Caesalpinia sappan Linn. Ameliorates Allergic Nasal Inflammation by Upregulating the Keap1/Nrf2/HO-1 Pathway in an Allergic Rhinitis Mouse Model and Nasal Epithelial Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation and Chemical Profiling of CSLW
2.2. Animals
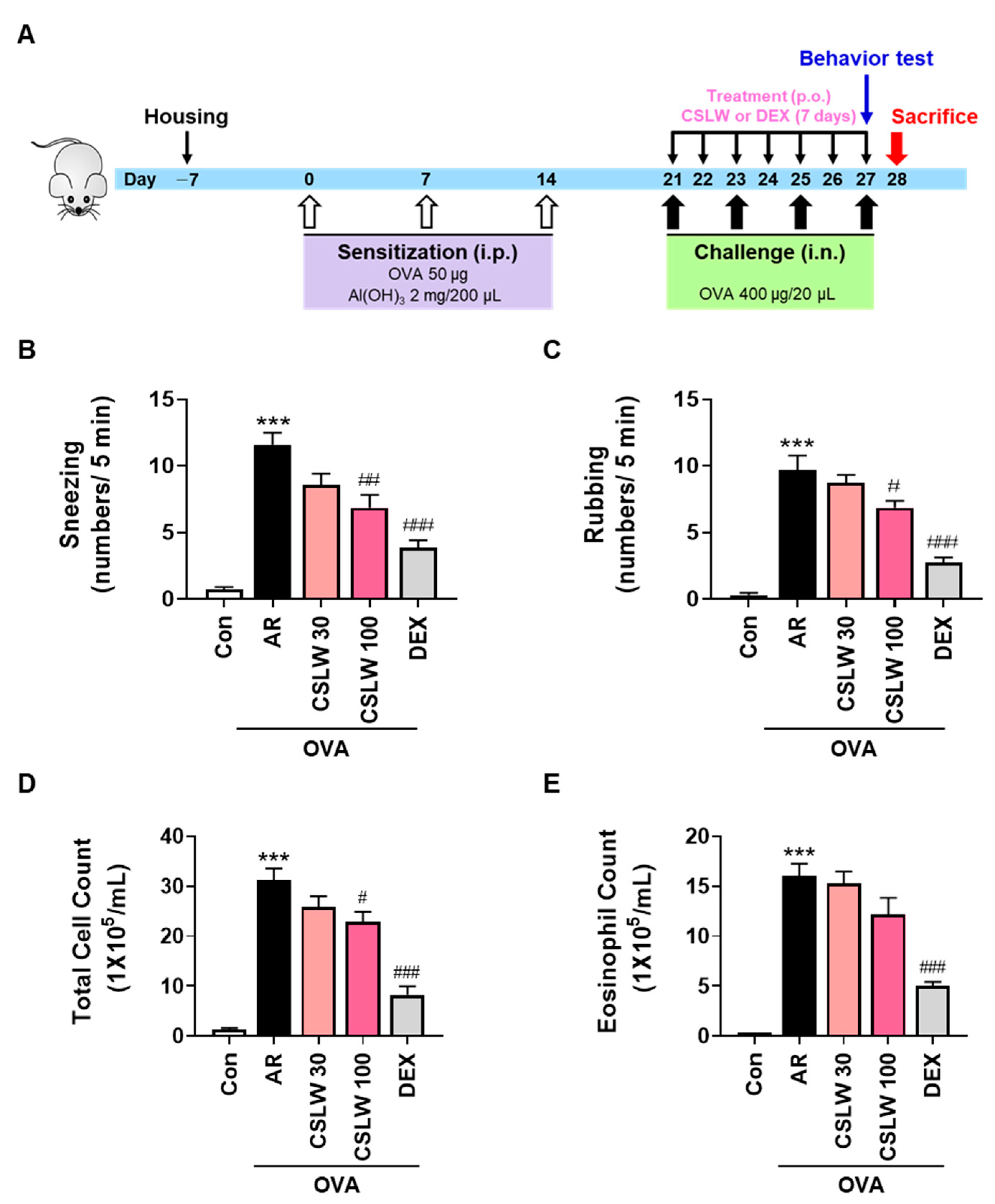
2.3. OVA-Induced AR Mouse Model and Treatment
2.4. Determination of Nasal Symptoms
2.5. Nasal Lavage Fluid (NALF) Collection and Cell Counting
2.6. Serum Collection and Measurement of Serum OVA-Specific IgE, Histamine, IL-5, and IL-13 Levels
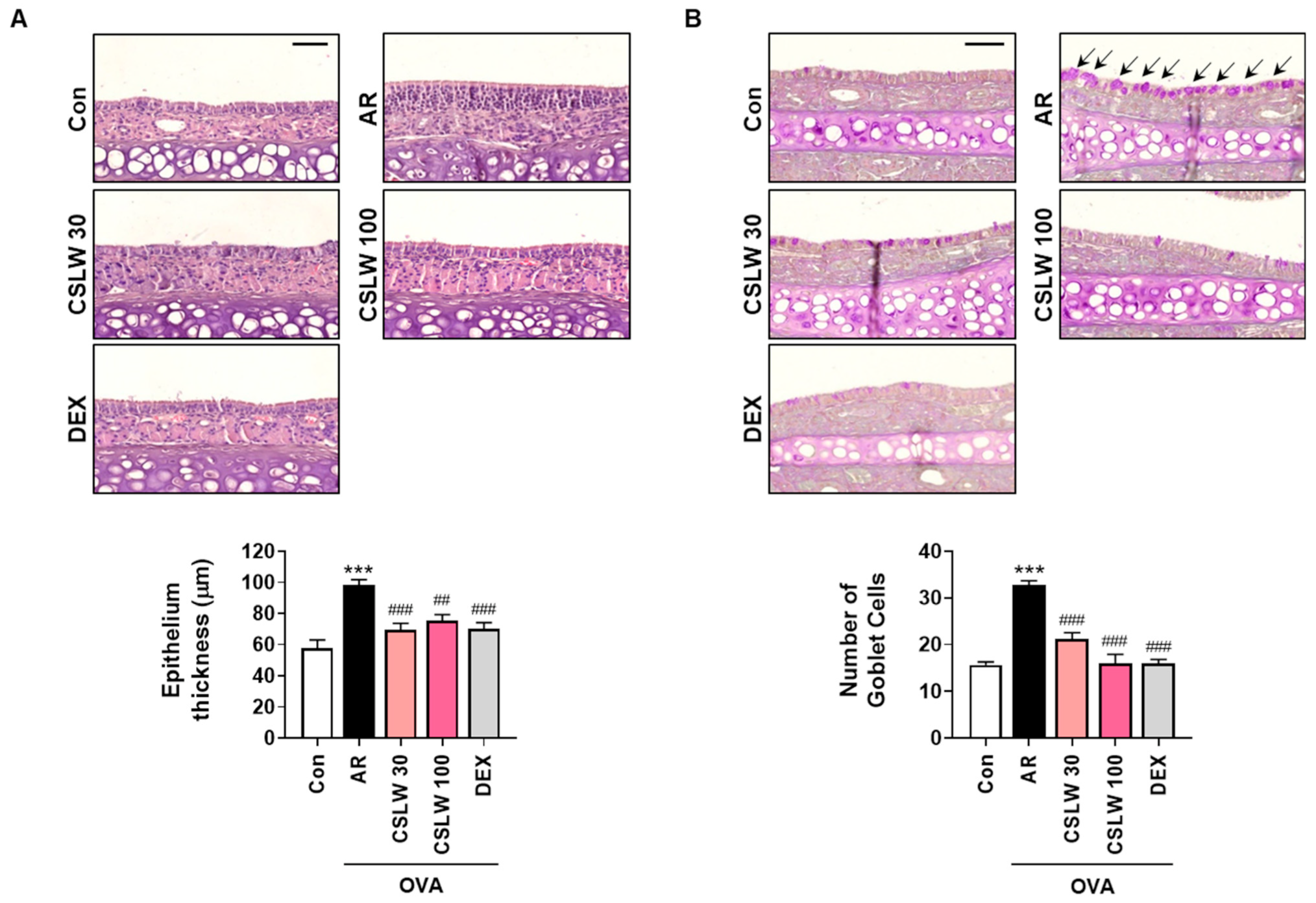
2.7. Histopathological Analysis of Nasal Tissues
2.8. Immunohistochemical Analysis of Nasal Tissues
2.9. Cell Culture
2.10. Cell Viability
2.11. Measurement of Eotaxin-3, Periostin, and MUC5AC Levels
2.12. Immunofluorescence Staining
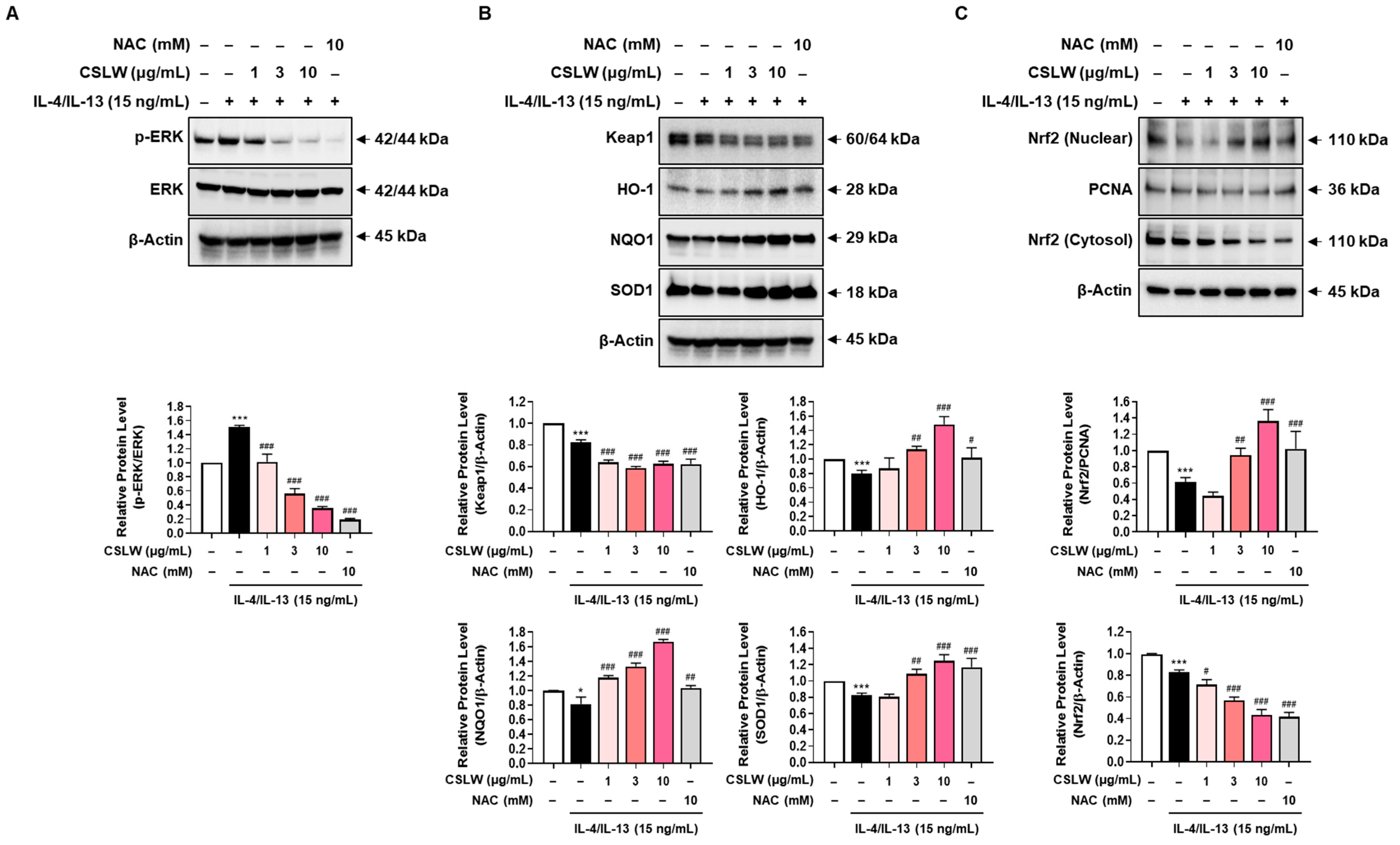
2.13. Determination of Protein Levels
2.14. Statistical Analysis
3. Results
3.1. CSLW Alleviates Nasal Allergy Symptoms and Reduces Immune Cell Accumulation of NALF in OVA-Induced AR Mice
3.2. CSLW Inhibits OVA-Specific IgE, Histamine, and Th2 Inflammatory Cytokine Levels in OVA-Induced AR Mice
3.3. CSLW Inhibits Nasal Mucosa Thickness and Goblet Cell Levels in the Nasal Tissues of OVA-Induced AR Mice
3.4. CSLW Inhibits Eosinophil Infiltration, Periostin and MUC5AC Levels, and Oxidative Damage of Nasal Tissue in OVA-Induced AR Mice
3.5. CSLW Inhibits Eotaxin-3, MUC5AC, and Periostin Expression by Reducing IL-4/IL-13-Induced ROS Production in HNEpCs
3.6. CSLW Inhibits the Production of IL-4/IL-13-Induced Inflammatory Mediators in HNEpCs via Regulation of the ERK-MAPK and Nrf2/HO-1 Signaling Pathways
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Watts, A.M.; Cripps, A.W.; West, N.P.; Cox, A.J. Modulation of allergic inflammation in the nasal mucosa of allergic rhinitis sufferers with topical pharmaceutical agents. Front. Pharmacol. 2019, 10, 294. [Google Scholar] [CrossRef] [PubMed]
