Treatment with Hydrogen-Rich Water Improves the Nociceptive and Anxio-Depressive-like Behaviors Associated with Chronic Inflammatory Pain in Mice
Abstract
:1. Introduction
2. Materials and Method
2.1. Animals
2.2. Generation of Inflammatory Pain
2.3. Mechanical Allodynia
2.4. Thermal Hyperalgesia
2.5. Depressive-like Behaviors
2.6. Anxiety-like Behaviors
2.7. Experimental Procedures
2.8. Drugs
2.9. Western Blot Analysis
2.10. Statistical Analysis
3. Results
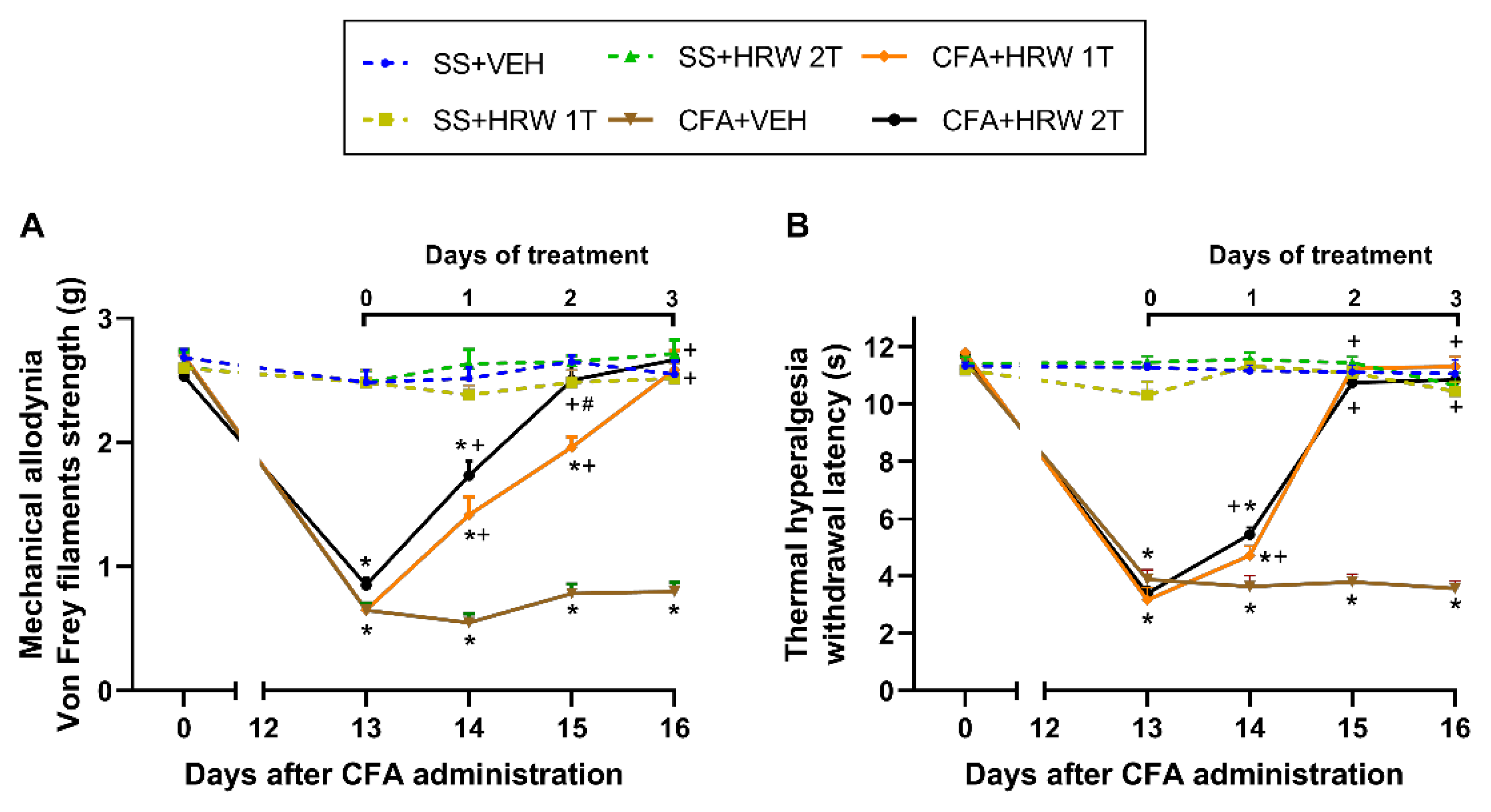
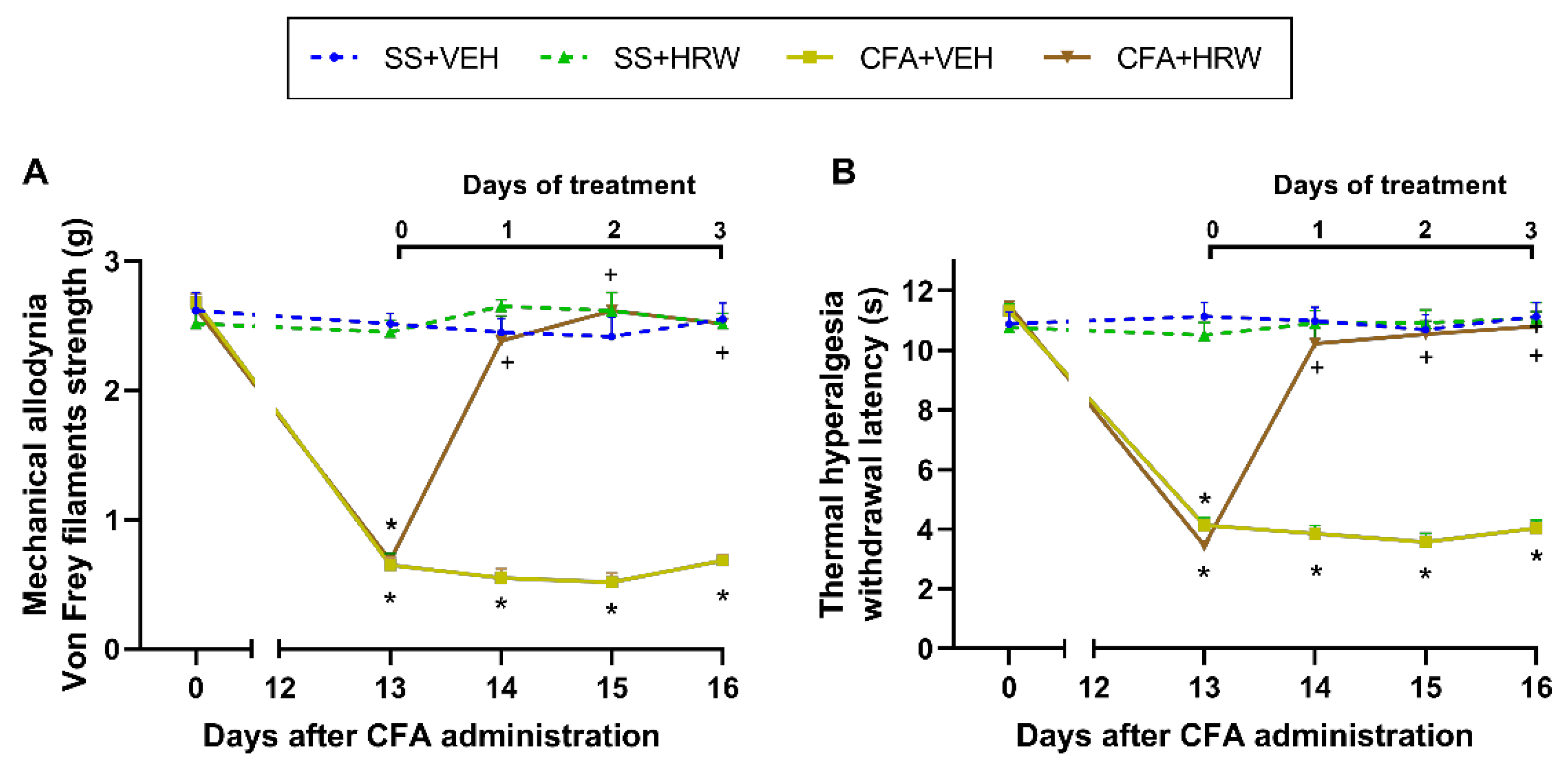
3.1. Effects of the Administration of HRW in the Mechanical Allodynia and Thermal Hyperalgesia Caused by CFA
3.2. Inhibition of the Depressive-like Behaviors Associated with Inflammatory Pain Produced by the Intraperitoneal Administration of HRW
3.3. Inhibition of the Anxiety-like Behaviors Associated with Inflammatory Pain Produced by the Intraperitoneal Administration of HRW
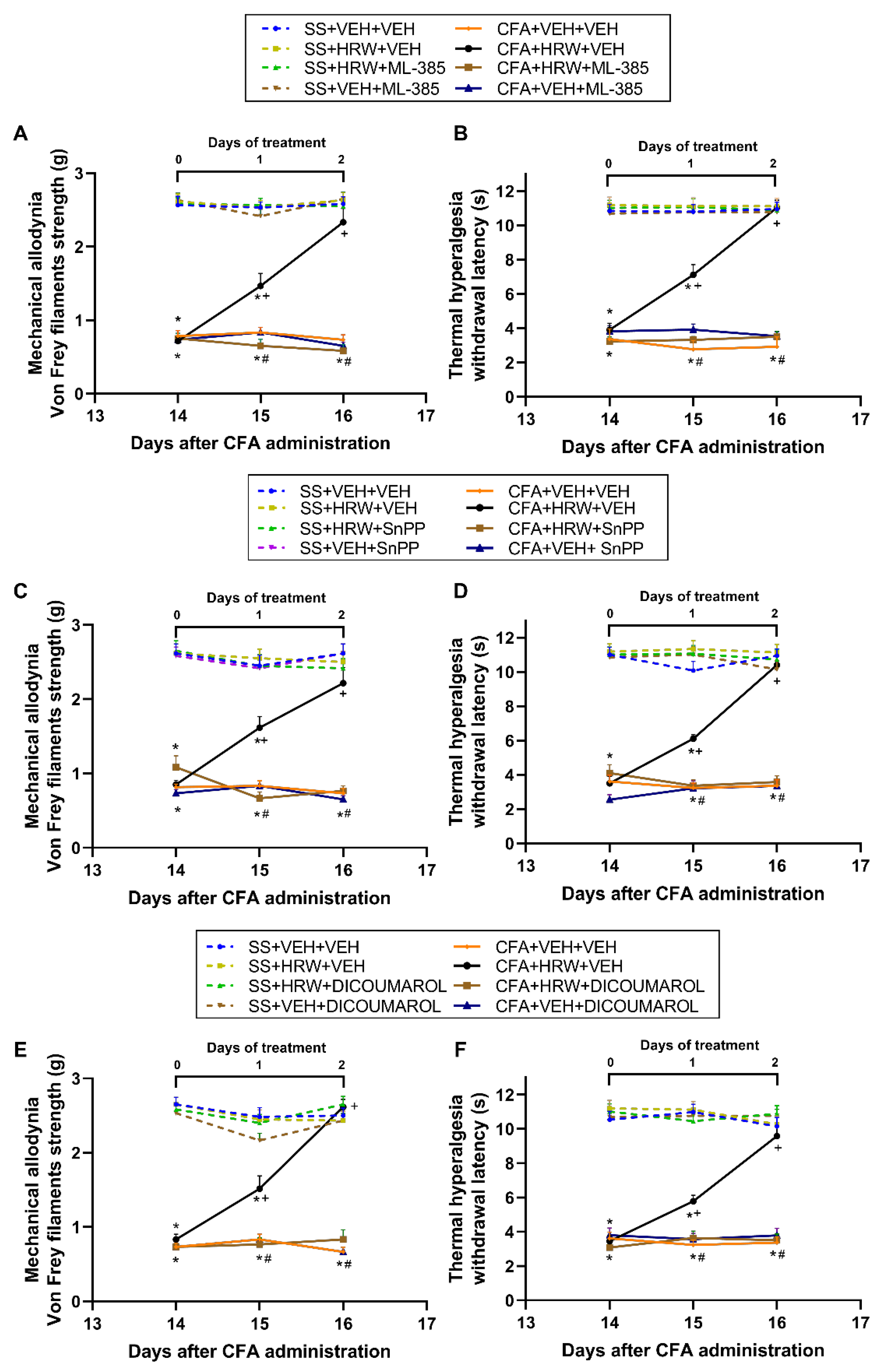
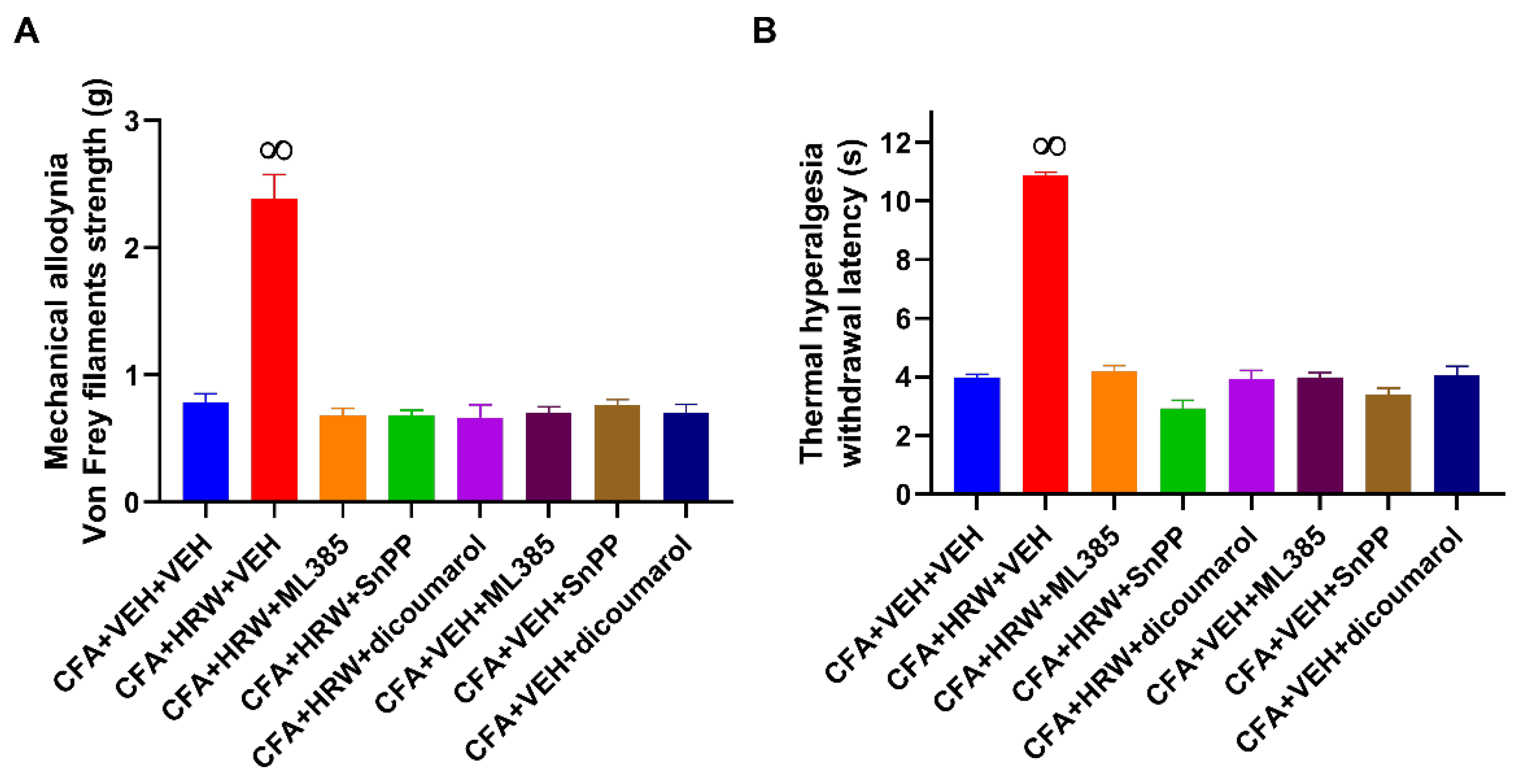
3.4. Reversion of the Antiallodynic and Antihyperalgesic Effects of HRW with Their Co-Treatment with Specific Inhibitors of the Nrf2/HO-1-NQO1 Signaling
3.5. Effects of Treatment with HRW on the Expression of 4-HNE, BAX, and p-IKBα in the Paws of Animals with Peripheral Inflammation
3.6. Effects of Treatment with HRW on the Expression of 4-HNE, BAX, and p-IKBα in the Amygdala of Animals with Paw Inflammation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Binder, A.; Baron, R. The Phar macological Therapy of Chronic Neuropathic Pain. Dtsch. Arztebl. Int. 2016, 113, 616–625. [Google Scholar]
- Narita, M.; Kaneko, C.; Miyoshi, K.; Nagumo, Y.; Kuzumaki, N.; Nakajima, M.; Nanjo, K.; Matsuzawa, K.; Yamazaki, M.; Suzuki, T. Chronic pain induces anxiety with concomitant changes in opioidergic function in the amygdala. Neuropsychopharmacology 2006, 31, 739–750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsu, Y.C.; Ma, K.H.; Guo, S.L.; Lin, B.F.; Tsai, C.S.; Yeh, C.C. The occurrence of pain- induced depression is different between rat models of inflammatory and neuropathic pain. J. Clin. Med. 2021, 10, 4016. