Impacts of Fortifying Nile Tilapia (Oreochromis niloticus) Diet with Different Strains of Microalgae on Its Performance, Fillet Quality and Disease Resistance to Aeromonas hydrophila Considering the Interplay between Antioxidant and Inflammatory Response
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethical Approval
2.2. Fish Maintenance
2.3. Microalgae and Diets Formulation
2.4. Experimental Design
2.5. Growth Performance
2.6. Blood and Tissue Sampling
2.7. Digestive and Liver Enzymes’ Activities
2.8. Fatty Acid Profile and Oxidative/Antioxidant Status in Serum and Muscle Tissues
2.9. Assessment of Serum Immune-Mediated Biomarkers
2.10. Gene Expression Analysis
2.11. Real-Time PCR for Quantitative Detection of Fish Bacterial Species
2.12. Challenge Test
2.13. Statistical Analysis
3. Results
3.1. Effect of NSS on Fish Growth Performance
3.2. Effect of NSS on Digestive and Liver Enzymes
3.3. Effect of NSS on Hematological, Immunological, and Antioxidant Status of Fish
3.4. Effect of NSS on Oxidative/Antioxidant Status and Fatty Acid Profile in Muscle Tissues
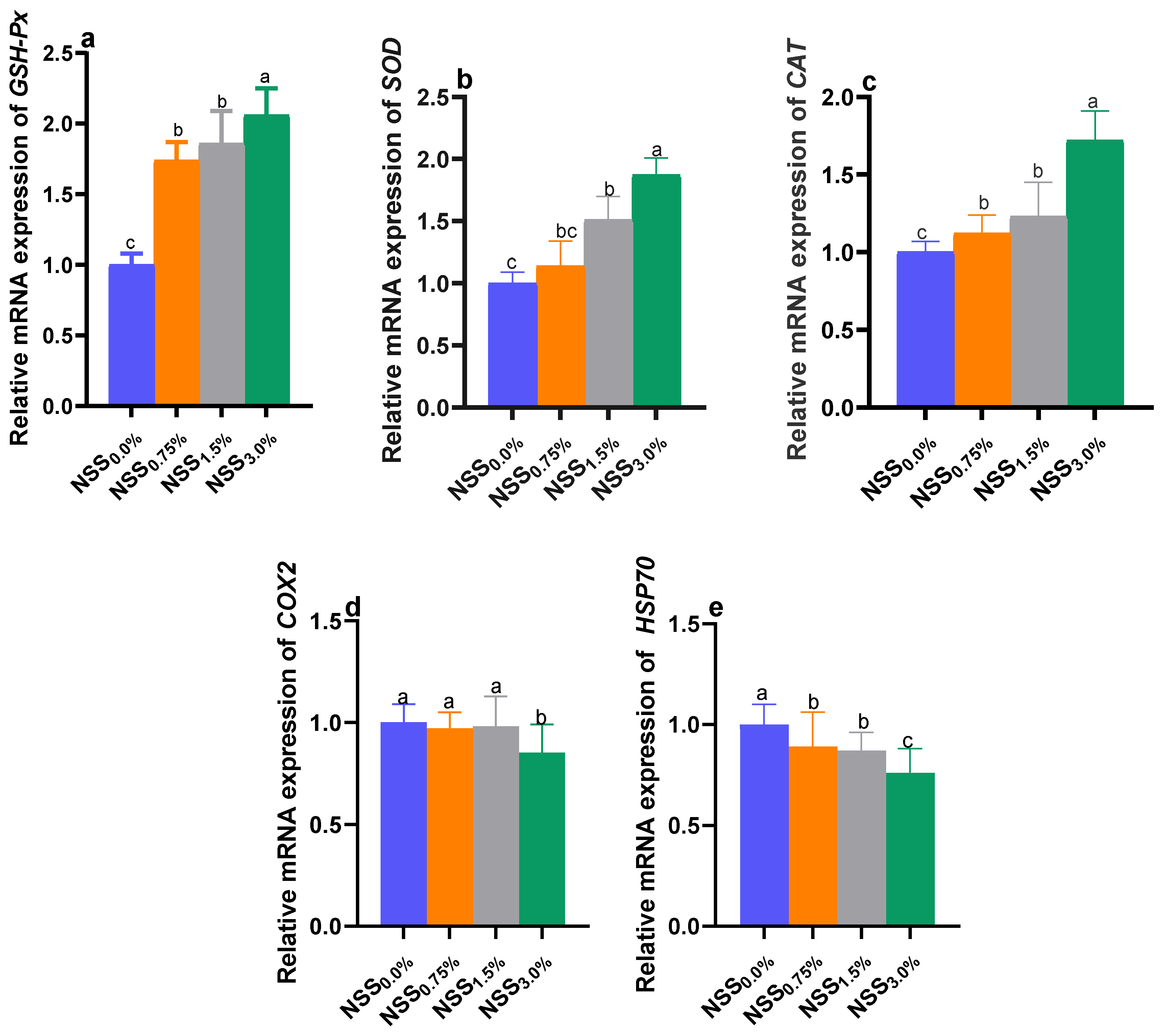
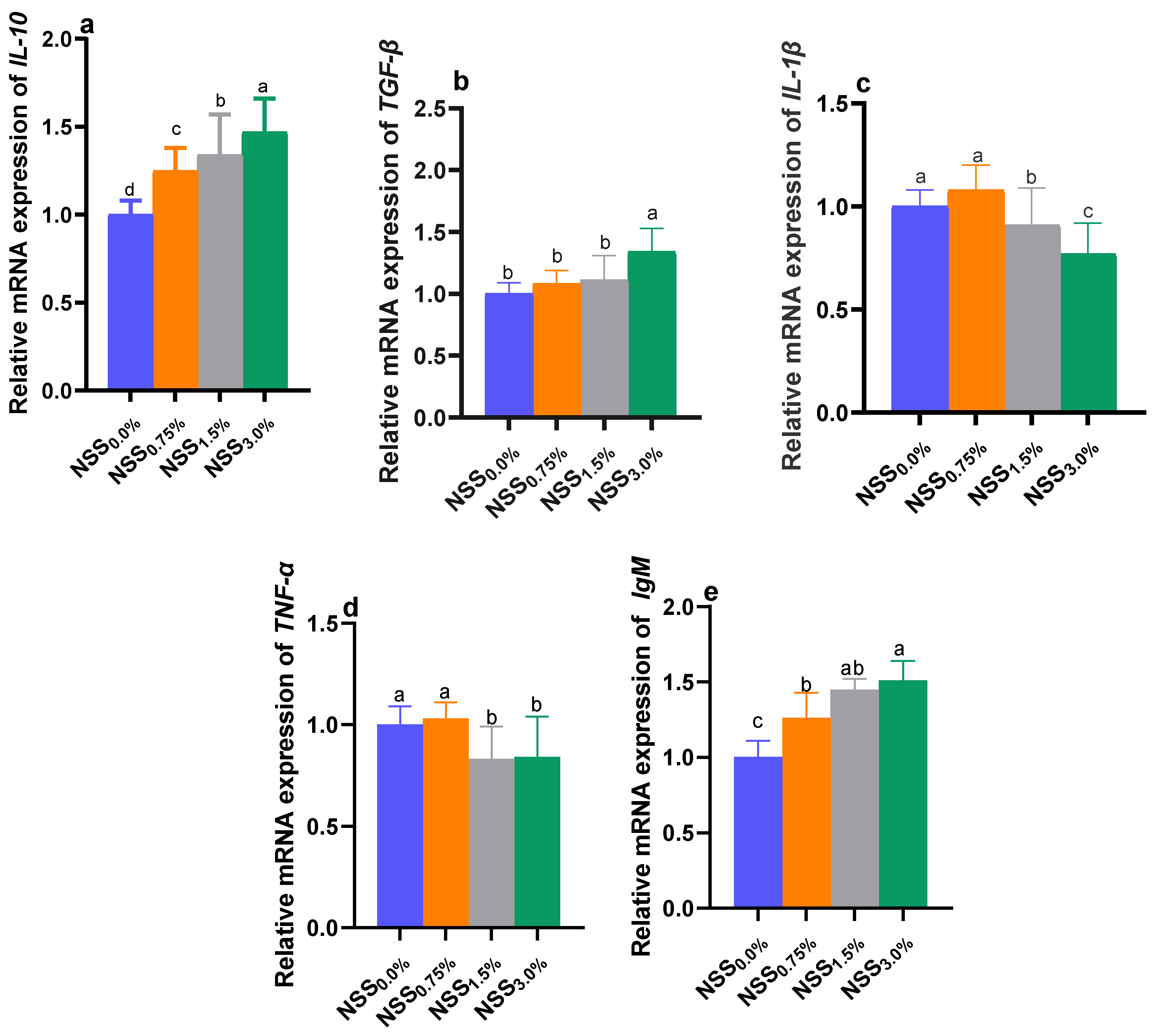
3.5. Modulation of Genes Expression by NSS
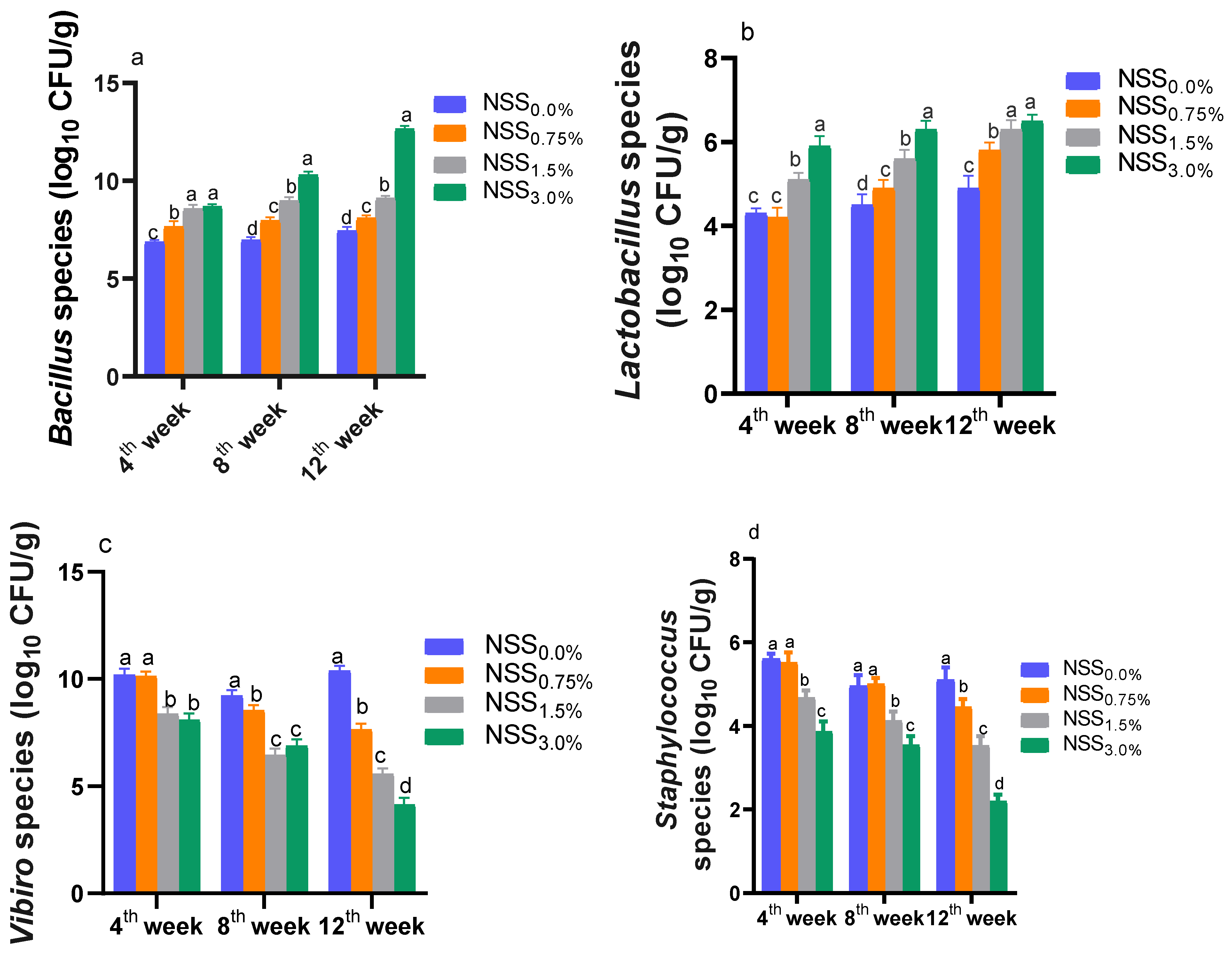
3.6. Effect of NNS on Some Intestinal Microbiota
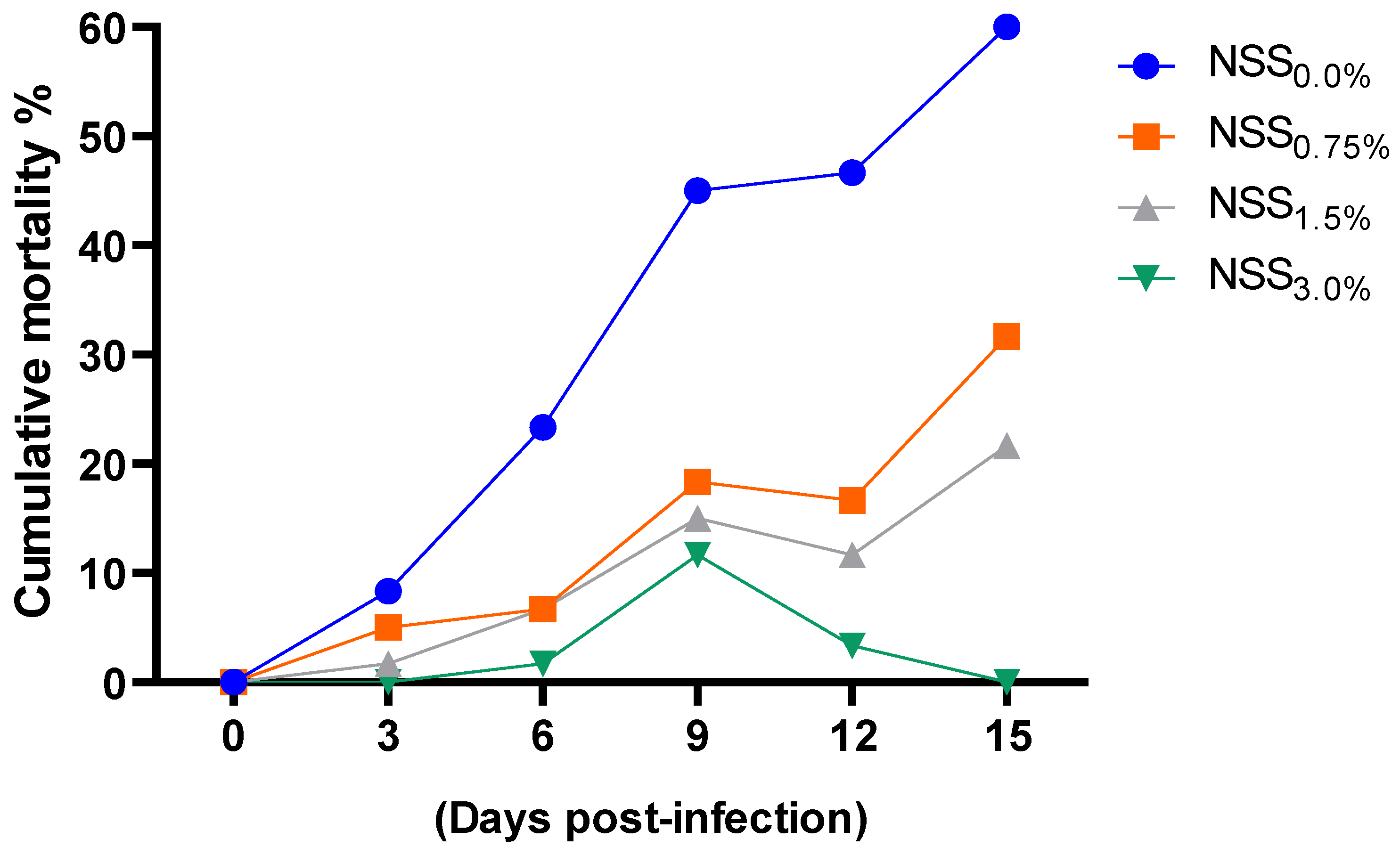
3.7. Effect of NNS on Aeromonas Hydrophila Population and Cumulative Mortality Rates
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lushchak, V.I. Environmentally induced oxidative stress in aquatic animals. Aquat. Toxicol. 2011, 101, 13–30. [Google Scholar] [CrossRef] [PubMed]
- Birnie-Gauvin, K.; Costantini, D.; Cooke, S.J.; Willmore, W.G. A comparative and evolutionary approach to oxidative stress in fish: A review. Fish Fish. 2017, 18, 928–942. [Google Scholar] [CrossRef]
- Nordberg, J.; Arnér, E.S. Reactive oxygen species, antioxidants, and the mammalian thioredoxin system. Free Radic. Biol. Med. 2001, 31, 1287–1312. [Google Scholar] [CrossRef]
- Hussain, T.; Tan, B.; Yin, Y.; Blachier, F.; Tossou, M.C.; Rahu, N. Oxidative stress and inflammation: What polyphenols can do for us? Oxid. Med. Cell. Longev. 2016, 2016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ibrahim, D.; Kishawy, A.T.; Khater, S.I.; Khalifa, E.; Ismail, T.A.; Mohammed, H.A.; Elnahriry, S.S.; Tolba, H.A.; Sherief, W.R.; Farag, M.F. Interactive effects of dietary quercetin nanoparticles on growth, flesh antioxidant capacity and transcription of cytokines and Aeromonas hydrophila quorum sensing orchestrating genes in Nile tilapia (Oreochromis niloticus). Fish Shellfish Immunol. 2021. [Google Scholar] [CrossRef]
- Mittal, M.; Siddiqui, M.R.; Tran, K.; Reddy, S.P.; Malik, A.B. Reactive oxygen species in inflammation and tissue injury. Antioxid. Redox Signal. 2014, 20, 1126–1167. [Google Scholar] [CrossRef] [Green Version]
- Ranneh, Y.; Ali, F.; Akim, A.M.; Hamid, H.A.; Khazaai, H.; Fadel, A. Crosstalk between reactive oxygen species and pro-inflammatory markers in developing various chronic diseases: A review. Appl. Biol. Chem. 2017, 60, 327–338. [Google Scholar] [CrossRef]
- Abd El-Hamid, M.I.; Ibrahim, S.M.; Eldemery, F.; El-Mandrawy, S.A.; Metwally, A.S.; Khalifa, E.; Elnahriry, S.S.; Ibrahim, D. Dietary cinnamaldehyde nanoemulsion boosts growth and transcriptomes of antioxidant and immune related genes to fight Streptococcus agalactiae infection in Nile tilapia (Oreochromis niloticus). Fish Shellfish Immunol. 2021, 113, 96–105. [Google Scholar] [CrossRef]
- Ibrahim, D.; Arisha, A.H.; Khater, S.I.; Gad, W.M.; Hassan, Z.; Abou-Khadra, S.H.; Mohamed, D.I.; Ahmed Ismail, T.; Gad, S.A.; Eid, S.A. Impact of Omega-3 Fatty Acids Nano-Formulation on Growth, Antioxidant Potential, Fillet Quality, Immunity, Autophagy-Related Genes and Aeromonas hydrophila Resistance in Nile Tilapia (Oreochromis niloticus). Antioxidants 2022, 11, 1523. [Google Scholar] [CrossRef]
- Abdel-Latif, H.M.; El-Ashram, S.; Yilmaz, S.; Naiel, M.A.; Kari, Z.A.; Hamid, N.K.A.; Dawood, M.A.; Nowosad, J.; Kucharczyk, D. The effectiveness of Arthrospira platensis and microalgae in relieving stressful conditions affecting finfish and shellfish species: An overview. Aquac. Rep. 2022, 24, 101135. [Google Scholar] [CrossRef]
- Sarker, P.K.; Kapuscinski, A.R.; Lanois, A.J.; Livesey, E.D.; Bernhard, K.P.; Coley, M.L. Towards sustainable aquafeeds: Complete substitution of fish oil with marine microalga Schizochytrium sp. improves growth and fatty acid deposition in juvenile Nile tilapia (Oreochromis niloticus). PLoS One 2016, 11, e0156684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shah, M.R.; Lutzu, G.A.; Alam, A.; Sarker, P.; Chowdhury, K.; Parsaeimehr, A.; Liang, Y.; Daroch, M. Microalgae in aquafeeds for a sustainable aquaculture industry. J. Appl. Phycol. 2018, 30, 197–213. [Google Scholar] [CrossRef]
- El-Sayed, H.S.; Ibrahim, H.A.; Beltagy, E.A.; Khairy, H.M. Effects of short term feeding of some marine microalgae on the microbial profile associated with Dicentrarchus labrax post larvae. Egypt. J. Aquat. Res. 2014, 40, 251–260. [Google Scholar] [CrossRef] [Green Version]
- Austin, B.; Baudet, E.; Stobie, M. Inhibition of bacterial fish pathogens by Tetraselmis suecica. J. Fish Dis. 1992, 15, 55–61. [Google Scholar] [CrossRef]
