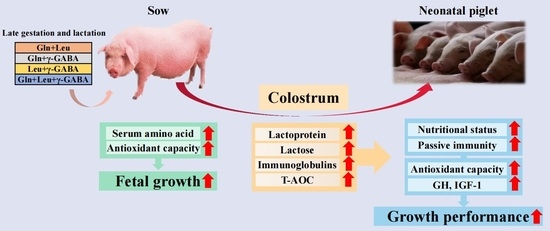
Maternal Amino Acid Mixtures Supplementation during Late Gestation and Lactation Improved Growth Performance of Piglets through Improving Colostrum Composition and Antioxidant Capacity
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Animals and Treatments
2.3. Litter Performance and the Feed Intake of Sows
2.4. Growth Performances of Neonatal Piglets
2.5. Sample Collection
2.6. Determination of Amino Acid
2.7. Assessment of Colostrum Composition
2.8. Determination of Serum Biochemical Parameters
2.9. Statistical Analysis
3. Results
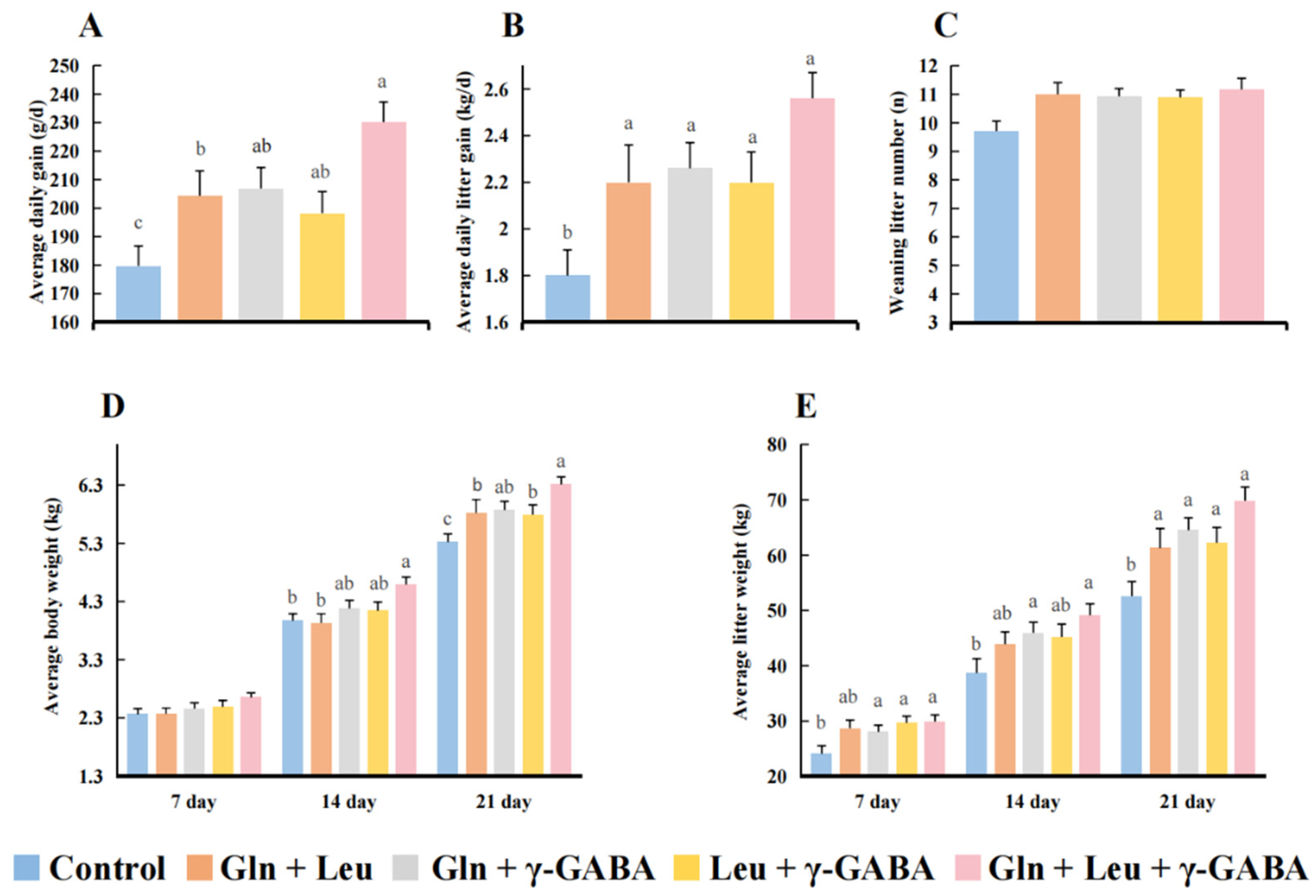
3.1. Effects of AAMs Supplementation on Litter Performance and the Feed Intake of Sows
3.2. Effects of AAMs Supplementation on Serum Free Amino Acid Concentrations of Sows
3.3. Effects of AAMs Supplementation on the Antioxidant Status of Sows
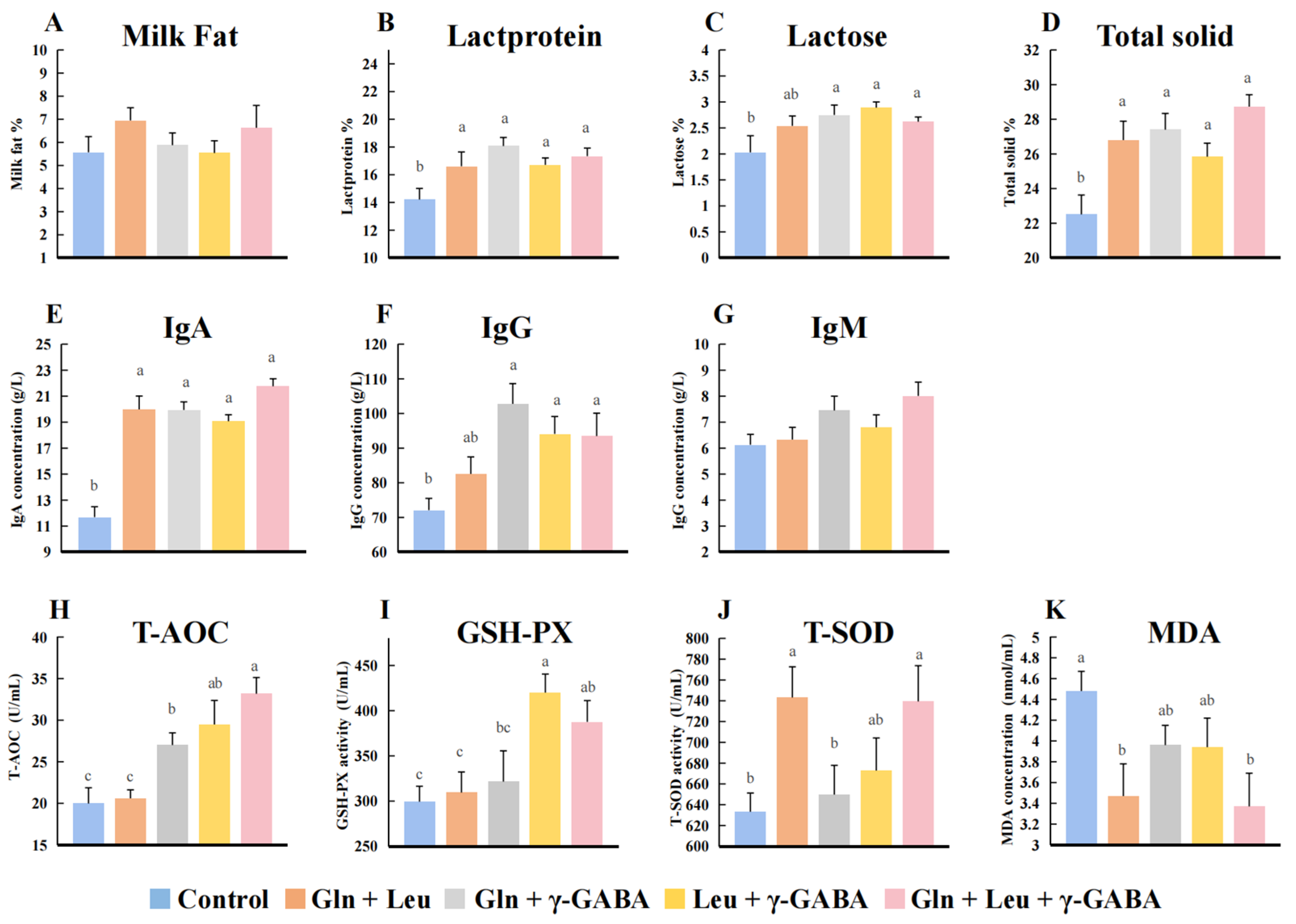
3.4. Effects of AAMs Supplementation on Colostrum Composition
3.5. Effects of AAMs Supplementation on the Growth Performance of Piglets
3.6. Effects of AAMs Supplementation on the Serum Biochemical Parameters of Piglets
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, J.; Wu, Z.; Li, D.; Li, N.; Dindot, S.V.; Sattereld, M.C.; Bazer, F.W.; Wu, G. Nutrition, epigenetics, and metabolic syndrome. Antioxid. Redox Sign. 2012, 17, 282–301. [Google Scholar] [CrossRef]
- Tan, C.; Wei, H.; Ao, J.; Long, G.; Peng, J. Inclusion of konjac flour in the gestation diet changes the gut microbiota, alleviates oxidative stress, and improves insulin sensitivity in sows. Appl. Environ. Microbiol. 2016, 82, 5899–5909. [Google Scholar] [CrossRef]
- Kim, S.W.; Weaver, A.C.; Shen, Y.B.; Zhao, Y. Improving efficiency of sow productivity: Nutrition and health. J. Anim. Sci. Biotechnol. 2013, 4, 26. [Google Scholar] [CrossRef]
- Berchieri-Ronchi, C.B.; Kim, S.W.; Zhao, Y.; Correa, C.R.; Yeum, K.J.; Ferreira, A.L. Oxidative stress status of highly prolific sows during gestation and lactation. Animal 2011, 5, 1774–1779. [Google Scholar] [CrossRef]
- Casanueva, E.; Viteri, F.E. Iron and oxidative stress in pregnancy. J. Nutr. 2003, 133, 1700S–1708S. [Google Scholar] [CrossRef]
- Cadenas, E. Basic mechanisms of antioxidant activity. Biofactors 1997, 6, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Valko, M.; Rhodes, C.J.; Moncol, J.; Izakovic, M.; Mazur, M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem. Biol. Interact. 2006, 160, 1–40. [Google Scholar] [CrossRef]
- Li, Q.; Yang, S.; Chen, F.; Guan, W.; Zhang, S. Nutritional strategies to alleviate oxidative stress in sows. Anim. Nutr. 2021, 9, 60–73. [Google Scholar] [CrossRef]
