Skeletal Muscle CSE Deficiency Leads to Insulin Resistance in Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Body Weight, Body Composition Measurement, and Skeletal Muscles Weight
2.3. Treadmill Exhaustive Exercise Capacity and Grip Strength
2.4. Glucose and Insulin Tolerance Tests
2.5. Exogenous H2S Supplementation
2.6. Aerobic Treadmill Exercise Protocol
2.7. HE Staining
2.8. Q-PCR
2.9. RNA-Seq Analysis
2.10. Western Blot Analysis
2.11. Statistical Analysis
3. Results
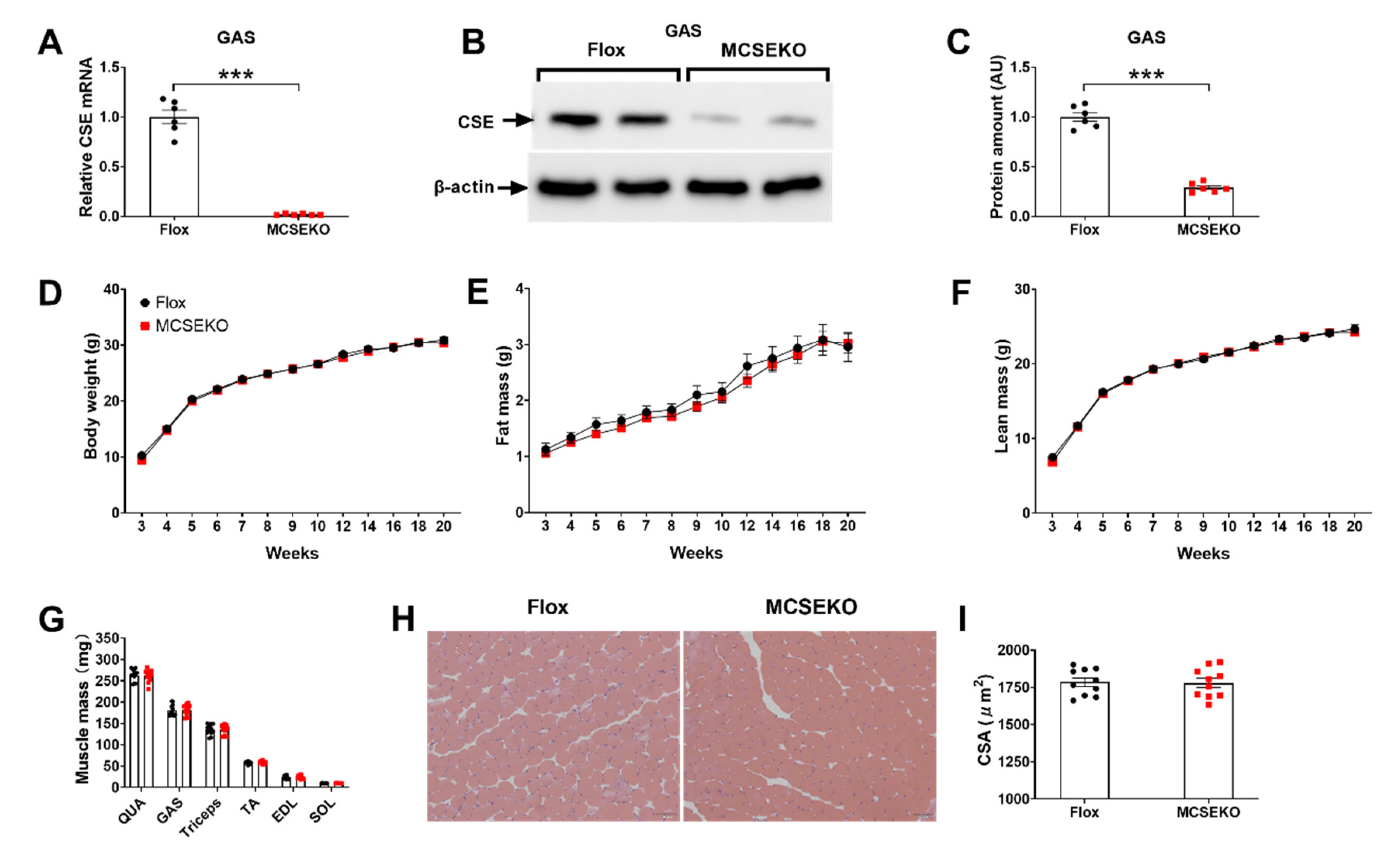
3.1. The MCSEKO Mice Show Normal Body Weight, Body Composition, Muscles Mass, and Musculature Morphology
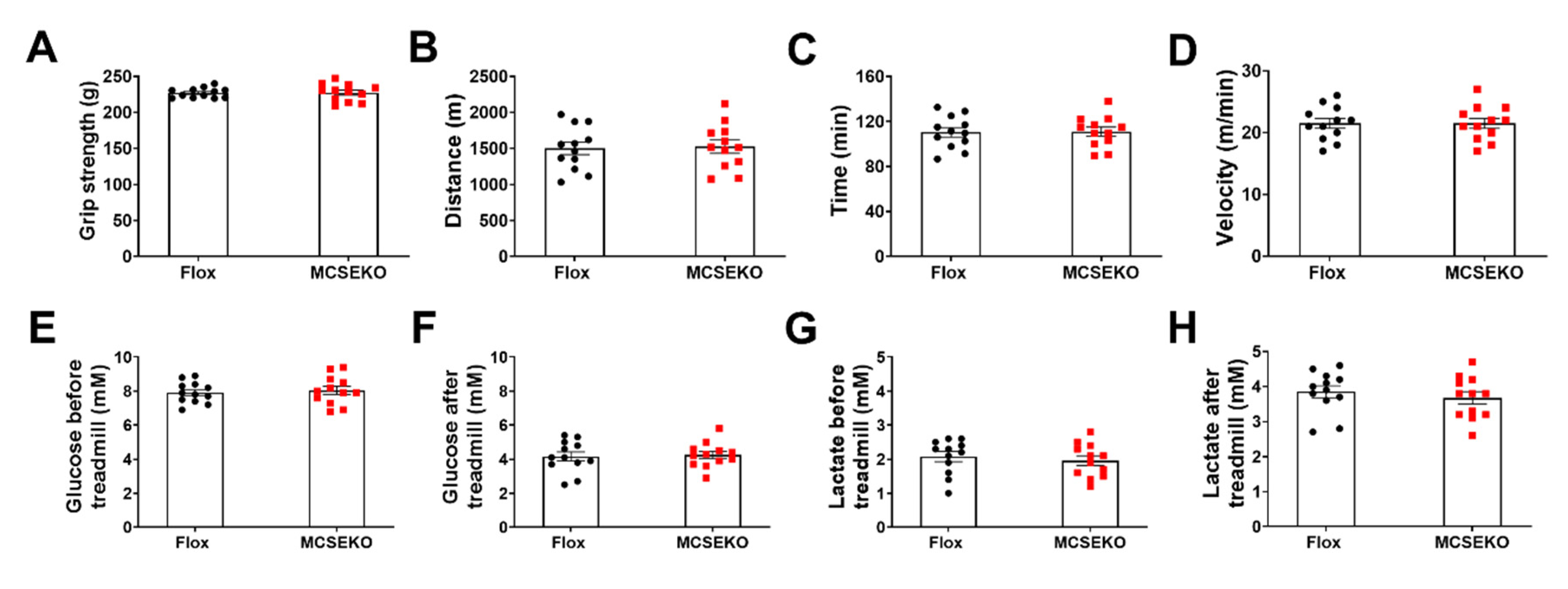
3.2. The MCSEKO Mice Show Normal Grip Strength and Exercise Capacity
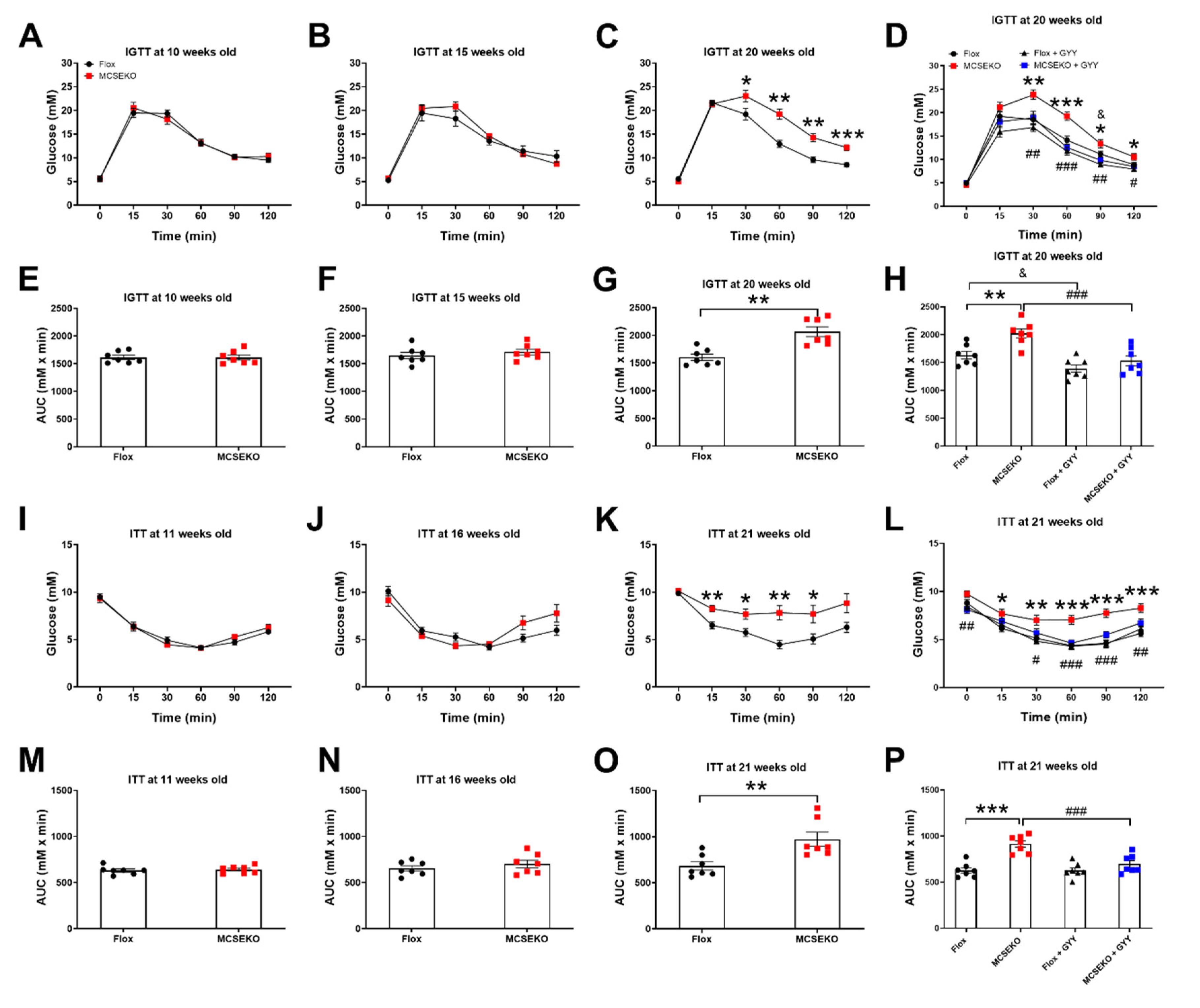
3.3. MCSEKO Mice Show Impaired Glucose Tolerance and Reduced Insulin Sensitivity with Age and GYY4137 Treatment Improved Them
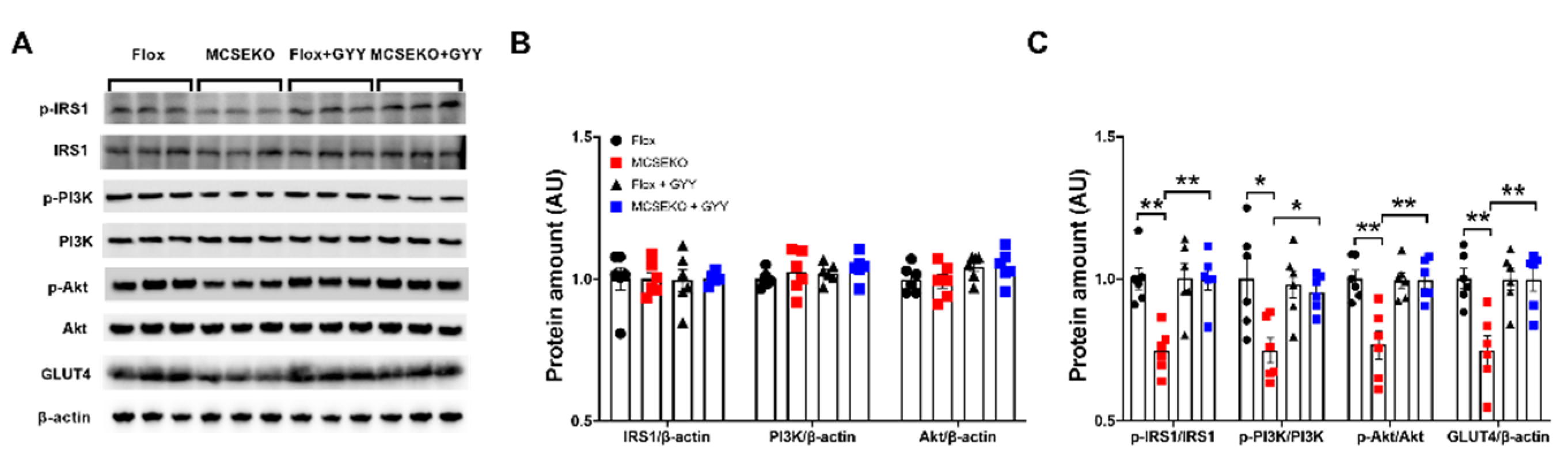
3.4. The Insulin Signal Pathway and GLUT4 Protein Level Are Down-Regulated in the Skeletal Muscle of the MCSEKO Mice and GYY4137 Treatment Ameliorates Them
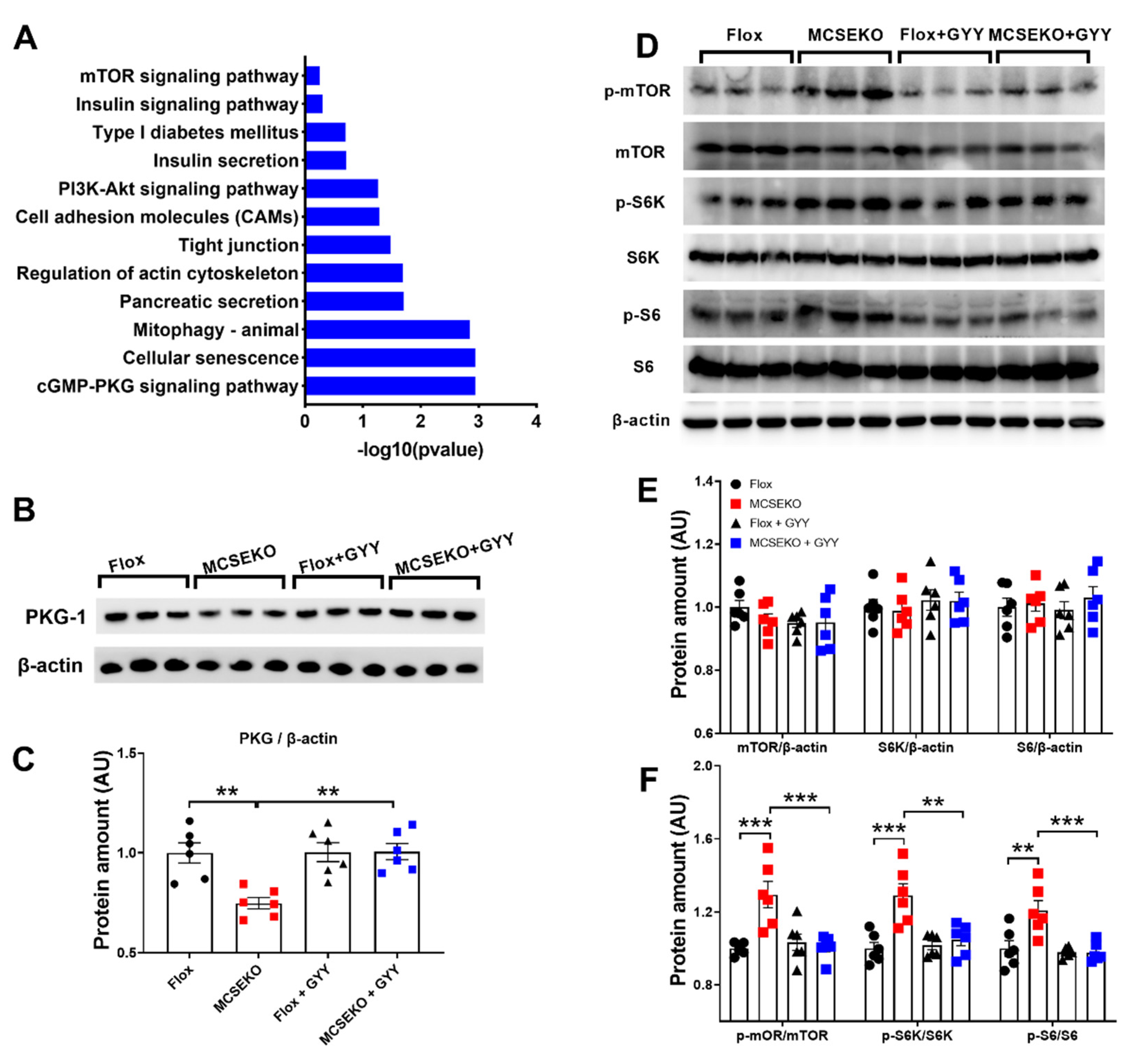
3.5. The PKG-1 Protein Level Was Down-Regulated and the mTOR Signal Pathway Was Up-Regulated in the Skeletal Muscle of the MCSEKO Mice and GYY4137 Treatment Attenuated Them
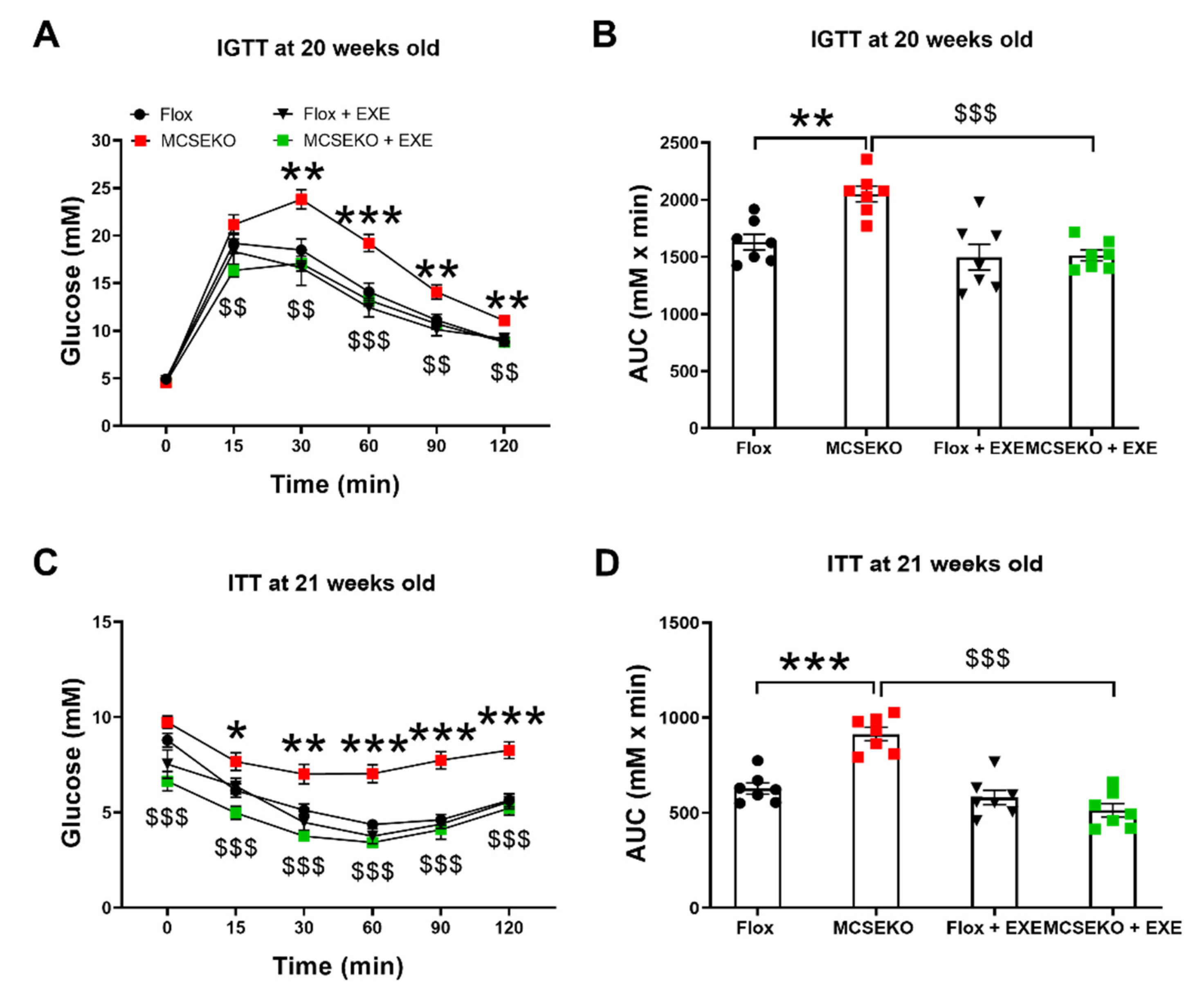
3.6. Aerobic Treadmill Exercise Improved Glucose Tolerance and Insulin Resistance in the MCSEKO Mice
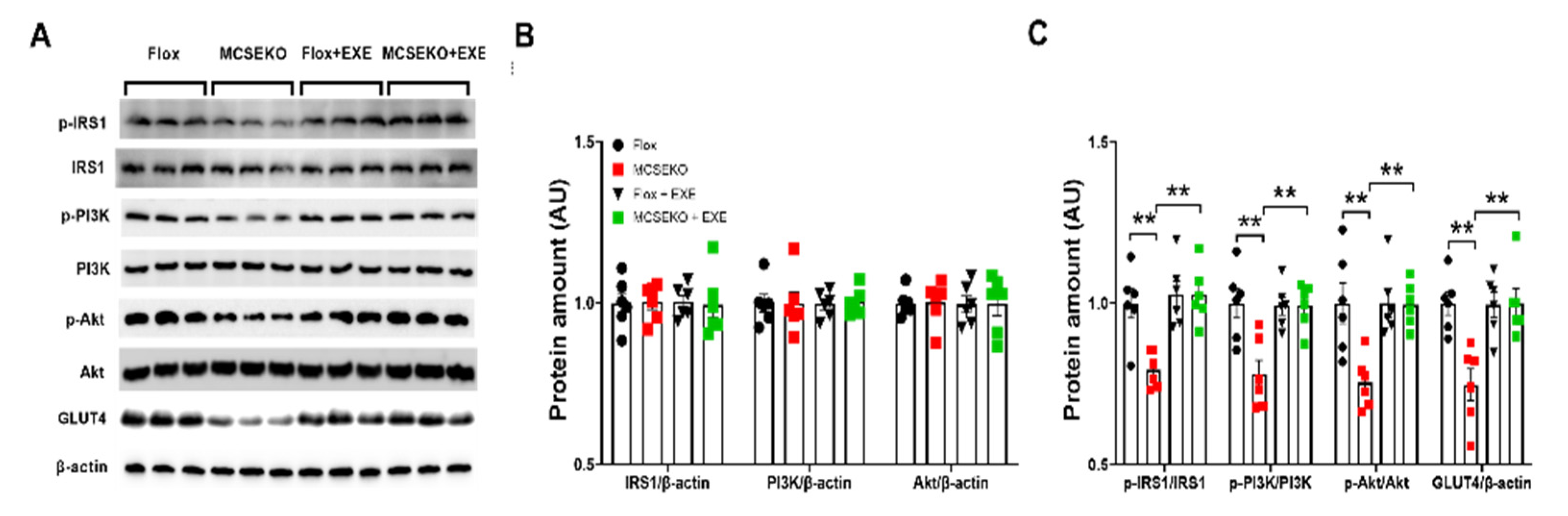
3.7. Aerobic Treadmill Exercise Increases GLUT4 Expression and Improves PKG-1, IRSI/PI3K/Akt and mTOR/S6K/S6 Signaling Pathways in MCSEKO Mice
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ismail, A.D. Resistance Training, Insulin Sensitivity & Metabolic Health. Ph.D. Thesis, University of Glasgow, Glasgow, UK, 2020. [Google Scholar]
- Fiorucci, S.; Distrutti, E.; Cirino, G.; Wallace, J.L. The emerging roles of hydrogen sulfide in the gastrointestinal tract and liver. Gastroenterology 2006, 131, 259–271. [Google Scholar] [CrossRef] [Green Version]
