Antioxidant Properties and Beneficial Cardiovascular Effects of a Natural Extract of Pomegranate in Healthy Volunteers: A Randomized Preliminary Single-Blind Controlled Study
Abstract
:1. Introduction
2. Materials and Methods
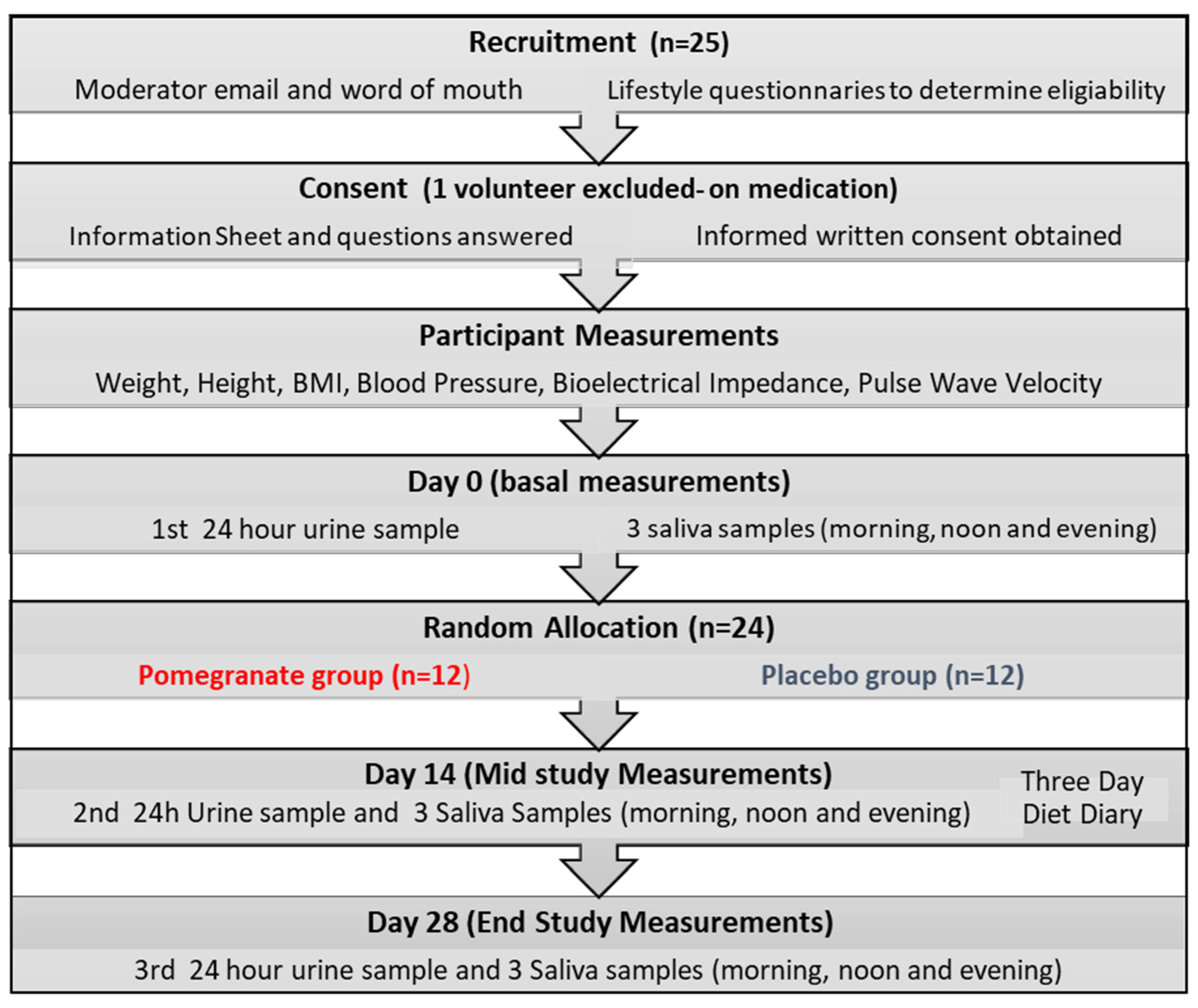
2.1. Design and Participants
2.2. Treatment
2.3. Study Procedures
2.3.1. Anthropometric, Body Composition and Aortic Stiffness Measurements
2.3.2. Collection of Saliva and Urine Samples
2.3.3. Determination of Glucocorticoids
2.3.4. Total Phenolic Products
2.3.5. Antioxidant Capacity and Lipid Peroxidation
2.4. Assessment of Nutritional Intake
2.5. Statsitical Analysis
3. Results
3.1. Study Population
3.2. Changes of Anthropometric, Body Composition, BP and PWV
3.3. Changes of Cortisol and Cortisone Levels in Salivary and Urine Samples