- Watts, A.M.; West, N.P.; Smith, P.K.; Cripps, A.W.; Cox, A.J. Adult allergic rhinitis sufferers have unique nasal mucosal and peripheral blood immune gene expression profiles: A case-control study. Immun. Inflamm. Dis. 2022, 10, 78–92. [Google Scholar] [CrossRef] [PubMed]
- Piao, C.H.; Fan, Y.J.; Nguyen, T.V.; Song, C.H.; Chai, O.H. Mangiferin alleviates ovalbumin-induced allergic rhinitis via Nrf2/HO-1/NF-kappaB signaling pathways. Int. J. Mol. Sci. 2020, 21, 3415. [Google Scholar] [CrossRef] [PubMed]
- Dong, F.L.; Tan, J.; Zheng, Y. Chlorogenic acid alleviates allergic inflammatory responses through regulating Th1/Th2 balance in ovalbumin-induced allergic rhinitis mice. Med. Sci. Monit. 2020, 26, e923358-1. [Google Scholar] [CrossRef] [PubMed]
- Pawankar, R.; Mori, S.; Ozu, C.; Kimura, S. Overview on the pathomechanisms of allergic rhinitis. Asia Pac. Allergy 2011, 1, 157–167. [Google Scholar] [CrossRef]
- Izuhara, K.; Nunomura, S.; Nanri, Y.; Ogawa, M.; Ono, J.; Mitamura, Y.; Yoshihara, T. Periostin in inflammation and allergy. Cell. Mol. Life Sci. 2017, 74, 4293–4303. [Google Scholar] [CrossRef]
- James, A.; Janson, C.; Malinovschi, A.; Holweg, C.; Alving, K.; Ono, J.; Ohta, S.; Ek, A.; Middelveld, R.; Dahlen, B.; et al. Serum periostin relates to type-2 inflammation and lung function in asthma: Data from the large population-based cohort Swedish GA(2)LEN. Allergy 2017, 72, 1753–1760. [Google Scholar] [CrossRef]
- Ito, Y.; Al Mubarak, R.; Roberts, N.; Correll, K.; Janssen, W.; Finigan, J.; Mishra, R.; Chu, H.W. IL-13 induces periostin and eotaxin expression in human primary alveolar epithelial cells: Comparison with paired airway epithelial cells. PLoS ONE 2018, 13, e0196256. [Google Scholar] [CrossRef]
- O’Dwyer, D.N.; Moore, B.B. The role of periostin in lung fibrosis and airway remodeling. Cell. Mol. Life Sci. 2017, 74, 4305–4314. [Google Scholar] [CrossRef]