- MacDonald, A.; Singh, R.H.; Rocha, J.C.; van Spronsen, F.J. Optimising amino acid absorption: Essential to improve nitrogen balance and metabolic control in phenylketonuria. Nutr. Res. Rev. 2019, 32, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Wu, G. Amino acids: Metabolism, functions, and nutrition. Amino Acids 2009, 37, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Welbourne, T.C. Ammonia production and glutamine incorporation into glutathione in the functioning rat kidney. Can. J. Biochem. 1979, 57, 233–237. [Google Scholar] [CrossRef]
- Wu, G.; Bazer, F.W.; Johnson, G.A.; Knabe, D.A.; Burghardt, R.C.; Spencer, T.E.; Li, X.L.; Wang, J.J. Triennial growth symposium: Important roles for l-glutamine in swine nutrition and production. J. Anim. Sci. 2011, 89, 2017–2030. [Google Scholar] [CrossRef]
- Zhu, Y.; Li, T.; Huang, S.; Wang, W.; Dai, Z.; Feng, C.; Wu, G.; Wang, J. Maternal l-glutamine supplementation during late gestation alleviates intrauterine growth restriction-induced intestinal dysfunction in piglets. Amino Acids 2018, 50, 1289–1299. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Feng, G.D.; Ao, X.; Li, Y.F.; Qian, H.X.; Liu, J.B.; Bai, G.Y.; He, Z.Z. Effects of L-glutamine on growth performance, antioxidant ability, immunity and expression of genes related to intestinal health in weanling pigs. Livest. Sci. 2016, 189, 102–109. [Google Scholar] [CrossRef]
- Duan, Y.; Li, F.; Li, Y.; Tang, Y.; Kong, X.; Feng, Z.; Anthony, T.G.; Watford, M.; Hou, Y.; Wu, G.; et al. The role of leucine and its metabolites in protein and energy metabolis. Amino Acids 2015, 48, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Katayama, S.; Mine, Y. Antioxidative activity of amino acids on tissue oxidative stress in human intestinal epithelial cell model. J. Agric. Food. Chem. 2007, 55, 8458–8464. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.J.; Li, F.N.; Duan, Y.H.; Kong, X.F.; Yan, Y.L.; Deng, J.P.; Tan, C.Q.; Wu, G.Y.; Yin, Y.L. Leucine alone or in combination with glutamic acid, but not with arginine, increases biceps femoris muscle and alters muscle aa transport and concentrations in fattening pigs. J. Anim. Physiol. Anim. Nutr. 2019, 103, 791–800. [Google Scholar] [CrossRef]
- Xu, W.; Bai, K.; He, J.; Su, W.; Dong, L.; Zhang, L.L.; Wang, T. Leucine improves growth performance of intrauterine growth retardation piglets by modifying gene and protein expression related to protein synthesis. Nutrition 2016, 32, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Liu, Y.; Duan, G.; Han, M.; Gong, S.; Yang, Z.; Duan, Y.; Guo, Q.; Chen, Q.; Li, F. The effect of dietary leucine supplementation on antioxidant capacity and meat quality of finishing pigs under heat stress. Antioxidants 2022, 11, 1373. [Google Scholar] [CrossRef] [PubMed]