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, B.; Verma, S. Comorbidities in chronic neuropathic pain. Pain Med. 2004, 5 (Suppl. 1), S9–S27. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Batallé, G.; Pol, O. The anxiolytic and antidepressant effects of diallyl disulfide and gyy4137 in animals with chronic neuropathic pain. Antioxidants 2021, 10, 1074. [Google Scholar] [CrossRef]
- Kremer, M.; Becker, L.J.; Barrot, M.; Yalcin, I. How to study anxiety and depression in rodent models of chronic pain? Eur. J. Neurosci. 2021, 53, 236–270. [Google Scholar] [CrossRef]
- Zhang, X.H.; Feng, C.C.; Pei, L.J.; Zhang, Y.N.; Chen, L.; Wei, X.Q.; Zhou, J.; Yong, Y.; Wang, K. Electroacupuncture Attenuates Neuropathic Pain and Comorbid Negative Behavior: The Involvement of the Dopamine System in the Amygdala. Front. Neurosci. 2021, 15, 657507. [Google Scholar] [CrossRef]
- Fang, A.; Li, Y.; Wu, X.; Wu, B.; Zhang, Y. Baicalin attenuates inflammatory pain associated depressive symptoms via Akt-mediated adult hippocampal neurogenesis. Metab. Brain Dis. 2020, 35, 1085–1093. [Google Scholar] [CrossRef]
- Huang, H.Y.; Liao, H.Y.; Lin, Y.W. Effects and Mechanisms of Electroacupuncture on Chronic Inflammatory Pain and Depression Comorbidity in Mice. Evid.-Based Complement. Alternat. Med. 2020, 2020, 495159. [Google Scholar] [CrossRef]
- Chen, J.; Song, Y.; Yang, J.; Zhang, Y.; Zhao, P.; Zhu, X.J.; Su, H.C. The contribution of TNF-α in the amygdala to anxiety in mice with persistent inflammatory pain. Neurosci. Lett. 2013, 541, 275–280. [Google Scholar] [CrossRef]
- Parent, A.J.; Beaudet, N.; Beaudry, H.; Bergeron, J.; Bérubé, P.; Drolet, G.; Sarret, P.; Gendron, L. Increased anxiety-like behaviors in rats experiencing chronic inflammatory pain. Behav. Brain Res. 2012, 229, 160–167. [Google Scholar] [CrossRef]
- Guan, S.Y.; Zhang, K.; Wang, X.S.; Yang, L.; Feng, B.; Tian, D.D.; Gao, M.R.; Liu, S.B.; Liu, A.; Zhao, M.G. Anxiolytic effects of polydatin through the blockade of neuroinflammation in a chronic pain mouse model. Mol. Pain 2020, 16, 1744806919900717. [Google Scholar] [CrossRef] [Green Version]
