Vitamin K2 (MK-7) Intercepts Keap-1/Nrf-2/HO-1 Pathway and Hinders Inflammatory/Apoptotic Signaling and Liver Aging in Naturally Aging Rat
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Experimental Design
2.2. Biochemical Analysis of Liver Function Tests
2.3. Liver HO-1 and TIMP Assay by ELISA
2.4. Real-Time RT-PCR Assay of Liver Nrf-2 and Keap-1
2.5. Histological Study
2.6. Immunohistochemical (IHC) Analysis of Tumor Necrosis Factor-α (TNF-α), COX-2, iNOS and TGF-β1
2.7. Transmission Electron Microscope
2.8. DNA Fragmentation
2.9. Statistical Analysis
3. Results
3.1. Biochemical Analysis of Liver Functions
3.2. Effect of VK2 on Aging-Induced Hepatic Cellular Structural and Ultrastructural Changes
3.3. Effect of VK2 on Hepatic Keap-1/Nrf-2/HO-1 Axis in Aged Rats
3.4. Effect of VK2 on Hepatic COX-2, iNOS and TNF-α
3.5. Effect of VK2 on Hepatic TIMP-1 and TGF-β in Aged Rats
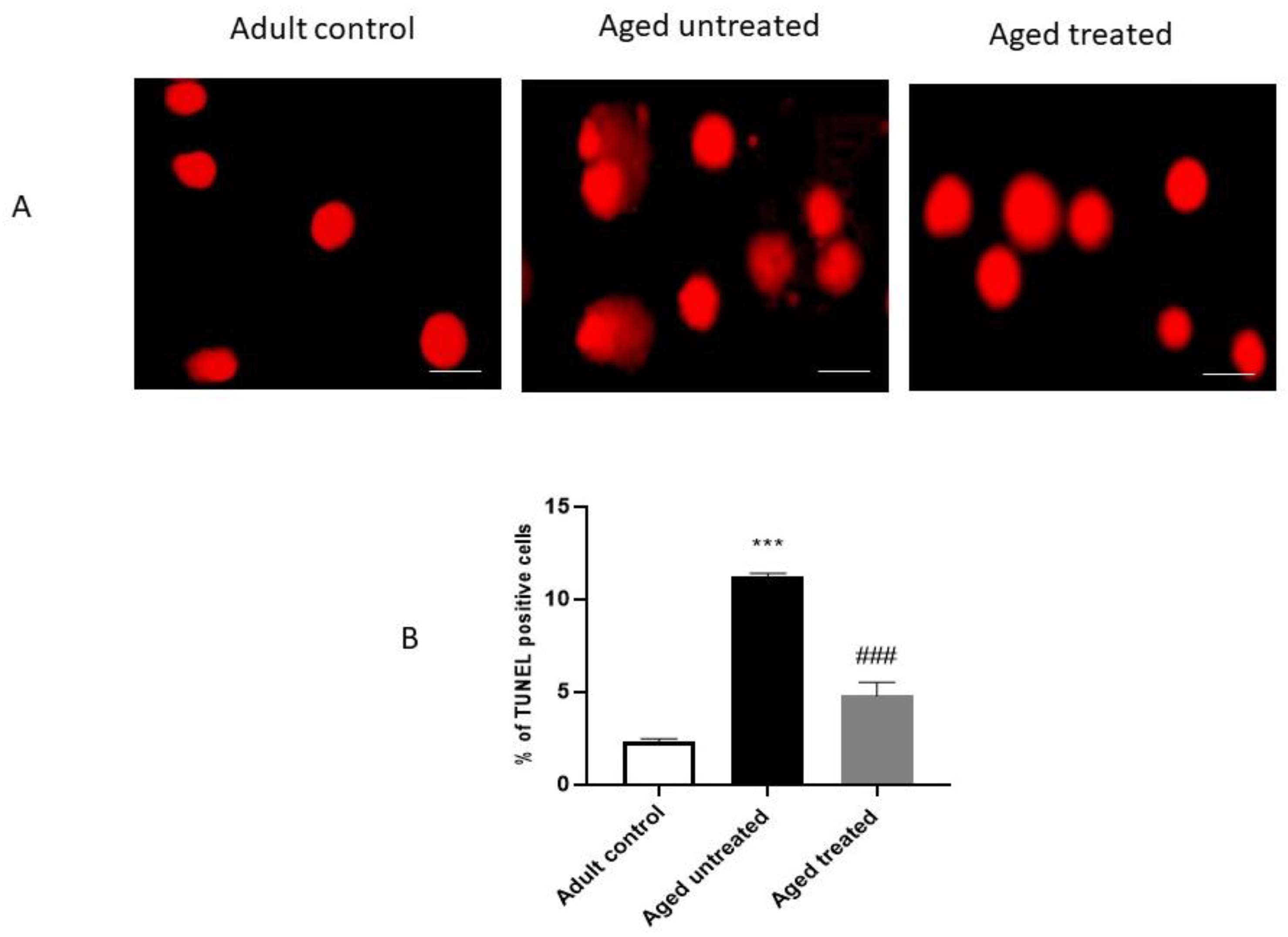
3.6. Effect of VK2 on Hepatic Apoptosis in Aged Rats
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rusu, M.E.; Georgiu, C.; Pop, A.; Mocan, A.; Kiss, B.; Vostinaru, O.; Fizesan, I.; Stefan, M.-G.; Gheldiu, A.-M.; Mates, L.; et al. Antioxidant Effects of Walnut (Juglans regia L.) Kernel and Walnut Septum Extract in a D-Galactose-Induced Aging Model and in Naturally Aged Rats. Antioxidants 2020, 9, 424. [Google Scholar] [CrossRef] [PubMed]
- Luo, D.; Li, J.; Chen, K.; Yin, Y.; Fang, Z.; Pang, H.; Rong, X.; Guo, J. Study on Metabolic Trajectory of Liver Aging and the Effect of Fufang Zhenzhu Tiaozhi on Aging Mice. Front. Pharmacol. 2019, 10, 926. [Google Scholar] [CrossRef] [PubMed]
- Łysek-Gładysińska, M.; Wieczorek, A.; Jóźwik, A.; Walaszczyk, A.; Jelonek, K.; Szczukiewicz-Markowska, G.; Horbańczuk, O.; Pietrowska, M.; Widłak, P.; Gabryś, D. Aging-Related Changes in the Ultrastructure of Hepatocytes and Cardiomyocytes of Elderly Mice Are Enhanced in ApoE-Deficient Animals. Cells 2021, 10, 502. [Google Scholar] [CrossRef] [PubMed]
- Marcos, R.; Correia-Gomes, C. Long live the liver: Immunohistochemical and stereological study of hepatocytes, liver sinusoidal endothelial cells, Kupffer cells and hepatic stellate cells of male and female rats throughout ageing. Cell Tissue Res. 2016, 366, 639–649. [Google Scholar] [CrossRef]
- Hunt, N.J.; Kang, S.W.S.; Lockwood, G.P.; Le Couteur, D.G.; Cogger, V.C. Hallmarks of Aging in the Liver. Comput. Struct. Biotechnol. J. 2019, 17, 1151–1161. [Google Scholar] [CrossRef]