- Vivas, J.; Carracedo, B.; Riano, J.; Razquin, B.E.; López-Fierro, P.; Acosta, F.; Naharro, G.; Villena, A.J. Behavior of an Aeromonas hydrophila aroA live vaccine in water microcosms. Appl. Environ. Microbiol. 2004, 70, 2702–2708. [Google Scholar] [CrossRef] [Green Version]
- Ardó, L.; Jeney, Z.; Adams, A.; Jeney, G. Immune responses of resistant and sensitive common carp families following experimental challenge with Aeromonas hydrophila. Fish Shellfish Immunol. 2010, 29, 111–116. [Google Scholar] [CrossRef]
- Ammar, A.M.; El-Naenaeey, E.-S.Y.; Abd El-Hamid, M.I.; El-Gedawy, A.A.; Elmalt, R.M. Campylobacter as a Major Foodborne Pathogen: A Review of Its Characteristics, Pathogenesis, Antimicrobial Resistance and Control. J. Microbiol. Biotechnol. Food Sci. 2021, 10, 609–619. [Google Scholar] [CrossRef]
- Ammar, A.M.; El-Naenaeey, E.-S.Y.; El-Malt, R.; El-Gedawy, A.A.; Khalifa, E.; Elnahriry, S.S.; El-Hamid, A.; Marwa, I. Prevalence, antimicrobial susceptibility, virulence and genotyping of Campylobacter jejuni with a special reference to the anti-virulence potential of Eugenol and beta-resorcylic acid on some multi-drug resistant isolates in Egypt. Animals 2021, 11, 3. [Google Scholar] [CrossRef]
- Ammar, A.; El-Hamid, M.; Eid, S.E.; El Oksh, A.S. Insight into antimicrobial resistance and virulence genes of emergent multidrug resistant avian pathogenic Escherichia coli in Egypt: How closely related are they. Rev. Med. Vet. 2015, 166, 304–314. [Google Scholar]
- Habte-Tsion, H.-M.; Kolimadu, G.D.; Rossi, W.; Filer, K.; Kumar, V. Effects of Schizochytrium and micro-minerals on immune, antioxidant, inflammatory and lipid-metabolism status of Micropterus salmoides fed high-and low-fishmeal diets. Sci. Rep. 2020, 10, 1–13. [Google Scholar] [CrossRef]
- Bélanger, A.; Sarker, P.K.; Bureau, D.P.; Chouinard, Y.; Vandenberg, G.W. Apparent digestibility of macronutrients and fatty acids from microalgae (Schizochytrium sp.) fed to rainbow trout (Oncorhynchus mykiss): A potential candidate for fish oil substitution. Animals 2021, 11, 456. [Google Scholar] [CrossRef] [PubMed]
- Cardona, E.; Segret, E.; Cachelou, Y.; Vanderesse, T.; Larroquet, L.; Hermann, A.; Surget, A.; Corraze, G.; Cachelou, F.; Bobe, J. Effect of micro-algae Schizochytrium sp. supplementation in plant diet on reproduction of female rainbow trout (Oncorhynchus mykiss): Maternal programming impact of progeny. J. Anim. Sci. Biotechnol. 2022, 13, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Priyadarshani, I.; Rath, B. Commercial and industrial applications of micro algae—A review. J. Algal Biomass Util. 2012, 3, 89–100. [Google Scholar]
- Zhang, F.; Man, Y.B.; Mo, W.Y.; Wong, M.H. Application of Spirulina in aquaculture: A review on wastewater treatment and fish growth. Rev. Aquac. 2020, 12, 582–599. [Google Scholar] [CrossRef]
- Wu, Q.; Liu, L.; Miron, A.; Klímová, B.; Wan, D.; Kuča, K. The antioxidant, immunomodulatory, and anti-inflammatory activities of Spirulina: An overview. Arch. Toxicol. 2016, 90, 1817–1840. [Google Scholar] [CrossRef]
- Sharifah, E.N.; Eguchi, M. The phytoplankton Nannochloropsis oculata enhances the ability of Roseobacter clade bacteria to inhibit the growth of fish pathogen Vibrio anguillarum. PLoS ONE 2011, 6, e26756. [Google Scholar] [CrossRef] [Green Version]
- Mounes, H.A.M.; Mansour, E.G.; Ahmed, K.M. Effect of Azolla pinnata and Nannochloropsis oculata on growth performance and immunoresponse of Nile tilapia (Oreochromis niloticus) and its resistance to bacterial infection. Egypt. J. Aquac. 2020, 10, 43–62. [Google Scholar] [CrossRef]
- Md, A.; Jin, F.; Jeong, U.-C.; Choi, J.-K.; Lee, D.-I.; Yu, H.S.; Kang, S.-J. Effects of Nannochloropsis concentration in diet on growth, survival and anti-inflammatory cytokine (Interleukin-10) production of the sea cucumber Apostichopus japonicus. Turkish J. Fish. Aquat. Sci. 2018, 18, 567–575. [Google Scholar]
- Abdelghany, M.F.; El-Sawy, H.B.; Abd El-Hameed, S.A.; Khames, M.K.; Abdel-Latif, H.M.; Naiel, M.A. Effects of dietary Nannochloropsis oculata on growth performance, serum biochemical parameters, immune responses, and resistance against Aeromonas veronii challenge in Nile tilapia (Oreochromis niloticus). Fish Shellfish Immunol. 2020, 107, 277–288. [Google Scholar] [CrossRef]
- Zahran, E.; Elbahnaswy, S.; Ibrahim, I.; Khaled, A.A. Nannochloropsis oculata enhances immune response, transcription of stress, and cytokine genes in Nile tilapia subjected to air exposure stress. Aquac. Rep. 2021, 21, 100911. [Google Scholar] [CrossRef]
- Ibrahim, D.; Nem, A.N.A.; Ibrahim, S.M.; Eissa, H.M.; Fawzey, M.; Mostafa, D.I.; Abd El-Kader, S.A.; Khater, S.; Khater, S.I. Dual effect of Selenium loaded Chitosan Nanoparticles on growth, antioxidant, immune related genes expression, transcriptomics modulation of caspase 1, cytochrome P450 and heat shock protein and Aeromonas hydrophila resistance of Nile Tilapia (Oreochromis niloticus). Fish Shellfish Immunol. 2021. [Google Scholar]
- Kishawy, A.T.; Mohammed, H.A.; Zaglool, A.W.; Attia, M.S.; Hassan, F.A.; Roushdy, E.M.; Ismail, T.A.; Ibrahim, D. Partial defatted black solider larvae meal as a promising strategy to replace fish meal protein in diet for Nile tilapia (Oreochromis niloticus): Performance, expression of protein and fat transporters, and cytokines related genes and economic efficiency. Aquaculture 2022, 555, 738195. [Google Scholar] [CrossRef]