- Miller, R.E.; Miller, R.J.; Malfait, A.M. Osteoarthritis joint pain: The cytokine connection. Cytokine 2014, 70, 185–193. [Google Scholar] [CrossRef] [Green Version]
- Sebba, A. Pain: A Review of Interleukin-6 and Its Roles in the Pain of Rheumatoid Arthritis. Open Access Rheumatol. Res. Rev. 2021, 13, 31–43. [Google Scholar] [CrossRef]
- Yang, Y.; Sheng, Q.; Nie, Z.; Liu, L.; Zhang, W.; Chen, G.; Ye, F.; Shi, L.; Lv, Z.; Xie, J.; et al. Daphnetin inhibits spinal glial activation via Nrf2/HO-1/NF-κB signaling pathway and attenuates CFA-induced inflammatory pain. Int. Immunopharmacol. 2021, 98, 107882. [Google Scholar] [CrossRef] [PubMed]
- Redondo, A.; Riego, G.; Pol, O. The Antinociceptive, Antioxidant and Anti-Inflammatory Effects of 5-Fluoro-2-Oxindole during Inflammatory Pain. Antioxidants 2020, 9, 1249. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-κB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Semmler, A.; Okulla, T.; Sastre, M.; Dumitrescu-Ozimek, L.; Heneka, M.T. Systemic inflammation induces apoptosis with variable vulnerability of different brain regions. J. Chem. Neuroanat. 2005, 30, 144–157. [Google Scholar] [CrossRef]
- Bhatt, S.; Nagappa, A.N.; Patil, C.R. Role of oxidative stress in depression. Drug Discov. Today 2020, 25, 1270–1276. [Google Scholar] [CrossRef]
- Hashimoto, K. Essential Role of Keap1-Nrf2 Signaling in Mood Disorders: Overview and Future Perspective. Front. Pharmacol. 2018, 9, 1182. [Google Scholar] [CrossRef]
- Ferreira-Chamorro, P.; Redondo, A.; Riego, G.; Leánez, S.; Pol, O. Sulforaphane inhibited the nociceptive responses, anxiety-And depressive-like behaviors associated with neuropathic pain and improved the anti-allodynic effects of morphine in mice. Front. Pharmacol. 2018, 9, 1332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Díaz, A.F.; Polo, S.; Gallardo, N.; Leánez, S.; Pol, O. Analgesic and antidepressant effects of oltipraz on neuropathic pain in mice by modulating microglial activation. J. Clin. Med. 2019, 8, 890. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yao, W.; Zhang, J.C.; Ishima, T.; Ren, Q.; Yang, C.; Dong, C.; Ma, M.; Saito, A.; Honda, T.; Hashimoto, K. Antidepressant effects of TBE-31 and MCE-1, the novel Nrf2 activators, in an inflammation model of depression. Eur. J. Pharmacol. 2016, 793, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Zhang, H.T.; Qin, S.C. Neuroprotective Effects of Molecular Hydrogen: A Critical Review. Neurosci. Bull. 2021, 37, 389–404. [Google Scholar] [CrossRef] [PubMed]