- Mahrouf-Yorgov, M.; de L’Hortet, A.C.; Cosson, C.; Slama, A.; Abdoun, E.; Guidotti, J.-E.; Fromenty, B.; Mitchell, C.; Gilgenkrantz, H. Increased susceptibility to liver fibrosis with age is correlated with an altered inflammatory response. Rejuvenation Res. 2011, 14, 353–363. [Google Scholar] [CrossRef] [PubMed]
- Saleh, D.O.; Mansour, D.F.; Hashad, I.M.; Bakeer, R.M. Effects of sulforaphane on D-galactose-induced liver aging in rats: Role of keap-1/nrf-2 pathway. Eur. J. Pharmacol. 2019, 855, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Younis, N.N.; Elsherbiny, N.M.; Shaheen, M.A.; Elseweidy, M.M. Modulation of NADPH oxidase and Nrf2/HO-1 pathway by vanillin in cisplatin-induced nephrotoxicity in rats. J. Pharm. Pharmacol. 2020, 72, 1546–1555. [Google Scholar] [CrossRef] [PubMed]
- Alsheblak, M.M.; Elsherbiny, N.M.; El-Karef, A.; El-Shishtawy, M.M. Protective effects of L-carnosine on CCl4 -induced hepatic injury in rats. Eur. Cytokine Netw. 2016, 27, 6–15. [Google Scholar] [CrossRef]
- Bellezza, I.; Giambanco, I.; Minelli, A.; Donato, R. Nrf2-Keap1 signaling in oxidative and reductive stress. Biochim. Biophys. Acta Mol. Cell Res. 2018, 1865, 721–733. [Google Scholar] [CrossRef]
- Afanas’ev, I. Superoxide and nitric oxide in senescence and aging. Front Biosci. 2009, 14, 3899–3912. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Omidkhoda, S.F.; Mehri, S.; Heidari, S.; Hosseinzadeh, H. Protective Effects of Crocin Against Hepatic Damages in D-galactose Aging Model in Rats. Iran J. Pharm. Res. 2020, 19, 440–450. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.M.; Chen, X.M.; Wu, D.; Shi, S.-Z.; Yin, Z.; Ding, R.; Lü, Y. Expression of tissue inhibitor of matrix metalloproteinases-1 during aging in rat liver. World J. Gastroenterol. 2005, 11, 3696–3700. [Google Scholar] [CrossRef]
- Li, Y.; Fan, W.; Link, F.; Wang, S.; Dooley, S. Transforming growth factor β latency: A mechanism of cytokine storage and signalling regulation in liver homeostasis and disease. JHEP Rep. 2022, 4, 100397. [Google Scholar] [CrossRef]
- Fabregat, I.; Caballero-Díaz, D. Transforming Growth Factor-β-Induced Cell Plasticity in Liver Fibrosis and Hepatocarcinogenesis. Front. Oncol. 2018, 8, 357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Braasch-Turi, M.; Crans, D.C. Synthesis of Naphthoquinone Derivatives: Menaquinones, Lipoquinones and Other Vitamin K Derivatives. Molecules 2020, 25, 4477. [Google Scholar] [CrossRef]