- Bi, C.; Yin, J.; Yang, W.; Shi, B.; Shan, A. Effects of dietary γ-aminobutyric acid supplementation on antioxidant status, blood hormones and meat quality in growing-finishing pigs undergoing transport stress. J. Anim. Physiol. Anim. Nutr. 2020, 104, 590–596. [Google Scholar] [CrossRef] [PubMed]
- Brameld, J.M.; Gilmour, R.S.; Buttery, P.J. Glucose and amino acids interact with hormones to control expression of insulin-like growth factor-Ⅰ and growth hormone receptor mrna in cultured pig hepatocytes. J. Nutr. 1999, 129, 1298–1306. [Google Scholar] [CrossRef] [PubMed]
- Chia, D.J. Minireview: Mechanisms of growth hormone-mediated gene regulation. Mol. Endocrinol. 2014, 28, 1012–1025. [Google Scholar] [CrossRef] [PubMed]
- Biri, A.; Bozkurt, N.; Turp, A.; Kavutcu, M.; Himmetoglu, O.; Durak, I. Role of oxidative stress in intrauterine growth restriction. Gynecol. Obstet. Investig. 2007, 64, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Mao, H.; Zhang, Y.; Ji, W.; Yun, Y.; Wei, X.; Cui, Y.; Wang, C. Leucine protects bovine intestinal epithelial cells from hydrogen peroxide-induced apoptosis by alleviating oxidative damage. J. Sci. Food. Agric. 2022, 102, 5903–5912. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Dai, S.; Li, J.; Wen, A.; Bai, X. Glutamine improves heat stress-induced oxidative damage in the broiler thigh muscle by activating the nuclear factor erythroid 2-related 2/Kelch-like ECH-associated protein 1 signaling pathway. Poult. Sci. 2020, 99, 1454–1461. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Jiang, L.; Pang, J.; Wu, Y.; Pi, Y.; Zang, J.; Wang, J.; Han, D. Maternal dietary supplementation with γ-Aminobutyric acid alleviated oxidative stress in gestating sows and their offspring by regulating GABRP. Animals 2022, 12, 2539. [Google Scholar] [CrossRef]
- Larqué, E.; Ruiz-Palacios, M.; Koletzko, B. Placental regulation of fetal nutrient supply. Curr. Opin. Clin. Nutr. 2013, 16, 292–297. [Google Scholar] [CrossRef] [PubMed]
- Cui, C.; Wu, C.; Wang, J.; Zheng, X.; Ma, Z.; Zhu, P.; Guan, W.; Zhang, S.; Chen, F. Leucine supplementation during late gestation globally alters placental metabolism and nutrient transport via modulation of the PI3K/AKT/mTOR signaling pathway in sows. Food Funct. 2022, 13, 2083–2097. [Google Scholar] [CrossRef] [PubMed]
- Marí, M.; Morales, A.; Colell, A.; García-Ruiz, C.; Fernández-Checa, J.C. Mitochondrial glutathione, a key survival antioxidant. Antioxid. Redox. Signal. 2009, 11, 2685–2700. [Google Scholar] [CrossRef] [PubMed]
