biochemical and Mass Spectrometric Approaches
Abstract
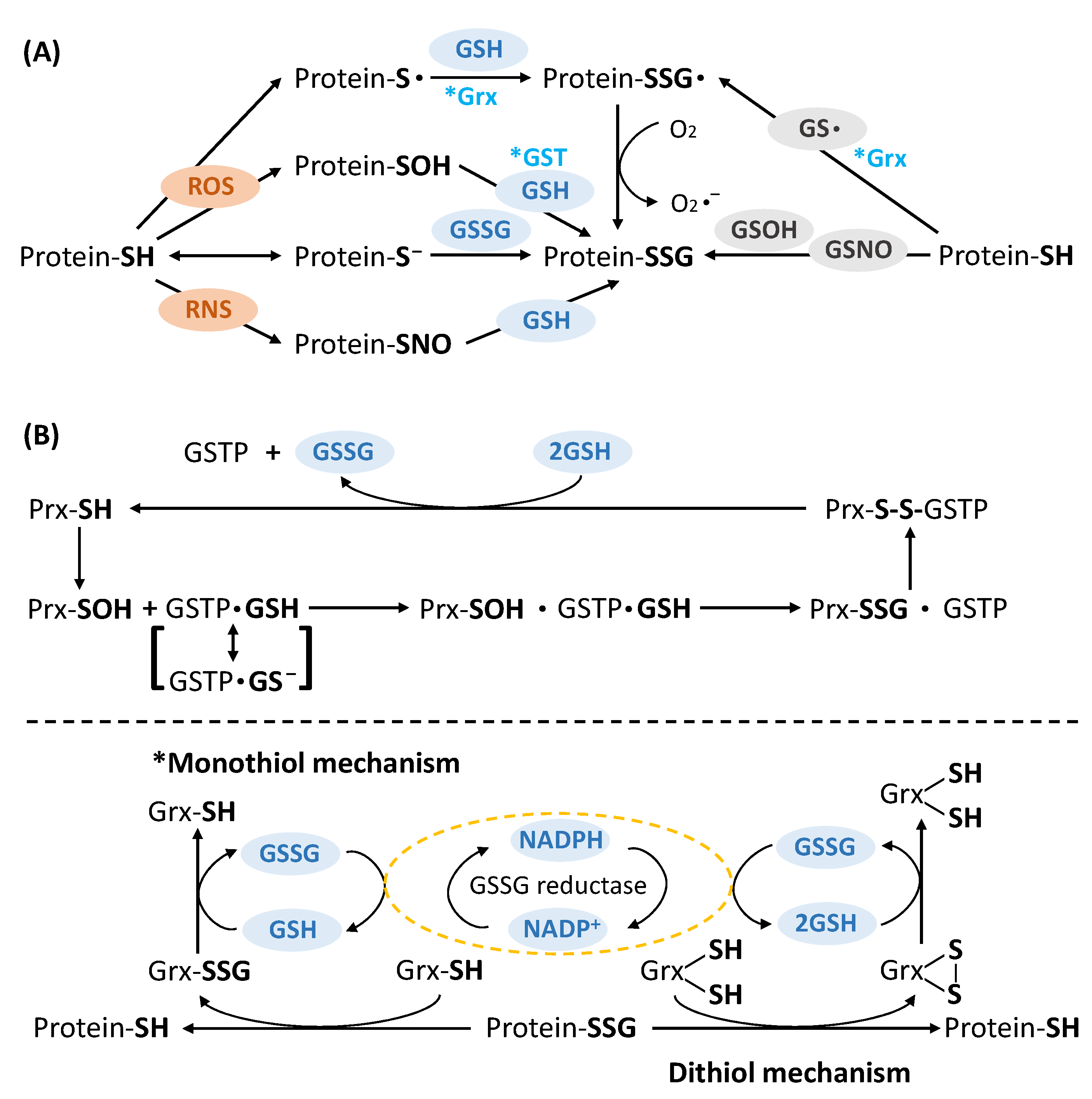
:1. Introduction
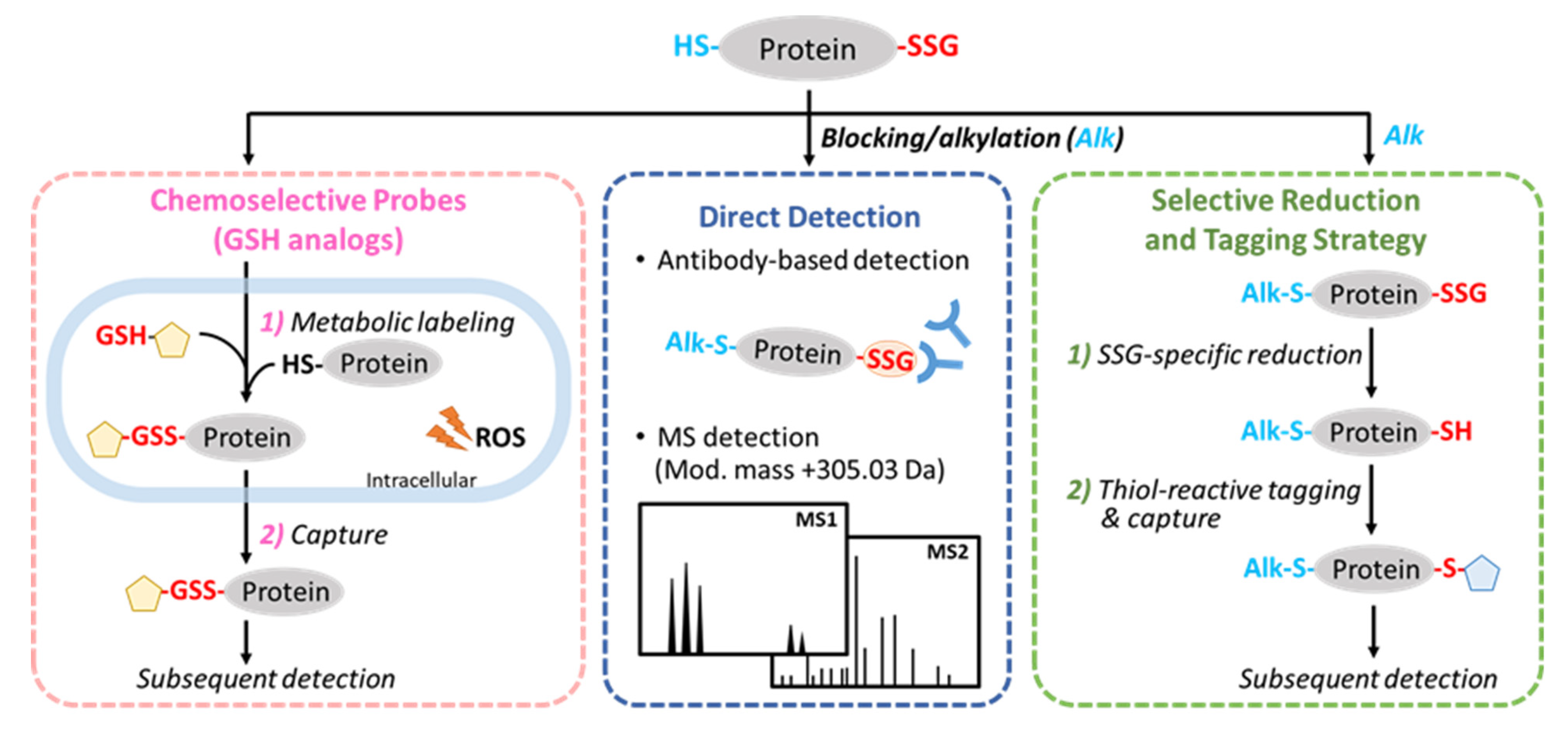
2. General Strategies for the Detection of Protein S-Glutathionylation
3. Methods for Characterizing Protein S-Glutathionylation
3.1. Direct Detection
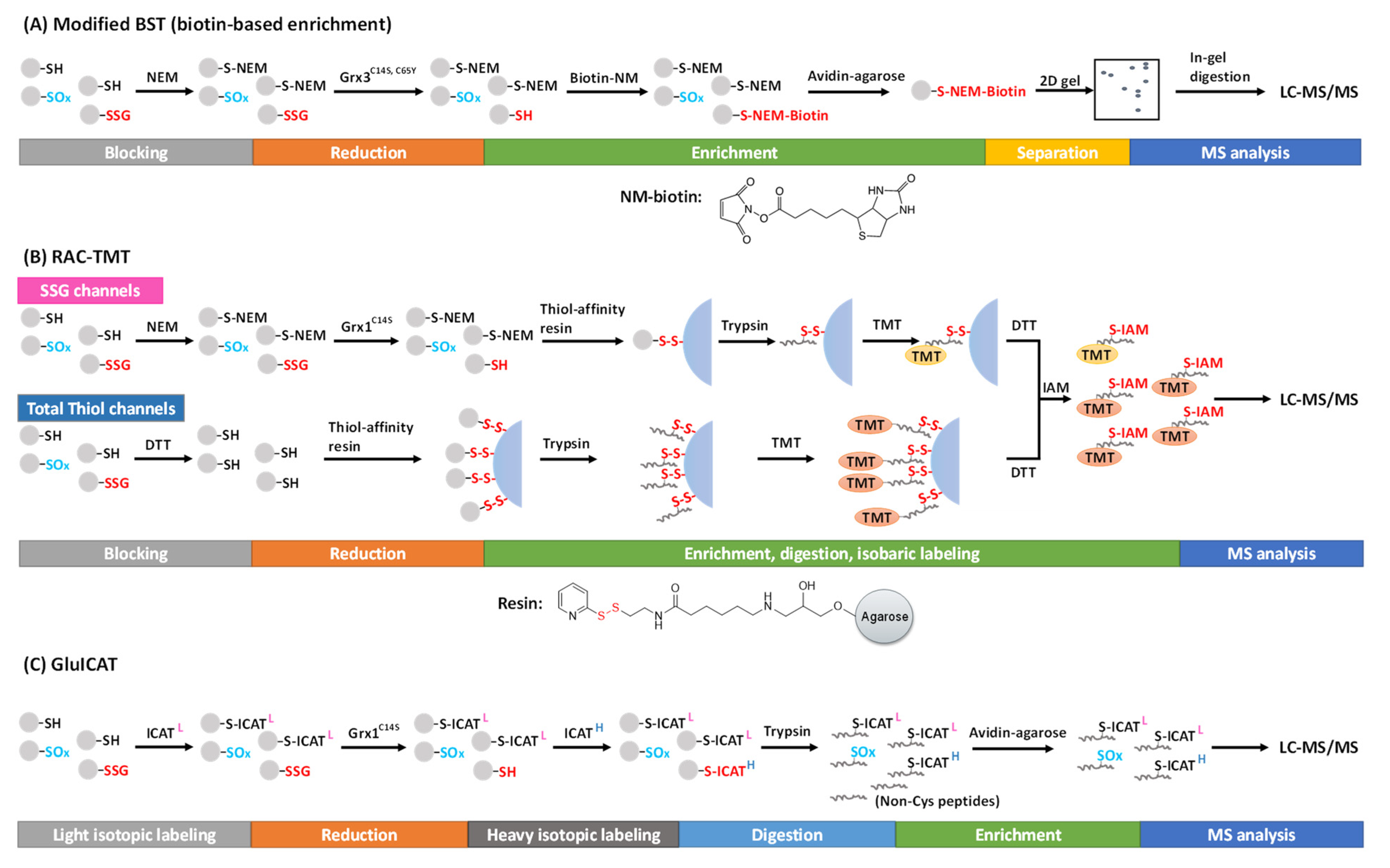
3.2. Selective Reduction and Tagging Approaches
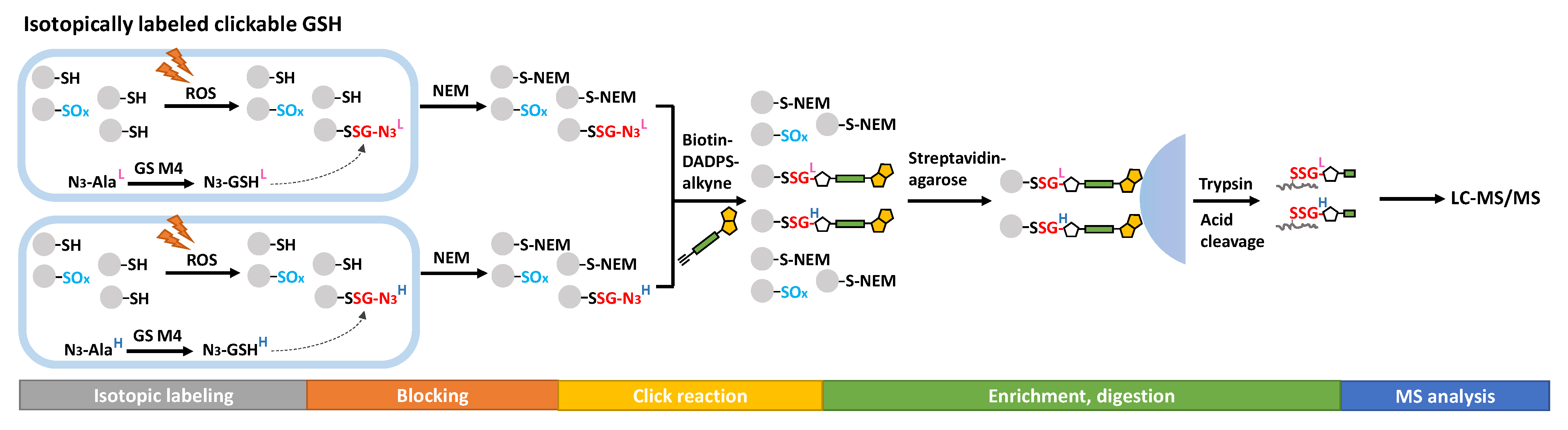
3.3. Chemoselective Probes
3.4. Global Profiling of the SSG Proteome
4. Functional Roles of S-Glutathionylation
5. Conclusions and Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Ursini, F.; Maiorino, M.; Forman, H.J. Redox homeostasis: The Golden Mean of healthy living. Redox Biol. 2016, 8, 205–215. [Google Scholar] [CrossRef] [PubMed]
- Snezhkina, A.V.; Kudryavtseva, A.V.; Kardymon, O.L.; Savvateeva, M.V.; Melnikova, N.V.; Krasnov, G.S.; Dmitriev, A.A. ROS Generation and Antioxidant Defense Systems in Normal and Malignant Cells. Oxid. Med. Cell. Longev. 2019, 2019, 6175804. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Wang, X.; Vikash, V.; Ye, Q.; Wu, D.; Liu, Y.; Dong, W. ROS and ROS-Mediated Cellular Signaling. Oxid. Med. Cell. Longev. 2016, 2016, 4350965. [Google Scholar] [CrossRef] [Green Version]
- Juan, C.; de la Lastra, J.P.; Plou, F.; Pérez-Lebeña, E. The Chemistry of Reactive Oxygen Species (ROS) Revisited: Outlining Their Role in Biological Macromolecules (DNA, Lipids and Proteins) and Induced Pathologies. Int. J. Mol. Sci. 2021, 22, 4642. [Google Scholar] [CrossRef]
- Marengo, B.; Nitti, M.; Furfaro, A.L.; Colla, R.; Ciucis, C.D.; Marinari, U.M.; Pronzato, M.A.; Traverso, N.; Domenicotti, C. Redox Homeostasis and Cellular Antioxidant Systems: Crucial Players in Cancer Growth and Therapy. Oxid. Med. Cell. Longev. 2016, 2016, 6235641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, L.; He, T.; Farrar, S.; Ji, L.; Liu, T.; Ma, X. Antioxidants Maintain Cellular Redox Homeostasis by Elimination of Reactive Oxygen Species. Cell. Physiol. Biochem. 2017, 44, 532–553. [Google Scholar] [CrossRef] [PubMed]
- Schafer, F.Q.; Buettner, G.R. Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free Radic. Biol. Med. 2001, 30, 1191–1212. [Google Scholar] [CrossRef]
- Duan, J.; Kodali, V.K.; Gaffrey, M.J.; Guo, J.; Chu, R.K.; Camp, D.G.; Smith, R.D.; Thrall, B.D.; Qian, W.J. Quantitative Profiling of Protein S-Glutathionylation Reveals Redox-Dependent Regulation of Macrophage Function during Nanoparticle-Induced Oxidative Stress. ACS Nano 2016, 10, 524–538. [Google Scholar] [CrossRef] [Green Version]
- Guo, Y.; Liu, Y.; Zhao, S.; Xu, W.; Li, Y.; Zhao, P.; Wang, D.; Cheng, H.; Ke, Y.; Zhang, X. Oxidative stress-induced FABP5 S-glutathionylation protects against acute lung injury by suppressing inflammation in macrophages. Nat. Commun. 2021, 12, 7094. [Google Scholar] [CrossRef]
- Kyte, J. Structure in Protein Chemistry; Garland Science: New York, NY, USA, 2006. [Google Scholar]
- Cumming, R.C.; Andon, N.L.; Haynes, P.A.; Park, M.; Fischer, W.H.; Schubert, D. Protein Disulfide Bond Formation in the Cytoplasm during Oxidative Stress. J. Biol. Chem. 2004, 279, 21749–21758. [Google Scholar] [CrossRef] [PubMed]
- Poole, L.B. The basics of thiols and cysteines in redox biology and chemistry. Free Radic. Biol. Med. 2015, 80, 148–157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsui, R.; Ferran, B.; Oh, A.; Croteau, D.; Shao, D.; Han, J.; Pimentel, D.R.; Bachschmid, M.M. Redox Regulation via Glutaredoxin-1 and Protein S-Glutathionylation. Antioxid. Redox Signal. 2020, 32, 677–700. [Google Scholar] [CrossRef] [PubMed]
- Kortemme, T.; Creighton, T.E. Ionisation of Cysteine Residues at the Termini of Model α-Helical Peptides. Relevance to Unusual Thiol pKaValues in Proteins of the Thioredoxin Family. J. Mol. Biol. 1995, 253, 799–812. [Google Scholar] [CrossRef] [PubMed]
- Martyniuk, C.J.; Fang, B.; Koomen, J.M.; Gavin, T.; Zhang, L.; Barber, D.S.; Lopachin, R.M. Molecular mechanism of glyceraldehyde-3-phosphate dehydrogenase inactivation by α,β-unsaturated carbonyl derivatives. Chem. Res. Toxicol. 2011, 24, 2302–2311. [Google Scholar] [CrossRef] [Green Version]
- Ravichandran, V.; Seres, T.; Moriguchi, T.; Thomas, J.A.; Johnston, R.B. S-thiolation of glyceraldehyde-3-phosphate dehydrogenase induced by the phagocytosis-associated respiratory burst in blood monocytes. J. Biol. Chem. 1994, 269, 25010–25015. [Google Scholar] [CrossRef]
- Barinova, K.V.; Serebryakova, M.V.; Muronetz, V.I.; Schmalhausen, E.V. S-glutathionylation of glyceraldehyde-3-phosphate dehydrogenase induces formation of C150-C154 intrasubunit disulfide bond in the active site of the enzyme. Biochim. Biophys. Acta (BBA)—Gen. Subj. 2017, 1861, 3167–3177. [Google Scholar] [CrossRef]
- Bedhomme, M.; Adamo, M.; Marchand, C.H.; Couturier, J.; Rouhier, N.; Lemaire, S.D.; Zaffagnini, M.; Trost, P. Glutathionylation of cytosolic glyceraldehyde-3-phosphate dehydrogenase from the model plant Arabidopsis thaliana is reversed by both glutaredoxins and thioredoxins in vitro. Biochem. J. 2012, 445, 337–347. [Google Scholar] [CrossRef] [Green Version]