- Fang, W.; Tang, L.; Wang, G.; Lin, J.; Liao, W.; Pan, W.; Xu, J. Molecular Hydrogen Protects Human Melanocytes from Oxidative Stress by Activating Nrf2 Signaling. J. Investig. Dermatol. 2020, 140, 2230–2241.e9. [Google Scholar] [CrossRef]
- Ross, D.; Siegel, D. The diverse functionality of NQO1 and its roles in redox control. Redox Biol. 2021, 41, 101950. [Google Scholar] [CrossRef]
- Zhao, C.; Yu, S.; Li, J.; Xu, W.; Ge, R. Changes in IL-4 and IL-13 expression in allergic-rhinitis treated with hydrogen-rich saline in guinea-pig model. Allergol. Immunopathol. 2017, 45, 350–355. [Google Scholar] [CrossRef]
- Fang, S.; Li, X.; Wei, X.; Zhang, Y.; Ma, Z.; Wei, Y.; Wang, W. Beneficial effects of hydrogen gas inhalation on a murine model of allergic rhinitis. Exp. Ther. Med. 2018, 16, 5178–5184. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Su, W.J.; Chen, Y.; Wu, T.Y.; Gong, H.; Shen, X.L.; Wang, Y.X.; Sun, X.J.; Jiang, C.L. Effects of hydrogen-rich water on depressive-like behavior in mice. Sci. Rep. 2016, 6, 23742. [Google Scholar] [CrossRef] [Green Version]
- Mizuno, K.; Sasaki, A.T.; Ebisu, K.; Tajima, K.; Kajimoto, O.; Nojima, J.; Kuratsune, H.; Hori, H.; Watanabe, Y. Hydrogen-rich water for improvements of mood, anxiety, and autonomic nerve function in daily life. Med. Gas Res. 2018, 388, 247–255. [Google Scholar]
- Ge, Y.; Wu, F.; Sun, X.; Xiang, Z.; Yang, L.; Huang, S.; Lu, Z.; Sun, Y.; Yu, W.F. Intrathecal infusion of hydrogen-rich normal saline attenuates neuropathic pain via inhibition of activation of spinal astrocytes and microglia in rats. PLoS ONE. 2014, 9, e97436. [Google Scholar] [CrossRef]
- Kawaguchi, M.; Satoh, Y.; Otsubo, Y.; Kazama, T. Molecular hydrogen attenuates neuropathic pain in mice. PLoS ONE 2014, 9, e100352. [Google Scholar] [CrossRef]