- Zajkowska, M.; Mroczko, B. From allergy to cancer-clinical usefulness of eotaxins. Cancers 2021, 13, 128. [Google Scholar] [CrossRef]
- Suzaki, I.; Kawano, S.; Komiya, K.; Tanabe, T.; Akaba, T.; Asano, K.; Suzaki, H.; Izuhara, K.; Rubin, B.K. Inhibition of IL-13-induced periostin in airway epithelium attenuates cellular protein expression of MUC5AC. Respirology 2017, 22, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Chung, Y.W.; Cha, J.; Han, S.; Chen, Y.; Gucek, M.; Cho, H.J.; Nakahira, K.; Choi, A.M.K.; Ryu, J.H.; Yoon, J.H. Apolipoprotein E and periostin are potential biomarkers of nasal mucosal inflammation. A parallel approach of in vitro and in vivo secretomes. Am. J. Respir. Cell Mol. Biol. 2020, 62, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Knoop, K.A.; Newberry, R.D. Goblet cells: Multifaceted players in immunity at mucosal surfaces. Mucosal Immunol. 2018, 11, 1551–1557. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.D.; Wypych, T.P. Cellular and functional heterogeneity of the airway epithelium. Mucosal Immunol. 2021, 14, 978–990. [Google Scholar] [CrossRef]
- Guo, J.; Xu, S. Astragaloside IV suppresses histamine-induced inflammatory factors and mucin 5 subtype AC overproduction in nasal epithelial cells via regulation of inflammation-related genes. Bioengineered 2021, 12, 6045–6056. [Google Scholar] [CrossRef]
- Calven, J.; Ax, E.; Radinger, M. The Airway Epithelium-A Central Player in Asthma Pathogenesis. Int. J. Mol. Sci. 2020, 21, 8907. [Google Scholar] [CrossRef]
- Gohy, S.; Hupin, C.; Ladjemi, M.Z.; Hox, V.; Pilette, C. Key role of the epithelium in chronic upper airways diseases. Clin. Exp. Allergy 2020, 50, 135–146. [Google Scholar] [CrossRef]
- Lim, J.O.; Song, K.H.; Lee, I.S.; Lee, S.J.; Kim, W.I.; Pak, S.W.; Shin, I.S.; Kim, T. Cimicifugae rhizoma extract attenuates oxidative stress and airway inflammation via the upregulation of Nrf2/HO-1/NQO1 and downregulation of NF-kappaB phosphorylation in ovalbumin-induced asthma. Antioxidants 2021, 10, 1626. [Google Scholar] [CrossRef]