- Licata, A.; Zerbo, M.; Como, S.; Cammilleri, M.; Soresi, M.; Montalto, G.; Giannitrapani, L. The Role of Vitamin Deficiency in Liver Disease: To Supplement or Not Supplement? Nutrients 2021, 13, 4014. [Google Scholar] [CrossRef]
- Capozzi, A.; Scambia, G.; Migliaccio, S.; Lello, S. Role of vitamin K2 in bone metabolism: A point of view and a short reappraisal of the literature. Gynecol. Endocrinol. 2020, 36, 285–288. [Google Scholar] [CrossRef]
- Villa, J.K.D.; Diaz, M.A.N.; Pizziolo, V.R.; Martino, H.S.D. Effect of vitamin K in bone metabolism and vascular calcification: A review of mechanisms of action and evidences. Crit. Rev. Food Sci. Nutr. 2017, 57, 3959–3970. [Google Scholar] [CrossRef]
- Harshman, S.G.; Shea, M.K. The Role of Vitamin K in Chronic Aging Diseases: Inflammation, Cardiovascular Disease, and Osteoarthritis. Curr. Nutr. Rep. 2016, 5, 90–98. [Google Scholar] [CrossRef]
- Li, J.; Lin, J.C.; Wang, H.; Peterson, J.W.; Furie, B.C.; Furie, B.; Booth, S.L.; Volpe, J.J.; Rosenberg, P.A. Novel role of vitamin k in preventing oxidative injury to developing oligodendrocytes and neurons. J. Neurosci. 2003, 23, 5816–5826. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Otsuka, M.; Kato, N.; Shao, R.-X.; Hoshida, Y.; Ijichi, H.; Koike, Y.; Taniguchi, H.; Moriyama, M.; Shiratori, Y.; Kawabe, T.; et al. Vitamin K2 inhibits the growth and invasiveness of hepatocellular carcinoma cells via protein kinase A activation. Hepatology 2004, 40, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Sultana, H.; Komai, M.; Shirakawa, H. The Role of Vitamin K in Cholestatic Liver Disease. Nutrients 2021, 13, 2515. [Google Scholar] [CrossRef] [PubMed]
- Habu, D.; Shiomi, S.; Tamori, A.; Takeda, T.; Tanaka, T.; Kubo, S.; Nishiguchi, S. Role of vitamin K2 in the development of hepatocellular carcinoma in women with viral cirrhosis of the liver. JAMA 2004, 292, 358–361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kakizaki, S.; Sohara, N.; Sato, K.; Suzuki, H.; Yanagisawa, M.; Nakajima, H.; Takagi, H.; Naganuma, A.; Otsuka, T.; Takahashi, H.; et al. Preventive effects of vitamin K on recurrent disease in patients with hepatocellular carcinoma arising from hepatitis C viral infection. J. Gastroenterol. Hepatol. 2007, 22, 518–522. [Google Scholar] [CrossRef] [PubMed]
- Simes, D.C.; Viegas, C.S.B.; Araújo, N.; Marreiros, C. Vitamin K as a Powerful Micronutrient in Aging and Age-Related Diseases: Pros and Cons from Clinical Studies. Int. J. Mol. Sci. 2019, 20, 4150. [Google Scholar] [CrossRef] [Green Version]