- Blaxhall, P.; Daisley, K. Routine haematological methods for use with fish blood. J. Fish Biol. 1973, 5, 771–781. [Google Scholar] [CrossRef]
- Jenkins, J.A.; Bart Jr, H.; Bowker, J.D.; Bowser, P.; MacMillan, J.; Nickum, J.; Rose, J.; Sorensen, P.; Whitledge, G.; Rachlin, J.W. Guidelines for the Use of Fishes in Research. American Fisheries Society: Bethesda, MD, USA, 2014. [Google Scholar]
- Reitman, S.; Frankel, S. A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am. J. Clin. Pathol. 1957, 28, 56–63. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in vitro. In Methods Enzymol; Elsevier: Amsterdam, The Netherlands, 1984; Volume 105, pp. 121–126. [Google Scholar]
- LeBel, C.P.; Ischiropoulos, H.; Bondy, S.C. Evaluation of the probe 2’,7’-dichlorofluorescin as an indicator of reactive oxygen species formation and oxidative stress. Chem. Res. Toxicol. 1992, 5, 227–231. [Google Scholar] [CrossRef] [Green Version]
- Loreto, F.; Velikova, V. Isoprene produced by leaves protects the photosynthetic apparatus against ozone damage, quenches ozone products, and reduces lipid peroxidation of cellular membranes. Plant Physiol. 2001, 127, 1781–1787. [Google Scholar] [CrossRef]
- Livingstone, D.; Martinez, P.G.; Michel, X.; Narbonne, J.; O’hara, S.; Ribera, D.; Winston, G. Oxyradical production as a pollution-mediated mechanism of toxicity in the common mussel, Mytilus edulis L., and other molluscs. Funct. Ecol. 1990, 415–424. [Google Scholar] [CrossRef]
- Shah, S.; Pal, A.; Kaushik, V.; Devi, S. Preparation and characterization of venlafaxine hydrochloride-loaded chitosan nanoparticles and in vitro release of drug. J. Appl. Polym. Sci. 2009, 112, 2876–2887. [Google Scholar] [CrossRef]
- Bryan, N.S.; Grisham, M.B. Methods to detect nitric oxide and its metabolites in biological samples. Free Radic. Biol. Med. 2007, 43, 645–657. [Google Scholar] [CrossRef] [Green Version]
- Grisham, M.B.; Johnson, G.G.; Lancaster, J.R., Jr. Quantitation of nitrate and nitrite in extracellular fluids. In Methods Enzymol; Elsevier: Amsterdam, The Netherlands, 1996; Volume 268, pp. 237–246. [Google Scholar]
- Suzuki, K.; Ota, H.; Sasagawa, S.; Sakatani, T.; Fujikura, T. Assay method for myeloperoxidase in human polymorphonuclear leukocytes. Anal. Biochem. 1983, 132, 345–352. [Google Scholar] [CrossRef]
- Sunyer, J.O.; Tort, L. Natural hemolytic and bactericidal activities of sea bream Chlorella vulgaris serum are effected by the alternative complement pathway. Vet. Immunol. Immunopathol. 1995, 45, 333–345. [Google Scholar] [CrossRef]
- Chen, X.-M.; Guo, G.-L.; Sun, L.; Yang, Q.-S.; Wang, G.-Q.; Qin, G.-X.; Zhang, D.-M. Effects of Ala-Gln feeding strategies on growth, metabolism, and crowding stress resistance of juvenile Cyprinus carpio var. Jian. Fish Shellfish Immunol. 2016, 51, 365–372. [Google Scholar] [CrossRef]
- Drieghe, S.A.; Alsaadi, H.; Tugirimana, P.L.; Delanghe, J.R. A new high-sensitive nephelometric method for assaying serum C-reactive protein based on phosphocholine interaction. Clin. Chem. Lab. Med. (CCLM) 2014, 52, 861–867. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- McOrist, A.L.; Jackson, M.; Bird, A.R. A comparison of five methods for extraction of bacterial DNA from human faecal samples. J. Microbiol. Methods 2002, 50, 131–139. [Google Scholar] [CrossRef]
- Solichová, K.; Němečková, I.; Šviráková, E.; Horáčková, Š. Novel identification methods including a species-specific PCR for hazardous Bacillus species. Acta Aliment. 2019, 48, 415–422. [Google Scholar] [CrossRef] [Green Version]
- Zhang, K.; Sparling, J.; Chow, B.L.; Elsayed, S.; Hussain, Z.; Church, D.L.; Gregson, D.B.; Louie, T.; Conly, J.M. New quadriplex PCR assay for detection of methicillin and mupirocin resistance and simultaneous discrimination of Staphylococcus aureus from coagulase-negative staphylococci. J. Clin. Microbiol. 2004, 42, 4947–4955. [Google Scholar] [CrossRef] [Green Version]
- Thompson, J.R.; Randa, M.A.; Marcelino, L.A.; Tomita-Mitchell, A.; Lim, E.; Polz, M.F. Diversity and dynamics of a North Atlantic coastal Vibrio community. Appl. Environ. Microbiol. 2004, 70, 4103–4110. [Google Scholar] [CrossRef] [Green Version]
- Sebastião, F.; Lemos, E.M.; Pilarski, F. Development of an absolute quantitative real-time PCR (qPCR) for the diagnosis of Aeromonas hydrophila infections in fish. Acta Sci. Microbiol. 2018, 1, 23–29. [Google Scholar] [CrossRef]
- Algammal, A.M.; Mohamed, M.F.; Tawfiek, B.A.; Hozzein, W.N.; El Kazzaz, W.M.; Mabrok, M. Molecular typing, antibiogram and PCR-RFLP based detection of Aeromonas hydrophila complex isolated from Oreochromis niloticus. Pathogens 2020, 9, 238. [Google Scholar] [CrossRef] [Green Version]
- El-Gohary, F.A.; Zahran, E.; Abd El-Gawad, E.A.; El-Gohary, A.H.M.; Abdelhamid, F.; El-Mleeh, A.; Elmahallawy, E.K.; Elsayed, M.M. Investigation of the prevalence, virulence genes, and antibiogram of motile Aeromonads isolated from Nile tilapia fish farms in Egypt and assessment of their water quality. Animals 2020, 10, 1432. [Google Scholar] [CrossRef] [PubMed]