- Robert, K.; Nehme, J.; Bourdon, E.; Pivert, G.; Friguet, B.; Delcayre, C.; Delabar, J.M.; Janel, N. Cystathionine beta synthase deficiency promotes oxidative stress, fibrosis, and steatosis in mice liver. Gastroenterology 2005, 128, 1405–1415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, N.C.; Yang, F.; Capecci, L.M.; Gu, Z.; Schafer, A.I.; Durante, W.; Yang, X.F.; Wang, H. Regulation of homocysteine metabolism and methylation in human and mouse tissues. FASEB J. 2010, 24, 2804–2817. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, G.; Ju, Y.; Fu, M.; Zhang, Y.; Pei, Y.; Racine, M.; Baath, S.; Merritt, T.J.S.; Wang, R.; Wu, L. Cystathionine gamma-lyase/hydrogen sulfide system is essential for adipogenesis and fat mass accumulation in mice. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2018, 1863, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Ishii, I.; Akahoshi, N.; Yamada, H.; Nakano, S.; Izumi, T.; Suematsu, M. Cystathionine gamma-Lyase-deficient mice require dietary cysteine to protect against acute lethal myopathy and oxidative injury. J. Biol. Chem. 2010, 285, 26358–26368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, X.; Yao, T.; Wang, R.; Guo, S.; Wang, X.; Zhou, Z.; Zhang, Y.; Zhuo, X.; Wang, R.; Li, J.Z.; et al. IRF4 in Skeletal Muscle Regulates Exercise Capacity via PTG/Glycogen Pathway. Adv. Sci. 2020, 7, 2001502. [Google Scholar] [CrossRef] [PubMed]
- Powell, C.R.; Dillon, K.M.; Matson, J.B. A review of hydrogen sulfide (H2S) donors: Chemistry and potential therapeutic applications. Biochem. Pharmacol. 2018, 149, 110–123. [Google Scholar] [CrossRef]
- Schefer, V.; Talan, M.I. Oxygen consumption in adult and AGED C57BL/6J mice during acute treadmill exercise of different intensity. Exp. Gerontol. 1996, 31, 387–392. [Google Scholar] [CrossRef]
