Identification and Quantification of Key Phytochemicals, Phytohormones, and Antioxidant Properties in Coccinia grandis during Fruit Ripening
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Plant Materials
2.3. Identification of Phytochemicals
2.4. Quantification of Phytochemicals
2.5. Quantification of Phytohormones
2.6. Antioxidant Properties
2.7. Statistical Analysis
3. Results and Discussion
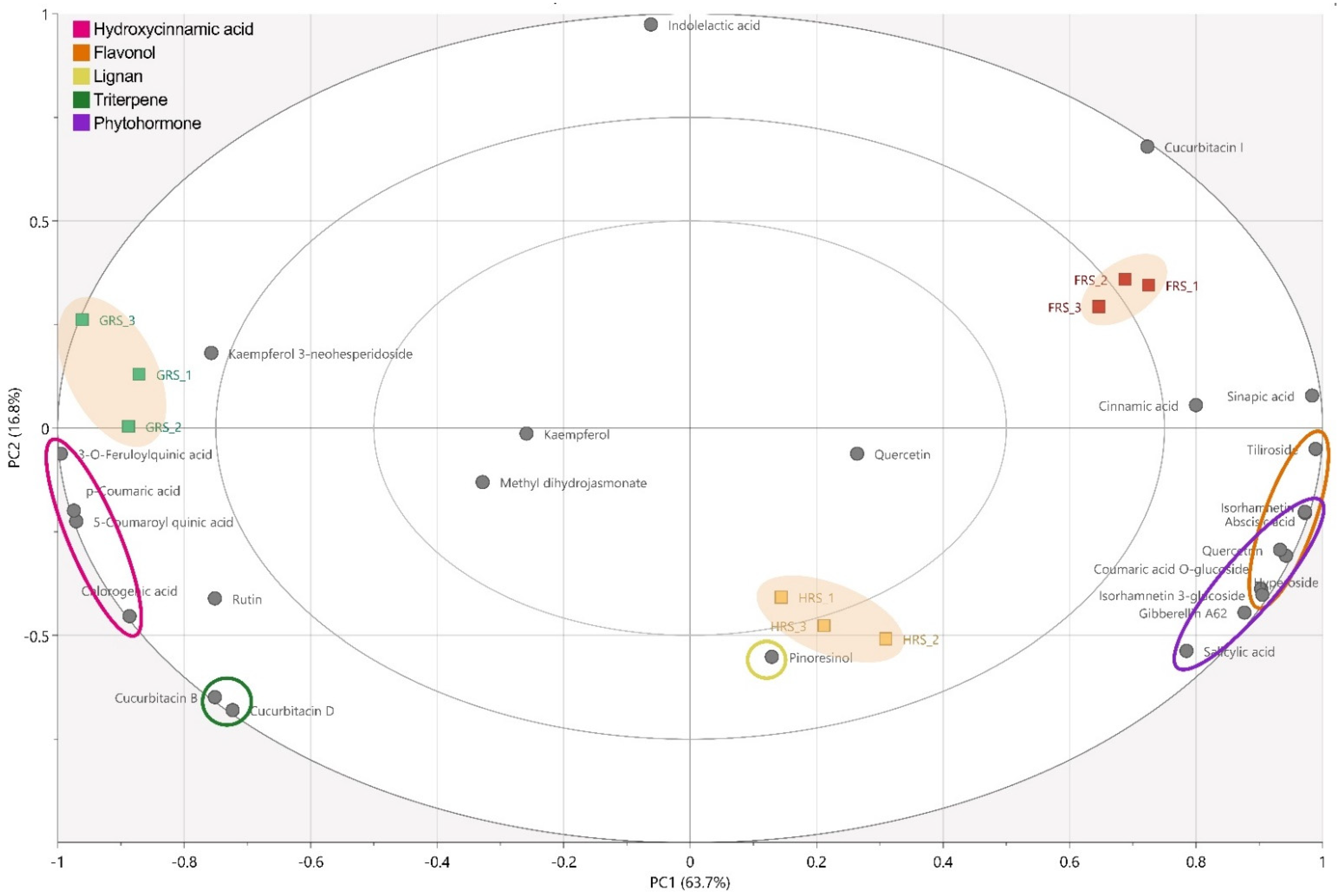
3.1. Phytochemical Composition
3.2. Quantification of the Phytochemicals
3.2.1. Hydroxycinnamic Acids
3.2.2. Flavonols
3.2.3. Lignan
3.2.4. Triterpenes
3.2.5. Phytohormones
4. Antioxidant Activities
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gordon, D.R.; Tancig, K.J.; Onderdonk, D.A.; Gantz, C.A. Assessing the invasive potential of biofuel species proposed for Florida and the United States using the Australian Weed Risk Assessment. Biomass Bioenergy 2011, 35, 74–79. [Google Scholar] [CrossRef]
- Kondhare, D.; Lade, H. Phytochemical profile, aldose reductase inhibitory, and antioxidant activities of Indian traditional medicinal Coccinia grandis (L.) fruit extract. 3 Biotech 2017, 7, 378. [Google Scholar] [CrossRef]
- Wasana, K.G.P.; Attanayake, A.P.; Weerarathna, T.P.; Jayatilaka, K.A.P.W. Efficacy and safety of a herbal drug of Coccinia grandis (Linn.) Voigt in patients with type 2 diabetes mellitus: A double blind randomized placebo controlled clinical trial. Phytomedicine 2021, 81, 153431. [Google Scholar] [CrossRef]
- Albrahim, T.; Alnasser, M.M.; Al-Anazi, M.R.; ALKahtani, M.D.; Alkahtani, S.; Al-Qahtani, A.A. Potential anti-inflammatory and anti-apoptotic effect of Coccinia grandis plant extract in LPS stimulated-THP-1 cells. Environ. Sci. Pollut. Res. 2020, 27, 21892–21904. [Google Scholar] [CrossRef]
- Singh, G.; Gupta, P.; Rawat, P.; Puri, A.; Bhatia, G.; Maurya, R. Antidyslipidemic activity of polyprenol from Coccinia grandis in high-fat diet-fed hamster model. Phytomedicine 2007, 14, 792–798. [Google Scholar] [CrossRef]
- Mukherjee, P.K.; Singha, S.; Kar, A.; Chanda, J.; Banerjee, S.; Dasgupta, B.; Haldar, P.K.; Sharma, N. Therapeutic importance of Cucurbitaceae: A medicinally important family. J. Ethnopharmacol. 2022, 282, 114599. [Google Scholar] [CrossRef]
- Wojdyło, A.; Oszmiański, J. Antioxidant activity modulated by polyphenol contents in apple and leaves during fruit development and ripening. Antioxidants 2020, 9, 567. [Google Scholar] [CrossRef]