- Tossounian, M.-A.; Zhang, B.; Gout, I. The Writers, Readers, and Erasers in Redox Regulation of GAPDH. Antioxidants 2020, 9, 1288. [Google Scholar] [CrossRef]
- Casagrande, S.; Bonetto, V.; Fratelli, M.; Gianazza, E.; Eberini, I.; Massignan, T.; Salmona, M.; Chang, G.; Holmgren, A.; Ghezzi, P. Glutathionylation of human thioredoxin: A possible crosstalk between the glutathione and thioredoxin systems. Proc. Natl. Acad. Sci. USA 2002, 99, 9745–9749. [Google Scholar] [CrossRef] [Green Version]
- Mieyal, J.J.; Chock, P.B. Posttranslational modification of cysteine in redox signaling and oxidative stress: Focus on s-glutathionylation. Antioxid. Redox Signal. 2012, 16, 471–475. [Google Scholar] [CrossRef]
- Pal, D.; Rai, A.; Checker, R.; Patwardhan, R.S.; Singh, B.; Sharma, D.; Sandur, S.K. Role of protein S-Glutathionylation in cancer progression and development of resistance to anti-cancer drugs. Arch. Biochem. Biophys. 2021, 704, 108890. [Google Scholar] [CrossRef] [PubMed]
- Mieyal, J.J.; Gallogly, M.M.; Qanungo, S.; Sabens, E.A.; Shelton, M.D. Molecular mechanisms and clinical implications of reversible protein S-glutathionylation. Antioxid. Redox Signal. 2008, 10, 1941–1988. [Google Scholar] [CrossRef] [PubMed]
- Cha, S.J.; Kim, H.; Choi, H.-J.; Lee, S.; Kim, K. Protein Glutathionylation in the Pathogenesis of Neurodegenerative Diseases. Oxid. Med. Cell. Longev. 2017, 2017, 2818565. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chia, S.B.; Elko, E.A.; Aboushousha, R.; Manuel, A.M.; Wetering, C.v.d.; Druso, J.E.; Velden, J.v.d.; Seward, D.J.; Anathy, V.; Irvin, C.G.; et al. Dysregulation of the glutaredoxin/S-glutathionylation redox axis in lung diseases. Am. J. Physiol.-Cell Physiol. 2020, 318, C304–C327. [Google Scholar] [CrossRef] [PubMed]
- Shelton, M.D.; Mieyal, J.J. Regulation by reversible S-glutathionylation: Molecular targets implicated in inflammatory diseases. Mol. Cells 2008, 25, 332–346. [Google Scholar] [PubMed]
- Gilbert, H.F. [2] Thiol/Disulfide Exchange Equilibria and Disulfidebond Stability. In Methods in Enzymology; Academic Press: Cambridge MA, USA, 1995; Volume 251, pp. 8–28. [Google Scholar]
- Gilbert, H.F. Molecular and Cellular Aspects of Thiol–Disulfide Exchange. In Advances in Enzymology and Related Areas of Molecular Biology; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 1990; pp. 69–172. [Google Scholar] [CrossRef]
- Gallogly, M.M.; Mieyal, J.J. Mechanisms of reversible protein glutathionylation in redox signaling and oxidative stress. Curr. Opin. Pharmacol. 2007, 7, 381–391. [Google Scholar] [CrossRef]
- Klatt, P.; Molina, E.P.; de Lacoba, M.G.; Padilla, C.A.; Martínez-Galisteo, E.; Barcena, J.; Lamas, S. Redox regulation of c-Jun DNA binding by reversible S-glutathiolation. FASEB J. 1999, 13, 1481–1490. [Google Scholar] [CrossRef]
- Zhang, J.; Ye, Z.-w.; Singh, S.; Townsend, D.M.; Tew, K.D. An evolving understanding of the S-glutathionylation cycle in pathways of redox regulation. Free Radic. Biol. Med. 2018, 120, 204–216. [Google Scholar] [CrossRef]
- Kalinina, E.; Novichkova, M. Glutathione in Protein Redox Modulation through S-Glutathionylation and S-Nitrosylation. Molecules 2021, 26, 435. [Google Scholar] [CrossRef]
- Giustarini, D.; Milzani, A.; Aldini, G.; Carini, M.; Rossi, R.; Dalle-Donne, I. S-Nitrosation versus S-Glutathionylation of Protein Sulfhydryl Groups by S-Nitrosoglutathione. Antioxid. Redox Signal. 2005, 7, 930–939. [Google Scholar] [CrossRef]
- Klatt, P.; Molina, E.P.; Lamas, S. Nitric Oxide Inhibits c-Jun DNA Binding by Specifically TargetedS-Glutathionylation. J. Biol. Chem. 1999, 274, 15857–15864. [Google Scholar] [CrossRef] [Green Version]
- Uemura, T.; Tsaprailis, G.; Gerner, E.W. GSTΠ stimulates caveolin-1-regulated polyamine uptake via actin remodeling. Oncotarget 2019, 10, 5713–5723. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Ye, Z.-w.; Chen, W.; Manevich, Y.; Mehrotra, S.; Ball, L.; Janssen-Heininger, Y.; Tew, K.D.; Townsend, D.M. S-Glutathionylation of estrogen receptor α affects dendritic cell function. J. Biol. Chem. 2018, 293, 4366–4380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wetzelberger, K.; Baba, S.P.; Thirunavukkarasu, M.; Ho, Y.-S.; Maulik, N.; Barski, O.A.; Conklin, D.J.; Bhatnagar, A. Postischemic Deactivation of Cardiac Aldose Reductase: ROLE OF GLUTATHIONE S-TRANSFERASE P AND GLUTAREDOXIN IN REGENERATION OF REDUCED THIOLS FROM SULFENIC ACIDS. J. Biol. Chem. 2010, 285, 26135–26148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ye, Z.-W.; Zhang, J.; Ancrum, T.; Manevich, Y.; Townsend, D.M.; Tew, K.D. Glutathione S-Transferase P-Mediated Protein S-Glutathionylation of Resident Endoplasmic Reticulum Proteins Influences Sensitivity to Drug-Induced Unfolded Protein Response. Antioxid. Redox Signal. 2017, 26, 247–261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Townsend, D.M.; Manevich, Y.; He, L.; Hutchens, S.; Pazoles, C.J.; Tew, K.D. Novel Role for Glutathione S-Transferase π: Regulator of Protein S-Glutathionylation Following Oxidative and Nitrosative Stress. J. Biol. Chem. 2009, 284, 436–445. [Google Scholar] [CrossRef] [Green Version]
