Effect of Empagliflozin on Thioacetamide-Induced Liver Injury in Rats: Role of AMPK/SIRT-1/HIF-1α Pathway in Halting Liver Fibrosis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Chemicals
2.3. Experimental Design
- Group 1: The control group: Rats were injected intraperitoneally with water containing 0.1% Tween 80 thrice weekly for six weeks.
- Group 2: The TAA group: Rats were intraperitoneally injected with TAA (100 mg/kg, IP) thrice weekly for six weeks.
- Groups 3 and 4: Rats received EMPA (3 and 6 mg/kg) [17] orally, daily for six weeks at the same time with TAA injection.
2.4. Body Weight Change
2.5. Blood Glucose
2.6. Blood and Tissue Samples
2.7. Assessment of Serum Biochemical Analysis
2.8. Determination of Tissue Protein
2.9. Preparation of Hepatic Tissue Homogenate
2.10. Assessment of Oxidative Stress Biomarkers
2.11. Assessment of Cell Regulatory and Inflammatory Markers
2.12. Histological Examination
2.13. Immunohistochemical Examination
2.14. qRT-PCR Analysis for IL-6 Expression in the Liver Tissues
2.15. Statistical Analysis
3. Results
3.1. Effect of Empagliflozin on the Percentage Change in Body Weight in Thioacetamide-Intoxicated Rats
3.2. Effect of Empagliflozin on Blood Glucose in Thioacetamide-Intoxicated Rats
3.3. Effect of Empagliflozin on Serum Hepatic Function in Thioacetamide-Intoxicated Rats
3.4. Effect of Empagliflozin on Lipid Profile in Thioacetamide-Intoxicated Rats
3.5. Effect of Empagliflozin on Oxidative Stress Biomarkers in Thioacetamide-Intoxicated Rats
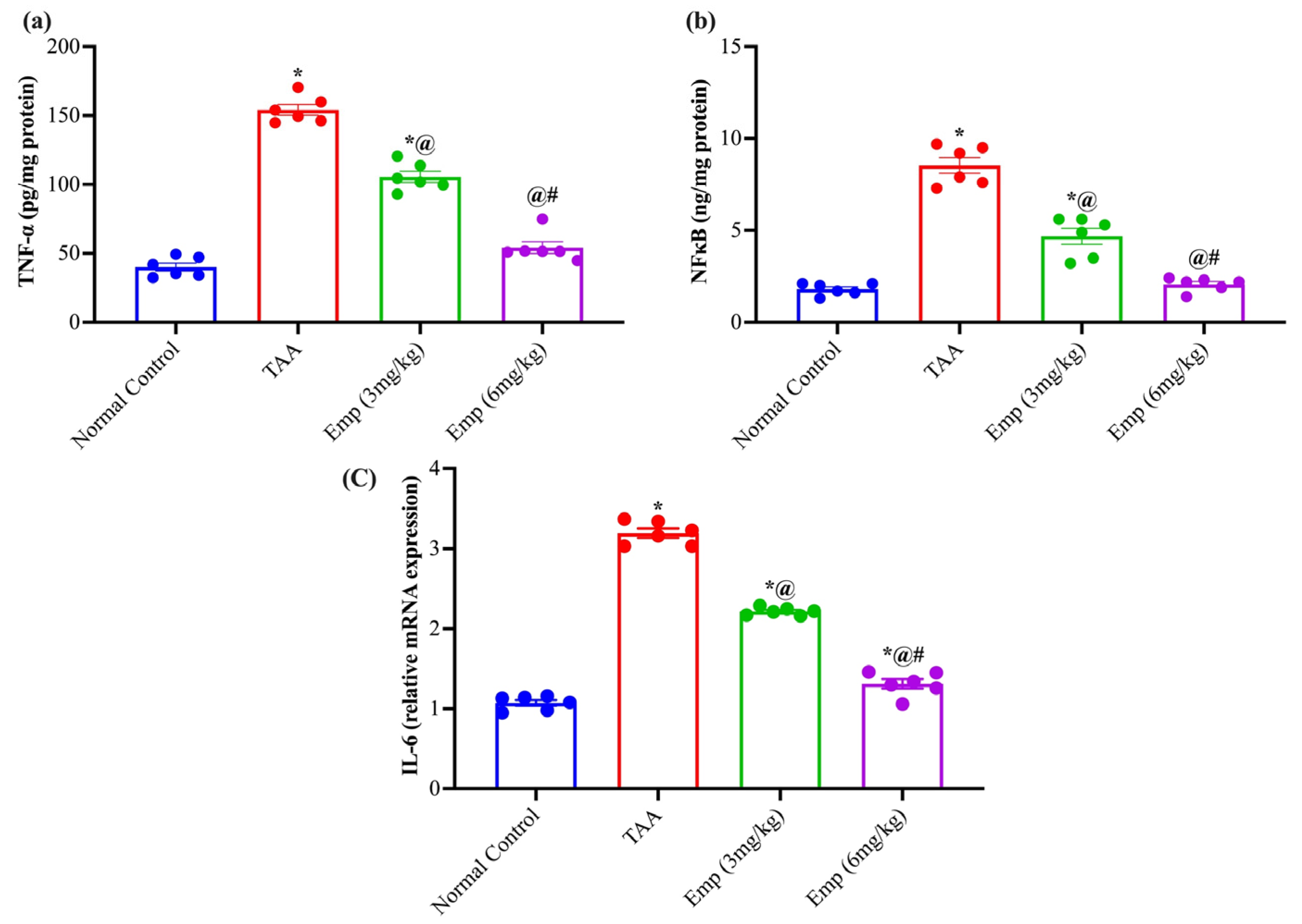
3.6. Effect of Empagliflozin on Hepatic Inflammatory Biomarkers in Thioacetamide-Intoxicated Rats
3.7. Effect of Empagliflozin on Hepatic AMP-Activated Protein Kinase, HIF-1α, and Sirtuin-1 in Thioacetamide-Intoxicated Rats
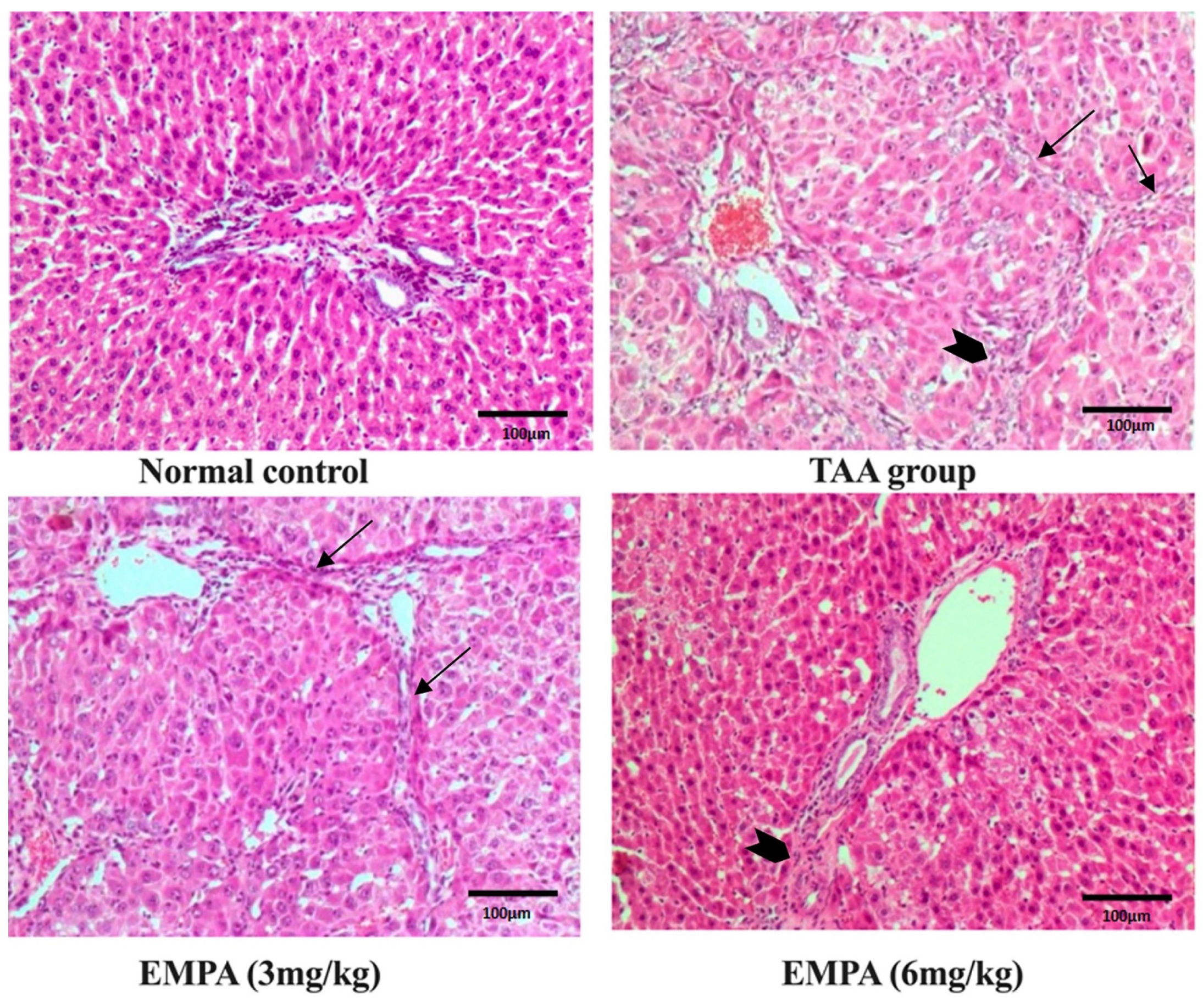
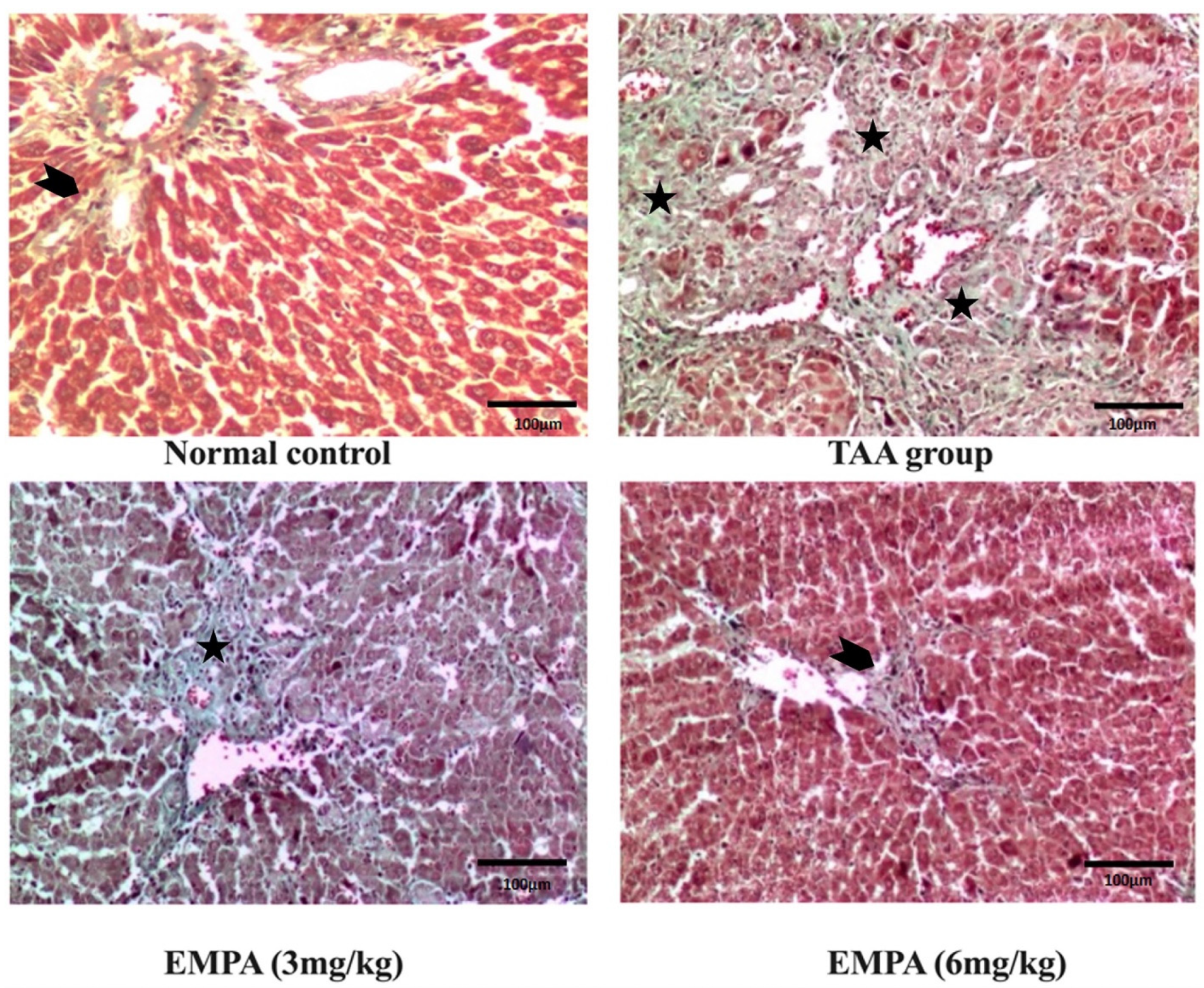
3.8. Histological Examination
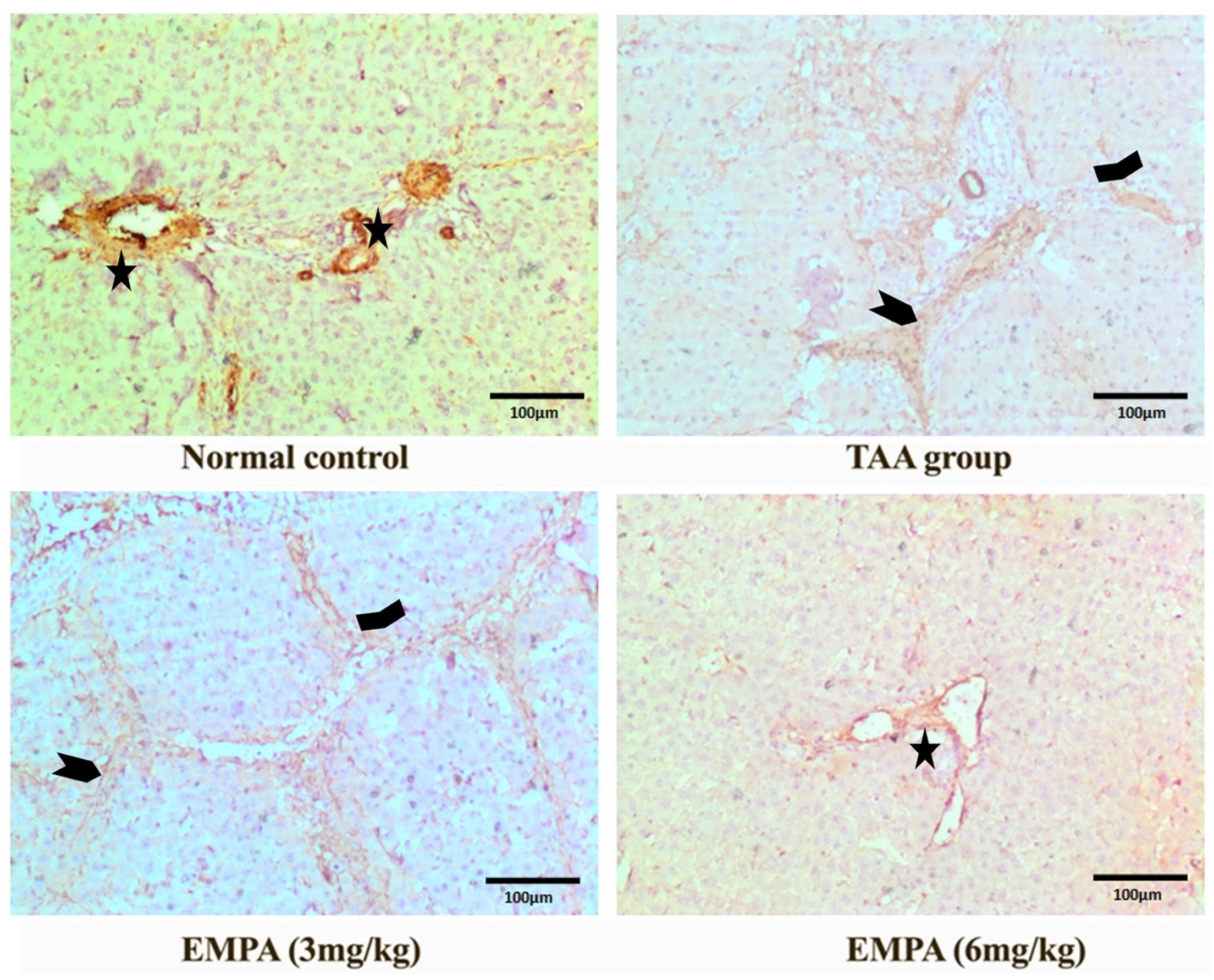
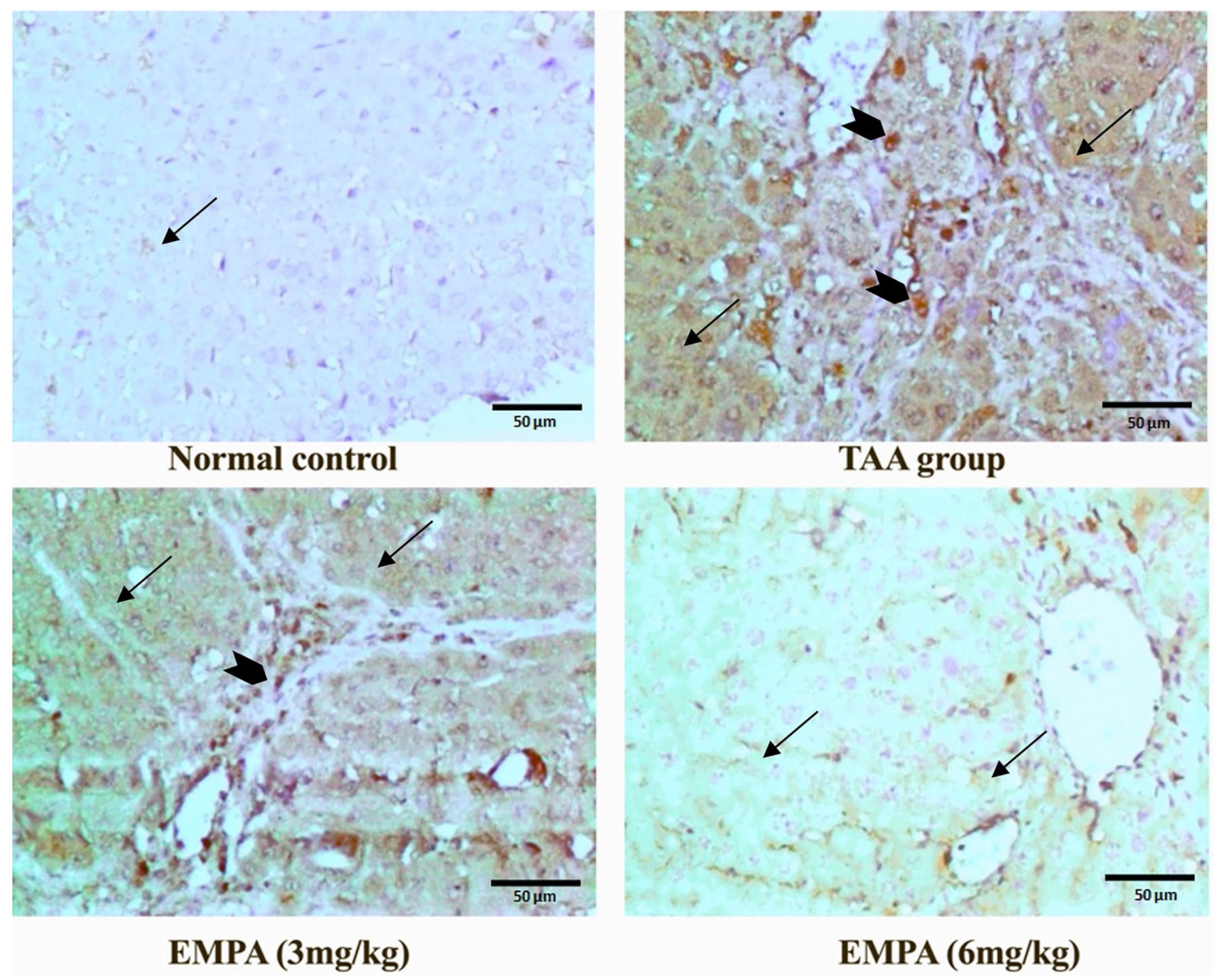
3.9. Immunohistochemical Examination
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Roehlen, N.; Crouchet, E.; Baumert, T.F. Liver Fibrosis: Mechanistic Concepts and Therapeutic Perspectives. Cells 2020, 9, 875. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Higashi, T.; Friedman, S.L.; Hoshida, Y. Hepatic Stellate Cells as Key Target in Liver Fibrosis. Adv. Drug Deliv. Rev. 2017, 121, 27–42. [Google Scholar] [CrossRef] [PubMed]