- Kiselova-Kaneva, Y.; Galunska, B.; Nikolova, M.; Dincheva, I.; Badjakov, I. High resolution LC-MS/MS characterization of polyphenolic composition and evaluation of antioxidant activity of Sambucus ebulus fruit tea traditionally used in Bulgaria as a functional food. Food Chem. 2022, 367, 130759. [Google Scholar] [CrossRef]
- Checkouri, E.; Reignier, F.; Robert-Da Silva, C.; Meilhac, O. Evaluation of polyphenol content and antioxidant capacity of aqueous extracts from eight medicinal plants from reunion island: Protection against oxidative stress in red blood cells and preadipocytes. Antioxidants 2020, 9, 959. [Google Scholar] [CrossRef]
- Mukherjee, A.; Gaurav, A.K.; Singh, S.; Yadav, S.; Bhowmick, S.; Abeysinghe, S.; Verma, J.P. The Bioactive potential of phytohormones: A review. Biotechnol. Rep. 2022, 35, e00748. [Google Scholar] [CrossRef]
- Belwal, T.; Pandey, A.; Bhatt, I.D.; Rawal, R.S.; Luo, Z. Trends of polyphenolics and anthocyanins accumulation along ripening stages of wild edible fruits of Indian Himalayan region. Sci. Rep. 2019, 9, 5894. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Ma, S.S.; Ibrahim, S.A.; Li, E.H.; Yang, H.; Huang, W. Identification and antioxidant properties of polyphenols in lotus seed epicarp at different ripening stages. Food Chem. 2015, 185, 159–164. [Google Scholar] [CrossRef]
- Arancibia-Avila, P.; Toledo, F.; Park, Y.S.; Jung, S.T.; Kang, S.G.; Heo, B.G.; Lee, S.H.; Sajewicz, M.; Kowalska, T.; Gorinstein, S. Antioxidant properties of durian fruit as influenced by ripening. LWT-Food Sci. Technol. 2008, 41, 2118–2125. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, L.; Zhao, A.; Karrar, E.; Zhang, H.; Jin, Q.; Wu, G.; Yang, X.; Chen, L.; Wang, X. Quality characteristics and antioxidant activity during fruit ripening of three monovarietal olive oils cultivated in China. J. Am. Oil. Chem. Soc. 2021, 98, 229–240. [Google Scholar] [CrossRef]
- Salem, M.A.; Yoshida, T.; Perez de Souza, L.; Alseekh, S.; Bajdzienko, K.; Fernie, A.R.; Giavalisco, P. An improved extraction method enables the comprehensive analysis of lipids, proteins, metabolites and phytohormones from a single sample of leaf tissue under water-deficit stress. Plant J. 2020, 103, 1614–1632. [Google Scholar] [CrossRef]
- Braca, A.; De Tommasi, N.; Di Bari, L.; Pizza, C.; Politi, M.; Morelli, I. Antioxidant principles from bauhinia tarapotensis. J. Nat. Prod. 2001, 64, 892–895. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Kumar, S.; Singh, A.; Kumar, B. Identification and characterization of phenolics and terpenoids from ethanolic extracts of Phyllanthus species by HPLC-ESI-QTOF-MS/MS. J. Pharm. Anal. 2017, 7, 214–222. [Google Scholar] [CrossRef]