3.4. Total Phenolic Compounds, Antioxidant Capacity and Lipid Peroxidation
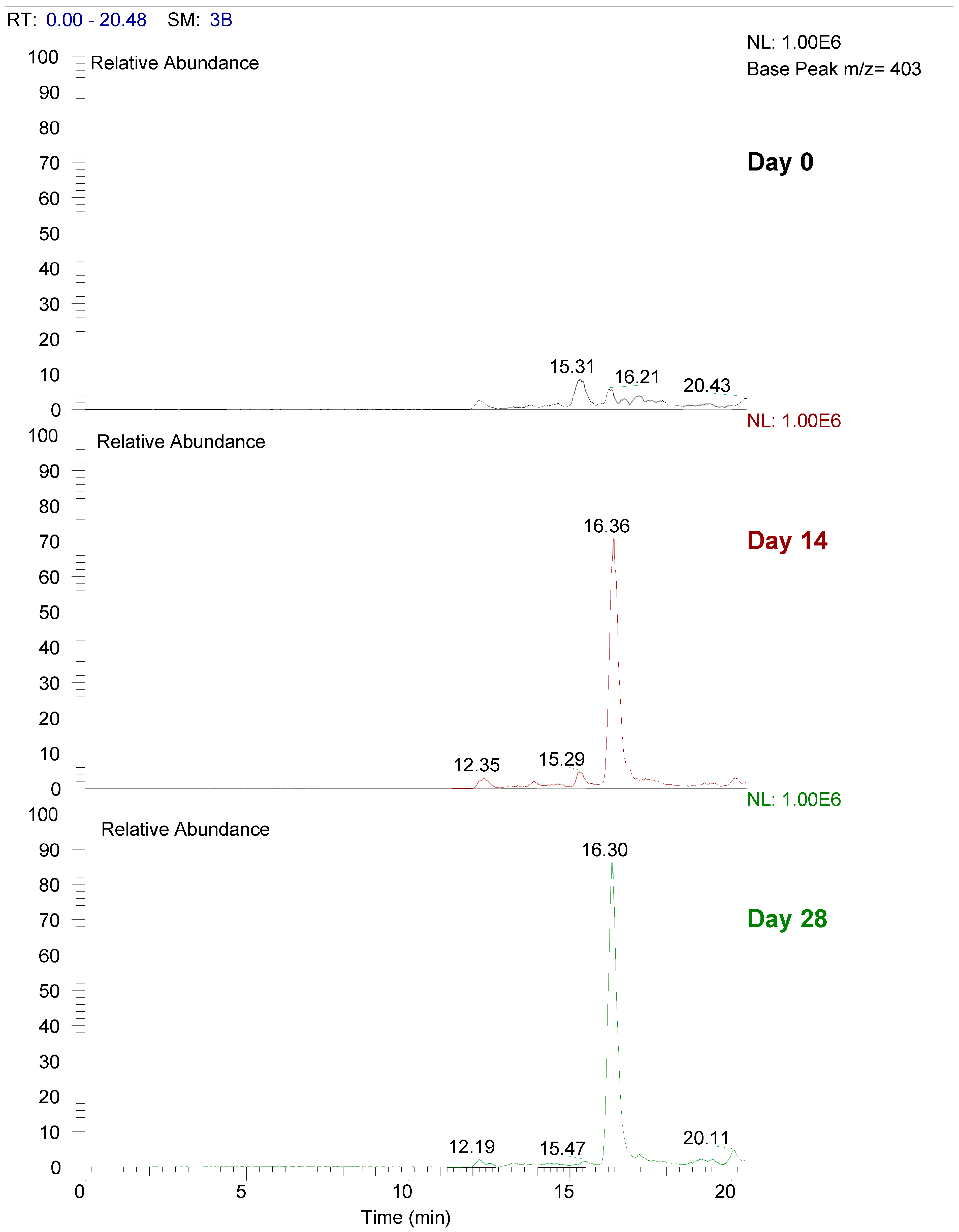
3.5. Bioavailability of Urolithin A Glucuronide
3.6. Nutritional Intake
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Cardiovascular Diseases (CVDs). Key Facts. 11 June 2021. Available online: https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds) (accessed on 5 May 2022).
- GBD 2019 Diseases and Injuries Collaborators. Global burden of 369 diseases and injuries in 204 countries and territories, 1990-2019: A systematic analysis of the Global Burden of Disease Study. Lancet 2020, 396, 1204–1222. [Google Scholar] [CrossRef]
- European Cardiovascular Disease Statistics 2017. Available online: https://ehnheart.org/cvd-statistics.html (accessed on 5 May 2022).