- Graminski, G.F.; Kubo, Y.; Armstrong, R.N. Spectroscopic and kinetic evidence for the thiolate anion of glutathione at the active site of glutathione S-transferase. Biochemistry 1989, 28, 3562–3568. [Google Scholar] [CrossRef]
- Manevich, Y.; Feinstein, S.I.; Fisher, A.B. Activation of the antioxidant enzyme 1-CYS peroxiredoxin requires glutathionylation mediated by heterodimerization with πGST. Proc. Natl. Acad. Sci. USA 2004, 101, 3780–3785. [Google Scholar] [CrossRef] [Green Version]
- Ralat, L.A.; Manevich, Y.; Fisher, A.B.; Colman, R.F. Direct Evidence for the Formation of a Complex between 1-Cysteine Peroxiredoxin and Glutathione S-Transferase π with Activity Changes in Both Enzymes. Biochemistry 2006, 45, 360–372. [Google Scholar] [CrossRef]
- Mannervik, B.; Axelsson, K. Role of cytoplasmic thioltransferase in cellular regulation by thiol-disulphide interchange. Biochem. J. 1980, 190, 125–130. [Google Scholar] [CrossRef] [Green Version]
- Gladyshev, V.N.; Liu, A.; Novoselov, S.V.; Krysan, K.; Sun, Q.-A.; Kryukov, V.M.; Kryukov, G.V.; Lou, M.F. Identification and Characterization of a New Mammalian Glutaredoxin (Thioltransferase), Grx2. J. Biol. Chem. 2001, 276, 30374–30380. [Google Scholar] [CrossRef] [Green Version]
- Menon, D.; Board, P.G. A Role for Glutathione Transferase Omega 1 (GSTO1-1) in the Glutathionylation Cycle. J. Biol. Chem. 2013, 288, 25769–25779. [Google Scholar] [CrossRef] [Green Version]
- Park, J.W.; Mieyal, J.J.; Rhee, S.G.; Chock, P.B. Deglutathionylation of 2-Cys Peroxiredoxin Is Specifically Catalyzed by Sulfiredoxin*. J. Biol. Chem. 2009, 284, 23364–23374. [Google Scholar] [CrossRef] [Green Version]
- Gallogly, M.M.; Starke, D.W.; Leonberg, A.K.; Ospina, S.M.E.; Mieyal, J.J. Kinetic and Mechanistic Characterization and Versatile Catalytic Properties of Mammalian Glutaredoxin 2: Implications for Intracellular Roles. Biochemistry 2008, 47, 11144–11157. [Google Scholar] [CrossRef] [Green Version]
- Musaogullari, A.; Chai, Y.-C. Redox Regulation by Protein S-Glutathionylation: From Molecular Mechanisms to Implications in Health and Disease. Int. J. Mol. Sci. 2020, 21, 8113. [Google Scholar] [CrossRef]
- Wu, C.; Parrott, A.M.; Fu, C.; Liu, T.; Marino, S.M.; Gladyshev, V.N.; Jain, M.R.; Baykal, A.T.; Li, Q.; Oka, S.; et al. Thioredoxin 1-Mediated Post-Translational Modifications: Reduction, Transnitrosylation, Denitrosylation, and Related Proteomics Methodologies. Antioxid. Redox Signal. 2011, 15, 2565–2604. [Google Scholar] [CrossRef] [Green Version]
- Guo, J.; Gaffrey, M.J.; Su, D.; Liu, T.; Camp, D.G., II; Smith, R.D.; Qian, W.J. Resin-assisted enrichment of thiols as a general strategy for proteomic profiling of cysteine-based reversible modifications. Nat. Protoc. 2014, 9, 64–75. [Google Scholar] [CrossRef] [Green Version]
- Carroll, L.; Jiang, S.; Irnstorfer, J.; Beneyto, S.; Ignasiak, M.T.; Rasmussen, L.M.; Rogowska-Wrzesinska, A.; Davies, M.J. Oxidant-induced glutathionylation at protein disulfide bonds. Free Radic. Biol. Med. 2020, 160, 513–525. [Google Scholar] [CrossRef]
- Jiang, S.; Carroll, L.; Rasmussen, L.M.; Davies, M.J. Oxidation of protein disulfide bonds by singlet oxygen gives rise to glutathionylated proteins. Redox Biol. 2021, 38, 101822. [Google Scholar] [CrossRef]
- Wolhuter, K.; Whitwell, H.J.; Switzer, C.H.; Burgoyne, J.R.; Timms, J.F.; Eaton, P. Evidence against Stable Protein S-Nitrosylation as a Widespread Mechanism of Post-translational Regulation. Mol. Cell 2018, 69, 438–450.e435. [Google Scholar] [CrossRef]
- Guo, J.; Nguyen, A.Y.; Dai, Z.; Su, D.; Gaffrey, M.J.; Moore, R.J.; Jacobs, J.M.; Monroe, M.E.; Smith, R.D.; Koppenaal, D.W.; et al. Proteome-wide Light/Dark Modulation of Thiol Oxidation in Cyanobacteria Revealed by Quantitative Site-specific Redox Proteomics. Mol. Cell. Proteom. 2014, 13, 3270–3285. [Google Scholar] [CrossRef] [Green Version]
- Gaffrey, M.J.; Day, N.J.; Li, X.; Qian, W.J. Resin-Assisted Capture Coupled with Isobaric Tandem Mass Tag Labeling for Multiplexed Quantification of Protein Thiol Oxidation. J. Vis. Exp. 2021, 172, e62671. [Google Scholar] [CrossRef]
- Lu, S.; Fan, S.-B.; Yang, B.; Li, Y.-X.; Meng, J.-M.; Wu, L.; Li, P.; Zhang, K.; Zhang, M.-J.; Fu, Y.; et al. Mapping native disulfide bonds at a proteome scale. Nat. Methods 2015, 12, 329–331. [Google Scholar] [CrossRef]
- Michelet, L.; Zaffagnini, M.; Vanacker, H.; Le Maréchal, P.; Marchand, C.; Schroda, M.; Lemaire, S.D.; Decottignies, P. In Vivo Targets of S-Thiolation in Chlamydomonas reinhardtii. J.Bio. Chem. 2008, 283, 21571–21578. [Google Scholar] [CrossRef]