- Ling, Y.; Lin, Z.; Zha, W.; Lian, T.; You, S. Rapid detection and characterisation of triterpene saponins from the root of Pulsatilla chinensis (bunge) regel by HPLC-ESI-QTOF-MS/MS. Phytochem. Anal. 2016, 27, 174–183. [Google Scholar] [CrossRef]
- Ling, Y.; Fu, Z.; Zhang, Q.; Xu, L.; Liao, L. Identification and structural elucidation of steroidal saponins from the root of Paris polyphylla by HPLC-ESI-QTOF-MS/MS. Nat. Prod. Res. 2015, 29, 1798–1803. [Google Scholar] [CrossRef]
- Gordon, A.; Cruz, A.P.G.; Cabral, L.M.C.; de Freitas, S.C.; Taxi, C.M.A.D.; Donangelo, C.M.; Mattietto, R.A.; Friedrich, M.; da Matta, V.M.; Marx, F. Chemical characterization and evaluation of antioxidant properties of Açaí fruits (Euterpe oleraceae Mart.) during ripening. Food Chem. 2012, 133, 256–263. [Google Scholar] [CrossRef]
- Polbuppha, I.; Suthiphasilp, V.; Maneerat, T.; Charoensup, R.; Limtharakul, T.; Cheenpracha, S.; Pyne, S.G.; Laphookhieo, S. Macluracochinones AE, antimicrobial flavonoids from Maclura cochinchinensis (Lour.) Corner. Phytochemistry 2021, 187, 112773. [Google Scholar] [CrossRef]
- Adeyemi, D.O.; Ukwenya, V.O.; Obuotor, E.M.; Adewole, S.O. Anti-hepatotoxic activities of Hibiscus sabdariffa L. in animal model of streptozotocin diabetes-induced liver damage. BMC Complement. Altern. Med. 2014, 14, 277. [Google Scholar] [CrossRef] [Green Version]
- Ye, Q.; Ma, X.Q.; Hu, C.L.; Lin, B.; Xu, L.S.; Zheng, C.J.; Qin, L.P. Antiosteoporotic activity and constituents of Podocarpium podocarpum. Phytomedicine 2015, 22, 94–102. [Google Scholar] [CrossRef]
- Yoon, K.D.; Chin, Y.W.; Yang, M.H.; Kim, J. Separation of anti-ulcer flavonoids from Artemisia extracts by high-speed countercurrent chromatography. Food Chem. 2011, 129, 679–683. [Google Scholar] [CrossRef]
- Masad, R.J.; Haneefa, S.M.; Mohamed, Y.A.; Al-Sbiei, A.; Bashir, G.; Fernandez-Cabezudo, M.J.; Al-Ramadi, B.K. The immunomodulatory effects of honey and associated flavonoids in cancer. Nutrients 2021, 13, 1269. [Google Scholar] [CrossRef]
- Ramos, S. Effects of dietary flavonoids on apoptotic pathways related to cancer chemoprevention. J. Nutr. Biochem. 2007, 18, 427–442. [Google Scholar] [CrossRef] [Green Version]
- Vázquez, G.; Fernández-Agulló, A.; Gómez-Castro, C.; Freire, M.S.; Antorrena, G.; González-Álvarez, J. Response surface optimization of antioxidants extraction from chestnut (Castanea sativa) bur. Ind. Crop. Prod. 2012, 35, 126–134. [Google Scholar] [CrossRef]
- Farah, A.; Monteiro, M.; Donangelo, C.M.; Lafay, S. Chlorogenic acids from green coffee extract are highly bioavailable in humans. J. Nutr. 2008, 138, 2309–2315. [Google Scholar] [CrossRef] [Green Version]