- Cichoż-Lach, H. Oxidative Stress as a Crucial Factor in Liver Diseases. World J. Gastroenterol. 2014, 20, 8082. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Rahman, R.F.; Fayed, H.M.; Asaad, G.F.; Ogaly, H.A.; Hessin, A.F.; Salama, A.A.A.; Abd El-Rahman, S.S.; Arbid, M.S.; Mohamed, M.A.E. The Involvement of TGF-Β1/FAK/α-SMA Pathway in the Antifibrotic Impact of Rice Bran Oil on Thioacetamide-Induced Liver Fibrosis in Rats. PLoS ONE 2021, 16, e0260130. [Google Scholar] [CrossRef]
- Vokálová, L.; Lauková, L.; Čonka, J.; Melišková, V.; Borbélyová, V.; Bábíčková, J.; Tóthová, L.; Hodosy, J.; Vlková, B.; Celec, P. Deoxyribonuclease Partially Ameliorates Thioacetamide-Induced Hepatorenal Injury. Am. J. Physiol. Liver Physiol. 2017, 312, G457–G463. [Google Scholar] [CrossRef] [Green Version]
- Ren, R.; Wang, Z.; Wu, M.; Wang, H. Emerging Roles of SIRT1 in Alcoholic Liver Disease. Int. J. Biol. Sci. 2020, 16, 3174–3183. [Google Scholar] [CrossRef]
- Nassir, F.; Ibdah, J.A. Sirtuins and Nonalcoholic Fatty Liver Disease. World J. Gastroenterol. 2016, 22, 10084. [Google Scholar] [CrossRef]
- De Gregorio, E.; Colell, A.; Morales, A.; Marí, M. Relevance of SIRT1-NF-ΚB Axis as Therapeutic Target to Ameliorate Inflammation in Liver Disease. Int. J. Mol. Sci. 2020, 21, 3858. [Google Scholar] [CrossRef]
- Ezhilarasan, D.; Najimi, M. Role of Sirtuins in Liver Diseases. In Sirtuin Biology in Medicine; Elsevier: Amsterdam, The Netherlands, 2021; pp. 329–340. [Google Scholar]
- Roth, K.J.; Copple, B.L. Role of Hypoxia-Inducible Factors in the Development of Liver Fibrosis. Cell. Mol. Gastroenterol. Hepatol. 2015, 1, 589–597. [Google Scholar] [CrossRef] [Green Version]
- Novikov, A.; Vallon, V. Sodium Glucose Cotransporter 2 Inhibition in the Diabetic Kidney. Curr. Opin. Nephrol. Hypertens. 2016, 25, 50–58. [Google Scholar] [CrossRef]
- Ndefo, U.A.; Anidiobi, N.O.; Basheer, E.; Eaton, A.T. Empagliflozin (Jardiance): A Novel SGLT2 Inhibitor for the Treatment of Type-2 Diabetes. Pharm. Ther. 2015, 40, 364–368. [Google Scholar]
- Lu, Q.; Li, X.; Liu, J.; Sun, X.; Rousselle, T.; Ren, D.; Tong, N.; Li, J. AMPK Is Associated with the Beneficial Effects of Antidiabetic Agents on Cardiovascular Diseases. Biosci. Rep. 2019, 39, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Taheri, H.; Malek, M.; Ismail-Beigi, F.; Zamani, F.; Sohrabi, M.; Reza babaei, M.; Khamseh, M.E. Effect of Empagliflozin on Liver Steatosis and Fibrosis in Patients With Non-Alcoholic Fatty Liver Disease Without Diabetes: A Randomized, Double-Blind, Placebo-Controlled Trial. Adv. Ther. 2020, 37, 4697–4708. [Google Scholar] [CrossRef] [PubMed]