- Timmis, A.; Townsend, N.; Gale, C.P.; Torbica, A.; Lettino, M.; Petersen, S.E.; Vardas, P. European Society of Cardiology. European Society of Cardiology: Cardiovascular Disease Statistics 2019. Eur. Heart J. 2020, 41, 12–85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yusuf, S.; Joseph, P.; Rangarajan, S.; Islam, S.; Mente, A.; Hystad, P.; Dagenais, G. Modifiable risk factors, cardiovascular disease, and mortality in 155,722 individuals from 21 high-income, middle-income, and low-income countries (PURE): A prospective cohort study. Lancet 2020, 395, 795–808. [Google Scholar] [CrossRef] [Green Version]
- Cannon, C.P. Cardiovascular disease and modifiable cardiometabolic risk factors. Clin. Cornerstone 2008, 9, 24–41. [Google Scholar] [CrossRef]
- Almoosawi, S.; Fyfe, L.; Ho, C.; Al-Dujaili, E. The effect of polyphenol-rich dark chocolate on fasting capillary whole blood glucose, total cholesterol, blood pressure and glucocorticoids in healthy overweight and obese subjects. Br. J. Nutr. 2010, 103, 842–850. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walker, B.R.; Connacher, A.A.; Webb, D.J.; Edwards, C.R. Glucocorticoids and blood pressure: A role for the cortisol/cortisone shuttle in the control of vascular tone in man. Clin. Sci. 1992, 83, 171–178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Virani, S.S.; Alonso, A.; Benjamin, E.J.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. Heart Disease and Stroke Statistics-2020 Update: A Report From the American Heart Association. Circulation 2020, 141, e139–e596. [Google Scholar]
- Dubois-Deruy., E.; Peugnet, V.; Turkieh, A.; Pinet, F. Oxidative stress in cardiovascular diseases. Antioxidants 2020, 9, 864. [Google Scholar] [CrossRef]
- Yung, L.M.; Leung, F.P.; Yao, X.; Chen, Z.Y.; Huang, Y. Reactive oxygen species in vascular wall. Cardiovasc. Hematol. Disord. Drug Targets 2006, 6, 1–19. [Google Scholar] [CrossRef]
- Blake, G.J.; Ridker, P.M. Inflammatory biomarkers and cardiovascular risk prediction. J. Intern. Med. 2002, 252, 283–294. [Google Scholar] [CrossRef]
- Pai, J.K.; Pischon, T.; Ma, J.; Manson, J.E.; Hankinson, S.E.; Joshipura, K.; Rimm, E.B. Inflammatory markers and the risk of coronary heart disease in men and women. N. Engl. J. Med. 2004, 351, 2599–2610. [Google Scholar] [CrossRef]
- Ogita, H.; Liao, J. Endothelial function and oxidative stress. Endothelium 2004, 11, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Yu, E.; Malik, V.S.; Hu, F.B. Cardiovascular disease prevention by diet modification: JACC Health Promotion Series. J. Am. Coll. Cardiol. 2018, 72, 914–926. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Diet, nutrition and the prevention of chronic diseases: Report of a joint WHO/FAO expert consultation. In Proceedings of the Joint WHO/FAO Expert Consultation on Diet, Nutrition and the Prevention of Chronic Diseases, Geneva, Switzerland, 28 January–1 February 2002. [Google Scholar]
- Wang, D.D.; Li, Y.; Bhupathiraju, S.N.; Rosner, B.A.; Sun, Q.; Giovannucci, E.L.; Hu, F.B. Fruit and vegetable intake and mortality: Results from 2 prospective cohort studies of US men and women and a meta-analysis of 26 cohort studies. Circulation 2021, 143, 1642–1654. [Google Scholar] [CrossRef]
- Turati, F.; Rossi, M.; Pelucchi, C.; Levi, F.; La Vecchia, C. Fruit and vegetables and cancer risk: A review of southern European studies. Br. J. Nutr. 2015, 113 (Suppl. S2), S102–S110. [Google Scholar] [CrossRef] [PubMed]
- Al-Dujaili, E.A.S. Functional foods need more mechanistic studies. EC Nutr. 2016, 4.1, 770–771. [Google Scholar]