- Elkattawy, H.A.; Ghoneim, F.M.; Eladl, M.A.; Said, E.; Ebrahim, H.A.; El-Shafey, M.; Asseri, S.M.; El-Sherbiny, M.; Alsalamah, R.H.; Elsherbiny, N.M.; et al. Vitamin K2 (Menaquinone-7) Reverses Age-Related Structural and Cognitive Deterioration in Naturally Aging Rats. Antioxidants 2022, 11, 514. [Google Scholar] [CrossRef]
- Popa, D.S.; Bigman, G.; Rusu, M.E. The Role of Vitamin K in Humans: Implication in Aging and Age-Associated Diseases. Antioxidants 2021, 10, 566. [Google Scholar] [CrossRef]
- Kim, I.H.; Kisseleva, T.; Brenner, D.A. Aging and liver disease. Curr. Opin. Gastroenterol. 2015, 31, 184–191. [Google Scholar] [CrossRef] [Green Version]
- Le Couteur, D.G.; Warren, A.; Cogger, V.C.; Smedsrød, B.; Sørensen, K.K.; De Cabo, R.; Fraser, R.; Mccuskey, R.S. Old age and the hepatic sinusoid. Anat. Rec. 2008, 291, 672–683. [Google Scholar] [CrossRef]
- Floreani, A. Liver diseases in the elderly: An update. Dig. Dis. 2007, 25, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Sheedfar, F.; Di Biase, S.; Koonen, D.; Vinciguerra, M. Liver diseases and aging: Friends or foes? Aging Cell 2013, 12, 950–954. [Google Scholar] [CrossRef] [PubMed]
- Sanz, N.; Díez-Fernández, C.; Alvarez, A.M.; Fernández-Simón, L.; Cascales, M. Age-related changes on parameters of experimentally-induced liver injury and regeneration. Toxicol. Appl. Pharmacol. 1999, 154, 40–49. [Google Scholar] [CrossRef]
- Höhn, A.; Grune, T. Lipofuscin: Formation, effects and role of macroautophagy. Redox Biol. 2013, 1, 140–144. [Google Scholar] [CrossRef] [Green Version]
- Hu, S.-J.; Jiang, S.-S.; Zhang, J.; Luo, D.; Yu, B.; Yang, L.-Y.; Zhong, H.; Yang, M.-W.; Liu, L.-Y.; Hong, F.-F.; et al. Effects of apoptosis on liver aging. World J. Clin. Cases 2019, 7, 691–704. [Google Scholar] [CrossRef]
- Poulose, N.; Raju, R. Aging and injury: Alterations in cellular energetics and organ function. Aging Dis. 2014, 5, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Navarro, A.; Boveris, A. The mitochondrial energy transduction system and the aging process. Am. J. Physiol. Cell Physiol. 2007, 292, C670–C686. [Google Scholar] [CrossRef]
- Joaquin, A.M.; Gollapudi, S. Functional decline in aging and disease: A role for apoptosis. J. Am. Geriatr. Soc. 2001, 49, 1234–1240. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Q.Q.; Guo, X.H.; Zhang, H.Y.; Liu, L.X. IGFBPrP1 induces liver fibrosis by inducing hepatic stellate cell activation and hepatocyte apoptosis via Smad2/3 signaling. World J. Gastroenterol. 2014, 20, 6523–6533. [Google Scholar] [CrossRef]