- Packer, M. Cardioprotective Effects of Sirtuin-1 and Its Downstream Effectors. Circ. Heart Fail. 2020, 13, e007197. [Google Scholar] [CrossRef]
- Abdel-latif, R.G.; Rifaai, R.A.; Amin, E.F. Empagliflozin Alleviates Neuronal Apoptosis Induced by Cerebral Ischemia/Reperfusion Injury through HIF-1α/VEGF Signaling Pathway. Arch. Pharm. Res. 2020, 43, 514–525. [Google Scholar] [CrossRef] [PubMed]
- Malik, S.; Suchal, K.; Khan, S.I.; Bhatia, J.; Kishore, K.; Dinda, A.K.; Arya, D.S. Apigenin Ameliorates Streptozotocin-Induced Diabetic Nephropathy in Rats via MAPK-NF-ĸB-TNF-α and TGF-Β1-MAPK-Fibronectin Pathways. Am. J. Physiol.-Ren. Physiol. 2017, 313, F414–F422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mansour, D.F.; Nada, S.A.; El-Denshary, E.S.; Omara, E.A.; Asaad, G.F.; Abdel-Rahman, R.F. Milk Whey Proteins Modulate Endotoxemia-Induced Hepatotoxicity in Rats. Int. J. Pharm. Pharm. Sci. 2015, 7, 65–71. [Google Scholar]
- Abdel-Rahman, R.F.; Alqasoumi, S.I.; Ogaly, H.A.; Abd-Elsalam, R.M.; El-Banna, H.A.; Soliman, G.A. Propolis Ameliorates Cerebral Injury in Focal Cerebral Ischemia/Reperfusion (I/R) Rat Model via Upregulation of TGF-Β1. Saudi Pharm. J. 2020, 28, 116–126. [Google Scholar] [CrossRef]
- El Awdan, S.A.; Abdel Rahman, R.F.; Ibrahim, H.M.; Hegazy, R.R.; El Marasy, S.A.; Badawi, M.; Arbid, M.S. Regression of Fibrosis by Cilostazol in a Rat Model of Thioacetamide-Induced Liver Fibrosis: Up Regulation of Hepatic CAMP, and Modulation of Inflammatory, Oxidative Stress and Apoptotic Biomarkers. PLoS ONE 2019, 14, e0216301. [Google Scholar] [CrossRef]
- Bancroft, J.D.; Gamble, M. Bancroft’s Theory and Practice of Histological Techniques; Elsevier: Amsterdam, The Netherlands, 2019; ISBN 9780702068874. [Google Scholar]
- Hsu, S.M.; Raine, L.; Fanger, H. The Use of Antiavidin Antibody and Avidin-Biotin-Peroxidase Complex in Immunoperoxidase Technics. Am. J. Clin. Pathol. 1981, 75, 816–821. [Google Scholar] [CrossRef]
- Carvalho, J.R.; Machado, M.V. New Insights about Albumin and Liver Disease. Ann. Hepatol. 2018, 17, 547–560. [Google Scholar] [CrossRef] [PubMed]
- Hessin, A.; Hegazy, R.R.; Hassan, A.A.; Yassin, N.Z.; Kenawy, S.A.B. Resveratrol Prevents Liver Fibrosis via Two Possible Pathways: Modulation of Alpha Fetoprotein Transcriptional Levels and Normalization of Protein Kinase C Responses. Indian J. Pharmacol. 2017, 49, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Ra, S.H.; Shin, R.H.; Ri, H.C.; Ri, J.H.; Ri, H.C.; Ri, A.J. Effect of Lesimarin against Thioacetamide-Induced Liver Cirrhosis in Rat. Braz. J. Pharm. Sci. 2019, 55, 17821. [Google Scholar] [CrossRef]