- Derakhshan, Z.; Ferrante, M.; Tadi, M.; Ansari, F.; Heydari, A.; Hosseini, M.S.; Sadrabad, E.K. Antioxidant activity and total phenolic content of ethanolic extract of pomegranate peels, juice and seeds. Food Chem. Toxicol. 2018, 114, 108–111. [Google Scholar] [CrossRef]
- Mastrodi Salgado, J.; Baroni Ferreira, T.R.; de Oliveira Biazotto, F.; Dos Santos Dias, C.T. Increased antioxidant content in juice enriched with dried extract of pomegranate (Punica granatum) peel. Plant Foods Hum. Nutr. 2012, 67, 39–43. [Google Scholar] [CrossRef]
- Ruis, A.R. Pomegranate and the meditation of balance in early medicine. Gastronomica 2015, 15, 22–33. [Google Scholar] [CrossRef]
- Al-Muammar, M.N.; Khan, F. Obesity: The preventive role of the pomegranate (Punica granatum). Nutrition 2012, 28, 595–604. [Google Scholar] [CrossRef]
- Stockton, A.; ASAl-Dujaili, E.; McDougall, G.J.; Davidson, I.; Drummond, S.; Wyness, L. Effect of pomegranate extract consumption on cardiovascular disease risk factors, stress hormones, and quality of life in human volunteers: An exploratory randomised, double-blind, placebo-controlled trial. EC Nutr. 2015, 2.4., 396–411. [Google Scholar]
- Aviram, M.; Dornfeld, L. Pomegranate juice consumption inhibits serum angiotensin converting enzyme activity and reduces systolic blood pressure. Atherosclerosis 2001, 158, 195–198. [Google Scholar] [CrossRef]
- Aviram, M.; Rosenblat, M.; Gaitini, D.; Nitecki, S.; Hoffman, A.; Dornfeld, L.; Hayek, T. Pomegranate juice consumption for 3 years by patients with carotid artery stenosis reduces common carotid intima-media thickness, blood pressure and LDL oxidation. Clin. Nutr. 2004, 23, 423–433. [Google Scholar] [CrossRef] [PubMed]
- Aviram, M.; Dornfeld, L.; Rosenblat, M.; Volkova, N.; Kaplan, M.; Coleman, R.; Fuhrman, B. Pomegranate juice consumption reduces oxidative stress, atherogenic modifications to LDL, and platelet aggregation: Studies in humans and in atherosclerotic apolipoprotein E-deficient mice. Am. J. Clin. Nutr. 2000, 71, 1062–1076. [Google Scholar] [CrossRef] [Green Version]
- Rosenblat, M.; Hayek, T.; Aviram, M. Anti-oxidative effects of pomegranate juice (PJ) consumption by diabetic patients on serum and on macrophages. Atherosclerosis 2006, 187, 363–371. [Google Scholar] [CrossRef] [PubMed]
- Stowe, C.B. The effects of pomegranate juice consumption on blood pressure and cardiovascular health. Complement Clin. Pr. 2011, 17, 113–115. [Google Scholar] [CrossRef]
- Tsang, C.; Smail, N.F.; Almoosawi, S.; Davidson, I.; Al-Dujaili, E.A. Intake of polyphenol-rich pomegranate pure juice influences urinary glucocorticoids, blood pressure and homeostasis model assessment of insulin resistance in human volunteers. J. Nutr. Sci. 2012, 31, e9. [Google Scholar] [CrossRef] [Green Version]
- Stockton, A.; Farhat, G.; McDougall, G.J.; Al-Dujaili, E.A.S. Effect of pomegranate extract on blood pressure and anthropometry in adults: A double-blind placebo-controlled randomised clinical trial. J. Nutr. Sci. 2017, 6, e39. [Google Scholar] [CrossRef] [Green Version]
- Lukaski, H.C.; Johnson, P.E.; Bolonchuk, W.W.; Lykken, G.I. Assessment of fat-free mass using bioelectrical impedance measurements of the human body. Am. J. Clin. Nutr. 1985, 41, 810–817. [Google Scholar] [CrossRef] [Green Version]
- Warren, R.E.; Marshall, T.; Padfield, P.L.; Chrubasik., S. Variability of office, 24-hour ambulatory, and self-monitored blood pressure measurements. BR J. Gen. Pract 2010, 60, 675–680. [Google Scholar] [CrossRef] [Green Version]