- Harman, D. Aging: A theory based on free radical and radiation chemistry. J. Gerontol. 1956, 11, 298–300. [Google Scholar] [CrossRef]
- Collins, B.H.; Holzknecht, Z.E.; Lynn, K.A.; Sempowski, G.D.; Smith, C.C.; Liu, S.; Parker, W.; Rockey, D.C. Association of age-dependent liver injury and fibrosis with immune cell populations. Liver Int. 2013, 33, 1175–1186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, X.; Ma, P.; Kong, L.; Wang, X.; Wang, Y.; Jiang, L. Vitamin K2 Inhibits Hepatocellular Carcinoma Cell Proliferation by Binding to 17β-Hydroxysteroid Dehydrogenase 4. Front. Oncol. 2021, 11, 757603. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.; Schurgers, L.J.; Shiels, P.G.; Stenvinkel, P. Early vascular ageing in chronic kidney disease: Impact of inflammation, vitamin K, senescence and genomic damage. Nephrol. Dial. Transplant. 2020, 35 (Suppl. 2), ii31–ii37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elsherbiny, N.M.; Eisa, N.H.; El-Sherbiny, M.; Said, E. Chemo-preventive effect of crocin against experimentally-induced hepatocarcinogenesis via regulation of apoptotic and Nrf2 signaling pathways. Environ. Toxicol. Pharmacol. 2020, 80, 103494. [Google Scholar] [CrossRef]
- Fahmy, E.K.; El-Sherbiny, M.; Said, E.; Elkattawy, H.A.; Qushawy, M.; Elsherbiny, N. Tranilast ameliorated subchronic silver nanoparticles-induced cerebral toxicity in rats: Effect on TLR4/NLRP3 and Nrf-2. Neurotoxicology 2021, 82, 167–176. [Google Scholar] [CrossRef]
- El-Sherbiny, M.; Fahmy, E.K.; Eisa, N.H.; Said, E.; Elkattawy, H.A.; Ebrahim, H.A.; Elsherbiny, N.M.; Ghoneim, F.M. Nanogold Particles Suppresses 5-Flurouracil-Induced Renal Injury: An Insight into the Modulation of Nrf-2 and Its Downstream Targets, HO-1 and γ-GCS. Molecules 2021, 26, 7684. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, J.; Duan, H.; Li, R.; Peng, W.; Wu, C. Activation of Nrf2/HO-1 signaling: An important molecular mechanism of herbal medicine in the treatment of atherosclerosis via the protection of vascular endothelial cells from oxidative stress. J. Adv. Res. 2021, 34, 43–63. [Google Scholar] [CrossRef]
- Mukai, K.; Itoh, S.; Morimoto, H. Stopped-flow kinetic study of vitamin E regeneration reaction with biological hydroquinones (reduced forms of ubiquinone, vitamin K, and tocopherolquinone) in solution. J. Biol. Chem. 1992, 267, 22277–22281. [Google Scholar] [CrossRef]