- Sugiura, H.; Ichinose, M. Oxidative and nitrative stress in bronchial asthma. Antioxid. Redox Signal. 2008, 10, 785–797. [Google Scholar] [CrossRef]
- Lopes, R.A.; Neves, K.B.; Tostes, R.C.; Montezano, A.C.; Touyz, R.M. Downregulation of nuclear factor erythroid 2-related factor and associated antioxidant genes contributes to redox-sensitive vascular dysfunction in hypertension. Hypertension 2015, 66, 1240–1250. [Google Scholar] [CrossRef]
- Wasik, U.; Milkiewicz, M.; Kempinska-Podhorodecka, A.; Milkiewicz, P. Protection against oxidative stress mediated by the Nrf2/Keap1 axis is impaired in primary biliary cholangitis. Sci. Rep. 2017, 7, 44769. [Google Scholar] [CrossRef] [PubMed]
- Seo, H.Y.; Lee, S.H.; Lee, J.H.; Hwang, J.S.; Kim, M.K.; Jang, B.K. Kahweol activates the Nrf2/HO-1 pathway by decreasing Keap1 expression independently of p62 and autophagy pathways. PLoS ONE 2020, 15, e0240478. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Cheng, L.; Wu, H.; He, P.; Zhang, Y.; Yang, Y.; Chen, J.; Chen, M. Activation of the KEAP1NRF2ARE signaling pathway reduces oxidative stress in Hep2 cells. Mol. Med. Rep. 2018, 18, 2541–2550. [Google Scholar] [CrossRef]
- Yi, L.; Cui, J.; Wang, W.; Tang, W.; Teng, F.; Zhu, X.; Qin, J.; Wuniqiemu, T.; Sun, J.; Wei, Y.; et al. Formononetin Attenuates Airway In fl ammation and Oxidative Stress in Murine Allergic Asthma. Front. Pharmacol. 2020, 11, 533841. [Google Scholar] [CrossRef] [PubMed]
- Han, M.; Lee, D.; Lee, S.H.; Kim, T.H. Oxidative stress and antioxidant pathway in allergic rhinitis. Antioxidants 2021, 10, 1266. [Google Scholar] [CrossRef] [PubMed]
- Jung, E.G.; Han, K.I.; Hwang, S.G.; Kwon, H.J.; Patnaik, B.B.; Kim, Y.H.; Han, M.D. Brazilin isolated from Caesalpinia sappan L. inhibits rheumatoid arthritis activity in a type-II collagen induced arthritis mouse model. BMC Complement. Altern. Med. 2015, 15, 124. [Google Scholar] [CrossRef]