- Laurent, S.; Boutouyrie, P.; Asmar, R.; Gautier, I.; Laloux, B.; Guize, L.; Benetos, A. Aortic stiffness is an independent predictor of all-cause and cardiovascular mortality in hypertensive patients. Hypertension 2001, 37, 1236–1241. [Google Scholar] [CrossRef] [Green Version]
- Al-Dujaili, E.A.; Baghdadi, H.H.; Howie, F.; Mason, J.I. Validation and application of a highly specific and sensitive ELISA for the estimation of cortisone in saliva, urine and in vitro cell-culture media by using a novel antibody. Steroids 2012, 77, 703–709. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.; Rossi, A. Colorimetric of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Al-Dujaili, E.A.S. Validation and application of a microplate method for the determination of ferric reducing antioxidant power (FRAP) in urine samples. Ann. Nutr. Metab. 2013, 63 (Suppl. S1), 341–342. [Google Scholar]
- Buege, J.A.; Aust, S.D. Microsomal lipid peroxidation. Methods Enzym. 1978, 52, 302–310. [Google Scholar]
- Trabulsi, J.; Schoeller, D.A. Evaluation of dietary assessment instruments against doubly labeled water, a biomarker of habitual energy intake. Am. J. Physiol. Endocrinol. Metab. 2001, 281, E891–E899. [Google Scholar] [CrossRef] [Green Version]
- Hintzpeter, J.; Stapelfeld, C.; Loerz, C.; Martin, H.J.; Maser, E. Green tea and one of its constituents, Epigallocatechine-3-gallate, are potent inhibitors of human 11β-hydroxysteroid dehydrogenase type 1. PLoS ONE 2014, 9, e84468. [Google Scholar] [CrossRef] [PubMed]
- Aviram, M.; Rosenblat, M. Pomegranate protection against cardiovascular diseases. Evid. Based Complement Altern. Med. 2012, 2012, 382763. [Google Scholar] [CrossRef]
- Basu, A.; Penugonda, K. Pomegranate juice: A heart-healthy fruit juice. Nutr. Rev. 2009, 67, 49–56. [Google Scholar] [CrossRef]
- Kershaw, E.E.; Flier, J.S. Adipose tissue as an endocrine organ. J. Clin. Endocrinol. Metab. 2004, 89, 2548–2556. [Google Scholar] [CrossRef]
- Abidov, M.; Ramazanov, Z.; Seifulla, R.; Grachev, S. The effects of Xanthigen in the weight management of obese premenopausal women with non-alcoholic fatty liver disease and normal liver fat. Diabetes Obes. Metab. 2010, 12, 72–81. [Google Scholar] [CrossRef]
- Vroegrijk, I.O.; van Diepen, J.A.; van den Berg, S.; Westbroek, I.; Keizer, H.; Gambelli, L.; Voshol, P.J. Pomegranate seed oil, a rich source of punicic acid, prevents diet-induced obesity and insulin resistance in mice. Food. Chem. Toxicol. 2011, 49, 1426–1430. [Google Scholar] [CrossRef] [PubMed]
- Ok, E.; Do, G.M.; Lim, Y.; Park, J.E.; Park, Y.J.; Kwon, O. Pomegranate vinegar attenuates adiposity in obese rats through coordinated control of AMPK signaling in the liver and adipose tissue. Lipids Health Dis. 2013, 12, 163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McDougall, J.; Kulkarni, N.N.; Stewart, D. Berry polyphenols inhibit pancreatic lipase activity in vitro. Food Chem. 2009, 14, 491–496. [Google Scholar] [CrossRef]
- Asgary, S.; Keshvari, M.; Sahebkar, A.; Hashemi, M.; Rafieian-Kopaei, M. Clinical investigation of the acute effects of pomegranate juice on blood pressure and endothelial function in hypertensive individuals. ARYA Atheroscler. 2013, 9, 326–331. [Google Scholar] [PubMed]
- Lynn, A.; Hamadeh, H.; Leung, W.C.; Russell, J.M.; Barker, M.E. Effects of pomegranate juice supplementation on pulse wave velocity and blood pressure in healthy young and middle-aged men and women. Plant Foods Hum. Nutr. 2012, 67, 309–314. [Google Scholar] [CrossRef]