- Ali, R.; Nagalli, S. Hyperammonemia; StatPearls Publishing: Tampa, FL, USA, 2022. [Google Scholar]
- Afshinnia, F.; Wong, K.K.; Sundaram, B.; Ackermann, R.J.; Pennathur, S. Hypoalbuminemia and Osteoporosis: Reappraisal of a Controversy. J. Clin. Endocrinol. Metab. 2016, 101, 167–175. [Google Scholar] [CrossRef] [Green Version]
- Zargar, S.; Wani, T.A.; Alamro, A.A.; Ganaie, M.A. Amelioration of Thioacetamide-Induced Liver Toxicity in Wistar Rats by Rutin. Int. J. Immunopathol. Pharmacol. 2017, 30, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Meyer, C.; Xu, C.; Weng, H.; Hellerbrand, C.; ten Dijke, P.; Dooley, S. Animal Models of Chronic Liver Diseases. Am. J. Physiol.-Gastrointest. Liver Physiol. 2013, 304, G449–G468. [Google Scholar] [CrossRef] [Green Version]
- Nasiri-Ansari, N.; Nikolopoulou, C.; Papoutsi, K.; Kyrou, I.; Mantzoros, C.S.; Kyriakopoulos, G.; Chatzigeorgiou, A.; Kalotychou, V.; Randeva, M.S.; Chatha, K.; et al. Empagliflozin Attenuates Non-Alcoholic Fatty Liver Disease (NAFLD) in High Fat Diet Fed ApoE(−/−) Mice by Activating Autophagy and Reducing ER Stress and Apoptosis. Int. J. Mol. Sci. 2021, 22, 818. [Google Scholar] [CrossRef]
- Jojima, T.; Tomotsune, T.; Iijima, T.; Akimoto, K.; Suzuki, K.; Aso, Y. Empagliflozin (an SGLT2 Inhibitor), Alone or in Combination with Linagliptin (a DPP-4 Inhibitor), Prevents Steatohepatitis in a Novel Mouse Model of Non-Alcoholic Steatohepatitis and Diabetes. Diabetol. Metab. Syndr. 2016, 8, 45. [Google Scholar] [CrossRef] [Green Version]
- Perakakis, N.; Chrysafi, P.; Feigh, M.; Veidal, S.S.; Mantzoros, C.S. Empagliflozin Improves Metabolic and Hepatic Outcomes in a Non-Diabetic Obese Biopsy-Proven Mouse Model of Advanced Nash. Int. J. Mol. Sci. 2021, 22, 6332. [Google Scholar] [CrossRef]
- Al-Attar, A.M.; Shawush, N.A. Influence of Olive and Rosemary Leaves Extracts on Chemically Induced Liver Cirrhosis in Male Rats. Saudi J. Biol. Sci. 2015, 22, 157–163. [Google Scholar] [CrossRef] [Green Version]
- Staňková, P.; Kučera, O.; Lotková, H.; Roušar, T.; Endlicher, R.; Červinková, Z. The Toxic Effect of Thioacetamide on Rat Liver in Vitro. Toxicol. Vitr. 2010, 24, 2097–2103. [Google Scholar] [CrossRef] [PubMed]