- Phumkhachorn, P.; Rattanachaikunsopon, P. Use of Bacteriophage to Control Experimental Aeromonas hydrophila Infection in Tilapia (Oreochromis niloticus). Pak. J. Biol. Sci. PJBS 2020, 23, 1659–1665. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Hamid, M.; Abd El-Aziz, N.; Ali, H. Protective potency of clove oil and its transcriptional down-regulation of Aeromonas sobria virulence genes in African catfish (Clarias gariepinus L.). Cell. Mol. Biol. 2016, 62, 49–54. [Google Scholar] [PubMed]
- Nakharuthai, C.; Rodrigues, P.M.; Schrama, D.; Kumkhong, S.; Boonanuntanasarn, S. Effects of different dietary vegetable lipid sources on health status in Nile tilapia (Oreochromis niloticus): Haematological indices, immune response parameters and plasma proteome. Animals 2020, 10, 1377. [Google Scholar] [CrossRef] [PubMed]
- Kris-Etherton, P.M.; Harris, W.S.; Appel, L.J. Fish consumption, fish oil, omega-3 fatty acids, and cardiovascular disease. Circulation 2002, 106, 2747–2757. [Google Scholar] [CrossRef] [PubMed]
- Pulz, O.; Gross, W. Valuable products from biotechnology of microalgae. Appl. Microbiol. Biotechnol. 2004, 65, 635–648. [Google Scholar] [CrossRef]
- Wells, M.L.; Potin, P.; Craigie, J.S.; Raven, J.A.; Merchant, S.S.; Helliwell, K.E.; Smith, A.G.; Camire, M.E.; Brawley, S.H. Algae as nutritional and functional food sources: Revisiting our understanding. J. Appl. Phycol. 2017, 29, 949–982. [Google Scholar] [CrossRef]
- Olofsson, M.; Lamela, T.; Nilsson, E.; Bergé, J.-P.; Del Pino, V.; Uronen, P.; Legrand, C. Combined effects of nitrogen concentration and seasonal changes on the production of lipids in Nannochloropsis oculata. Mar. Drugs 2014, 12, 1891–1910. [Google Scholar] [CrossRef] [Green Version]
- Peet, M.; Stokes, C. Omega-3 fatty acids in the treatment of psychiatric disorders. Drugs 2005, 65, 1051–1059. [Google Scholar] [CrossRef]
- Brasky, T.M.; Till, C.; White, E.; Neuhouser, M.L.; Song, X.; Goodman, P.; Thompson, I.M.; King, I.B.; Albanes, D.; Kristal, A.R. Serum phospholipid fatty acids and prostate cancer risk: Results from the prostate cancer prevention trial. Am. J. Epidemiol. 2011, 173, 1429–1439. [Google Scholar] [CrossRef] [Green Version]
- Pascon, G.; Messina, M.; Petit, L.; Valente, L.M.P.; Oliveira, B.; Przybyla, C.; Dutto, G.; Tulli, F. Potential application and beneficial effects of a marine microalgal biomass produced in a high-rate algal pond (HRAP) in diets of European sea bass, Dicentrarchus labrax. Environ. Sci. Pollut. Res. 2021, 28, 62185–62199. [Google Scholar] [CrossRef] [PubMed]
- Sarker, P.K.; Kapuscinski, A.R.; McKuin, B.; Fitzgerald, D.S.; Nash, H.M.; Greenwood, C. Microalgae-blend tilapia feed eliminates fishmeal and fish oil, improves growth, and is cost viable. Sci. Rep. 2020, 10, 1–14. [Google Scholar]
- Elmowalid, G.A.E.; Ahmad, A.A.M.; El-Hamid, M.I.A.; Ibrahim, D.; Wahdan, A.; El Oksh, A.S.; Yonis, A.E.; Elkady, M.A.; Ismail, T.A.; Alkhedaide, A.Q. Nigella sativa Extract Potentially Inhibited Methicillin Resistant Staphylococcus aureus Induced Infection in Rabbits: Potential Immunomodulatory and Growth Promoting Properties. Animals 2022, 12, 2635. [Google Scholar] [CrossRef]
- Hashem, Y.M.; Abd El-Hamid, M.I.; Awad, N.F.; Ibrahim, D.; Elshater, N.S.; El-Malt, R.M.; Hassan, W.H.; Abo-Shama, U.H.; Nassan, M.A.; El-Bahy, S.M. Insights into growth-promoting, anti-inflammatory, immunostimulant, and antibacterial activities of Toldin CRD as a novel phytobiotic in broiler chickens experimentally infected with Mycoplasma gallisepticum. Poult. Sci. 2022, 101, 102154. [Google Scholar] [CrossRef]
- Ibrahim, D.; Eldemery, F.; Metwally, A.S.; Abd-Allah, E.M.; Mohamed, D.T.; Ismail, T.A.; Hamed, T.A.; Al Sadik, G.M.; Neamat-Allah, A.N.; Abd El-Hamid, M.I. Dietary eugenol nanoemulsion potentiated performance of broiler chickens: Orchestration of digestive enzymes, intestinal barrier functions and cytokines related gene expression with a consequence of attenuating the severity of E. coli O78 infection. Front. Vet. Sci. 2022, 9. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, D.; Ismail, T.A.; Khalifa, E.; El-Kader, A.; Shaimaa, A.; Mohamed, D.I.; Mohamed, D.T.; Shahin, S.E.; El-Hamid, A.; Marwa, I. Supplementing Garlic Nanohydrogel Optimized Growth, Gastrointestinal Integrity and Economics and Ameliorated Necrotic Enteritis in Broiler Chickens Using a Clostridium perfringens Challenge Model. Animals 2021, 11, 2027. [Google Scholar] [CrossRef]
- Wei, H.-K.; Deng, Z.; Jiang, S.-Z.; Song, T.-X.; Zhou, Y.-F.; Peng, J.; Tao, Y.-X. Eicosapentaenoic acid abolishes inhibition of insulin-induced mTOR phosphorylation by LPS via PTP1B downregulation in skeletal muscle. Mol. Cell. Endocrinol. 2017, 439, 116–125. [Google Scholar] [CrossRef]
- Gingras, A.A.; White, P.J.; Chouinard, P.Y.; Julien, P.; Davis, T.A.; Dombrowski, L.; Couture, Y.; Dubreuil, P.; Myre, A.; Bergeron, K. Long-chain omega-3 fatty acids regulate bovine whole-body protein metabolism by promoting muscle insulin signalling to the Akt–mTOR–S6K1 pathway and insulin sensitivity. J. Physiol. 2007, 579, 269–284. [Google Scholar] [CrossRef]
- Stoneham, T.R.; Kuhn, D.D.; Taylor, D.P.; Neilson, A.P.; Smith, S.A.; Gatlin, D.M.; Chu, H.S.S.; O’Keefe, S.F. Production of omega-3 enriched tilapia through the dietary use of algae meal or fish oil: Improved nutrient value of fillet and offal. PLoS ONE 2018, 13, e0194241. [Google Scholar]
- Burdge, G. Metabolism of α-linolenic acid in humans. Prostaglandins Leukot. Essent. Fat. Acids 2006, 75, 161–168. [Google Scholar] [CrossRef]