- Muley, M.M.; Krustev, E.; Mcdougall, J.J. Preclinical Assessment of Inflammatory Pain. CNS Neurosci. Ther. 2016, 22, 88–101. [Google Scholar] [CrossRef]
- Redondo, A.; Chamorro, P.A.F.; Riego, G.; Leánez, S.; Pol, O. Treatment with sulforaphane produces antinociception and improves morphine effects during inflammatory pain in mice. J. Pharmacol. Exp. Ther. 2017, 363, 293–302. [Google Scholar] [CrossRef] [Green Version]
- Steru, L.; Chermat, R.; Thierry, B.; Simon, P. The tail suspension test: A new method for screening antidepressants in mice. Psychopharmacology 1985, 85, 367–370. [Google Scholar] [CrossRef]
- Porsolt, R.D.; Le Pichon, M.; Jalfre, M. Depression: A new animal model sensitive to antidepressant treatments. Nature 1977, 266, 730–732. [Google Scholar] [CrossRef]
- La Porta, C.; Lara-Mayorga, I.M.; Negrete, R.; Maldonado, R. Effects of pregabalin on the nociceptive, emotional and cognitive manifestations of neuropathic pain in mice. Eur. J. Pain. 2016, 20, 1454–1466. [Google Scholar] [CrossRef] [Green Version]
- Carcolé, M.; Zamanillo, D.; Merlos, M.; Fernández-Pastor, B.; Cabañero, D.; Maldonado, R. Blockade of the Sigma-1 Receptor Relieves Cognitive and Emotional Impairments Associated to Chronic Osteoarthritis Pain. Front. Pharmacol. 2019, 10, 468. [Google Scholar] [CrossRef] [Green Version]
- Kraeuter, A.K.; Guest, P.C.; Sarnyai, Z. The Elevated Plus Maze Test for Measuring Anxiety-like Behavior in Rodents. In Pre-Clinical Models; Guest, P., Ed.; Humana Press: New York, NY, USA, 2018; Volume 1916, pp. 69–74. [Google Scholar]
- Kraeuter, A.K.; Guest, P.C.; Sarnyai, Z. The Open Field Test for Measuring Locomotor Activity and Anxiety-like Behavior. In Pre-Clinical Models; Guest, P., Ed.; Humana Press: New York, NY, USA, 2018; Volume 1916, pp. 99–103. [Google Scholar]
- Martínez-Serrat, M.; Martínez-Martel, I.; Coral-Pérez, S.; Bai, X.; Batallé, G.; Pol, O. Hydrogen-Rich Water as a Novel Therapeutic Strategy for the Affective Disorders Linked with Chronic Neuropathic Pain in Mice. Antioxidants 2022, 11, 1826. [Google Scholar] [CrossRef]