- Delgado, R.; Abad-Guamán, R.; Nicodemus, N.; Diaz-Perales, A.; García, J.; Carabao, R.; Menoyo, D. Effect of pre- and post-weaning dietary supplementation with arginine and glutamine on rabbit performance and intestinal health. BMC Vet. Res. 2019, 15, 199–210. [Google Scholar] [CrossRef]
- Li, Y.H.; Li, F.; Liu, M.; Yin, J.J.; Cheng, B.J.; Shi, B.M.; Shan, A.S. Effect of γ-aminobutyric acid on growth performance, behavior and plasma hormones in weaned pigs. Can. J. Anim. Sci. 2015, 95, 165–171. [Google Scholar] [CrossRef]
- Chang, Y.; Cai, H.; Liu, G.; Chang, W.; Zheng, A.; Zhang, S.; Liao, R.; Liu, W.; Li, Y.; Tian, J. Effects of dietary leucine supplementation on the gene expression of mammalian target of rapamycin signaling pathway and intestinal development of broilers. Anim. Nutr. 2015, 1, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Trottier, N.L.; Shipley, C.F.; Easter, R.A. Plasma amino acid uptake by the mammary gland of the lactating sow. J. Anim. Sci. 1997, 75, 1266–1278. [Google Scholar] [CrossRef] [PubMed]
- Spincer, J.; Rook, J.A.; Towers, K.G. The uptake of plasma constituents by the mammary gland of the sow. Biochem. J. 1969, 111, 727–732. [Google Scholar] [CrossRef]
- NRC. Nutrient Requirements of Swine, 10th ed.; National Academy Press: Washington, DC, USA, 1998. [Google Scholar]
- Kim, S.W.; Wu, G. Regulatory role for amino acids in mammary gland growth and milk synthesis. Amino Acids 2009, 37, 89–95. [Google Scholar] [CrossRef]
- Yin, J.; Ren, W.; Liu, G.; Duan, J.; Yang, G.; Wu, L.; Li, T.; Yin, Y. Birth oxidative stress and the development of an antioxidant system in newborn piglets. Free Radic. Res. 2013, 47, 1027–1035. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Huang, S.; Jiang, L.; Dai, Z.; Li, T.; Han, D.; Wang, J. Characterization of the early life microbiota development and predominant lactobacillus species at distinct gut segments of low- and normal-birth-weight piglets. Front. Microbiol. 2019, 10, 797. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, B.O.; Bäckhed, F. Signals from the gut microbiota to distant organs in physiology and disease. Nat. Med. 2016, 22, 1079–1089. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhu, Y.; Feng, C.; Lin, G.; Wu, G.; Li, D.; Wang, J. Innate differences and colostrum-induced alterations of jejunal mucosal proteins in piglets with intra-uterine growth restriction. Br. J. Nutr. 2018, 119, 119734–119747. [Google Scholar] [CrossRef] [PubMed]