- Han, D.; Hanawa, N.; Saberi, B.; Kaplowitz, N. Mechanisms of Liver Injury. III. Role of Glutathione Redox Status in Liver Injury. Am. J. Physiol. Liver Physiol. 2006, 291, G1–G7. [Google Scholar] [CrossRef] [PubMed]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.D.; Mazur, M.; Telser, J. Free Radicals and Antioxidants in Normal Physiological Functions and Human Disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef]
- Nohl, H.; Kozlov, A.V.; Gille, L.; Staniek, K. Cell Respiration and Formation of Reactive Oxygen Species: Facts and Artefacts. Biochem. Soc. Trans. 2003, 31, 1308–1311. [Google Scholar] [CrossRef] [PubMed]
- Hinchy, E.C.; Gruszczyk, A.V.; Willows, R.; Navaratnam, N.; Hall, A.R.; Bates, G.; Bright, T.P.; Krieg, T.; Carling, D.; Murphy, M.P. Mitochondria-Derived ROS Activate AMP-Activated Protein Kinase (AMPK) Indirectly. J. Biol. Chem. 2018, 293, 17208–17217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, N.; Heo, Y.J.; Choi, S.E.; Jeon, J.Y.; Han, S.J.; Kim, D.J.; Kang, Y.; Lee, K.W.; Kim, H.J. Hepatoprotective Effects of Gemigliptin and Empagliflozin in a Murine Model of Diet-Induced Non-Alcoholic Fatty Liver Disease. Biochem. Biophys. Res. Commun. 2022, 588, 154–160. [Google Scholar] [CrossRef]
- Moghadamnia, D.; Mokhtari, M.; Khatamsaz, S. Comparison of Protective Effects of Omega3 Fish Oil and Aqueous Extract of Glycyrrhiza Glabra Root on Thioacetamide Induced Lipid Toxicity in Male Rats. Biomed. Pharmacol. J. 2016, 9, 111–119. [Google Scholar] [CrossRef]
- Ali, A.M.; El-Tawil, O.S.; Al-Mokaddem, A.K.; Abd El-Rahman, S.S. Promoted Inhibition of TLR4/MiR-155/ NFkB P65 Signaling by Cannabinoid Receptor 2 Agonist (AM1241), Aborts Inflammation and Progress of Hepatic Fibrosis Induced by Thioacetamide. Chem. Biol. Interact. 2021, 336, 109398. [Google Scholar] [CrossRef]
- Nicholson, D. Caspase Structure, Proteolytic Substrates, and Function during Apoptotic Cell Death. Cell Death Differ. 1999, 6, 1028–1042. [Google Scholar] [CrossRef] [Green Version]
- Bantel, H.; Lügering, A.; Heidemann, J.; Volkmann, X.; Poremba, C.; Strassburg, C.P.; Manns, M.P.; Schulze-Osthoff, K. Detection of Apoptotic Caspase Activation in Sera from Patients with Chronic HCV Infection Is Associated with Fibrotic Liver Injury. Hepatology 2004, 40, 1078–1087. [Google Scholar] [CrossRef]
- Eissa, L.A.; Kenawy, H.I.; El-Karef, A.; Elsherbiny, N.M.; El-Mihi, K.A. Antioxidant and Anti-Inflammatory Activities of Berberine Attenuate Hepatic Fibrosis Induced by Thioacetamide Injection in Rats. Chem. Biol. Interact. 2018, 294, 91–100. [Google Scholar] [CrossRef] [PubMed]