- Singh, A.; Venkannagari, S.; Oh, K.H.; Zhang, Y.Q.; Rohde, J.M.; Liu, L.; Nimmagadda, S.; Sudini, K.; Brimacombe, K.R.; Gajghate, S.; et al. Small Molecule Inhibitor of NRF2 Selectively Intervenes Therapeutic Resistance in KEAP1-Deficient NSCLC Tumors. ACS Chem. Biol. 2016, 11, 3214–3225. [Google Scholar] [CrossRef] [Green Version]
- Tseng, C.K.; Lin, C.K.; Wu, Y.H.; Chen, Y.H.; Chen, W.C.; Young, K.C.; Lee, J.C. Human heme oxygenase 1 is a potential host cell factor against dengue virus replication. Sci. Rep. 2016, 6, 32176. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.T.; Hu, J.L.; Ren, J.H.; Yu, H.B.; Zhong, S.; Wai Wong, V.K.; Kwan Law, B.Y.; Chen, W.X.; Xu, H.M.; Zhang, Z.Z.; et al. Dicoumarol, an NQO1 inhibitor, blocks cccDNA transcription by promoting degradation of HBx. J. Hepatol. 2021, 74, 522–534. [Google Scholar] [CrossRef] [PubMed]
- Porta, A.; Rodríguez, L.; Bai, X.; Batallé, G.; Roch, G.; Pouso-Vázquez, E.; Balboni, G.; Pol, O. Hydrogen sulfide inhibits inflammatory pain and enhances the analgesic properties of delta opioid receptors. Antioxidants 2021, 10, 1977. [Google Scholar] [CrossRef] [PubMed]
- Iketani, M.; Ohsawa, I. Molecular Hydrogen as a Neuroprotective Agent. Curr. Neuropharmacol. 2017, 15, 324–331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tian, Y.; Zhang, Y.; Wang, Y.; Chen, Y.; Fan, W.; Zhou, J.; Qiao, J.; Wei, Y. Hydrogen, a Novel Therapeutic Molecule, Regulates Oxidative Stress, Inflammation, and Apoptosis. Front. Physiol. 2021, 12, 789507. [Google Scholar] [CrossRef]
- Sheng, J.; Liu, S.; Wang, Y.; Cui, R.; Zhang, X. The Link between Depression and Chronic Pain: Neural Mechanisms in the Brain. Neural Plast. 2017, 2017, 9724371. [Google Scholar] [CrossRef] [Green Version]
- Cunha, A.M.; Pereira-Mendes, J.; Almeida, A.; Guimarães, M.R.; Leite-Almeida, H. Chronic pain impact on rodents’ behavioral repertoire. Neurosci. Biobehav. Rev. 2020, 119, 101–127. [Google Scholar] [CrossRef]
- Roch, G.; Batallé, G.; Bai, X.; Pouso-Vázquez, E.; Rodríguez, L.; Pol, O. The Beneficial Effects of Heme Oxygenase 1 and Hydrogen Sulfide Activation in the Management of Neuropathic Pain, Anxiety- and Depressive-like Effects of Paclitaxel in Mice. Antioxidants 2022, 11, 122. [Google Scholar] [CrossRef]