- Yu, E.S.; Min, H.J.; An, S.Y.; Won, H.Y.; Hong, J.H.; Hwang, E.S. Regulatory mechanisms of IL-2 and IFNγ suppression by quercetin in T helper cells. Biochem. Pharmacol. 2008, 76, 70–78. [Google Scholar] [CrossRef]
- Rocabado, G.O.; Bedoya, L.M.; Abad, M.J.; Bermejo, P. Rubus-a review of its phytochemical and pharmacological profile. Nat. Prod. Commun. 2008, 3, 423–436. [Google Scholar] [CrossRef] [Green Version]
- Grochowski, D.M.; Locatelli, M.; Granica, S.; Cacciagrano, F.; Tomczyk, M. A review on the dietary flavonoid tiliroside. Compr. Rev. Food Sci. Food Saf. 2018, 17, 1395–1421. [Google Scholar] [CrossRef] [Green Version]
- Abou Baker, D.H. An ethnopharmacological review on the therapeutical properties of flavonoids and their mechanisms of actions: A comprehensive review based on up to date knowledge. Toxicol. Rep. 2022, 9, 445–469. [Google Scholar] [CrossRef] [PubMed]
- Palafox-Carlos, H.; Yahia, E.M.; González-Aguilar, G.A. Identification and quantification of major phenolic compounds from mango (Mangifera indica, cv. Ataulfo) fruit by HPLC–DAD–MS/MS-ESI and their individual contribution to the antioxidant activity during ripening. Food Chem. 2012, 135, 105–111. [Google Scholar] [CrossRef]
- Adlercreutz, H. Lignans and human health. Crit. Rev. Clin. Lab. Sci. 2007, 44, 483–525. [Google Scholar] [CrossRef] [PubMed]
- Peterson, J.; Dwyer, J.; Adlercreutz, H.; Scalbert, A.; Jacques, P.; McCullough, M.L. Dietary lignans: Physiology and potential for cardiovascular disease risk reduction. Nutr. Rev. 2010, 68, 571–603. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schroeder, F.C.; Del Campo, M.L.; Grant, J.B.; Weibel, D.B.; Smedley, S.R.; Bolton, K.L.; Meinwald, J.; Eisner, T. Pinoresinol: A lignol of plant origin serving for defense in a caterpillar. Proc. Natl. Acad. Sci. USA 2006, 103, 15497–15501. [Google Scholar] [CrossRef] [Green Version]
- Maldini, M.; Montoro, P.; Piacente, S.; Pizza, C. Phenolic compounds from Bursera simaruba Sarg. bark: Phytochemical investigation and quantitative analysis by tandem mass spectrometry. Phytochemistry 2009, 70, 641–649. [Google Scholar] [CrossRef]
- Miró, M. Cucurbitacins and their pharmacological effects. Phytother. Res. 1995, 9, 159–168. [Google Scholar] [CrossRef]
- Attard, E.; Cuschieri, A.; Scicluna-Spiteri, A.; Brincat, M.P. Cytotoxicity of cucurbitacin E extracted from Ecballium elaterium in vitro. J. Nat. Remedies 2004, 4, 137–144. Available online: https://www.um.edu.mt/library/oar//handle/123456789/19462 (accessed on 1 September 2022).