- Sugawara, J.; Hayashi, K.; Yokoi, T.; Cortez-Cooper, M.Y.; DeVan, A.E.; Anton, M.A.; Tanaka, H. Brachial-ankle pulse wave velocity: An index of central arterial stiffness? J. Hum. Hypertens 2005, 19, 401–406. [Google Scholar] [CrossRef] [Green Version]
- Sutton-Tyrrell, K.; Najjar, S.S.; Boudreau, R.M.; Venkitachalam, L.; Kupelian, V.; Simonsick, E.M.; Newman, A. Elevated aortic pulse wave velocity, a marker of arterial stiffness, predicts cardiovascular events in well-functioning older adults. Circulation 2005, 28, 3384–3390. [Google Scholar] [CrossRef]
- De Nigris, F.; Williams-Ignarro, S.; Lerman, L.O.; Crimi, E.; Botti, C.; Mansueto, G.; Napoli, C. Beneficial effects of pomegranate juice on oxidation-sensitive genes and endothelial nitric oxide synthase activity at sites of perturbed shear stress. Proc. Natl. Acad. Sci. USA 2005, 29, 4896–4901. [Google Scholar] [CrossRef] [Green Version]
- Shema-Didi, L.; Kristal, B.; Sela, S.; Geron, R.; Ore, L. Does Pomegranate intake attenuate cardiovascular risk factors in hemodialysis patients? Nutr. J. 2014, 13, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kidambi, S.; Kotchen, J.M.; Grim, C.E.; Raff, H.; Mao, J.; Singh, R.J.; Kotchen, T.A. Association of adrenal steroids with hypertension and the metabolic syndrome in blacks. Hypertension 2007, 49, 704–711. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gil, M.I.; Tomás-Barberán, F.A.; Hess-Pierce, B.; Holcroft, D.M.; Kader, A.A. Antioxidant activity of pomegranate juice and its relationship with phenolic composition and processing. J. Agric. Food Chem. 2000, 48, 4581–4589. [Google Scholar] [CrossRef] [PubMed]
- Perron, N.R.; Brumaghim, J.L. A Review of the Antioxidant Mechanisms of Polyphenol Compounds Related to Iron Binding. Cell Biochem. Biophys. 2009, 53, 75–100. [Google Scholar] [CrossRef] [PubMed]
- Yiannakopoulou, E.C. Targeting oxidative stress response by green tea polyphenols: Clinical imply cations. Free Radic Res. 2013, 47, 667–671. [Google Scholar] [CrossRef]
- Nani, A.; Murtaza, B.; Sayed Khan, A.; Khan, N.A.; Hichami, A. Antioxidant and Anti-Inflammatory Potential of Polyphenols Contained in Mediterranean Diet in Obesity: Molecular Mechanisms. Molecules 2021, 26, 985. [Google Scholar] [CrossRef]
- Arcusa, R.; Carrillo, J.Á.; Cerdá, B.; Durand, T.; Gil-Izquierdo, Á.; Medina, S.; Galano, J.-M.; Villaño Valencia, D.; Marhuenda, J.; Zafrilla, P. Anti-Inflammatory and Antioxidant Capacity of a Fruit and Vegetable-Based Nutraceutical Measured by Urinary Oxylipin Concentration in a Healthy Population: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Antioxidants 2022, 11, 1342. [Google Scholar] [CrossRef]
- Andriantsitohaina, R.; Auger, C.; Chataigneau, T.; Étienne-Selloum, N.; Li, H.; Martínez, M.C.; Schini-Kerth, V.B.; Laher, I. Molecular mechanisms of the cardiovascular protective effects of polyphenols. Br J. Nutr. 2012, 108, 1532–1549. [Google Scholar] [CrossRef] [Green Version]
- Heber, D.; Seeram, N.P.; Wyatt, H.; Henning, S.M.; Zhang, Y.; Ogden, L.G.; Hill, J.O. Safety and antioxidant activity of a pomegranate ellagitannin-enriched polyphenol dietary supplement in overweight individuals with increased waist size. J. Agric. Food Chem. 2007, 55, 10050–10054. [Google Scholar] [CrossRef]
- Seeram, N.P.; Henning, S.M.; Zhang, Y.; Suchard, M.; Li, Z.; Heber, D. Pomegranate juice ellagitannin metabolites are present in human plasma and some persist in urine for up to 48 hours. J. Nutr. 2006, 136, 2481–2485. [Google Scholar] [CrossRef] [Green Version]