- Gao, Q.; Song, H.; Wang, X.T.; Liang, Y.; Xi, Y.J.; Gao, Y.; Guo, Q.J.; LeBaron, T.; Luo, Y.X.; Li, S.C.; et al. Molecular hydrogen increases resilience to stress in mice. Sci. Rep. 2017, 7, 9625. [Google Scholar] [CrossRef]
- Wen, D.; Zhao, P.; Hui, R.; Wang, J.; Shen, Q.; Gong, M.; Guo, H.; Cong, B.; Ma, C. Hydrogen-rich saline attenuates anxiety-like behaviors in morphine-withdrawn mice. Neuropharmacology 2017, 118, 199–208. [Google Scholar] [CrossRef]
- Lillywhite, A.; Woodhams, S.G.; Goncalves, S.V.; Watson, D.J.G.; Li, L.; Burston, J.J.; Gowler, P.R.W.; Canals, M.; Walsh, D.A.; Hathway, G.J.; et al. Anxiety enhances pain in a model of osteoarthritis and is associated with altered endogenous opioid function and reduced opioid analgesia. Pain Rep. 2021, 6, e956. [Google Scholar] [CrossRef]
- Li, Y.; Shen, C.; Zhou, X.; Zhang, J.; Lai, X.; Zhang, Y. Local Treatment of Hydrogen-Rich Saline Promotes Wound Healing in Vivo by Inhibiting Oxidative Stress via Nrf-2/HO-1 Pathway. Oxidative Med. Cell. Longev. 2022, 2022, 2949824. [Google Scholar] [CrossRef]
- Batallé, G.; Bai, X.; Pouso-Vázquez, E.; Roch, G.; Rodríguez, L.; Pol, O. The recovery of cognitive and affective deficiencies linked with chronic osteoarthritis pain and implicated pathways by slow-releasing hydrogen sulfide treatment. Antioxidants 2021, 10, 1632. [Google Scholar] [CrossRef]
- Imagawa, K.; de Andrés, M.C.; Hashimoto, K.; Pitt, D.; Itoi, E.; Goldring, M.B.; Roach, H.I.; Oreffo, R.O. The epigenetic effect of glucosamine and a nuclear factor-kappa B (NF-kB) inhibitor on primary human chondrocytes--implications for osteoarthritis. Biochem. Biophys. Res. Commun. 2011, 405, 362–367. [Google Scholar] [CrossRef] [Green Version]
- Lee, K.M.; Kang, B.S.; Lee, H.L.; Son, S.J.; Hwang, S.H.; Kim, D.S.; Park, J.S.; Cho, H.J. Spinal NF-kB activation induces COX-2 upregulation and contributes to inflammatory pain hypersensitivity. Eur. J. Neurosci. 2004, 12, 3375–3381. [Google Scholar] [CrossRef]
- Xia, Z.B.; Yuan, Y.J.; Zhang, Q.H.; Li, H.; Dai, J.L.; Min, J.K. Salvianolic Acid B Suppresses Inflammatory Mediator Levels by Downregulating NF-κB in a Rat Model of Rheumatoid Arthritis. Med. Sci. Monit. 2018, 24, 2524–2532. [Google Scholar] [CrossRef] [Green Version]