- Gong, X.; Gutala, R.; Jaiswal, A.K. Quinone oxidoreductases and vitamin K metabolism. Vitam. Horm. 2008, 78, 85–101. [Google Scholar] [CrossRef]
- Vos, M.; Esposito, G.; Edirisinghe, J.N.; Vilain, S.; Haddad, D.M.; Slabbaert, J.R.; Van Meensel, S.; Schaap, O.; De Strooper, B.; Meganathan, R.; et al. Vitamin K2 is a mitochondrial electron carrier that rescues pink1 deficiency. Science 2012, 336, 1306–1310. [Google Scholar] [CrossRef]
- Alisi, L.; Cao, R.; De Angelis, C.; Cafolla, A.; Caramia, F.; Cartocci, G.; Librando, A.; Fiorelli, M. The Relationships Between Vitamin K and Cognition: A Review of Current Evidence. Front. Neurol. 2019, 10, 239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kołakowski, A.; Kurzyna, P.F.; Bzdęga, W.; Żywno, H.; Harasim-Symbor, E.; Chabowski, A.; Konstantynowicz-Nowicka, K. Influence of vitamin K2 on lipid precursors of inflammation and fatty acids pathway activities in HepG2 cells. Eur. J. Cell Biol. 2021, 100, 151188. [Google Scholar] [CrossRef] [PubMed]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Sherbiny, M.; Atef, H.; Helal, G.M.; Al-Serwi, R.H.; Elkattawy, H.A.; Shaker, G.A.; Said, E.; Abulfaraj, M.; Albalawi, M.A.; Elsherbiny, N.M. Vitamin K2 (MK-7) Intercepts Keap-1/Nrf-2/HO-1 Pathway and Hinders Inflammatory/Apoptotic Signaling and Liver Aging in Naturally Aging Rat. Antioxidants 2022, 11, 2150. https://doi.org/10.3390/antiox11112150
El-Sherbiny M, Atef H, Helal GM, Al-Serwi RH, Elkattawy HA, Shaker GA, Said E, Abulfaraj M, Albalawi MA, Elsherbiny NM. Vitamin K2 (MK-7) Intercepts Keap-1/Nrf-2/HO-1 Pathway and Hinders Inflammatory/Apoptotic Signaling and Liver Aging in Naturally Aging Rat. Antioxidants. 2022; 11(11):2150. https://doi.org/10.3390/antiox11112150
Chicago/Turabian StyleEl-Sherbiny, Mohamed, Hoda Atef, Ghada M. Helal, Rasha Hamed Al-Serwi, Hany A. Elkattawy, Gehan Ahmed Shaker, Eman Said, Moaz Abulfaraj, Marzough A. Albalawi, and Nehal M. Elsherbiny. 2022. "Vitamin K2 (MK-7) Intercepts Keap-1/Nrf-2/HO-1 Pathway and Hinders Inflammatory/Apoptotic Signaling and Liver Aging in Naturally Aging Rat" Antioxidants 11, no. 11: 2150. https://doi.org/10.3390/antiox11112150
APA StyleEl-Sherbiny, M., Atef, H., Helal, G. M., Al-Serwi, R. H., Elkattawy, H. A., Shaker, G. A., Said, E., Abulfaraj, M., Albalawi, M. A., & Elsherbiny, N. M. (2022). Vitamin K2 (MK-7) Intercepts Keap-1/Nrf-2/HO-1 Pathway and Hinders Inflammatory/Apoptotic Signaling and Liver Aging in Naturally Aging Rat. Antioxidants, 11(11), 2150. https://doi.org/10.3390/antiox11112150