- Fratelli, M.; Demol, H.; Puype, M.; Casagrande, S.; Villa, P.; Eberini, I.; Vandekerckhove, J.; Gianazza, E.; Ghezzi, P. Identification of proteins undergoing glutathionylation in oxidatively stressed hepatocytes and hepatoma cells. Proteomics 2003, 3, 1154–1161. [Google Scholar] [CrossRef]
- Schuppe-Koistinen, I.; Moldeus, P.; Bergman, T.; Cotgreave, I.A. S-Thiolation of human endothelial cell glyceraldehyde-3-phosphate dehydrogenase after hydrogen peroxide treatment. Eur. J. Biochem. 1994, 221, 1033–1037. [Google Scholar] [CrossRef]
- Rokutan, K.; Johnston, R.B.; Kawai, K. Oxidative stress induces S-thiolation of specific proteins in cultured gastric mucosal cells. Am. J. Physiol.-Gastrointest. Liver Physiol. 1994, 266, G247–G254. [Google Scholar] [CrossRef]
- Ward, N.E.; Stewart, J.R.; Ioannides, C.G.; O’Brian, C.A. Oxidant-Induced S-Glutathiolation Inactivates Protein Kinase C-α (PKC-α): A Potential Mechanism of PKC Isozyme Regulation. Biochemistry 2000, 39, 10319–10329. [Google Scholar] [CrossRef]
- Shenton, D.; Grant, C.M. Protein S-thiolation targets glycolysis and protein synthesis in response to oxidative stress in the yeast Saccharomyces cerevisiae. Biochem. J. 2003, 374, 513–519. [Google Scholar] [CrossRef]
- Dalle-Donne, I.; Giustarini, D.; Colombo, R.; Milzani, A.; Rossi, R. S-glutathionylation in human platelets by a thiol-disulfide exchange-independent mechanism. Free Radic. Biol. Med. 2005, 38, 1501–1510. [Google Scholar] [CrossRef]
- Fratelli, M.; Gianazza, E.; Ghezzi, P. Redox proteomics: Identification and functional role of glutathionylated proteins. Expert Rev. Proteom. 2004, 1, 365–376. [Google Scholar] [CrossRef]
- Domenico, F.D.; Cenini, G.; Sultana, R.; Perluigi, M.; Uberti, D.; Memo, M.; Butterfield, D.A. Glutathionylation of the Pro-apoptotic Protein p53 in Alzheimer’s Disease Brain: Implications for AD Pathogenesis. Neurochem. Res. 2009, 34, 727–733. [Google Scholar] [CrossRef]
- Kil, I.S.; Kim, S.Y.; Park, J.-W. Glutathionylation regulates IκB. Biochem. Biophys. Res. Commun. 2008, 373, 169–173. [Google Scholar] [CrossRef]
- Carvalho, A.N.; Marques, C.; Guedes, R.C.; Castro-Caldas, M.; Rodrigues, E.; van Horssen, J.; Gama, M.J. S-Glutathionylation of Keap1: A new role for glutathione S-transferase pi in neuronal protection. FEBS Lett. 2016, 590, 1455–1466. [Google Scholar] [CrossRef] [Green Version]
- Jin, Y.; Diffee, G.M.; Colman, R.J.; Anderson, R.M.; Ge, Y. Top-down Mass Spectrometry of Sarcomeric Protein Post-translational Modifications from Non-human Primate Skeletal Muscle. J. Am. Soc. Mass Spectrom. 2019, 30, 2460–2469. [Google Scholar] [CrossRef]
- Wei, L.; Gregorich, Z.R.; Lin, Z.; Cai, W.; Jin, Y.; McKiernan, S.H.; McIlwain, S.; Aiken, J.M.; Moss, R.L.; Diffee, G.M.; et al. Novel Sarcopenia-related Alterations in Sarcomeric Protein Post-translational Modifications (PTMs) in Skeletal Muscles Identified by Top-down Proteomics. Mol. Cell. Proteom. 2018, 17, 134–145. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Day, N.J.; Feng, S.; Gaffrey, M.J.; Lin, T.D.; Paurus, V.L.; Monroe, M.E.; Moore, R.J.; Yang, B.; Xian, M.; et al. Mass spectrometry-based direct detection of multiple types of protein thiol modifications in pancreatic beta cells under endoplasmic reticulum stress. Redox Biol. 2021, 46, 102111. [Google Scholar] [CrossRef]
- Jaffrey, S.R.; Snyder, S.H. The Biotin Switch Method for the Detection of S-Nitrosylated Proteins. Sci. STKE 2001, 2001, pl1. [Google Scholar] [CrossRef]
- Lind, C.; Gerdes, R.; Hamnell, Y.; Schuppe-Koistinen, I.; von Löwenhielm, H.B.; Holmgren, A.; Cotgreave, I.A. Identification of S-glutathionylated cellular proteins during oxidative stress and constitutive metabolism by affinity purification and proteomic analysis. Arch. Biochem. Biophys. 2002, 406, 229–240. [Google Scholar] [CrossRef]
- Su, D.; Gaffrey, M.J.; Guo, J.; Hatchell, K.E.; Chu, R.K.; Clauss, T.R.W.; Aldrich, J.T.; Wu, S.; Purvine, S.; Camp, D.G.; et al. Proteomic identification and quantification of S-glutathionylation in mouse macrophages using resin-assisted enrichment and isobaric labeling. Free Radic. Biol. Med. 2014, 67, 460–470. [Google Scholar] [CrossRef] [Green Version]
- Bushweller, J.H.; Aaslund, F.; Wuethrich, K.; Holmgren, A. Structural and functional characterization of the mutant Escherichia coli glutaredoxin (C14.fwdarw.S) and its mixed disulfide with glutathione. Biochemistry 1992, 31, 9288–9293. [Google Scholar] [CrossRef] [PubMed]
- Reynaert, N.L.; Ckless, K.; Guala, A.S.; Wouters, E.F.M.; van der Vliet, A.; Janssen-Heininger, Y.M.W. In situ detection of S-glutathionylated proteins following glutaredoxin-1 catalyzed cysteine derivatization. Biochim. Biophys. Acta (BBA)—Gen. Subj. 2006, 1760, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Qian, W.-J.; Strittmatter, E.F.; Camp, D.G.; Anderson, G.A.; Thrall, B.D.; Smith, R.D. High-Throughput Comparative Proteome Analysis Using a Quantitative Cysteinyl-peptide Enrichment Technology. Anal. Chem. 2004, 76, 5345–5353. [Google Scholar] [CrossRef]