- Bartalis, J.; Halaweish, F.T. Relationship between cucurbitacins reversed-phase high-performance liquid chromatography hydrophobicity index and basal cytotoxicity on HepG2 cells. J. Chromatogr. B 2005, 818, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, N.; Yoshida, Y.; Sugiura, T.; Matsuno, K.; Fujino, A.; Yamashita, U. Cucurbitacin D isolated from Trichosanthes kirilowii induces apoptosis in human hepatocellular carcinoma cells in vitro. Int. Immunopharmacol. 2009, 9, 508–513. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Ding, N.; Kanazawa, T.; Yamashita, U.; Yoshida, Y. Cucurbitacin D is a new inflammasome activator in macrophages. Int. Immunopharmacol. 2013, 17, 1044–1050. [Google Scholar] [CrossRef] [PubMed]
- Chanda, J.; Biswas, S.; Kar, A.; Mukherjee, P.K. Determination of cucurbitacin E in some selected herbs of ayurvedic importance through RP-HPLC. J. Ayurveda Integr. Med. 2020, 11, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Klee, H.J.; Giovannoni, J.J. Genetics and control of tomato fruit ripening and quality attributes. Annu. Rev. Genet. 2011, 45, 41–59. [Google Scholar] [CrossRef]
- Seymour, G.B.; Ryder, C.D.; Cevik, V.; Hammond, J.P.; Popovich, A.; King, G.J.; Vreblaov, J.; Giovannoi, J.J.; Manning, K. A Sepallata gene is involved in the development and ripening of strawberry (Fragaria × ananassa Duch.) fruit, a non-climacteric tissue. J. Exp. Bot. 2011, 62, 1179–1188. [Google Scholar] [CrossRef]
- Asghari, M. Impact of jasmonates on safety, productivity and physiology of food crops. Trends. Food Sci. Technol. 2019, 91, 169–183. [Google Scholar] [CrossRef]
- Lin, L.; Tan, R.X. Cross-kingdom actions of phytohormones: A functional scaffold exploration. Chem. Rev. 2011, 111, 2734–2760. [Google Scholar] [CrossRef]
- Sakthivel, P.; Sharma, N.; Klahn, P.; Gereke, M.; Bruder, D. Abscisic acid: A phytohormone and mammalian cytokine as novel pharmacon with potential for future development into clinical applications. Curr. Med. Chem. 2016, 23, 1549–1570. [Google Scholar] [CrossRef]
- Voller, J.; Maková, B.; Kadlecová, A.; Gonzalez, G.; Strnad, M. Plant hormone cytokinins for modulating human aging and age-related diseases. In Hormones in Ageing and Longevity, 6th ed.; Springer: Berlin/Heidelberg, Germany, 2017; pp. 311–335. [Google Scholar] [CrossRef]
- Sakr, S.A.; Shalaby, S.Y. Effect of gibberellin-A3 on metamorphosis in the Egyptian toad Bufo regularis. J. Basic Appl. Zool. 2012, 65, 153–156. [Google Scholar] [CrossRef]
- Zocchi, E.; Hontecillas, R.; Leber, A.; Einerhand, A.; Carbo, A.; Bruzzone, S.; Tubau-Juni, N.; Philpson, N.; Zoccoli-Rodriguez, V.; Sturla, L.; et al. Abscisic acid: A novel nutraceutical for glycemic control. Front. Nutr. 2017, 4, 24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Magnone, M.; Sturla, L.; Guida, L.; Spinelli, S.; Begani, G.; Bruzzone, S.; Fresia, C.; Zocchi, E. Abscisic acid: A conserved hormone in plants and humans and a promising aid to combat prediabetes and the metabolic syndrome. Nutrients 2020, 12, 1724. [Google Scholar] [CrossRef] [PubMed]