- Heo, J.W.; No, M.H.; Cho, J.; Choi, Y.; Cho, E.J.; Park, D.H.; Kim, T.W.; Kim, C.J.; Seo, D.Y.; Han, J.; et al. Moderate aerobic exercise training ameliorates impairment of mitochondrial function and dynamics in skeletal muscle of high-fat diet-induced obese mice. FASEB J. 2021, 35, e21340. [Google Scholar] [CrossRef]
- Fernando, P.; Bonen, A.; Hoffman-Goetz, L. Predicting submaximal oxygen consumption during treadmill running in mice. Can J. Physiol. Pharmacol. 1993, 71, 854–857. [Google Scholar] [CrossRef]
- Guo, X.; Sun, W.; Luo, G.; Wu, L.; Xu, G.; Hou, D.; Hou, Y.; Guo, X.; Mu, X.; Qin, L.; et al. Panax notoginseng saponins alleviate skeletal muscle insulin resistance by regulating the IRS1-PI3K-AKT signaling pathway and GLUT4 expression. FEBS Open Bio 2019, 9, 1008–1019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parsanathan, R.; Jain, S. Cystathionine γ-lyase-hydrogen sulfide (H2S) deficiency downregulates muscle myokine Fndc5 / Irisin and glucose metabolism in C2C12 myotubes and gastrocnemius muscle of HFD-fed mice. Free Radic. Biol. Med. 2018, 128, S90. [Google Scholar] [CrossRef]
- Tian, Y.; Heng, D.; Xu, K.; Liu, W.; Weng, X.; Hu, X.; Zhang, C. cGMP/PKG-I Pathway-Mediated GLUT1/4 Regulation by NO in Female Rat Granulosa Cells. Endocrinology 2018, 159, 1147–1158. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Yoon, S.O.; Poulogiannis, G.; Yang, Q.; Ma, X.M.; Villen, J.; Kubica, N.; Hoffman, G.R.; Cantley, L.C.; Gygi, S.P.; et al. Phosphoproteomic analysis identifies Grb10 as an mTORC1 substrate that negatively regulates insulin signaling. Science 2011, 332, 1322–1326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rivas, D.A.; Yaspelkis, B.B., 3rd; Hawley, J.A.; Lessard, S.J. Lipid-induced mTOR activation in rat skeletal muscle reversed by exercise and 5’-aminoimidazole-4-carboxamide-1-beta-D-ribofuranoside. J. Endocrinol. 2009, 202, 441–451. [Google Scholar] [CrossRef] [Green Version]