- Kramer, P.A.; Duan, J.; Gaffrey, M.J.; Shukla, A.K.; Wang, L.; Bammler, T.K.; Qian, W.J.; Marcinek, D.J. Fatiguing contractions increase protein S-glutathionylation occupancy in mouse skeletal muscle. Redox Biol. 2018, 17, 367–376. [Google Scholar] [CrossRef]
- Duan, J.; Zhang, T.; Gaffrey, M.J.; Weitz, K.K.; Moore, R.J.; Li, X.; Xian, M.; Thrall, B.D.; Qian, W.J. Stochiometric quantification of the thiol redox proteome of macrophages reveals subcellular compartmentalization and susceptibility to oxidative perturbations. Redox Biol. 2020, 36, 101649. [Google Scholar] [CrossRef]
- Zhang, T.; Gaffrey, M.J.; Li, X.; Qian, W.J. Characterization of cellular oxidative stress response by stoichiometric redox proteomics. Am. J. Physiol. Cell Physiol. 2021, 320, C182–C194. [Google Scholar] [CrossRef]
- Day, N.J.; Gaffrey, M.J.; Qian, W.J. Stoichiometric Thiol Redox Proteomics for Quantifying Cellular Responses to Perturbations. Antioxidants 2021, 10, 499. [Google Scholar] [CrossRef]
- McGarry, D.J.; Chen, W.; Chakravarty, P.; Lamont, D.L.; Wolf, C.R.; Henderson, C.J. Proteome-wide identification and quantification of S-glutathionylation targets in mouse liver. Biochem. J. 2015, 469, 25–32. [Google Scholar] [CrossRef]
- Campbell, M.D.; Duan, J.; Samuelson, A.T.; Gaffrey, M.J.; Merrihew, G.E.; Egertson, J.D.; Wang, L.; Bammler, T.K.; Moore, R.J.; White, C.C.; et al. Improving mitochondrial function with SS-31 reverses age-related redox stress and improves exercise tolerance in aged mice. Free Radic. Biol. Med. 2019, 134, 268–281. [Google Scholar] [CrossRef]
- Zhang, T.; Day, N.J.; Gaffrey, M.; Weitz, K.K.; Attah, K.; Mimche, P.N.; Paine, R., III; Qian, W.J.; Helms, M.N. Regulation of hyperoxia-induced neonatal lung injury via post-translational cysteine redox modifications. Redox Biol. 2022, 55, 102405. [Google Scholar] [CrossRef]
- Leichert, L.I.; Gehrke, F.; Gudiseva, H.V.; Blackwell, T.; Ilbert, M.; Walker, A.K.; Strahler, J.R.; Andrews, P.C.; Jakob, U. Quantifying changes in the thiol redox proteome upon oxidative stress in vivo. Proc. Natl. Acad. Sci. USA 2008, 105, 8197–8202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, L.; Chan, J.C.Y.; Chupin, S.; Gueguen, N.; Desquiret-Dumas, V.; Koh, S.K.; Li, J.; Gao, Y.; Deng, L.; Verma, C.; et al. Increased Protein S-Glutathionylation in Leber’s Hereditary Optic Neuropathy (LHON). Int. J. Mol. Sci. 2020, 21, 3027. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.C.Y.; Soh, A.C.K.; Kioh, D.Y.Q.; Li, J.; Verma, C.; Koh, S.K.; Beuerman, R.W.; Zhou, L.; Chan, E.C.Y. Reactive Metabolite-induced Protein Glutathionylation: A Potentially Novel Mechanism Underlying Acetaminophen Hepatotoxicity. Mol. Cell. Proteom. 2018, 17, 2034–2050. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, H.; Jedrychowski, M.P.; Schweppe, D.K.; Huttlin, E.L.; Yu, Q.; Heppner, D.E.; Li, J.; Long, J.; Mills, E.L.; Szpyt, J.; et al. A Quantitative Tissue-Specific Landscape of Protein Redox Regulation during Aging. Cell 2020, 180, 968–983.e24. [Google Scholar] [CrossRef]
- Shakir, S.; Vinh, J.; Chiappetta, G. Quantitative analysis of the cysteine redoxome by iodoacetyl tandem mass tags. Anal. Bioanal. Chem. 2017, 409, 3821–3830. [Google Scholar] [CrossRef]
- Demasi, M.; Silva, G.M.; Netto, L.E. 20 S proteasome from Saccharomyces cerevisiae is responsive to redox modifications and is S-glutathionylated. J. Biol. Chem. 2003, 278, 679–685. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dixon, D.P.; Skipsey, M.; Grundy, N.M.; Edwards, R. Stress-Induced Protein S-Glutathionylation in Arabidopsis. Plant Physiol. 2005, 138, 2233–2244. [Google Scholar] [CrossRef] [Green Version]
- Brennan, J.P.; Miller, J.I.A.; Fuller, W.; Wait, R.; Begum, S.; Dunn, M.J.; Eaton, P. The Utility of N,N-Biotinyl Glutathione Disulfide in the Study of Protein S-Glutathiolation. Mol. Cell. Proteom. 2006, 5, 215–225. [Google Scholar] [CrossRef] [Green Version]
- Sullivan, D.M.; Wehr, N.B.; Fergusson, M.M.; Levine, R.L.; Finkel, T. Identification of Oxidant-Sensitive Proteins: TNF-α Induces Protein Glutathiolation. Biochemistry 2000, 39, 11121–11128. [Google Scholar] [CrossRef]
- Samarasinghe, K.T.G.; Munkanatta Godage, D.N.P.; Zhou, Y.; Ndombera, F.T.; Weerapana, E.; Ahn, Y.-H. A clickable glutathione approach for identification of protein glutathionylation in response to glucose metabolism. Mol. BioSystems 2016, 12, 2471–2480. [Google Scholar] [CrossRef] [Green Version]
- Kekulandara, D.N.; Samarasinghe, K.T.G.; Godage, D.N.P.M.; Ahn, Y.-H. Clickable glutathione using tetrazine-alkene bioorthogonal chemistry for detecting protein glutathionylation. Org. Biomol. Chem. 2016, 14, 10886–10893. [Google Scholar] [CrossRef] [PubMed]
- VanHecke, G.C.; Abeywardana, M.Y.; Ahn, Y.-H. Proteomic Identification of Protein Glutathionylation in Cardiomyocytes. J. Proteome Res. 2019, 18, 1806–1818. [Google Scholar] [CrossRef] [PubMed]