- Mueller, M.; Weinmann, D.; Toegel, S.; Holzer, W.; Unger, F.M.; Viernstein, H. Compounds from Caesalpinia sappan with anti-inflammatory properties in macrophages and chondrocytes. Food Funct. 2016, 7, 1671–1679. [Google Scholar] [CrossRef]
- Niu, Y.; Wang, S.F.; Li, C.Q.; Wang, J.M.; Liu, Z.H.; Kang, W.Y. Effective compounds from Caesalpinia sappan L. on the tyrosinase in vitro and in vivo. Nat. Prod. Commun 2020, 15. [Google Scholar] [CrossRef]
- Baek, N.I.; Jeon, S.G.; Ahn, E.M.; Hahn, J.T.; Bahn, J.H.; Jang, J.S.; Cho, S.W.; Park, J.K.; Choi, S.Y. Anticonvulsant compounds from the wood of Caesalpinia sappan L. Arch. Pharm Res. 2000, 23, 344–348. [Google Scholar] [CrossRef]
- Nirmal, N.P.; Rajput, M.S.; Prasad, R.G.; Ahmad, M. Brazilin from Caesalpinia sappan heartwood and its pharmacological activities: A review. Asian Pac. J. Trop Med. 2015, 8, 421–430. [Google Scholar] [CrossRef]
- Choi, D.H.; Hwang, H.S. Anti-inflammation activity of brazilin in TNF-α induced human psoriasis dermatitis skin model. Appl. Biol. Chem. 2019, 62, 46. [Google Scholar] [CrossRef]
- Hwang, Y.H.; Jang, S.A.; Kim, T.; Ha, H. Anti-osteoporotic and anti-adipogenic effects of Rhus chinensis nutgalls in ovariectomized mice fed with a high-fat diet. Planta Med. 2019, 85, 1128–1135. [Google Scholar] [CrossRef] [PubMed]
- Hulme, A.N.; McNab, H.; Peggie, D.A.; Quye, A. Negative ion electrospray mass spectrometry of neoflavonoids. Phytochemistry 2005, 66, 2766–2770. [Google Scholar] [CrossRef] [PubMed]
- Tong, X.Z.; Zhu, H.; Shi, Y.; Xu, H.T.; Wang, B.; Zhao, J.H. An LC/MS/MS method for simultaneous quantitation of two homoisoflavones: Protosappanin B and brazilin with hypoglycemic activity in rat plasma and its application to a comparative pharmacokinetic study in normal and streptozotocin-treated rats. J. Ethnopharmacol. 2013, 148, 682–690. [Google Scholar] [CrossRef] [PubMed]