- Fu, L.; Liu, X.; Niu, Y.; Yuan, H.; Zhang, N.; Lavi, E. Effects of high-fat diet and regular aerobic exercise on global gene expression in skeletal muscle of C57BL/6 mice. Metabolism 2012, 61, 146–152. [Google Scholar] [CrossRef]
- Niu, Y.; Yuan, H.; Fu, L. Aerobic exercise’s reversal of insulin resistance by activating AMPKalpha-ACC-CPT1 signaling in the skeletal muscle of C57BL/6 mice. Int. J. Sport Nutr. Exerc. Metab. 2010, 20, 370–380. [Google Scholar] [CrossRef] [PubMed]
- Boluyt, M.O.; Brevick, J.L.; Rogers, D.S.; Randall, M.J.; Scalia, A.F.; Li, Z.B. Changes in the rat heart proteome induced by exercise training: Increased abundance of heat shock protein hsp20. Proteomics 2006, 6, 3154–3169. [Google Scholar] [CrossRef] [Green Version]
- Lu, F.; Lu, B.; Zhang, L.; Wen, J.; Wang, M.; Zhang, S.; Li, Q.; Shu, F.; Sun, Y.; Liu, N.; et al. Hydrogen sulphide ameliorating skeletal muscle atrophy in db/db mice via Muscle RING finger 1 S-sulfhydration. J. Cell. Mol. Med. 2020, 24, 9362–9377. [Google Scholar] [CrossRef] [PubMed]
- Parsanathan, R.; Jain, S.K. Hydrogen sulfide increases glutathione biosynthesis, and glucose uptake and utilisation in C2C12 mouse myotubes. Free Radic. Res. 2018, 52, 288–303. [Google Scholar] [CrossRef] [PubMed]
- Achari, A.E.; Jain, S.K. L-Cysteine supplementation increases adiponectin synthesis and secretion, and GLUT4 and glucose utilization by upregulating disulfide bond A-like protein expression mediated by MCP-1 inhibition in 3T3-L1 adipocytes exposed to high glucose. Mol. Cell. Biochem. 2016, 414, 105–113. [Google Scholar] [CrossRef]
- Cai, J.; Shi, X.; Wang, H.; Fan, J.; Feng, Y.; Lin, X.; Yang, J.; Cui, Q.; Tang, C.; Xu, G.; et al. Cystathionine gamma lyase-hydrogen sulfide increases peroxisome proliferator-activated receptor gamma activity by sulfhydration at C139 site thereby promoting glucose uptake and lipid storage in adipocytes. Biochim. Biophys. Acta 2016, 1861, 419–429. [Google Scholar] [CrossRef] [PubMed]