- VanHecke, G.C.; Yapa Abeywardana, M.; Huang, B.; Ahn, Y.-H. Isotopically Labeled Clickable Glutathione to Quantify Protein S-Glutathionylation. ChemBioChem 2020, 21, 853–859. [Google Scholar] [CrossRef]
- Chardonnet, S.; Sakr, S.; Cassier-Chauvat, C.; Le Maréchal, P.; Chauvat, F.; Lemaire, S.D.; Decottignies, P. First Proteomic Study of S-Glutathionylation in Cyanobacteria. J. Proteome Res. 2015, 14, 59–71. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, C.; Li, C.; Zhou, J.; Xu, X.; Peng, X.; Zhou, X. S-glutathionylation proteome profiling reveals a crucial role of a thioredoxin-like protein in interspecies competition and cariogenecity of Streptococcus mutans. PLOS Pathog. 2020, 16, e1008774. [Google Scholar] [CrossRef]
- Pastore, A.; Piemonte, F. S-Glutathionylation signaling in cell biology: Progress and prospects. Eur. J. Pharm. Sci. 2012, 46, 279–292. [Google Scholar] [CrossRef]
- Lermant, A.; Murdoch, C.E. Cysteine Glutathionylation Acts as a Redox Switch in Endothelial Cells. Antioxidants 2019, 8, 315. [Google Scholar] [CrossRef] [Green Version]
- Yi, M.; Ma, Y.; Zhu, S.; Luo, C.; Chen, Y.; Wang, Q.; Deng, H. Comparative proteomic analysis identifies biomarkers for renal aging. Aging 2020, 12, 21890–21903. [Google Scholar] [CrossRef]
- Anathy, V.; Lahue, K.G.; Chapman, D.G.; Chia, S.B.; Casey, D.T.; Aboushousha, R.; van der Velden, J.L.J.; Elko, E.; Hoffman, S.M.; McMillan, D.H.; et al. Reducing protein oxidation reverses lung fibrosis. Nat. Med. 2018, 24, 1128–1135. [Google Scholar] [CrossRef]
- Tsukahara, Y.; Ferran, B.; Minetti, E.T.; Chong, B.S.H.; Gower, A.C.; Bachschmid, M.M.; Matsui, R. Administration of Glutaredoxin-1 Attenuates Liver Fibrosis Caused by Aging and Non-Alcoholic Steatohepatitis. Antioxidants 2022, 11, 867. [Google Scholar] [CrossRef]
- Newman, S.F.; Sultana, R.; Perluigi, M.; Coccia, R.; Cai, J.; Pierce, W.M.; Klein, J.B.; Turner, D.M.; Butterfield, D.A. An increase in S-glutathionylated proteins in the Alzheimer’s disease inferior parietal lobule, a proteomics approach. J. Neurosci. Res. 2007, 85, 1506–1514. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, H.; Gong, W.; Liu, Z.; Wu, H.; Hu, W.; Chen, X.; Wang, L.; Wu, S.; Chen, C.; et al. S-Glutathionylation of human inducible Hsp70 reveals a regulatory mechanism involving the C-terminal α-helical lid. J. Biol. Chem. 2020, 295, 8302–8324. [Google Scholar] [CrossRef] [Green Version]
- Loescher, C.M.; Breitkreuz, M.; Li, Y.; Nickel, A.; Unger, A.; Dietl, A.; Schmidt, A.; Mohamed, B.A.; Kötter, S.; Schmitt, J.P.; et al. Regulation of titin-based cardiac stiffness by unfolded domain oxidation (UnDOx). Proc. Natl. Acad. Sci. USA 2020, 117, 24545–24556. [Google Scholar] [CrossRef]
- Li, S.; Wang, L.; Xu, Z.; Huang, Y.; Xue, R.; Yue, T.; Xu, L.; Gong, F.; Bai, S.; Wu, Q.; et al. ASC deglutathionylation is a checkpoint for NLRP3 inflammasome activation. J. Exp. Med. 2021, 218, e20202637. [Google Scholar] [CrossRef]
- Watanabe, Y.; Watanabe, K.; Fujioka, D.; Nakamura, K.; Nakamura, T.; Uematsu, M.; Bachschmid, M.M.; Matsui, R.; Kugiyama, K. Protein S-glutathionylation stimulate adipogenesis by stabilizing C/EBPβ in 3T3L1 cells. FASEB J. 2020, 34, 5827–5837. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Ye, Z.-w.; Chen, W.; Culpepper, J.; Jiang, H.; Ball, L.E.; Mehrotra, S.; Blumental-Perry, A.; Tew, K.D.; Townsend, D.M. Altered redox regulation and S-glutathionylation of BiP contribute to bortezomib resistance in multiple myeloma. Free Radic. Biol. Med. 2020, 160, 755–767. [Google Scholar] [CrossRef]
- Zaffagnini, M.; Marchand, C.H.; Malferrari, M.; Murail, S.; Bonacchi, S.; Genovese, D.; Montalti, M.; Venturoli, G.; Falini, G.; Baaden, M.; et al. Glutathionylation primes soluble glyceraldehyde-3-phosphate dehydrogenase for late collapse into insoluble aggregates. Proc. Natl. Acad. Sci. USA 2019, 116, 26057–26065. [Google Scholar] [CrossRef]
- Kaus-Drobek, M.; Mücke, N.; Szczepanowski, R.H.; Wedig, T.; Czarnocki-Cieciura, M.; Polakowska, M.; Herrmann, H.; Wysłouch-Cieszyńska, A.; Dadlez, M. Vimentin S-glutathionylation at Cys328 inhibits filament elongation and induces severing of mature filaments in vitro. FEBS J. 2020, 287, 5304–5322. [Google Scholar] [CrossRef]
- Dutka, T.L.; Mollica, J.P.; Lamboley, C.R.; Weerakkody, V.C.; Greening, D.W.; Posterino, G.S.; Murphy, R.M.; Lamb, G.D. S-nitrosylation and S-glutathionylation of Cys134 on troponin I have opposing competitive actions on Ca2+ sensitivity in rat fast-twitch muscle fibers. Am. J. Physiol.-Cell Physiol. 2017, 312, C316–C327. [Google Scholar] [CrossRef]
- Mohr, S.; Hallak, H.; de Boitte, A.; Lapetina, E.G.; Brüne, B. Nitric Oxide-induced S-Glutathionylation and Inactivation of Glyceraldehyde-3-phosphate Dehydrogenase. J. Biol. Chem. 1999, 274, 9427–9430. [Google Scholar] [CrossRef] [Green Version]