- Liao, D.; Lv, C.; Cao, L.; Yao, D.; Wu, Y.; Long, M.; Liu, N.; Jiang, P. Curcumin Attenuates Chronic Unpredictable Mild Stress-Induced Depressive-Like Behaviors via Restoring Changes in Oxidative Stress and the Activation of Nrf2 Signaling Pathway in Rats. Oxidative Med. Cell. Longev. 2020, 2020, 9268083. [Google Scholar] [CrossRef]
- Cazuza, R.A.; Batallé, G.; Bai, X.; Leite-Panissi, C.R.A.; Pol, O. Effects of treatment with a carbon monoxide donor and an activator of heme oxygenase 1 on the nociceptive, apoptotic and/or oxidative alterations induced by persistent inflammatory pain in the central nervous system of mice. Brain Res. Bull. 2022, 188, 169–178. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coral-Pérez, S.; Martínez-Martel, I.; Martínez-Serrat, M.; Batallé, G.; Bai, X.; Leite-Panissi, C.R.A.; Pol, O. Treatment with Hydrogen-Rich Water Improves the Nociceptive and Anxio-Depressive-like Behaviors Associated with Chronic Inflammatory Pain in Mice. Antioxidants 2022, 11, 2153. https://doi.org/10.3390/antiox11112153
Coral-Pérez S, Martínez-Martel I, Martínez-Serrat M, Batallé G, Bai X, Leite-Panissi CRA, Pol O. Treatment with Hydrogen-Rich Water Improves the Nociceptive and Anxio-Depressive-like Behaviors Associated with Chronic Inflammatory Pain in Mice. Antioxidants. 2022; 11(11):2153. https://doi.org/10.3390/antiox11112153
Chicago/Turabian StyleCoral-Pérez, Santiago, Ignacio Martínez-Martel, Maria Martínez-Serrat, Gerard Batallé, Xue Bai, Christie R. A. Leite-Panissi, and Olga Pol. 2022. "Treatment with Hydrogen-Rich Water Improves the Nociceptive and Anxio-Depressive-like Behaviors Associated with Chronic Inflammatory Pain in Mice" Antioxidants 11, no. 11: 2153. https://doi.org/10.3390/antiox11112153
APA StyleCoral-Pérez, S., Martínez-Martel, I., Martínez-Serrat, M., Batallé, G., Bai, X., Leite-Panissi, C. R. A., & Pol, O. (2022). Treatment with Hydrogen-Rich Water Improves the Nociceptive and Anxio-Depressive-like Behaviors Associated with Chronic Inflammatory Pain in Mice. Antioxidants, 11(11), 2153. https://doi.org/10.3390/antiox11112153