- Manna, P.; Jain, S.K. Hydrogen sulfide and l-cysteine increase phosphatidylinositol 3,4,5-trisphosphate (PIP3) and glucose utilization by inhibiting phosphatase and tensin homolog (PTEN) protein and activating phosphoinositide 3-kinase (PI3K)/serine/threonine protein kinase (AKT)/protein kinase Czeta/lambda (PKCzeta/lambda) in 3T3l1 adipocytes. J. Biol. Chem. 2011, 286, 39848–39859. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saltiel, A.R.; Kahn, C.R. Insulin signalling and the regulation of glucose and lipid metabolism. Nature 2001, 414, 799–806. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, A.H.; Pessin, J.E. Insulin regulation of glucose uptake: A complex interplay of intracellular signalling pathways. Diabetologia 2002, 45, 1475–1483. [Google Scholar] [CrossRef] [PubMed]
- Xue, R.; Hao, D.D.; Sun, J.P.; Li, W.W.; Zhao, M.M.; Li, X.H.; Chen, Y.; Zhu, J.H.; Ding, Y.J.; Liu, J.; et al. Hydrogen sulfide treatment promotes glucose uptake by increasing insulin receptor sensitivity and ameliorates kidney lesions in type 2 diabetes. Antioxid. Redox Signal. 2013, 19, 5–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, S.; Duan, C.; Gao, G.; Wang, X.; Yang, H. Alpha-synuclein overexpression negatively regulates insulin receptor substrate 1 by activating mTORC1/S6K1 signaling. Int. J. Biochem. Cell. Biol. 2015, 64, 25–33. [Google Scholar] [CrossRef]
- Koketsu, Y.; Sakoda, H.; Fujishiro, M.; Kushiyama, A.; Fukushima, Y.; Ono, H.; Anai, M.; Kikuchi, T.; Fukuda, T.; Kamata, H.; et al. Hepatic overexpression of a dominant negative form of raptor enhances Akt phosphorylation and restores insulin sensitivity in K/KAy mice. Am. J. Physiol. Endocrinol. Metab. 2008, 294, E719–E725. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Sui, Y.; Wu, Q.; Xie, B.; Sun, Z. Attenuated mTOR Signaling and Enhanced Glucose Homeostasis by Dietary Supplementation with Lotus Seedpod Oligomeric Procyanidins in Streptozotocin (STZ)-Induced Diabetic Mice. J. Agric. Food Chem. 2017, 65, 3801–3810. [Google Scholar] [CrossRef] [PubMed]