- Sinclair, A.J.; Attar-Bashi, N.M.; Li, D. What is the role of α-linolenic acid for mammals? Lipids 2002, 37, 1113–1123. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, S.K.A.; Schorer, M.; Moura, G.d.S.; Lanna, E.A.T.; Pedreira, M.M. Evaluation of growth and fatty acid profile of Nile tilapia (Oreochromis niloticus) fed with Schizochytrium sp. Aquac. Res. 2019, 50, 1068–1074. [Google Scholar] [CrossRef]
- Dayras, P.; Bialais, C.; Sadovskaya, I.; Lee, M.-C.; Lee, J.-S.; Souissi, S. Microalgal Diet Influences the Nutritive Quality and Reproductive Investment of the Cyclopoid Copepod Paracyclopina nana. Front. Mar. Sci. 2021, 1147. [Google Scholar] [CrossRef]
- Eryalçın, K.M.; Yıldız, M. Effects of long-term feeding with dried microalgae added microdiets on growth and fatty acid composition of gilthead sea bream (Chlorella vulgaris L., 1758). Turkish J. Fish. Aquat. Sci. 2015, 15, 905–915. [Google Scholar]
- Mozanzadeh, M.T.; Marammazi, J.G.; Yavari, V.; Agh, N.; Mohammadian, T.; Gisbert, E. Dietary n− 3 LC-PUFA requirements in silvery-black porgy juveniles (Sparidentex hasta). Aquaculture 2015, 448, 151–161. [Google Scholar] [CrossRef]
- Peng, M.; Xu, W.; Tan, P.; Du, J.; Mai, K.; Zhou, H.; Zhang, Y.; Nian, R.; Macq, B.; Ai, Q. Effect of dietary fatty acid composition on growth, fatty acids composition and hepatic lipid metabolism in juvenile turbot (Scophthalmus maximus L.) fed diets with required n3 LC-PUFAs. Aquaculture 2017, 479, 591–600. [Google Scholar] [CrossRef]
- Kumar, N.; Chandan, N.K.; Gupta, S.K.; Bhushan, S.; Patole, P.B. Omega-3 fatty acids effectively modulate growth performance, immune response, and disease resistance in fish against multiple stresses. Aquaculture 2022, 547, 737506. [Google Scholar] [CrossRef]
- Wilhelm Filho, D.; Tribess, T.; Gaspari, C.; Claudio, F.; Torres, M.; Magalhaes, A. Seasonal changes in antioxidant defenses of the digestive gland of the brown mussel (Perna perna). Aquaculture 2001, 203, 149–158. [Google Scholar] [CrossRef]
- Winston, G.W.; Di Giulio, R.T. Prooxidant and antioxidant mechanisms in aquatic organisms. Aquat. Toxicol. 1991, 19, 137–161. [Google Scholar] [CrossRef]
- Vickers, N.J. Animal communication: When i’m calling you, will you answer too? Curr. Biol. 2017, 27, R713–R715. [Google Scholar] [CrossRef]
- Martínez-Álvarez, R.M.; Morales, A.E.; Sanz, A. Antioxidant defenses in fish: Biotic and abiotic factors. Rev. Fish Biol. Fish. 2005, 15, 75–88. [Google Scholar] [CrossRef]
- Mahfouz, R.; Sharma, R.; Sharma, D.; Sabanegh, E.; Agarwal, A. Diagnostic value of the total antioxidant capacity (TAC) in human seminal plasma. Fertil. Steril. 2009, 91, 805–811. [Google Scholar] [CrossRef] [PubMed]
- Qiao, H.; Hu, D.; Ma, J.; Wang, X.; Wu, H.; Wang, J. Feeding effects of the microalga Nannochloropsis sp. on juvenile turbot (Scophthalmus maximus L.). Algal Res. 2019, 41, 101540. [Google Scholar] [CrossRef]
- Castro, C.; Coutinho, F.; Iglesias, P.; Oliva-Teles, A.; Couto, A. Chlorella sp. and Nannochloropsis sp. inclusion in plant-based diets modulate the intestine and liver antioxidant mechanisms of European sea bass juveniles. Front. Vet. Sci. 2020, 7, 607575. [Google Scholar] [CrossRef]
- Wu, K.; Cleveland, B.M.; Portman, M.; Sealey, W.M.; Lei, X.G. Supplemental microalgal DHA and astaxanthin affect astaxanthin metabolism and redox status of Juvenile Rainbow trout. Antioxidants 2020, 10, 16. [Google Scholar] [CrossRef]
- Palmegiano, G.B.; Gai, F.; Daprà, F.; Gasco, L.; Pazzaglia, M.; Peiretti, P.G. Effects of Spirulina and plant oil on the growth and lipid traits of white sturgeon (Acipenser transmontanus) fingerlings. Aquac. Res. 2008, 39, 587–595. [Google Scholar] [CrossRef]
- Gaweł, S.; Wardas, M.; Niedworok, E.; Wardas, P. Malondialdehyde (MDA) as a lipid peroxidation marker. Wiad. Lek. Wars. Pol. 2004, 57, 453–455. [Google Scholar]
- Sharma, S.; Shah, E.; Davla, D.; Dixit, G.; Patel, A.; Kumar, A.K. Effect of microalga-based diet on oxidative stress enzymes of African catfish, Clarias gariepinus. Int. Aquat. Res. 2019, 11, 377–387. [Google Scholar] [CrossRef]
- Ahmad, M.T.; Shariff, M.; Md. Yusoff, F.; Goh, Y.M.; Banerjee, S. Applications of microalga Chlorella vulgaris in aquaculture. Rev. Aquac. 2020, 12, 328–346. [Google Scholar] [CrossRef]
- Safafar, H.; Van Wagenen, J.; Møller, P.; Jacobsen, C. Carotenoids, phenolic compounds and tocopherols contribute to the antioxidative properties of some microalgae species grown on industrial wastewater. Mar. Drugs 2015, 13, 7339–7356. [Google Scholar] [CrossRef] [Green Version]
- Tan, B.L.; Norhaizan, M.E.; Liew, W.-P.-P.; Sulaiman Rahman, H. Antioxidant and oxidative stress: A mutual interplay in age-related diseases. Front. Pharmacol. 2018, 9, 1162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, H.Y.; Kim, K.-S.; Lee, Y.-C.; Moon, H.-I.; Lee, J.-H. Oleifolioside A, a new active compound, attenuates LPS-stimulated iNOS and COX-2 expression through the downregulation of NF-κB and MAPK activities in RAW 264.7 macrophages. Evid. Based Complement. Alternat. Med. 2012, 2012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Finkel, T.; Holbrook, N.J. Oxidants, oxidative stress and the biology of ageing. Nature 2000, 408, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Uribe, C.; Folch, H.; Enríquez, R.; Moran, G. Innate and adaptive immunity in teleost fish: A review. Vet. Med. 2011, 56, 486. [Google Scholar] [CrossRef] [Green Version]