- Le, A.; Lane, A.N.; Hamaker, M.; Bose, S.; Gouw, A.; Barbi, J.; Tsukamoto, T.; Rojas, C.J.; Slusher, B.S.; Zhang, H.; et al. Glucose-independent glutamine metabolism via TCA cycling for proliferation and survival in B cells. Cell Metab. 2012, 15, 110–121. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Hao, L.; Chandra, P.; Jones, D.P.; Willams, I.R.; Gewirtz, A.T.; Ziegler, T.R. Dietary glutamine and oral antibiotics each improve indexes of gut barrier function in rat short bowel syndrome. Am. J. Physiol. Gastrointest. Liver. Physiol. 2009, 296, G348–G355. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zou, X.T.; Li, H.; Dong, X.Y.; Zhao, W. Effect of dietary γ-aminobutyric acid on laying performance, egg quality, immune activity and endocrine hormone in heat-stressed Roman hens. Anim. Sci. J. 2012, 83, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Donald, K.; Petersen, C.; Turvey, S.E.; Finlay, B.B.; Azad, M.B. Secretory IgA: Linking microbes, maternal health, and infant health through human milk. Cell Host Microbe 2022, 30, 650–659. [Google Scholar] [CrossRef] [PubMed]
- Caballero-Flores, G.; Sakamoto, K.; Zeng, M.Y.; Wang, Y.; Hakim, J.; Matus-Acuña, V.; Inohara, N.; Núñez, G. Maternal immunization confers protection to the offspring against an attaching and effacing pathogen through delivery of IgG in breast milk. Cell Host Microbe 2019, 25, 313–323. [Google Scholar] [CrossRef] [PubMed]

| Items | Gestation | Lactation |
|---|---|---|
| Ingredient amount (%) | ||
| Corn | 64.84 | 64.97 |
| Soybean meal | 16.70 | 16.00 |
| Wheat bran | 10.00 | 10.50 |
| Soybean oil | 4.00 | 4.00 |
| Salt | 0.30 | 0.30 |
| Premix 1 | 4.00 | 4.00 |
| L-lysine (98 %) | 0.10 | 0.15 |
| L-threonine (98 %) | 0.04 | 0.05 |
| L-tryptophane (98 %) | 0.02 | 0.03 |
| Total | 100 | 100 |
| Nutrition level (calculated value 2) | ||
| Digestible energy (Mcal/kg) | 3.38 | 3.40 |
| Crude protein (%) | 14.41 | 15.21 |
| Calcium (%) | 0.83 | 0.72 |
| Total phosphorus (%) | 0.64 | 0.62 |
| L-lysine (%) | 0.75 | 0.84 |
| L- methionine (%) | 0.22 | 0.23 |
| L- methionine+ L-cysteine, % | 0.47 | 0.49 |
| L-threonine (%) | 0.52 | 0.56 |
| L-tryptophane (%) | 0.16 | 0.18 |
| Items | Treatments | p-Value | ||||
|---|---|---|---|---|---|---|
| Control | Gln + Leu | Gln + γ-GABA | Leu + γ-GABA | Gln + Leu + γ-GABA | ||
| Average of parity | 1.08 ± 0.08 | 1.00 ± 0.00 | 1.14 ± 0.10 | 1.18 ± 0.18 | 1.17 ± 0.08 | 0.788 |
| Number of piglets born | 11.31 ± 0.92 | 12.91 ± 0.67 | 11.78 ± 0.69 | 11.72 ± 0.67 | 12.50 ± 0.52 | 0.546 |
| Number of piglets born alive | 11.15 ± 0.90 | 12.82 ± 0.60 | 11.71 ± 0.71 | 11.36 ± 0.63 | 12.00 ± 0.51 | 0.530 |