- Um, S.H.; Frigerio, F.; Watanabe, M.; Picard, F.; Joaquin, M.; Sticker, M.; Fumagalli, S.; Allegrini, P.R.; Kozma, S.C.; Auwerx, J.; et al. Absence of S6K1 protects against age- and diet-induced obesity while enhancing insulin sensitivity. Nature 2004, 431, 200–205. [Google Scholar] [CrossRef] [PubMed]
- Etgen, G.J., Jr.; Fryburg, D.A.; Gibbs, E.M. Nitric oxide stimulates skeletal muscle glucose transport through a calcium/contraction- and phosphatidylinositol-3-kinase-independent pathway. Diabetes 1997, 46, 1915–1919. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bergandi, L.; Silvagno, F.; Russo, I.; Riganti, C.; Anfossi, G.; Aldieri, E.; Ghigo, D.; Trovati, M.; Bosia, A. Insulin stimulates glucose transport via nitric oxide/cyclic GMP pathway in human vascular smooth muscle cells. Arter. Thromb. Vasc. Biol. 2003, 23, 2215–2221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frigolet, M.E.; Thomas, G.; Beard, K.; Lu, H.; Liu, L.; Fantus, I.G. The bradykinin-cGMP-PKG pathway augments insulin sensitivity via upregulation of MAPK phosphatase-5 and inhibition of JNK. Am. J. Physiol. Endocrinol. Metab. 2017, 313, E321–E334. [Google Scholar] [CrossRef] [Green Version]
- Bassot, A.; Chauvin, M.A.; Bendridi, N.; Ji-Cao, J.; Vial, G.; Monnier, L.; Bartosch, B.; Alves, A.; Cottet-Rousselle, C.; Gouriou, Y.; et al. Regulation of Mitochondria-Associated Membranes (MAMs) by NO/sGC/PKG Participates in the Control of Hepatic Insulin Response. Cells 2019, 8, 1319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rains, J.L.; Jain, S.K. Oxidative stress, insulin signaling, and diabetes. Free Radic. Biol. Med. 2011, 50, 567–575. [Google Scholar] [CrossRef] [Green Version]
- Kimura, Y.; Goto, Y.; Kimura, H. Hydrogen sulfide increases glutathione production and suppresses oxidative stress in mitochondria. Antioxid. Redox Signal. 2010, 12, 1–13. [Google Scholar] [CrossRef]