- Yodha, A.W.M.; Abdillah, M.; Indalifiany, A.; Elfahmi, E. Isolation and identification of antioxidant compounds from methanol extract of sappan wood (Caesalpinia sappan). J. Farm. Sains Praktis 2021, 7, 214–223. [Google Scholar] [CrossRef]
- Ren, J.J.; Yu, Z.; Yang, F.L.; Lv, D.; Hung, S.; Zhang, J.; Lin, P.; Liu, S.X.; Zhang, N.; Bachert, C. Effects of Bifidobacterium breve feeding strategy and delivery modes on experimental allergic rhinitis mice. PLoS ONE 2015, 10, e0140018. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Xu, O.; Wang, J.; Shan, C.; Ren, X. Luteolin ameliorates inflammation and Th1/Th2 imbalance via regulating the TLR4/NF-kappaB pathway in allergic rhinitis rats. Immunopharmacol. Immunotoxicol. 2021, 43, 319–327. [Google Scholar] [CrossRef]
- Fan, Y.; Nguyen, T.V.; Piao, C.H.; Shin, H.S.; Song, C.H.; Chai, O.H. Fructus Amomi extract attenuates nasal inflammation by restoring Th1/Th2 balance and down-regulation of NF-kappaB phosphorylation in OVA-induced allergic rhinitis. Biosci. Rep. 2022, 42, BSR20212681. [Google Scholar] [CrossRef]
- Fan, Y.; Piao, C.H.; Hyeon, E.; Jung, S.Y.; Eom, J.E.; Shin, H.S.; Song, C.H.; Chai, O.H. Gallic acid alleviates nasal inflammation via activation of Th1 and inhibition of Th2 and Th17 in a mouse model of allergic rhinitis. Int. Immunopharmacol. 2019, 70, 512–519. [Google Scholar] [CrossRef]
- Shao, Y.Y.; Zhou, Y.M.; Hu, M.; Li, J.Z.; Chen, C.J.; Wang, Y.J.; Shi, X.Y.; Wang, W.J.; Zhang, T.T. The anti-allergic rhinitis effect of traditional chinese medicine of shenqi by regulating mast cell degranulation and Th1/Th2 cytokine balance. Molecules 2017, 22, 504. [Google Scholar] [CrossRef]
- Ebihara, N.; Takahashi, K.; Takemura, H.; Akanuma, Y.; Asano, K.; Sunagawa, M. Suppressive effect of quercetin on nitric oxide production from nasal epithelial cells in vitro. Evid.-Based Complement. Alternat. Med. 2018, 2018, 6097625. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.P.; Zeng, M.; Deng, Y.H.; Zhao, J.M.; Zhou, X.X.; Trudeau, J.B.; Goldschmidt, E.; Moore, J.A.; Chu, H.W.; Zhang, W.T.; et al. 15-Lipoxygenase 1 in nasal polyps promotes CCL26/eotaxin 3 expression through extracellular signal-regulated kinase activation. J. Allergy Clin. Immun. 2019, 144, 1228–1241. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Choi, Y.H.; Xian, Z.; Zheng, M.; Piao, H.; Yan, G. Aloperine suppresses allergic airway inflammation through NF-kappaB, MAPK, and Nrf2/HO-1 signaling pathways in mice. Int. Immunopharmacol. 2018, 65, 571–579. [Google Scholar] [CrossRef] [PubMed]
- Bai, D.; Sun, T.; Lu, F.; Shen, Y.; Zhang, Y.; Zhang, B.; Yu, G.; Li, H.; Hao, J. Eupatilin suppresses OVA-induced asthma by inhibiting NF-kappaB and MAPK and activating Nrf2 signaling pathways in mice. Int. J. Mol. Sci. 2022, 23, 1582. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, X.; Vikash, V.; Ye, Q.; Wu, D.; Liu, Y.; Dong, W. ROS and ROS-mediated cellular signaling. Oxid. Med. Cell. Longev. 2016, 2016, 4350965. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Ge, A.; Zhu, W.; Liu, Y.N.; Ji, N.F.; Zha, W.J.; Zhang, J.X.; Zeng, X.N.; Huang, M. Morin attenuates ovalbumin-induced airway inflammation by modulating oxidative stress-responsive MAPK signaling. Oxid. Med. Cell. Longev. 2016, 2016, 5843672. [Google Scholar] [CrossRef]
- Nguyen, T.; Nioi, P.; Pickett, C.B. The Nrf2-antioxidant response element signaling pathway and its activation by oxidative stress. J. Biol. Chem. 2009, 284, 13291–13295. [Google Scholar] [CrossRef]
- Motohashi, H.; Yamamoto, M. Nrf2-Keap1 defines a physiologically important stress response mechanism. Trends Mol. Med. 2004, 10, 549–557. [Google Scholar] [CrossRef]
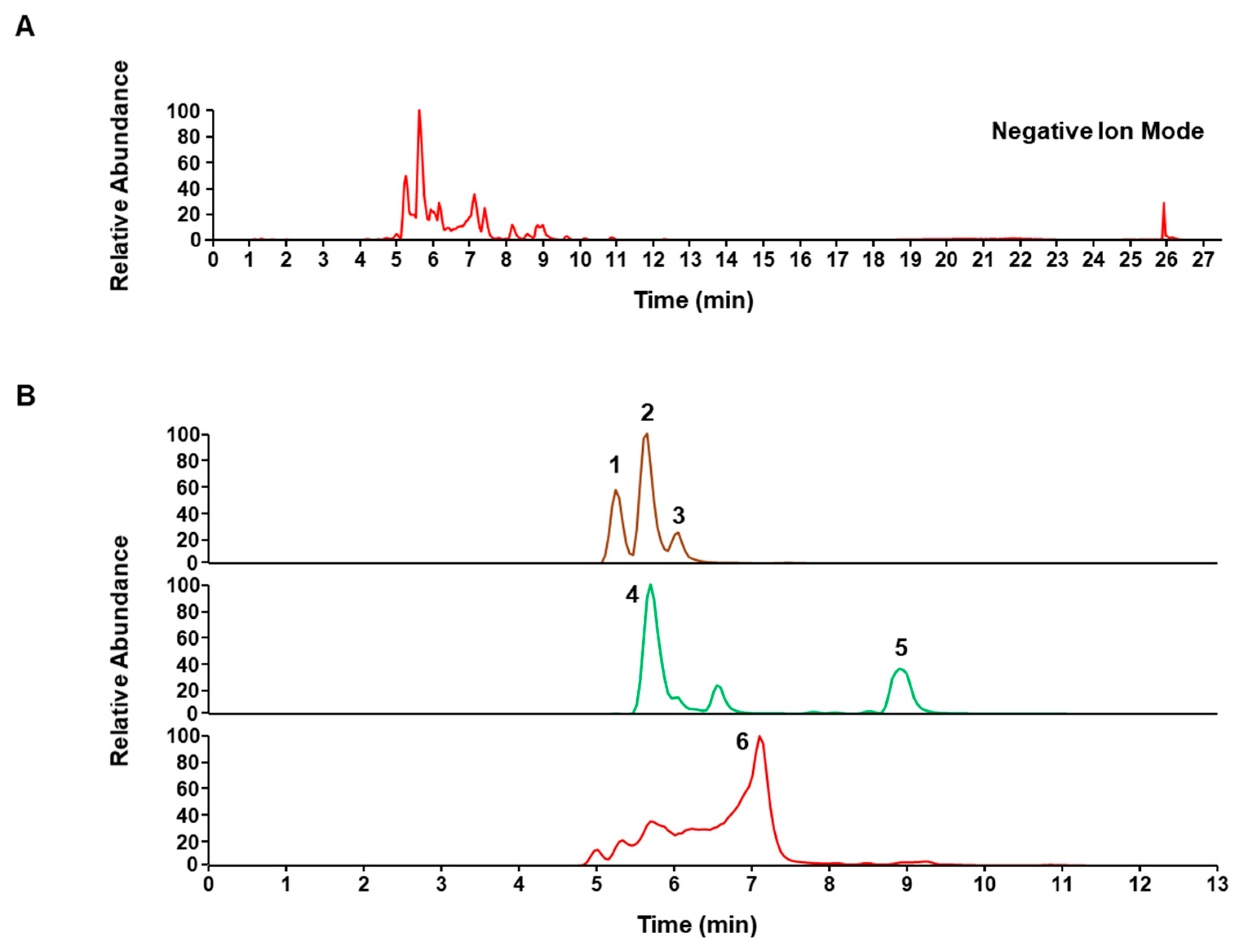



| No. | Rt (min) | Calculated (m/z) | Estimated (m/z) | Error (ppm) | Adduct | Formula | MS2 Fragments (m/z) | Identification |
|---|---|---|---|---|---|---|---|---|
| 1 | 5.26 | 303.0874 | 303.0874 | −0.0253 | [M − H]− | C16H16O6 | 123, 163, 229 | Episappanol |