- Espín, J.C.; Larrosa, M.; García-Conesa, M.T.; Tomás-Barberán, F. Biological significance of urolithins, the gut microbial ellagic Acid-derived metabolites: The evidence so far. Evid. Based Complement Altern. Med. 2013, 270418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Larrosa, M.; García-Conesa, M.T.; Espín, J.C.; Tomás-Barberán, F.A. Ellagitannins, ellagic acid and vascular health. Mol. Asp. Med. 2010, 31, 513–539. [Google Scholar] [CrossRef] [PubMed]
- Cerdá, B.; Espín, J.C.; Parra, S.; Martínez, P.; Tomás-Barberán, F.A. The potent in vitro antioxidant ellagitannins from pomegranate juice are metabolised into bioavailable but poor antioxidant hydroxy-6H-dibenzopyran-6-one derivatives by the colonic microflora of healthy humans. Eur. J. Nutr. 2004, 43, 205–220. [Google Scholar] [CrossRef] [PubMed]
- Al-Dujaili, E.A.; Good, G.; Tsang, C. Consumption of Pomegranate Juice Attenuates Exercise-Induced Oxidative Stress, Blood Pressure and Urinary Cortisol/Cortisone Ratio in Human Adults. EC Nutr. 2016, 4, 982–995. [Google Scholar]
- Sellitto, C.; Corbi, G.; Stefanelli, B.; Manzo, V.; Trucillo, M.; Charlier, B.; Mensitieri, F.; Izzo, V.; Lucariello, A.; Perna, A.; et al. Antioxidant Supplementation Hinders the Role of Exercise Training as a Natural Activator of SIRT1. Nutrients 2022, 14, 2092. [Google Scholar] [CrossRef]
- d’Unienville, N.; Blake, H.; Coates1, A.; Hill, A.; Nelson, M.; Buckley, J. Effect of food sources of nitrate, polyphenols, L-arginine and L-citrulline on endurance exercise performance: A systematic review and meta-analysis of randomised controlled trials. J. Int. Soc. Sports Nutr. 2021, 18, 76. [Google Scholar] [CrossRef]


| Variables | Pomegranate (n = 12) | Placebo (n = 12) | p-Value |
|---|---|---|---|
| Age, years | 23.5 ± 1.8 | 24 ± 2.4 | 0.632 |
| Body weight, kg | 69.8 ± 14.6 | 71.6 ± 14.1 | 0.695 |
| Height, cm | 1.7 ± 0.1 | 1.7 ± 0.2 | 0.960 |
| BMI, kg/m2 | 24.1 ± 2.7 | 24.7 ± 2.9 | 0.446 |
| Fat mass, % | 24.2 ± 5.0 | 23.2 ± 8.5 | 0.680 |
| Fat mass, kg | 16.4 ± 2.9 | 15.8 ± 5.1 | 0.875 |
| Lean mass, kg | 54.4 ± 14.1 | 55.1 ± 15.2 | 0.364 |
| SBP, mmHg | 118.1 ± 6.6 | 118.2 ± 11.5 | 0.854 |
| DBP, mmHg | 68.9 ± 5.1 | 71.2 ± 7.2 | 0.750 |
| PWV, m/s | 6.5 ± 0.6 | 6.5 ± 0.5 | 0.763 |
| Cortisol, urinary, nmol/day | 125.2 ± 63.1 | 112.6 ± 99.2 | 0.481 |
| Cortisone, urinary, nmol/day | 109.7 ± 48.7 | 96.6 ± 30.7 | 0.355 |
| Cortisol/cortisone ratio, urinary | 1.14 ± 0.68 | 1.16 ± 0.75 | 0.729 |
| Cortisol, salivary, ng/mL | 5.66 ± 1.3 | 4.996 ± 3.5 | 0.283 |
| Cortisone, salivary, ng/mL | 4.83 ± 1.47 | 4.649 ± 3.1 | 0.315 |
| Cortisol/cortisone ratio, salivary | 1.22 ± 0.27 | 1.075 ± 0.4 | 0.089 |
| Total phenolics, mg GAE/day | 436.2 ± 94.9 | 430.1 ± 236.3 | 0.293 |
| Antioxidant capacity, FRAP, mmol Fe+2/day | 4.7 ± 1.2 | 5.2 ± 1.9 | 0.285 |
| Lipid peroxidation, TBARS, µmoL MDA/day | 2.46 ± 0.27 | 2.4 ± 0.3 | 0.543 |
| Variables | Pomegranate (n = 12) | Placebo (n = 12) | ||||
|---|---|---|---|---|---|---|
| Day 0 | Day 28 | p-Value | Day 0 | Day 28 | p-Value | |
| Weight, kg | 70.9 ± 14 | 70.6 ± 13.9 | 0.170 | 71.6 ± 13.9 | 71.5 ± 14.2 | 0.977 |
| BMI, kg/m2 | 23.6 ± 2.9 | 23.4 ± 2.9 | 0.157 | 24.7 ± 2.9 | 24.7 ± 3.1 | 0.848 |
| Fat mass, % | 24.1 ± 4.1 | 23.5 ± 3.8 | 0.002 | 23.2 ± 8.5 | 22.8 ± 8.4 | 0.069 |
| Fat mass, kg | 16.4 ± 2.9 | 15.7 ± 2.9 | 0.005 | 15.8 ± 5.1 | 15.0 ± 4.8 | 0.119 |
| Lean mass, kg | 54.4 ± 14.1 | 55.8 ± 13.9 | 0.025 | 55.1 ± 14.9 | 55.5 ± 15.6 | 0.075 |
| SBP, mmHg | 118.1 ± 6.6 | 113.9 ± 9.4 | 0.014 | 118.2 ± 11.5 | 116.8 ± 11.2 | 0.444 |