- Rodnick, K.J.; Holloszy, J.O.; Mondon, C.E.; James, D.E. Effects of exercise training on insulin-regulatable glucose-transporter protein levels in rat skeletal muscle. Diabetes 1990, 39, 1425–1429. [Google Scholar] [CrossRef]
- Kraniou, G.N.; Cameron-Smith, D.; Hargreaves, M. Acute exercise and GLUT4 expression in human skeletal muscle: Influence of exercise intensity. J. Appl. Physiol. 2006, 101, 934–937. [Google Scholar] [CrossRef] [Green Version]
- Richter, E.A.; Hargreaves, M. Exercise, GLUT4, and skeletal muscle glucose uptake. Physiol. Rev. 2013, 93, 993–1017. [Google Scholar] [CrossRef] [Green Version]
- Frosig, C.; Rose, A.J.; Treebak, J.T.; Kiens, B.; Richter, E.A.; Wojtaszewski, J.F. Effects of endurance exercise training on insulin signaling in human skeletal muscle: Interactions at the level of phosphatidylinositol 3-kinase, Akt, and AS160. Diabetes 2007, 56, 2093–2102. [Google Scholar] [CrossRef]
- Christ-Roberts, C.Y.; Pratipanawatr, T.; Pratipanawatr, W.; Berria, R.; Belfort, R.; Kashyap, S.; Mandarino, L.J. Exercise training increases glycogen synthase activity and GLUT4 expression but not insulin signaling in overweight nondiabetic and type 2 diabetic subjects. Metabolism 2004, 53, 1233–1242. [Google Scholar] [CrossRef]
- Dela, F.; Larsen, J.J.; Mikines, K.J.; Ploug, T.; Petersen, L.N.; Galbo, H. Insulin-stimulated muscle glucose clearance in patients with NIDDM. Effects of one-legged physical training. Diabetes 1995, 44, 1010–1020. [Google Scholar] [CrossRef]
- Zierath, J.R. Invited review: Exercise training-induced changes in insulin signaling in skeletal muscle. J. Appl. Physiol. 2002, 93, 773–781. [Google Scholar] [CrossRef] [Green Version]
- Niu, Y.M.; Yuan, H.; Zhang, N.; Fu, L. The relationship between mTor/S6K1 signaling pathway and insulin resistance and the study of aerobic exercise on this pathway. Zhongguo Ying Yong Sheng Li Xue Za Zhi 2010, 26, 399–403. [Google Scholar]