- El-Mihi, K.A.; Kenawy, H.I.; El-Karef, A.; Elsherbiny, N.M.; Eissa, L.A. Naringin Attenuates Thioacetamide-Induced Liver Fibrosis in Rats through Modulation of the PI3K/Akt Pathway. Life Sci. 2017, 187, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Fang, T.; Xu, L.; Liu, X.; Li, X.; Xue, M.; Yu, X.; Sun, B.; Chen, L. Empagliflozin Alleviates Hepatic Steatosis by Activating the AMPK-TET2-Autophagy Pathway in Vivo and in Vitro. Front. Pharmacol. 2021, 11, 622153. [Google Scholar] [CrossRef]
- Shin, M.-R.; Lee, S.H.; Roh, S.-S. The Potential Hepatoprotective Effect of Paeoniae Radix Alba in Thioacetamide-Induced Acute Liver Injury in Rats. Evid. Based Complement. Altern. Med. 2022, 2022, 7904845. [Google Scholar] [CrossRef]
- Ruderman, N.B.; Julia Xu, X.; Nelson, L.; Cacicedo, J.M.; Saha, A.K.; Lan, F.; Ido, Y. AMPK and SIRT1: A Long-Standing Partnership? Am. J. Physiol. Metab. 2010, 298, E751–E760. [Google Scholar] [CrossRef]
- Spano, M.; Di Matteo, G.; Ingallina, C.; Ambroselli, D.; Carradori, S.; Gallorini, M.; Giusti, A.M.; Salvo, A.; Grosso, M.; Mannina, L. Modulatory Properties of Food and Nutraceutical Components Targeting NLRP3 Inflammasome Activation. Nutrients 2022, 14, 490. [Google Scholar] [CrossRef]
- Chen, L.; Lan, Z. Polydatin Attenuates Potassium Oxonate-Induced Hyperuricemia and Kidney Inflammation by Inhibiting NF-ΚB/NLRP3 Inflammasome Activation via the AMPK/SIRT1 Pathway. Food Funct. 2017, 8, 1785–1792. [Google Scholar] [CrossRef]
- Park, S.-J.; Ahmad, F.; Philp, A.; Baar, K.; Williams, T.; Luo, H.; Ke, H.; Rehmann, H.; Taussig, R.; Brown, A.L.; et al. Resveratrol Ameliorates Aging-Related Metabolic Phenotypes by Inhibiting CAMP Phosphodiesterases. Cell 2012, 148, 421–433. [Google Scholar] [CrossRef] [Green Version]
- Al-Hashem, F.; Al-Humayed, S.; Amin, S.N.; Kamar, S.S.; Mansy, S.S.; Hassan, S.; Abdel-Salam, L.O.; Ellatif, M.A.; Alfaifi, M.; Haidara, M.A.; et al. Metformin Inhibits MTOR–HIF-1α Axis and Profibrogenic and Inflammatory Biomarkers in Thioacetamide-induced Hepatic Tissue Alterations. J. Cell. Physiol. 2019, 234, 9328–9337. [Google Scholar] [CrossRef] [PubMed]
- Ryu, D.R.; Yu, M.R.; Kong, K.H.; Kim, H.; Kwon, S.H.; Jeon, J.S.; Han, D.C.; Noh, H. Sirt1-Hypoxia-Inducible Factor-1α Interaction Is a Key Mediator of Tubulointerstitial Damage in the Aged Kidney. Aging Cell 2019, 18, e12904. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, S.; Naz, N.; Malik, I.; Ramadori, P.; Amanzada, A.; Ramadori, G.; Moriconi, F. Role of the Hypoxia Inducible Factors HIF-1α and HIF-2α during Hepatic Repair in a Rat Model of Thioacetamide (TAA)-Induced Chronic Liver Damage. Z. Gastroenterol. 2014, 52. [Google Scholar] [CrossRef]