| Number of healthy piglets | 8.54 ± 0.83 | 10.55 ± 0.70 | 10.64 ± 0.67 | 10.72 ± 0.37 | 10.83 ± 0.76 | 0.053 |
| Birth weight (kg) | 1.33 ± 0.09 | 1.30 ± 0.05 | 1.40 ± 0.06 | 1.42 ± 0.05 | 1.35 ± 0.05 | 0.723 |
| Birth litter weight (kg) | 14.44 ± 1.04 | 16.73 ± 1.03 | 16.48 ± 0.77 | 15.97 ± 1.06 | 16.14 ± 0.98 | 0.507 |
| Lactation ADFI (kg/d) | 4.51 ± 0.14 b | 5.06 ± 0.18 ab | 4.77 ± 0.21 b | 4.97 ± 0.29 b | 5.67 ± 0.25 a | 0.007 |
| Items (µmol/L) | Treatments | p-Value | ||||
|---|---|---|---|---|---|---|
| Control | Gln + Leu | Gln + γ-GABA | Leu + γ-GABA | Gln + Leu + γ-GABA | ||
| Glutamine | 1180.54 ± 48.83 | 1306.69 ± 39.02 | 1367.57 ± 132.74 | 1221.93 ± 91.70 | 1552.03 ± 100.39 | 0.056 |
| Leucine | 183.64 ± 6.87 c | 229.58 ± 12.81 b | 196.47 ± 4.03 c | 268.86 ± 13.42 a | 247.55 ± 12.40 ab | <0.01 |
| γ-aminobutyric acid | 96.51 ± 4.82 | 103.87 ± 3.15 | 116.19 ± 5.09 | 106.25 ± 5.25 | 106.42 ± 2.13 | 0.052 |
| Glycine | 483.98 ± 38.61 | 484.95 ± 62.64 | 619.09 ± 72.24 | 464.77 ± 44.28 | 539.58 ± 56.71 | 0.317 |
| Alanine | 504.95 ± 36.07 | 405.32 ± 45.98 | 395.16 ± 56.36 | 414.85 ± 33.42 | 494.37 ± 53.19 | 0.301 |
| Serine | 116.42 ± 16.00 | 110.08 ± 13.13 | 118.90 ± 18.20 | 102.48 ± 12.74 | 88.19 ± 5.83 | 0.535 |
| Proline | 266.90 ± 45.05 | 276.97 ± 28.45 | 310.04 ± 52.28 | 387.01 ± 56.41 | 328.98 ± 30.56 | 0.343 |
| Valine | 260.53 ± 28.92 | 175.61 ± 12.68 | 219.13 ± 38.62 | 200.31 ± 22.45 | 191.18 ± 21.10 | 0.218 |
| Threonine | 1458.00 ± 49.69 | 1260.26 ± 43.36 | 1368.32 ± 106.02 | 1474.89 ± 129.42 | 1368.18 ± 66.62 | 0.425 |
| Glutamic acid | 120.58 ± 5.41 c | 139.26 ± 8.55 bc | 124.71 ± 7.61 c | 155.01 ± 12.36 b | 178.89 ± 11.19 ab | 0.001 |
| Methionine | 40.41 ± 1.24 | 45.73 ± 2.41 | 46.54 ± 1.51 | 43.94 ± 1.84 | 50.49 ± 3.49 | 0.055 |
| Histidine | 16.90 ± 0.17 | 15.82 ± 0.52 | 17.43 ± 0.90 | 16.44 ± 0.90 | 16.69 ± 0.61 | 0.562 |
| Phenylalanine | 66.91 ± 4.20 b | 70.92 ± 7.72 b | 75.34 ± 4.16 b | 72.65 ± 3.00 b | 90.21 ± 3.39 a | 0.022 |
| Arginine | 186.00 ± 7.28 b | 195.83 ± 9.83 b | 201.90 ± 7.39 b | 250.34 ± 9.78 a | 193.58 ± 6.30 b | <0.01 |
| Tyrosine | 122.11 ± 5.87 | 112.30 ± 6.08 | 120.64 ± 9.93 | 118.85 ± 8.78 | 101.17 ± 4.73 | 0.314 |
| Lysine | 65.45 ± 2.20 d | 78.98 ± 2.75 c | 80.72 ± 4.79 c | 108.73 ± 2.42 a | 92.40 ± 2.19 b | <0.01 |
| Items | Treatments | p-Value | ||||
|---|---|---|---|---|---|---|
| Control | Gln + Leu | Gln + γ-GABA | Leu + γ-GABA | Gln + Leu + γ-GABA | ||
| T-AOC (U/mL) | 5.90 ± 0.43 b | 6.99 ± 0.45 ab | 7.23 ± 0.44 a | 7.44 ± 0.20 a | 8.06 ± 0.37 a | 0.011 |
| GSH-PX (U/mL) | 684.40 ± 47.34 b | 788.99 ± 48.19 ab | 908.25 ± 25.06 a | 781.65 ± 49.43 ab | 873.39 ± 42.03 a | 0.011 |