- Glynn, E.L.; Lujan, H.L.; Kramer, V.J.; Drummond, M.J.; DiCarlo, S.E.; Rasmussen, B.B. A chronic increase in physical activity inhibits fed-state mTOR/S6K1 signaling and reduces IRS-1 serine phosphorylation in rat skeletal muscle. Appl. Physiol. Nutr. Metab. 2008, 33, 93–101. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Hiam, D.; Hong, Y.H.; Zulli, A.; Hayes, A.; Rattigan, S.; McConell, G.K. Nitric oxide is required for the insulin sensitizing effects of contraction in mouse skeletal muscle. J. Physiol. 2017, 595, 7427–7439. [Google Scholar] [CrossRef]
- Fan, G.C.; Kranias, E.G. Small heat shock protein 20 (HspB6) in cardiac hypertrophy and failure. J. Mol. Cell. Cardiol. 2011, 51, 574–577. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, G.C.; Zhou, X.; Wang, X.; Song, G.; Qian, J.; Nicolaou, P.; Chen, G.; Ren, X.; Kranias, E.G. Heat shock protein 20 interacting with phosphorylated Akt reduces doxorubicin-triggered oxidative stress and cardiotoxicity. Circ. Res. 2008, 103, 1270–1279. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, M.; Liu, X.; Bao, P.; Wang, Y.; Zhu, X.; Liu, Y.; Ni, X.; Lu, J. Skeletal Muscle CSE Deficiency Leads to Insulin Resistance in Mice. Antioxidants 2022, 11, 2216. https://doi.org/10.3390/antiox11112216
Xu M, Liu X, Bao P, Wang Y, Zhu X, Liu Y, Ni X, Lu J. Skeletal Muscle CSE Deficiency Leads to Insulin Resistance in Mice. Antioxidants. 2022; 11(11):2216. https://doi.org/10.3390/antiox11112216
Chicago/Turabian StyleXu, Miaomiao, Xiaoguang Liu, Peng Bao, Yanjie Wang, Xiaoyan Zhu, Yujian Liu, Xin Ni, and Jianqiang Lu. 2022. "Skeletal Muscle CSE Deficiency Leads to Insulin Resistance in Mice" Antioxidants 11, no. 11: 2216. https://doi.org/10.3390/antiox11112216
APA StyleXu, M., Liu, X., Bao, P., Wang, Y., Zhu, X., Liu, Y., Ni, X., & Lu, J. (2022). Skeletal Muscle CSE Deficiency Leads to Insulin Resistance in Mice. Antioxidants, 11(11), 2216. https://doi.org/10.3390/antiox11112216