- Davies, P.J. The plant hormones: Their nature, occurrence, and functions. In Plant Hormones; Springer: Berlin/Heidelberg, Germany, 2010; pp. 1–15. [Google Scholar] [CrossRef]
- Bari, R.; Jones, J.D. Role of plant hormones in plant defence responses. Plant Mol. Biol. 2009, 69, 473–488. [Google Scholar] [CrossRef]
- Hussein, W.F.; Farahat, F.Y.; Abass, M.A.; Shehata, A.S. Hepatotoxic potential of Gibberellic Acid (GA3) in adult male albino rats. J. Life Sci. 2011, 8, 373–383. [Google Scholar] [CrossRef]
- Liu, F.; Jiang, H.; Ye, S.; Chen, W.P.; Liang, W.; Xu, Y.; Sun, B.; Sun, J.; Wang, Q.; Cohen, J.D.; et al. The Arabidopsis P450 protein CYP82C2 modulates jasmonate-induced root growth inhibition, defense gene expression and indole glucosinolate biosynthesis. Cell Res. 2010, 20, 539–552. [Google Scholar] [CrossRef] [PubMed]
| # | Analyte Name | RT (min) | Molecular Formula | Molecular Weight | Adduct | m/z | Error (ppm) | ||
|---|---|---|---|---|---|---|---|---|---|
| GRS | HRS | FRS | |||||||
| Hydroxycinnamic acids | |||||||||
| 1 | Coumaric acid O-glucoside | 4.53 | C15H18O8 | 326.1002 | [M + HCOO]− | 371.0984 | 0.295 | 0.173 | 0.315 |
| 2 | Chlorogenic acid | 7.09 | C16H18O9 | 354.0951 | [M − H]− | 353.0878 | 0.159 | 0.090 | 0.053 |
| 3 | Sinapic acid | 8.69 | C11H12O5 | 224.0685 | [M + H]+ | 225.0757 | 0.859 | 0.737 | 0.899 |
| 4 | 5-Coumaroyl quinic acid | 10.66 | C16H18O8 | 338.1002 | [M − H]− | 337.0929 | 0.113 | 0.012 | 0.095 |
| 5 | p-Coumaric acid | 10.72 | C9H8O3 | 164.0473 | [M + H − H2O]+ | 147.0441 | 0.758 | 1.745 | 1.561 |
| 6 | 3-O-Feruloylquinic acid | 12.99 | C17H20O9 | 368.1107 | [M + H]+ | 369.1180 | 0.553 | 0.294 | 0.251 |
| 7 | Cinnamic acid | 30.87 | C9H8O2 | 148.0524 | [M + H]+ | 149.0597 | 1.199 | 1.270 | 0.139 |
| Flavonols | |||||||||
| 8 | Rutin | 15.94 | C27H30O16 | 610.1534 | [M − H]− | 609.1461 | 0.628 | 0.139 | 0.017 |
| 9 | Quercetin | 16.14 | C15H10O7 | 302.0427 | [M + H]+ | 303.0499 | 1.671 | 1.708 | 1.623 |
| 10 | Hyperoside | 16.20 | C21H20O12 | 464.0955 | [M − H]− | 463.0882 | 0.184 | 0.224 | 0.343 |
| 11 | Kaempferol 3-neohesperidoside | 17.05 | C27H30O15 | 594.1585 | [M − H]− | 593.1512 | 0.382 | 0.200 | 0.107 |
| 12 | Kaempferol | 17.28 | C15H10O6 | 286.0477 | [M + H]+ | 287.0550 | 1.754 | 2.019 | 1.913 |
| 13 | Quercitrin | 17.33 | C21H20O11 | 448.1006 | [M − H]− | 447.0933 | 0.091 | 0.002 | 0.890 |
| 14 | Isorhamnetin 3-glucoside | 17.68 | C22H22O12 | 478.1111 | [M − H]− | 477.1038 | 0.177 | 0.22 | 0.716 |
| 15 | Tiliroside | 20.80 | C30H26O13 | 594.1373 | [M − H]− | 593.1301 | 0.145 | 0.152 | 0.428 |
| 16 | Isorhamnetin | 22.86 | C16H12O7 | 316.0583 | [M − H]− | 315.0510 | - | 0.304 | 0.197 |
| Lignan | |||||||||
| 17 | Pinoresinol | 19.20 | C20H22O6 | 358.1416 | [M + H − H2O]+ | 341.1384 | 1.196 | 1.643 | 1.261 |
| Triterpenes | |||||||||
| 18 | Cucurbitacin I | 15.40 | C30H42O7 | 514.2930 | [M + H − H2O]+ | 497.2898 | - | - | 1.105 |
| 19 | Cucurbitacin D | 24.09 | C30H44O7 | 516.3087 | [M + H − H2O]+ | 499.3054 | 0.891 | 1.282 | - |
| 20 | Cucurbitacin B | 27.83 | C32H46O8 | 558.3193 | [M + HCOO]− | 603.3175 | 0.120 | 0.282 | - |