- Heo, Y.-J.; Lee, N.; Choi, S.-E.; Jeon, J.-Y.; Han, S.-J.; Kim, D.-J.; Kang, Y.; Lee, K.-W.; Kim, H.-J. Empagliflozin Reduces the Progression of Hepatic Fibrosis in a Mouse Model and Inhibits the Activation of Hepatic Stellate Cells via the Hippo Signalling Pathway. Biomedicines 2022, 10, 1032. [Google Scholar] [CrossRef]
- Komiya, C.; Tsuchiya, K.; Shiba, K.; Miyachi, Y.; Furuke, S.; Shimazu, N.; Yamaguchi, S.; Kanno, K.; Ogawa, Y. Ipragliflozin Improves Hepatic Steatosis in Obese Mice and Liver Dysfunction in Type 2 Diabetic Patients Irrespective of Body Weight Reduction. PLoS ONE 2016, 11, e0151511. [Google Scholar] [CrossRef] [Green Version]
- Devenny, J.J.; Godonis, H.E.; Harvey, S.J.; Rooney, S.; Cullen, M.J.; Pelleymounter, M.A. Weight Loss Induced by Chronic Dapagliflozin Treatment Is Attenuated by Compensatory Hyperphagia in Diet-Induced Obese (DIO) Rats. Obesity 2012, 20, 1645–1652. [Google Scholar] [CrossRef] [Green Version]
- Watanabe, Y.; Nakayama, K.; Taniuchi, N.; Horai, Y.; Kuriyama, C.; Ueta, K.; Arakawa, K.; Senbonmatsu, T.; Shiotani, M. Beneficial Effects of Canagliflozin in Combination with Pioglitazone on Insulin Sensitivity in Rodent Models of Obese Type 2 Diabetes. PLoS ONE 2015, 10, e0116851. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Li, Q.; Huang, W.; Han, Y.; Tan, H.; An, M.; Xiang, Q.; Zhou, R.; Yang, L.; Cheng, Y. Dapagliflozin Alleviates Hepatic Steatosis by Restoring Autophagy via the AMPK-MTOR Pathway. Front. Pharmacol. 2021, 12, 589273. [Google Scholar] [CrossRef]
- Zhang, E.; Zhao, Y.; Hu, H. Impact of Sodium Glucose Cotransporter 2 Inhibitors on Nonalcoholic Fatty Liver Disease Complicated by Diabetes Mellitus. Hepatol. Commun. 2021, 5, 736–748. [Google Scholar] [CrossRef]
- Androutsakos, T.; Nasiri-Ansari, N.; Bakasis, A.-D.; Kyrou, I.; Efstathopoulos, E.; Randeva, H.S.; Kassi, E. SGLT-2 Inhibitors in NAFLD: Expanding Their Role beyond Diabetes and Cardioprotection. Int. J. Mol. Sci. 2022, 23, 3107. [Google Scholar] [CrossRef]
- Wei, Q.; Xu, X.; Guo, L.; Li, J.; Li, L. Effect of SGLT2 Inhibitors on Type 2 Diabetes Mellitus With Non-Alcoholic Fatty Liver Disease: A Meta-Analysis of Randomized Controlled Trials. Front. Endocrinol. 2021, 12, 635556. [Google Scholar] [CrossRef]


| Groups | Albumin (g/dL) | GGT (nmol/mL) | ALT (U/L) | AST (U/L) | Ammonia (μg/mL) |