| T-SOD (U/mL) | 145.95 ± 3.12 b | 156.36 ± 4.10 ab | 146.63 ± 1.72 b | 159.85 ± 2.57 a | 155.67 ± 4.46 ab | 0.022 |
| MDA (nmol/mL) | 4.28 ± 0.28 a | 2.92 ± 0.43 bc | 3.64 ± 0.21 ab | 2.43 ± 0.28 c | 2.56 ± 0.38 bc | 0.002 |
| Items (µmol/L) | Treatments | p-Value | ||||
|---|---|---|---|---|---|---|
| Control | Gln + Leu | Gln + γ-GABA | Leu + γ-GABA | Gln + Leu + γ-GABA | ||
| Glutamine | 137.93 ± 17.48 | 203.66 ± 20.51 | 159.60 ± 12.16 | 154.58 ± 13.66 | 167.54 ± 13.27 | 0.064 |
| Leucine | 28.92 ± 2.0 c | 44.96 ± 5.49 ab | 37.57 ± 3.33 bc | 36.34 ± 2.55 b | 54.31 ± 4.63 a | <0.01 |
| γ-aminobutyric acid | 68.08 ± 1.44 | 74.55 ± 2.87 | 71.36 ± 0.66 | 71.04 ± 0.39 | 70.67 ± 0.30 | 0.062 |
| Glycine | 20.55 ± 1.19 c | 21.50 ± 1.44 c | 22.55 ± 1.69 bc | 32.02 ± 1.93 a | 27.06 ± 2.24 ab | <0.01 |
| Alanine | 43.47 ± 2.87 a | 37.60 ± 2.33 ab | 36.76 ± 3.20 ab | 33.00 ± 3.08 bc | 24.92 ± 2.77 c | 0.001 |
| Serine | 32.96 ± 1.65 b | 34.33 ± 2.76 ab | 36.40 ± 2.16 a | 31.51 ± 1.96 ab | 37.91 ± 3.04 a | 0.334 |
| Proline | 64.07 ± 3.52 | 76.60 ± 5.84 | 68.13 ± 4.68 | 64.22 ± 5.18 | 73.27 ± 5.51 | 0.312 |
| Valine | 34.70 ± 4.00 c | 36.78 ± 3.25 ab | 33.94 ± 4.46 | 30.60 ± 2.23 bc | 40.72 ± 3.79 a | 0.387 |
| Glutamic acid | 42.09 ± 3.29 b | 65.61 ± 5.05 a | 51.42 ± 3.97 b | 50.82 ± 3.29 b | 53.33 ± 5.96 ab | 0.015 |
| Methionine | 7.25 ± 0.40 | 6.93 ± 0.34 | 6.59 ± 0.25 | 6.42 ± 0.24 | 6.10 ± 0.37 | 0.141 |
| Histidine | 11.33 ± 0.46 | 11.50 ± 0.36 | 10.47 ± 0.13 | 10.63 ± 0.07 | 10.96 ± 0.25 | 0.079 |
| Phenylalanine | 26.23 ± 1.99 | 24.28 ± 2.07 | 21.83 ± 0.95 | 23.26 ± 1.55 | 20.62 ± 1.13 | 0.139 |
| Arginine | 6.28 ± 0.20 | 6.09 ± 0.21 | 6.11 ± 0.04 | 6.19 ± 0.09 | 6.17 ± 0.08 | 0.873 |
| Tyrosine | 27.83 ± 2.07 | 25.15 ± 2.41 | 23.89 ± 1.76 | 23.33 ± 1.26 | 24.80 ± 1.83 | 0.509 |
| Lysine | 20.99 ± 1.74 | 19.21 ± 0.46 | 18.37 ± 0.75 | 17.07 ± 0.60 | 18.49 ± 1.59 | 0.208 |
| Item (µmol/L) | Treatments | p-Value | ||||
|---|---|---|---|---|---|---|
| Control | Gln + Leu | Gln + γ-GABA | Leu + γ-GABA | Gln + Leu + γ-GABA | ||
| Glutamine | 1061.70 ± 56.65 b | 1897.68 ± 116.92 a | 1658.13 ± 228.91 a | 1662.83 ± 130.77 a | 1890.09 ± 119.61 a | 0.002 |
| Leucine | 143.62 ± 10.74 b | 188.95 ± 8.84 a | 172.77 ± 9.30 a | 171.01 ± 5.65 a | 173.29 ± 9.14 a | 0.024 |
| γ-aminobutyric acid | 61.72 ± 0.011 c | 84.81 ± 2.63 b | 97.48 ± 4.60 a | 91.43 ± 3.58 ab | 90.28 ± 2.39 ab | <0.01 |
| Glycine | 529.21 ± 27.89 c | 684.80 ± 58.56 bc | 563.33 ± 23.77 bc | 709.30 ± 71.29 ab | 776.79 ± 47.67 a | 0.013 |
| Alanine | 717.70 ± 68.70 a | 455.75 ± 18.05 b | 660.89 ± 32.21 a | 673.01 ± 15.72 a | 523.80 ± 28.43 b | <0.01 |