- Mustafa Rizvi, S.H.; Shao, D.; Tsukahara, Y.; Pimentel, D.R.; Weisbrod, R.M.; Hamburg, N.M.; McComb, M.E.; Matsui, R.; Bachschmid, M.M. Oxidized GAPDH transfers S-glutathionylation to a nuclear protein Sirtuin-1 leading to apoptosis. Free Radic. Biol. Med. 2021, 174, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Sirover, M.A. Moonlighting glyceraldehyde-3-phosphate dehydrogenase: Posttranslational modification, protein and nucleic acid interactions in normal cells and in human pathology. Crit. Rev. Biochem. Mol. Biol. 2020, 55, 354–371. [Google Scholar] [CrossRef] [PubMed]
- Alegre-Cebollada, J.; Kosuri, P.; Giganti, D.; Eckels, E.; Rivas-Pardo, J.A.; Hamdani, N.; Warren, C.M.; Solaro, R.J.; Linke, W.A.; Fernández, J.M. S-Glutathionylation of Cryptic Cysteines Enhances Titin Elasticity by Blocking Protein Folding. Cell 2014, 156, 1235–1246. [Google Scholar] [CrossRef] [Green Version]
- Leutert, M.; Entwisle, S.W.; Villén, J. Decoding Post-Translational Modification Crosstalk With Proteomics. Mol. Cell. Proteom. 2021, 20. [Google Scholar] [CrossRef]
- Budde, H.; Hassoun, R.; Tangos, M.; Zhazykbayeva, S.; Herwig, M.; Varatnitskaya, M.; Sieme, M.; Delalat, S.; Sultana, I.; Kolijn, D.; et al. The Interplay between S-Glutathionylation and Phosphorylation of Cardiac Troponin I and Myosin Binding Protein C in End-Stage Human Failing Hearts. Antioxidants 2021, 10, 1134. [Google Scholar] [CrossRef]
- Day, N.J.; Zhang, T.; Gaffrey, M.J.; Zhao, R.; Fillmore, T.L.; Moore, R.J.; Rodney, G.G.; Qian, W.-J. A deep redox proteome profiling workflow and its application to skeletal muscle of a Duchenne Muscular Dystrophy model. Free Radic. Biol. Med. 2022, 193, 373–384. [Google Scholar] [CrossRef]
- Kruyer, A.; Ball, L.E.; Townsend, D.M.; Kalivas, P.W.; Uys, J.D. Post-translational S-glutathionylation of cofilin increases actin cycling during cocaine seeking. PLoS ONE 2019, 14, e0223037. [Google Scholar] [CrossRef] [Green Version]
- Gao, X.; Li, L.; Parisien, M.; Wu, J.; Bederman, I.; Gao, Z.; Krokowski, D.; Chirieleison, S.M.; Abbott, D.W.; Wang, B.; et al. Discovery of a redox thiol switch: Implications for cellular energy metabolism. Mol. Cell. Proteom. 2020, 19, 852–870. [Google Scholar] [CrossRef] [Green Version]
- Behring, J.B.; van der Post, S.; Mooradian, A.D.; Egan, M.J.; Zimmerman, M.I.; Clements, J.L.; Bowman, G.R.; Held, J.M. Spatial and temporal alterations in protein structure by EGF regulate cryptic cysteine oxidation. Sci. Signal. 2020, 13, eaay7315. [Google Scholar] [CrossRef]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Žídek, A.; Potapenko, A.; et al. Highly accurate protein structure prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef]
- Tunyasuvunakool, K.; Adler, J.; Wu, Z.; Green, T.; Zielinski, M.; Zidek, A.; Bridgland, A.; Cowie, A.; Meyer, C.; Laydon, A.; et al. Highly accurate protein structure prediction for the human proteome. Nature 2021, 596, 590–596. [Google Scholar] [CrossRef]
- Huang, K.L.; Scott, A.D.; Zhou, D.C.; Wang, L.B.; Weerasinghe, A.; Elmas, A.; Liu, R.; Wu, Y.; Wendl, M.C.; Wyczalkowski, M.A.; et al. Spatially interacting phosphorylation sites and mutations in cancer. Nat. Commun. 2021, 12, 2313. [Google Scholar] [CrossRef] [PubMed]
- Alford, R.F.; Leaver-Fay, A.; Jeliazkov, J.R.; O’Meara, M.J.; DiMaio, F.P.; Park, H.; Shapovalov, M.V.; Renfrew, P.D.; Mulligan, V.K.; Kappel, K.; et al. The Rosetta All-Atom Energy Function for Macromolecular Modeling and Design. J. Chem. Theory Comput. 2017, 13, 3031–3048. [Google Scholar] [CrossRef] [PubMed]
- Audagnotto, M.; Dal Peraro, M. Protein post-translational modifications: In silico prediction tools and molecular modeling. Comput. Struct. Biotechnol. J. 2017, 15, 307–319. [Google Scholar] [CrossRef]
- Zhu, F.; Yang, S.; Meng, F.; Zheng, Y.; Ku, X.; Luo, C.; Hu, G.; Liang, Z. Leveraging Protein Dynamics to Identify Functional Phosphorylation Sites using Deep Learning Models. J. Chem. Inf. Model. 2022, 62, 3331–3345. [Google Scholar] [CrossRef] [PubMed]
- Siodlak, D. alpha, beta-Dehydroamino acids in naturally occurring peptides. Amino Acids 2015, 47, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Lyons, B.; Truscott, R.J.; Schey, K.L. Human protein aging: Modification and crosslinking through dehydroalanine and dehydrobutyrine intermediates. Aging Cell 2014, 13, 226–234. [Google Scholar] [CrossRef] [Green Version]
- Lai, K.-Y.; Galan, S.R.G.; Zeng, Y.; Zhou, T.H.; He, C.; Raj, R.; Riedl, J.; Liu, S.; Chooi, K.P.; Garg, N.; et al. LanCLs add glutathione to dehydroamino acids generated at phosphorylated sites in the proteome. Cell 2021, 184, 2680–2695.e2626. [Google Scholar] [CrossRef]
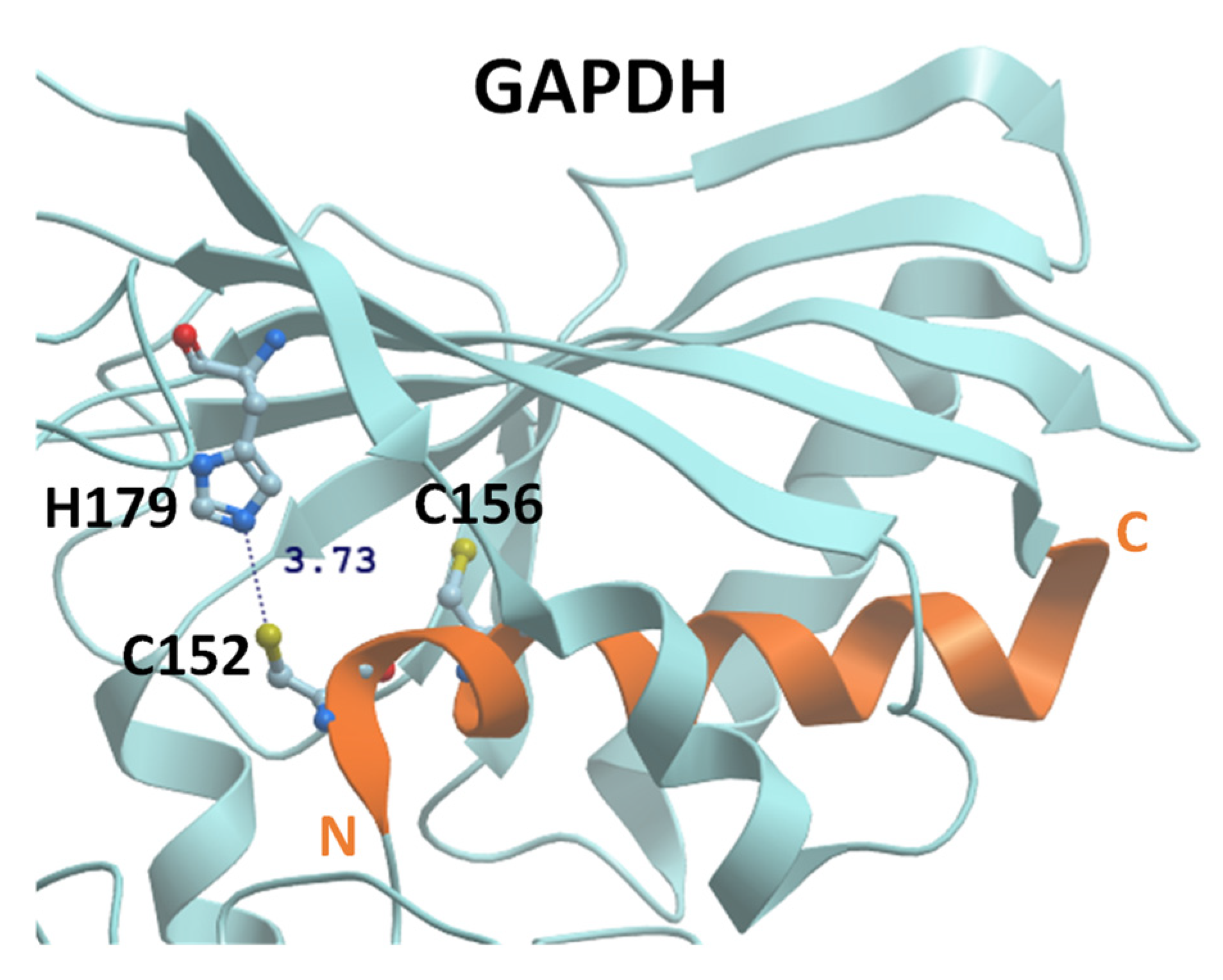
| Biological System | Method | Coverage of the SSG Proteome | Experiment Condition | Significance | Ref. |
|---|---|---|---|---|---|
| Mouse lung | RAC-TMT | ~7600 SSG sites. | Hyperoxia vs. basal; Neonatal wild-type vs. overexpression (β-ENaC) | Landscape view of SSG-modified proteome in mouse lung and the impact of hypoxia on the SSG proteome | [83] |