| Phytohormones | |||||||||
| 21 | Salicylic acid | 1.80 | C7H6O3 | 138.0317 | [M − H]− | 137.0244 | 0.637 | 0.653 | 0.857 |
| 22 | Indoleacetic acid | 4.12 | C10H9NO2 | 175.1839 | [M + H − H2O]+ | 188.0706 | 0.134 | 0.210 | 0.483 |
| 23 | Abscisic acid | 6.87 | C15H20O4 | 264.1362 | [M + H]+ | 264.1434 | 0.723 | 0.978 | 0.925 |
| 24 | Gibberellin A62 | 16.01 | C19H22O5 | 330.1467 | [M + HCOO]− | 375.1449 | 0.124 | 0.330 | 0.943 |
| 25 | Methyl dihydrojasmonate | 22.05 | C13H22O3 | 226.1569 | [M + H]+ | 227.1642 | 1.489 | 1.124 | 1.220 |
| Compounds | GRS | HRS | FRS | F-Value |
|---|---|---|---|---|
| Hydroxycinnamic acids | ||||
| Chlorogenic acid | 56.13 ± 2.29 a | 25.18 ± 0.09 b | 9.96 ± 0.18 c | 940.51 *** |
| p-Coumaric acid | 8.20 ± 0.81 a | 2.74 ± 0.21 b | 2.46 ± 0.03 b | 134.13 *** |
| Flavonols | ||||
| Quercetin | 0.64 ± 0.01 c | 1.84 ± 0.01 b | 2.03 ± 0.03 a | 4529.71 *** |
| Quercitrin | 0.52 ± 0.02 b | 1.57 ± 0.02 a | 0.44 ± 0.01 c | 4915.79 *** |
| Rutin | 152.40 ± 0.57 a | 146.45 ± 4.91 b | 114.12 ± 0.63 c | 153.89 *** |
| Tiliroside | 3.49 ± 0.03 c | 50.86 ± 0.88 b | 85.97 ± 0.13 a | 19,439.95 *** |
| Lignan | ||||
| Pinoresinol | 2.99 ± 0.00 c | 6.00 ± 0.02 a | 3.17 ± 0.02 b | 34,645.58 *** |
| Triterpenes | ||||
| Cucurbitacin B | 660.81 ± 11.88 a | 340.21 ± 7.35 b | 0.33 ± 0.02 c | 5029.44 *** |
| Cucurbitacin D | 25.95 ± 0.76 | 18.35 ± 0.43 | n.d. | 227.28 *** |
| Cucurbitacin I | n.d. | 0.01 ± 0.01 | n.d. | - |
| Compounds | GRS | HRS | FRS | F-Value |
|---|---|---|---|---|
| ABA | 205.70 b | 113.72 c | 265.23 a | 172.50 *** |
| GA3 | 1.13 a | 1.90 a | 0.95 a | 3.47 |
| GA4 | 48.89 a | 20.58 b | n.d. | 11.89 * |
| IAA | 2.29 c | 12.20 b | 51.90 a | 219.92 *** |
| JA | 6.14 a | n.d. | 3.21 b | 29.18 ** |
| SA | 209.69 c | 1451.69 b | 2879.24 a | 3124.91 *** |
| GRS | HRS | FRS | F-Value | |
|---|---|---|---|---|
| DPPH a | 5.87 ± 0.03 c | 29.88 ± 0.19 b | 83.97 ± 0.71 a | 26,295.98 *** |
| ABTS b | 2.09 ± 0.94 c | 1.25 ± 0.50 b | 0.70 ± 0.58 a | 860.87 *** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, I.Y.; Joo, N. Identification and Quantification of Key Phytochemicals, Phytohormones, and Antioxidant Properties in Coccinia grandis during Fruit Ripening. Antioxidants 2022, 11, 2218. https://doi.org/10.3390/antiox11112218
Lee IY, Joo N. Identification and Quantification of Key Phytochemicals, Phytohormones, and Antioxidant Properties in Coccinia grandis during Fruit Ripening. Antioxidants. 2022; 11(11):2218. https://doi.org/10.3390/antiox11112218
Chicago/Turabian StyleLee, In Young, and Nami Joo. 2022. "Identification and Quantification of Key Phytochemicals, Phytohormones, and Antioxidant Properties in Coccinia grandis during Fruit Ripening" Antioxidants 11, no. 11: 2218. https://doi.org/10.3390/antiox11112218
APA StyleLee, I. Y., & Joo, N. (2022). Identification and Quantification of Key Phytochemicals, Phytohormones, and Antioxidant Properties in Coccinia grandis during Fruit Ripening. Antioxidants, 11(11), 2218. https://doi.org/10.3390/antiox11112218