| DBP, mmHg | 68.9 ± 5.1 | 65.2 ± 6.1 | 0.009 | 71.2 ± 7.2 | 71.7 ± 6.9 | 0.756 |
| PWV, m/s | 6.6 ± 0.5 | 6.1 ± 0.5 | 0.013 | 6.5 ± 0.5 | 6.4 ± 0.4 | 0.203 |
| Study Groups and Time | Study Day | Cortisol, ng/mL | p-Value | Cortisone, ng/mL | p-Value |
|---|---|---|---|---|---|
| Pomegranate | |||||
| Morning | Day 0 | 8.02 ± 2.6 | 7.5 ± 2.8 | ||
| Day 14 | 6.42 ± 1.1 | 0.045 | 6.96 ± 2.4 | 0.35 | |
| Day 28 | 5.65 ± 1.9 | 0.002 | 8.11 ± 4.2 | 0.58 | |
| Noon | Day 0 | 5.39 ± 0.8 | 4.33 ± 1.2 | ||
| Day 14 | 4.57 ± 1.3 | 0.07 | 4.34 ± 1.2 | 0.98 | |
| Day 28 | 4.51 ± 1.9 | 0.12 | 6.08 ± 2.9 | 0.38 | |
| Evening | Day 0 | 3.6 ± 1.4 | 2.65 ± 1.1 | ||
| Day 14 | 3.45 ± 1.5 | 0.82 | 2.93 ± 0.8 | 0.48 | |
| Day 28 | 3.36 ± 1.8 | 0.61 | 3.26 ± 2.9 | 0.23 | |
| Placebo | |||||
| Morning | Day 0 | 7.15 ± 3.5 | 6.848 ± 3.1 | ||
| Day 14 | 7.28 ± 3.5 | 0.456 | 7.025 ± 3.8 | 0.186 | |
| Day 28 | 8.11 ± 2.9 | 0.340 | 6.435 ± 3.5 | 0.886 | |
| Noon | Day 0 | 4.66 ± 3.3 | 3.88 ± 0.8 | ||
| Day 14 | 4.59 ± 1.8 | 0.524 | 4.16 ± 1.6 | 0.247 | |
| Day 28 | 8.11 ± 3.6 | 0.752 | 4.734 ± 2.2 | 0.343 | |
| Evening | Day 0 | 3.17 ± 3.9 | 3.22 ± 2.4 | ||
| Day 14 | 3.24 ± 1.9 | 0.758 | 3.027 ± 2.6 | 0.394 | |
| Day 28 | 3.54 ± 1.5 | 0.794 | 3.384 ± 1.2 | 0.859 |
| Study Day | Pomegranate Group (n = 12) | Placebo Group (n = 12) | ||||
|---|---|---|---|---|---|---|
| Cortisol nmol/Day | Cortisone nmol/Day | Cortisol/ Cortisone Ratio | Cortisol nmol/Day | Cortisone nmol/Day | Cortisol/ Cortisone Ratio | |
| Day 0 | 125.9 ± 63.1 | 109.7 ± 48.8 | 1.14 ± 0.68 | 122.6 ± 99.6 | 96.4 ± 30.7 | 1.17 ± 0.85 |
| Day 14 | 99.87 ± 44.9 | 111.6 ± 39.9 | 0.89 ± 0.47 | 128.2 ± 99.4 | 116.1 ± 32.2 | 1.08 ± 0.82 |
| Day 28 | 99.1 ± 34.3 | 109.3 ± 43.3 | 0.906 ± 0.41 | 137.7 ± 61.7 | 110.6 ± 39.3 | 1.24 ± 1.17 |
| Days 14 vs. 0, p value | 0.003 | 0.859 | 0.243 | 0.174 | 0.069 | 0.953 |
| Days 28 vs. 0, p value | 0.043 | 0.716 | 0.219 | 0.434 | 0.433 | 0.078 |
| Variables | Pomegranate (n = 12) | Placebo (n = 12) | p Value |
|---|---|---|---|
| Energy, kcal | 1884.8 ± 741.2 | 2052.9 ± 825.1 | 0.467 |
| Proteins, g | 109.9 ± 64.7 | 107.9 ± 74.8 | 0.921 |
| Carbohydrates, g | 196.4 ± 53.9 | 223.7 ± 81.1 | <0.05 |
| Fat, g | 76.4 ± 44.4 | 84.6 ± 35.8 | 0.272 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Dujaili, E.A.S.; Casey, C.; Stockton, A. Antioxidant Properties and Beneficial Cardiovascular Effects of a Natural Extract of Pomegranate in Healthy Volunteers: A Randomized Preliminary Single-Blind Controlled Study. Antioxidants 2022, 11, 2124. https://doi.org/10.3390/antiox11112124
Al-Dujaili EAS, Casey C, Stockton A. Antioxidant Properties and Beneficial Cardiovascular Effects of a Natural Extract of Pomegranate in Healthy Volunteers: A Randomized Preliminary Single-Blind Controlled Study. Antioxidants. 2022; 11(11):2124. https://doi.org/10.3390/antiox11112124
Chicago/Turabian StyleAl-Dujaili, Emad A. S., Ciara Casey, and Angela Stockton. 2022. "Antioxidant Properties and Beneficial Cardiovascular Effects of a Natural Extract of Pomegranate in Healthy Volunteers: A Randomized Preliminary Single-Blind Controlled Study" Antioxidants 11, no. 11: 2124. https://doi.org/10.3390/antiox11112124
APA StyleAl-Dujaili, E. A. S., Casey, C., & Stockton, A. (2022). Antioxidant Properties and Beneficial Cardiovascular Effects of a Natural Extract of Pomegranate in Healthy Volunteers: A Randomized Preliminary Single-Blind Controlled Study. Antioxidants, 11(11), 2124. https://doi.org/10.3390/antiox11112124