| RAW 264.7 mouse macrophages | RAC-TMT | Occupancy for 4099 SSG sites | Basal condition | Proteome-wide quantification of both SSG and total oxidation occupancy under physiological conditions, revealing cellular compartmentation of redox homeostasis. | [78] |
| Mouse skeletal muscle | RAC-TMT | Occupancy for >2200 SSG sites and changes due to muscle fatiguing | Gastrocnemius muscle with and without fatiguing contractions | Increased muscle protein SSGs identified following a single bout of fatiguing contraction | [77] |
| HL-1 cardiomyocyte | Clickable GSH | 1398 SSG-peptides in isotopic duplex experiment | H2O2 treatment (1 mM) | In vivo isotopic tagging of protein SSGs for direct enrichment and quantitative detection; Validation (by Western blot and site mutation) of two structural proteins of interest. | [96] |
| Mouse liver | Modified RAC-TMT | 724 SSG-modified proteins | Basal condition, GSTP-nulled mice | The SSG proteome mediated enzymatically by GSTP | [81] |
| Synechocystis sp. PCC6803 | GSSG-Biotin | 349 proteins with SSG (protein level enrichment); 145 SSG sites (peptide level enrichment) | Lysate treated with GSSG-biotin | First SSG proteome profiling in cyanobacteria by GSSG-biotin and LC-MS/MS. | [97] |
| Streptococcus mutans UA159 | IodoTMT switch strategy | 357 SSG sites | Wild-type vs. mutants | SSG profiling in in bacteria and mutants; Site mutagenesis validation for SSG function on a thioredoxin-like protein. | [98] |
| Human skin fibroblasts | GluICAT | 2307 SSG sites | LHON patients vs. healthy controls | Quantify the ratio of SSG and free thiols with heavy or light ICAT | [85] |
| Protein | Cys Site | Biological System | Approaches | Structural/Functional Change | Potential Physiological Consequence | Ref. |
|---|---|---|---|---|---|---|
| Hsp70, human | C574, C603 | HeLa cells | Modified BST | Unfolding of the α-helical lid structure; Blocking substrate-binding site; Increasing ATPase activity | [105] | |
| FABP | C127 | Primary macrophages (mouse) | RAC-TMT, Anti-GSH antibodies (Co-IP) | Promote fatty acid binding function and nuclear translocation; Active PPARβ/δ | Inhibition of LPS-induced inflammation | [9] |
| Titin distal I-band (82Ig83 domain) | C13585 (cryptic cysteines) | Mouse hearts | OxICAT, Anti-GSH antibodies (Western blot) | Unfolded domain oxidation; Enhance titin phosphorylation | Decrease titin-based stiffness | [106] |
| ASC (apoptosis-associated speck-like protein containing a CARD) | C171 | Bone marrow–derived macrophages (mouse) | Anti-GSH antibodies (Western blot, proximity ligation assay) | Domain rotation leading to reduced area of CARD-CARD binding interface; Preventing oligomerization | Repress NLRP3 inflammasome activation | [107] |
| C/EBPβ | C201 and C296 | 3T3L1 preadipocyte | Anti-GSH antibodies (Western blot) | Decreased interaction with PIAS1; Stabilizing C/EBPβ | Promotion of adipogenesis | [108] |
| BiP | C41, C420 | Multiple myeloma cells | Anti-GSH antibodies | Decreased α-helix and increased β-sheets; Enhancing foldase activity; Decreasing ATPase activity | Proteasome inhibitor resistance | [109] |
| GAPDH | C149 | Arabidopsis thaliana | LC-MS/MS | Enzyme inactivation; Misfolding and destabilized conformation; Inducing aggregation and disulfide bond formation with C153 | [110] | |
| SMYD2 | C13 | H9c2 myocytes | Clickable GSH (Western blot) | Dissociation; Disrupts SMYD2–Hsp90–N2A(titin) interactions | Sarcomere destabilization | [93] |
| Vimentin | C328 | LC-MS/MS | Stabilizing pep2B region of vimentin tetramers; Impaired assembly into long filament | [111] | ||
| Troponin I | C134 | Skinned mammalian muscle fiber | BioGEE, anti-GSH antibodies, LC-MS/MS | Competitive action with SNO on the same Cys site | Increased Ca2+ sensitivity | [112] |
| Estrogen receptor α | C221, C245, C417, and C447 | Primary macrophages (mouse) | Anti-GSH antibodies, LC-MS/MS | Reduced binding potential: receptor density and affinity for 17β-estradiol | [36] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Zhang, T.; Day, N.J.; Feng, S.; Gaffrey, M.J.; Qian, W.-J. Defining the S-Glutathionylation Proteome by Biochemical and Mass Spectrometric Approaches. Antioxidants 2022, 11, 2272. https://doi.org/10.3390/antiox11112272
Li X, Zhang T, Day NJ, Feng S, Gaffrey MJ, Qian W-J. Defining the S-Glutathionylation Proteome by Biochemical and Mass Spectrometric Approaches. Antioxidants. 2022; 11(11):2272. https://doi.org/10.3390/antiox11112272
Chicago/Turabian StyleLi, Xiaolu, Tong Zhang, Nicholas J. Day, Song Feng, Matthew J. Gaffrey, and Wei-Jun Qian. 2022. "Defining the S-Glutathionylation Proteome by Biochemical and Mass Spectrometric Approaches" Antioxidants 11, no. 11: 2272. https://doi.org/10.3390/antiox11112272
APA StyleLi, X., Zhang, T., Day, N. J., Feng, S., Gaffrey, M. J., & Qian, W.-J. (2022). Defining the S-Glutathionylation Proteome by Biochemical and Mass Spectrometric Approaches. Antioxidants, 11(11), 2272. https://doi.org/10.3390/antiox11112272