| 2 | 5.61 | 303.0874 | 303.0874 | −0.0253 | [M − H]− | C16H16O6 | 231 | Protosappanin B * |
| 3 | 6.02 | 303.0874 | 303.0874 | −0.0253 | [M − H]− | C16H16O6 | 229, 257 | Sappanol |
| 4 | 5.7 | 285.0768 | 285.0768 | −0.0236 | [M − H]− | C16H14O5 | 163 | Brazilin |
| 5 | 8.9 | 285.0768 | 285.077 | −0.3214 | [M − H]− | C16H14O5 | 123, 165 | 3-deoxysappanone B |
| 6 | 7.1 | 283.0612 | 283.0612 | 0.1723 | [M − H]− | C16H12O5 | 240, 173 | Brazilein |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pyun, B.-J.; Jo, K.; Lee, J.Y.; Lee, A.; Jung, M.-A.; Hwang, Y.-H.; Jung, D.H.; Ji, K.-Y.; Choi, S.; Kim, Y.H.; et al. Caesalpinia sappan Linn. Ameliorates Allergic Nasal Inflammation by Upregulating the Keap1/Nrf2/HO-1 Pathway in an Allergic Rhinitis Mouse Model and Nasal Epithelial Cells. Antioxidants 2022, 11, 2256. https://doi.org/10.3390/antiox11112256
Pyun B-J, Jo K, Lee JY, Lee A, Jung M-A, Hwang Y-H, Jung DH, Ji K-Y, Choi S, Kim YH, et al. Caesalpinia sappan Linn. Ameliorates Allergic Nasal Inflammation by Upregulating the Keap1/Nrf2/HO-1 Pathway in an Allergic Rhinitis Mouse Model and Nasal Epithelial Cells. Antioxidants. 2022; 11(11):2256. https://doi.org/10.3390/antiox11112256
Chicago/Turabian StylePyun, Bo-Jeong, Kyuhyung Jo, Joo Young Lee, Ami Lee, Myung-A Jung, Youn-Hwan Hwang, Dong Ho Jung, Kon-Young Ji, Susanna Choi, Yun Hee Kim, and et al. 2022. "Caesalpinia sappan Linn. Ameliorates Allergic Nasal Inflammation by Upregulating the Keap1/Nrf2/HO-1 Pathway in an Allergic Rhinitis Mouse Model and Nasal Epithelial Cells" Antioxidants 11, no. 11: 2256. https://doi.org/10.3390/antiox11112256
APA StylePyun, B.-J., Jo, K., Lee, J. Y., Lee, A., Jung, M.-A., Hwang, Y.-H., Jung, D. H., Ji, K.-Y., Choi, S., Kim, Y. H., & Kim, T. (2022). Caesalpinia sappan Linn. Ameliorates Allergic Nasal Inflammation by Upregulating the Keap1/Nrf2/HO-1 Pathway in an Allergic Rhinitis Mouse Model and Nasal Epithelial Cells. Antioxidants, 11(11), 2256. https://doi.org/10.3390/antiox11112256