- Cerezuela, R.; Guardiola, F.A.; Meseguer, J.; Esteban, M. Enrichment of gilthead seabream (Chlorella vulgaris L.) diet with microalgae: Effects on the immune system. Fish Physiol. Biochem. 2012, 38, 1729–1739. [Google Scholar] [CrossRef]
- Ma, K.; Chen, S.; Wu, Y.; Ma, Y.; Qiao, H.; Fan, J.; Wu, H. Dietary supplementation with microalgae enhances the zebrafish growth performance by modulating immune status and gut microbiota. Appl. Microbiol. Biotechnol. 2022, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-Y.; Chen, J.-C.; Tayag, C.M.; Li, H.-F.; Putra, D.F.; Kuo, Y.-H.; Bai, J.-C.; Chang, Y.-H. Spirulina elicits the activation of innate immunity and increases resistance against Vibrio alginolyticus in shrimp. Fish Shellfish Immunol. 2016, 55, 690–698. [Google Scholar] [CrossRef]
- Zuo, R.; Ai, Q.; Mai, K.; Xu, W.; Wang, J.; Xu, H.; Liufu, Z.; Zhang, Y. Effects of dietary n-3 highly unsaturated fatty acids on growth, nonspecific immunity, expression of some immune related genes and disease resistance of large yellow croaker (Larmichthys crocea) following natural infestation of parasites (Cryptocaryon irritans). Fish Shellfish Immunol. 2012, 32, 249–258. [Google Scholar]
- Benítez-Dorta, V.; Caballero, M.J.; Izquierdo, M.; Manchado, M.; Infante, C.; Zamorano, M.J.; Montero, D. Total substitution of fish oil by vegetable oils in Senegalese sole (Solea senegalensis) diets: Effects on fish performance, biochemical composition, and expression of some glucocorticoid receptor-related genes. Fish Physiol. Biochem. 2013, 39, 335–349. [Google Scholar] [CrossRef]
- Kiron, V.; Thawonsuwan, J.; Panigrahi, A.; Scharsack, J.; Satoh, S. Antioxidant and immune defences of rainbow trout (Oncorhynchus mykiss) offered plant oils differing in fatty acid profiles from early stages. Aquacult. Nutr. 2011, 17, 130–140. [Google Scholar] [CrossRef]
- Gutiérrez, S.; Svahn, S.L.; Johansson, M.E. Effects of omega-3 fatty acids on immune cells. Int. J. Mol. Sci. 2019, 20, 5028. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Talukdar, S.; Bae, E.J.; Imamura, T.; Morinaga, H.; Fan, W.; Li, P.; Lu, W.J.; Watkins, S.M.; Olefsky, J.M. GPR120 is an omega-3 fatty acid receptor mediating potent anti-inflammatory and insulin-sensitizing effects. Cell 2010, 142, 687–698. [Google Scholar]
- Montero, D.; Mathlouthi, F.; Tort, L.; Afonso, J.; Torrecillas, S.; Fernández-Vaquero, A.; Negrin, D.; Izquierdo, M. Replacement of dietary fish oil by vegetable oils affects humoral immunity and expression of pro-inflammatory cytokines genes in gilthead sea bream Chlorella vulgaris. Fish Shellfish Immunol. 2010, 29, 1073–1081. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.; Al-Adul-Elah, K.; Azad, I.; Alzalzalah, A.; Alnuiami, S. High DHA Algae Meal as Cost-effective Alternative to High DHA Fish Oil in Finisher Feed for Sobaity Sea Bream (Sparidentex hasta). Anim. Feed Sci. Technol. 2022, 115209. [Google Scholar] [CrossRef]
- Scarpa, R.; Hutchinson, W.G.; Chilton, S.M.; Buongiorno, J. Importance of forest attributes in the willingness to pay for recreation: A contingent valuation study of Irish forests. For. Policy Econ. 2000, 1, 315–329. [Google Scholar] [CrossRef]
- Xie, J.; Fang, H.; Liao, S.; Guo, T.; Yin, P.; Liu, Y.; Tian, L.; Niu, J. Study on Schizochytrium sp. improving the growth performance and non-specific immunity of golden pompano (Trachinotus ovatus) while not affecting the antioxidant capacity. Fish Shellfish Immunol. 2019, 95, 617–623. [Google Scholar] [CrossRef]
- Wang, Y.; Li, M.; Filer, K.; Xue, Y.; Ai, Q.; Mai, K. Evaluation of Schizochytrium meal in microdiets of Pacific white shrimp (Litopenaeus vannamei) larvae. Aquac. Res. 2017, 48, 2328–2336. [Google Scholar] [CrossRef]
- Lin, F.; Xu, J.; Shi, J.; Li, H.; Li, B. Molecular cloning and characterization of a novel glyoxalase I gene TaGly I in wheat (Triticum aestivum L.). Mol. Biol. Rep. 2010, 37, 729–735. [Google Scholar] [CrossRef]
- Baharloei, M.; Heidari, B.; Zamani, H.; Ghafouri, H.; Hadavi, M. Effects of heat shock protein inducer on Hsp70 gene expression and immune parameters during Streptococcus iniae infection in a Persian sturgeon fry. Vet. Res. Forum 2021, 12, 473–479. [Google Scholar]
- Cerezuela, R.; Fumanal, M.; Tapia-Paniagua, S.T.; Meseguer, J.; Moriñigo, M.Á.; Esteban, M. Histological alterations and microbial ecology of the intestine in gilthead seabream (Chlorella vulgaris L.) fed dietary probiotics and microalgae. Cell Tissue Res. 2012, 350, 477–489. [Google Scholar] [CrossRef]
- Deane, E.E.; Li, J.; Woo, N.Y. Modulated heat shock protein expression during pathogenic Vibrio alginolyticus stress of sea bream. Dis. Aquat. Organ. 2004, 62, 205–215. [Google Scholar] [CrossRef] [PubMed]
- Li, E.; Xu, C.; Wang, X.; Wang, S.; Zhao, Q.; Zhang, M.; Qin, J.G.; Chen, L. Gut microbiota and its modulation for healthy farming of Pacific white shrimp Litopenaeus vannamei. Rev. Fish. Sci. Aquac. 2018, 26, 381–399. [Google Scholar] [CrossRef]
- Shi, Y.; Cao, X.; Ye, Z.; Xu, Y.; Wang, Y.; Li, Z.; Hang, W.; He, N. Role of dietary Schizochytrium sp. in improving disease resistance of zebrafish through metabolic and microbial analysis. Aquaculture 2021, 539, 736631. [Google Scholar] [CrossRef]