| Serine | 124.89 ± 6.36 | 169.56 ± 13.6 | 136.91 ± 11.01 | 149.22 ± 12.67 | 148.22 ± 19.28 | 0.204 |
| Proline | 339.15 ± 28.01 | 472.62 ± 16.01 | 353.71 ± 25.90 | 496.89 ± 99.89 | 474.75 ± 37.86 | 0.113 |
| Valine | 250.42 ± 13.64 | 251.96 ± 7.39 | 225.79 ± 17.53 | 223.59 ± 29.83 | 211.25 ± 19.03 | 0.487 |
| Threonine | 448.03 ± 39.17 | 502.12 ± 36.26 | 448.55 ± 19.07 | 572.15 ± 55.78 | 470.43 ± 30.02 | 0.149 |
| Glutamic acid | 150.77 ± 26.16 | 178.70 ± 7.71 | 118.13 ± 15.01 | 143.29 ± 15.51 | 153.07 ± 31.23 | 0.388 |
| Methionine | 36.30 ± 3.60 | 52.34 ± 3.94 | 53.10 ± 5.73 | 48.28 ± 6.34 | 56.13 ± 4.58 | 0.073 |
| Histidine | 59.65 ± 5.09 ab | 72.39 ± 3.79 a | 61.78 ± 4.24 ab | 54.15 ± 5.39 b | 72.75 ± 4.08 a | 0.029 |
| Phenylalanine | 83.46 ± 5.20 | 100.57 ± 3.21 | 99.71 ± 9.21 | 95.20 ± 4.87 | 102.54 ± 7.67 | 0.252 |
| Arginine | 8.60 ± 0.27 | 9.83 ± 0.33 | 9.91 ± 0.55 | 9.94 ± 0.46 | 9.85 ± 0.58 | 0.214 |
| Tyrosine | 108.91 ± 10.48 | 147.46 ± 9.52 | 124.96 ± 15.55 | 122.46 ± 13.62 | 156.42 ± 21.17 | 0.170 |
| Lysine | 116.31 ± 11.67 b | 169.32 ± 12.33 a | 152.80 ± 15.03 ab | 132.96 ± 12.67 ab | 163.12 ± 7.25 a | 0.026 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, X.; Zhang, X.; Wu, Y.; Che, D.; Ye, H.; Pi, Y.; Tao, S.; Wang, J.; Han, D. Maternal Amino Acid Mixtures Supplementation during Late Gestation and Lactation Improved Growth Performance of Piglets through Improving Colostrum Composition and Antioxidant Capacity. Antioxidants 2022, 11, 2144. https://doi.org/10.3390/antiox11112144
Yuan X, Zhang X, Wu Y, Che D, Ye H, Pi Y, Tao S, Wang J, Han D. Maternal Amino Acid Mixtures Supplementation during Late Gestation and Lactation Improved Growth Performance of Piglets through Improving Colostrum Composition and Antioxidant Capacity. Antioxidants. 2022; 11(11):2144. https://doi.org/10.3390/antiox11112144
Chicago/Turabian StyleYuan, Xiongkun, Xiangyu Zhang, Yujun Wu, Dongsheng Che, Hao Ye, Yu Pi, Shiyu Tao, Junjun Wang, and Dandan Han. 2022. "Maternal Amino Acid Mixtures Supplementation during Late Gestation and Lactation Improved Growth Performance of Piglets through Improving Colostrum Composition and Antioxidant Capacity" Antioxidants 11, no. 11: 2144. https://doi.org/10.3390/antiox11112144
APA StyleYuan, X., Zhang, X., Wu, Y., Che, D., Ye, H., Pi, Y., Tao, S., Wang, J., & Han, D. (2022). Maternal Amino Acid Mixtures Supplementation during Late Gestation and Lactation Improved Growth Performance of Piglets through Improving Colostrum Composition and Antioxidant Capacity. Antioxidants, 11(11), 2144. https://doi.org/10.3390/antiox11112144