|---|---|---|---|---|---|
| Normal control | 4.38 ± 0.133 | 3.12 ± 0.105 | 35.68 ± 3.154 | 63.35 ± 3.389 | 0.8 ± 0.134 |
| TAA | 1.73 ± 0.141 * | 28.02 ± 2.936 * | 181.68 ± 3.052 * | 218.3 ± 3.353 * | 3.6 ± 0.249 * |
| Emp (3 mg/kg) | 2.32 ± 0.138 *@ | 14.98 ± 1.337 *@ | 98.68 ± 3.718 *@ | 121.72 ± 7.958 *@ | 2.13 ± 0.092 *@ |
| Emp (6 mg/kg) | 3.8 ± 0.157 *@# | 6.22 ± 0.674 @# | 58 ± 4.261 *@# | 87.02 ± 4.661 *@# | 1.13 ± 0.093 @# |
| Groups | GSH (nmol/mg Protein) | ATP (pg/mg Protein) | AMP (pg/mg Protein) | MDA (nmol/mg Protein) | Triglycerides (mg/dL) | Total Cholesterol (nmol/mL) |
|---|---|---|---|---|---|---|
| Normal control | 1.7 ± 0.058 | 135.72 ± 8.765 | 215.78 ± 8.913 | 0.33 ± 0.026 | 87.68 ± 7.27 | 1.24 ± 0.078 |
| TAA | 0.48 ± 0.017 * | 35.15 ± 3.338 * | 72.23 ± 3.428 * | 1.88 ± 0.02 * | 185.3 ± 6.276 * | 3.48 ± 0.117 * |
| Emp (3 mg/kg) | 0.87 ± 0.042 *@ | 67.68 ± 2.272 *@ | 138.7 ± 8.108 *@ | 1.22 ± 0.043 *@ | 157.3 ± 4.157 *@ | 2.37 ± 0.046 *@ |
| Emp (6 mg/kg) | 1.65 ± 0.034 @# | 124.48 ± 2.836 @# | 208.5 ± 6.399 @# | 0.47 ± 0.047 @# | 100 ± 7.189 @# | 1.38 ± 0.053 @# |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
ElBaset, M.A.; Salem, R.S.; Ayman, F.; Ayman, N.; Shaban, N.; Afifi, S.M.; Esatbeyoglu, T.; Abdelaziz, M.; Elalfy, Z.S. Effect of Empagliflozin on Thioacetamide-Induced Liver Injury in Rats: Role of AMPK/SIRT-1/HIF-1α Pathway in Halting Liver Fibrosis. Antioxidants 2022, 11, 2152. https://doi.org/10.3390/antiox11112152
ElBaset MA, Salem RS, Ayman F, Ayman N, Shaban N, Afifi SM, Esatbeyoglu T, Abdelaziz M, Elalfy ZS. Effect of Empagliflozin on Thioacetamide-Induced Liver Injury in Rats: Role of AMPK/SIRT-1/HIF-1α Pathway in Halting Liver Fibrosis. Antioxidants. 2022; 11(11):2152. https://doi.org/10.3390/antiox11112152
Chicago/Turabian StyleElBaset, Marwan A., Rana S. Salem, Fairouz Ayman, Nadeen Ayman, Nooran Shaban, Sherif M. Afifi, Tuba Esatbeyoglu, Mahmoud Abdelaziz, and Zahraa S. Elalfy. 2022. "Effect of Empagliflozin on Thioacetamide-Induced Liver Injury in Rats: Role of AMPK/SIRT-1/HIF-1α Pathway in Halting Liver Fibrosis" Antioxidants 11, no. 11: 2152. https://doi.org/10.3390/antiox11112152
APA StyleElBaset, M. A., Salem, R. S., Ayman, F., Ayman, N., Shaban, N., Afifi, S. M., Esatbeyoglu, T., Abdelaziz, M., & Elalfy, Z. S. (2022). Effect of Empagliflozin on Thioacetamide-Induced Liver Injury in Rats: Role of AMPK/SIRT-1/HIF-1α Pathway in Halting Liver Fibrosis. Antioxidants, 11(11), 2152. https://doi.org/10.3390/antiox11112152