- Bravo-Tello, K.; Ehrenfeld, N.; Solís, C.J.; Ulloa, P.E.; Hedrera, M.; Pizarro-Guajardo, M.; Paredes-Sabja, D.; Feijóo, C.G. Effect of microalgae on intestinal inflammation triggered by soybean meal and bacterial infection in zebrafish. PLoS ONE 2017, 12, e0187696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kulshreshtha, A.; Jarouliya, U.; Bhadauriya, P.; Prasad, G.; Bisen, P. Spirulina in health care management. Curr. Pharm. Biotechnol. 2008, 9, 400–405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rahman, A.; Shefat, S.H.T.; Chowdhury, M.A. Effects of Probiotic Bacillus on Growth Performance, Immune Response and Disease Resistance in Aquaculture. Preprints 2021, 2021030075, 1–25. [Google Scholar]
- Bhowmik, D.; Dubey, J.; Mehra, S. Probiotic efficiency of Spirulina platensis-stimulating growth of lactic acid bacteria. World J. Dairy Food Sci. 2009, 4, 160–163. [Google Scholar]
- Mariey, Y.; Samak, H.; Ibrahem, M. Effect of using Spirulina platensis algae as afeed additive for poultry diets: 1-Productive and reproductive performances of local laying hens. Egypt. Poult. Sci. J. 2012, 32, 201–215. [Google Scholar]
- Kokou, F.; Makridis, P.; Kentouri, M.; Divanach, P. Antibacterial activity in microalgae cultures. Aquac. Res. 2012, 43, 1520–1527. [Google Scholar] [CrossRef]
- Kankaanpää, P.E.; Salminen, S.J.; Isolauri, E.; Lee, Y.K. The influence of polyunsaturated fatty acids on probiotic growth and adhesion. FEMS Microbiol. Lett. 2001, 194, 149–153. [Google Scholar] [CrossRef]
- Ringø, E.; Zhou, Z.; Vecino, J.G.; Wadsworth, S.; Romero, J.; Krogdahl, Å.; Olsen, R.E.; Dimitroglou, A.; Foey, A.; Davies, S. Effect of dietary components on the gut microbiota of aquatic animals. A never-ending story? Aquacult. Nutr. 2016, 22, 219–282. [Google Scholar] [CrossRef] [Green Version]
- Abdel-Raouf, N.; Ibraheem, I.B. Antibiotic activity of two Anabaena species against four fish pathogenic Aeromonas species. Afr. J. Biotechnol. 2008, 7, 2644–2648. [Google Scholar]
- Dopazo, C.; Lemos, M.; Lodeiros, C.; Bolinches, J.; Barja, J.; Toranzo, A.E. Inhibitory activity of antibiotic-producing marine bacteria against fish pathogens. J. Appl. Bacteriol. 1988, 65, 97–101. [Google Scholar] [CrossRef] [PubMed]
- Cardozo, K.H.; Guaratini, T.; Barros, M.P.; Falcão, V.R.; Tonon, A.P.; Lopes, N.P.; Campos, S.; Torres, M.A.; Souza, A.O.; Colepicolo, P. Metabolites from algae with economical impact. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2007, 146, 60–78. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Gao, Q.; Dong, S.; Zhou, J.; Ye, Z.; Lan, Y. Effects of dietary n-3 highly unsaturated fatty acids (HUFAs) on growth, fatty acid profiles, antioxidant capacity and immunity of sea cucumber Apostichopus japonicus (Selenka). Fish Shellfish Immunol. 2016, 54, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Wall, R.; Ross, R.P.; Fitzgerald, G.F.; Stanton, C. Fatty acids from fish: The anti-inflammatory potential of long-chain omega-3 fatty acids. Nutr. Rev. 2010, 68, 280–289. [Google Scholar] [CrossRef]
- Cheng, C.-L.; Huang, S.-J.; Wu, C.-L.; Gong, H.-Y.; Ken, C.-F.; Hu, S.-Y.; Wu, J.-L. Transgenic expression of omega-3 PUFA synthesis genes improves zebrafish survival during Vibrio vulnificus infection. J. Biomed. Sci. 2015, 22, 1–13. [Google Scholar] [CrossRef]

| Item | |
|---|---|
| Ingredient | % |
| Fish meal | 21.5 |
| Soybean meal | 24.00 |
| Yellow corn | 33.30 |
| Corn gluten | 5.50 |
| Rice bran | 10.00 |
| Fish oil | 2.80 |
| Lysine | 0.10 |
| DL-methionine (98%) | 0.20 |
| Threonine | 0.10 |
| Di-calcium phosphate | 1.20 |
| * Vitamins and minerals premix | 1.20 |
| Chemical analysis | |
| Digestible energy (kcal/kg) | 2904 |
| Crude protein, % | 32.00 |
| Ether extract, % | 7.91 |
| Nitrogen free extract, % | 45.81 |
| Calcium, % | 0.90 |
| Available phosphorus, % | 0.45 |
| Lysine, % | 2.00 |
| Methionine, % | 0.88 |
| Target Gene | Primer Sequence (5′–3′) | Accession Number/Reference |
|---|---|---|
| SOD | F-GACGTGACAACACAGGTTGC R-TACAGCCACCGTAACAGCAG | XM_003449940.5 |
| CAT | F-TCAGCACAGAAGACACAGACA R-GACCATTCCTCCACTCCAGAT | XM_031754288.1 |
| GSH-Px | F-F-CCAAGAGAACTGCAAGAACGA R-CAGGACACGTCATTCCTACAC | NM_001279711.1 |
| TGF-β | F-GTTTGAACTTCGGCGGTACTG R-TCCTGCTCATAGTCCCAGAGA | XM_003459454.2 |
| IL-10 | F-CTGCTAGATCAGTCCGTCGAA R-GCAGAACCGTGTCCAGGTAA | XM_013269189.3 |
| IgM | F: AGGAGACAGGACTGGAATGCACAA R: GGAGGCAGTATAGGTATCATCCTC | XM_025906584.1 |
| IL-1β | F-TGCTGAGCACAGAATTCCAG R-GCTGTGGAGAAGAACCAAGC | XM_019365841.2 |
| TNF-α | F-GAGGTCGGCGTGCCAAGA R-TGGTTTCCGTCCACAGCGT | NM_001279533.1 |
| HSP70 | F-TGGAGTCCTACGCCTTCAACA R-CAGGTAGCACCAGTGGGCAT | XM_003442456.5 |
| COX-2 | F-GGCCGGGTGTAGTCACAAAT R-CGACCACTACCTACACGCTC | XM_003445052 |
| β-actin | F-CAGCAAGCAGGAGTACGATG R-TGTGTGGTGTGTGGTTGTTTTG | XM_031749543.1 |
| 16s rRNA/genus Lactobacillus | F-TGGAAACAGGTGCTAATACCG R-CCATTGTGGAAGATTCCC | [48] |
| 16S-23S rRNA/Bacillus species | F-GCTGGTTAGAGCGCACGCCTGATA R-CATCCACCGTGCGCCCTTTCTAAC | [49] |
| 16S rRNA/genus Staphylococcus | F-AACTCTGTTATTAGGGAAGAACA R-CCACCTTCCTCCGGTTTGTCACC | [50] |
| 16S rRNA/genus Vibrio | F-GGCGTAAAGCGCATGCAGGT R-GAAATTCTACCCCCCTCTACAG | [51] |
| ahaI/Aeromonas hydrophila | F-GAGAAGGTGACCACCAAGAACA R-GAGATGTCAGCCTTGTAGAGCT | [52] |
| Parameter | Experimental Group | p Value | SEM | |||
|---|---|---|---|---|---|---|
| NSS0.0% | NSS0.75% | NSS1.5% | NSS3.0% | |||
| IBW (g/fish | 23.90 | 23.86 | 23.68 | 24.05 | 0.87 | 0.07 |
| FBW (g/fish) | 75.60 c | 76.93 c | 87.07 b | 96.03 a | <0.04 | 3.02 |
| WG (g/fish) | 51.70 c | 53.07 c | 63.38 b | 71.98 a | <0.001 | 7.50 |
| WG (%) | 216.36 b | 222.35 b | 267.60 a | 299.21 a | 0.001 | 16.43 |
| Feed intake (g/fish) | 84.96 a | 84.57 a | 85.13 a | 75.70 b | 0.03 | 4.01 |
| FCR | 1.65 a | 1.60 a | 1.34 b | 1.05 c | <0.006 | 0.01 |
| SGR (%) | 1.37 b | 1.39 b | 1.55 a | 1.65 a | <0.001 | 0.00 |
| PER | 1.90 b | 1.96 b | 2.32 b | 2.98 a | <0.001 | 0.02 |
| Parameter | Experimental Group | p Value | SEM | |||
|---|---|---|---|---|---|---|
| NSS0.0% | NSS0.75% | NSS1.5% | NSS3.0% | |||
| Chymotrypsin (U/L) | 24.60 c | 26.30 b | 27.57 a | 27.83 a | 0.008 | 0.03 |
| Amylase (U/L) | 28.23 c | 30.73 b | 32.33 a | 32.30 a | 0.009 | 0.26 |
| Lipase (U/L) | 25.67 b | 26.40 ab | 27.83 a | 28.43 a | <0.01 | 0.08 |
| Protease (U/L) | 28.47 d | 29.27 c | 30.53 b | 33.20 a | 0.02 | 0.17 |
| ALT (U/L) | 60.40 | 60.03 | 60.03 | 59.93 | 0.68 | 0.54 |
| AST(U/L) | 17.27 | 17.07 | 17.40 | 17.07 | 0.09 | 0.16 |
| Parameter | Experimental Group | p Value | SEM | |||
|---|---|---|---|---|---|---|
| NSS0.0% | NSS0.75% | NSS1.5% | NSS3.0% | |||
| RBCs (×106/μL) | 2.36 b | 2.38 b | 2.41 ab | 2.55 a | 0.02 | 0.07 |
| Ht (%) | 32.43 | 33.00 | 32.55 | 32.53 | 0.09 | 0.57 |
| Hb (g/dL) | 7.21 | 7.37 | 7.35 | 7.42 | 0.11 | 0.09 |
| WBCs (×103/μL) | 6.93 | 6.81 | 6.48 | 6.88 | 0.08 | 0.16 |
| Lysozyme (μg/mL) | 0.89 d | 1.16 c | 1.42 b | 1.55 a | <0.001 | 0.13 |
| NO (μmol/L) | 0.40 c | 0.68 b | 0.74 b | 0.88 a | <0.001 | 0.06 |
| ACH50 (u/mL) | 258.00 c | 328.67 b | 341.33 b | 382.00 a | <0.001 | 7.16 |
| MPO (μmoL/L) | 0.67 b | 0.64 b | 0.73 ab | 0.82 a | <0.001 | 0.25 |
| IgM (μg/mL) | 28.50 b | 28.27 b | 28.90 b | 29.78 a | <0.001 | 1.38 |
| MDA (nmoL/mL) | 9.50 a | 8.07 b | 6.23 c | 4.07 d | <0.001 | 0.06 |
| CAT (U/L) | 78.93 c | 90.63 b | 93.33 ab | 96.53 a | 0.02 | 0.96 |
| SOD (μ/mL) | 11.23 c | 14.80 b | 16.67 a | 17.70 a | 0.03 | 0.14 |
| GSH-Px (μmoL/mg) | 4.37 c | 4.43 c | 5.80 b | 7.90 a | <0.001 | 0.04 |
| CRP (ng/mL) | 8.97 a | 7.1333 b | 7.0333 b | 5.367 c | <0.001 | 0.09 |
| Cortisol (nmol/L) | 5.88 | 6.05 | 5.91 | 6.00 | 0.06 | 0.25 |
| Parameter | Experimental Groups | p Value | SEM | |||
|---|---|---|---|---|---|---|
| NSS0.0% | NSS0.75% | NSS1.5% | NSS3.0% | |||
| MDA (nmol/g tissue) | 23.63a | 22.83 ab | 21.80 b | 20.73 b | <0.01 | 0.09 |
| ROS | 90.00 a | 88.83 a | 81.63 b | 76.87 c | <0.01 | 0.90 |
| H2O2 (μmoL/g tissue) | 2.99 a | 2.86 b | 2.46 c | 2.22 d | 0.03 | 1.30 |
| T-AOC (U/mg prot) | 1.54 c | 1.80 b | 1.86 b | 2.38 a | 0.04 | 0.17 |
| Σ SFAs | 37.60 a | 35.11 b | 34.12 b | 31.92 c | <0.001 | 0.39 |
| Σ MUSFAs | 44.13 a | 38.59 b | 31.96 c | 29.10 d | 0.03 | 0.29 |
| Σ PUFAs | 48.69 c | 52.05 b | 55.62 a | 58.90 a | <0.01 | 0.16 |
| EPA | 0.76 d | 0.91 c | 1.86 b | 2.01 a | 0.04 | 1.34 |
| DHA | 1.23 d | 1.45 c | 3.27 b | 3.86 a | <0.01 | 0.96 |
| Σn−3 | 4.10 d | 5.30 c | 6.30 b | 8.10 a | 0.03 | 0.22 |
| Σn−6 | 42.69 a | 40.25 b | 38.69 c | 35.4 d | 0.01 | 0.35 |
| Σn−6/Σn−3 | 7.62 a | 5.51 b | 4.35 c | 2.85 d | 0.02 | 0.17 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ibrahim, D.; Abd El-Hamid, M.I.; Al-Zaban, M.I.; ElHady, M.; El-Azzouny, M.M.; ElFeky, T.M.; Al Sadik, G.M.; Samy, O.M.; Hamed, T.A.; Albalwe, F.M.; et al. Impacts of Fortifying Nile Tilapia (Oreochromis niloticus) Diet with Different Strains of Microalgae on Its Performance, Fillet Quality and Disease Resistance to Aeromonas hydrophila Considering the Interplay between Antioxidant and Inflammatory Response. Antioxidants 2022, 11, 2181. https://doi.org/10.3390/antiox11112181
Ibrahim D, Abd El-Hamid MI, Al-Zaban MI, ElHady M, El-Azzouny MM, ElFeky TM, Al Sadik GM, Samy OM, Hamed TA, Albalwe FM, et al. Impacts of Fortifying Nile Tilapia (Oreochromis niloticus) Diet with Different Strains of Microalgae on Its Performance, Fillet Quality and Disease Resistance to Aeromonas hydrophila Considering the Interplay between Antioxidant and Inflammatory Response. Antioxidants. 2022; 11(11):2181. https://doi.org/10.3390/antiox11112181
Chicago/Turabian StyleIbrahim, Doaa, Marwa I. Abd El-Hamid, Mayasar I. Al-Zaban, Mohamed ElHady, Mona M. El-Azzouny, Tamer Mohamed ElFeky, Gehan M. Al Sadik, Omima M. Samy, Thoria A. Hamed, Fauzeya Mateq Albalwe, and et al. 2022. "Impacts of Fortifying Nile Tilapia (Oreochromis niloticus) Diet with Different Strains of Microalgae on Its Performance, Fillet Quality and Disease Resistance to Aeromonas hydrophila Considering the Interplay between Antioxidant and Inflammatory Response" Antioxidants 11, no. 11: 2181. https://doi.org/10.3390/antiox11112181
APA StyleIbrahim, D., Abd El-Hamid, M. I., Al-Zaban, M. I., ElHady, M., El-Azzouny, M. M., ElFeky, T. M., Al Sadik, G. M., Samy, O. M., Hamed, T. A., Albalwe, F. M., Alenezi, M. A., & Omar, A. E. (2022). Impacts of Fortifying Nile Tilapia (Oreochromis niloticus) Diet with Different Strains of Microalgae on Its Performance, Fillet Quality and Disease Resistance to Aeromonas hydrophila Considering the Interplay between Antioxidant and Inflammatory Response. Antioxidants, 11(11), 2181. https://doi.org/10.3390/antiox11112181







