Roles of Ferredoxin-NADP+ Oxidoreductase and Flavodoxin in NAD(P)H-Dependent Electron Transfer Systems
Abstract
1. Introduction
2. Structure and Properties of the FNR, Fd and Fld
3. Catalytic Cycle of the FNR-Fd and FNR-Fld Systems
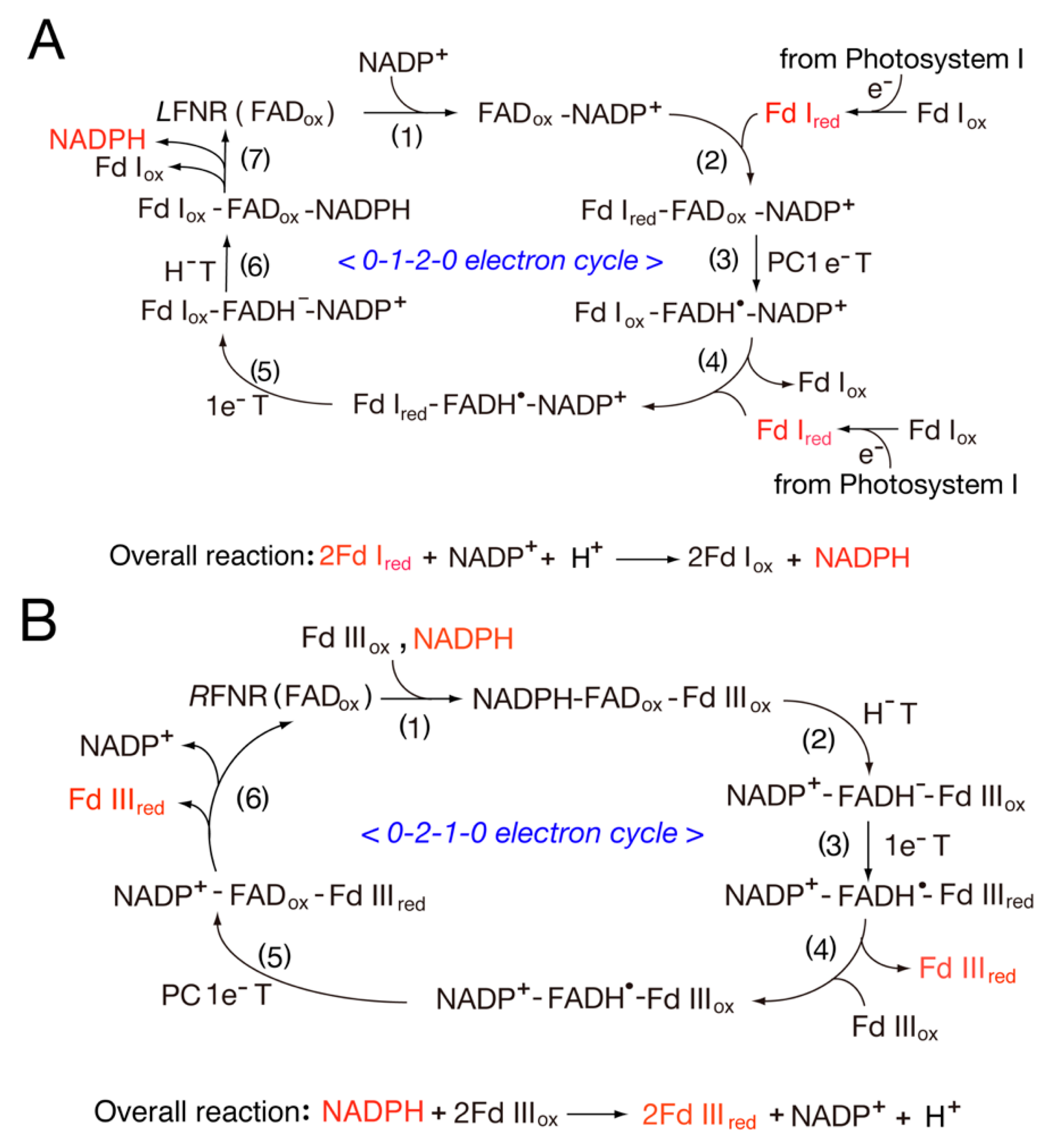
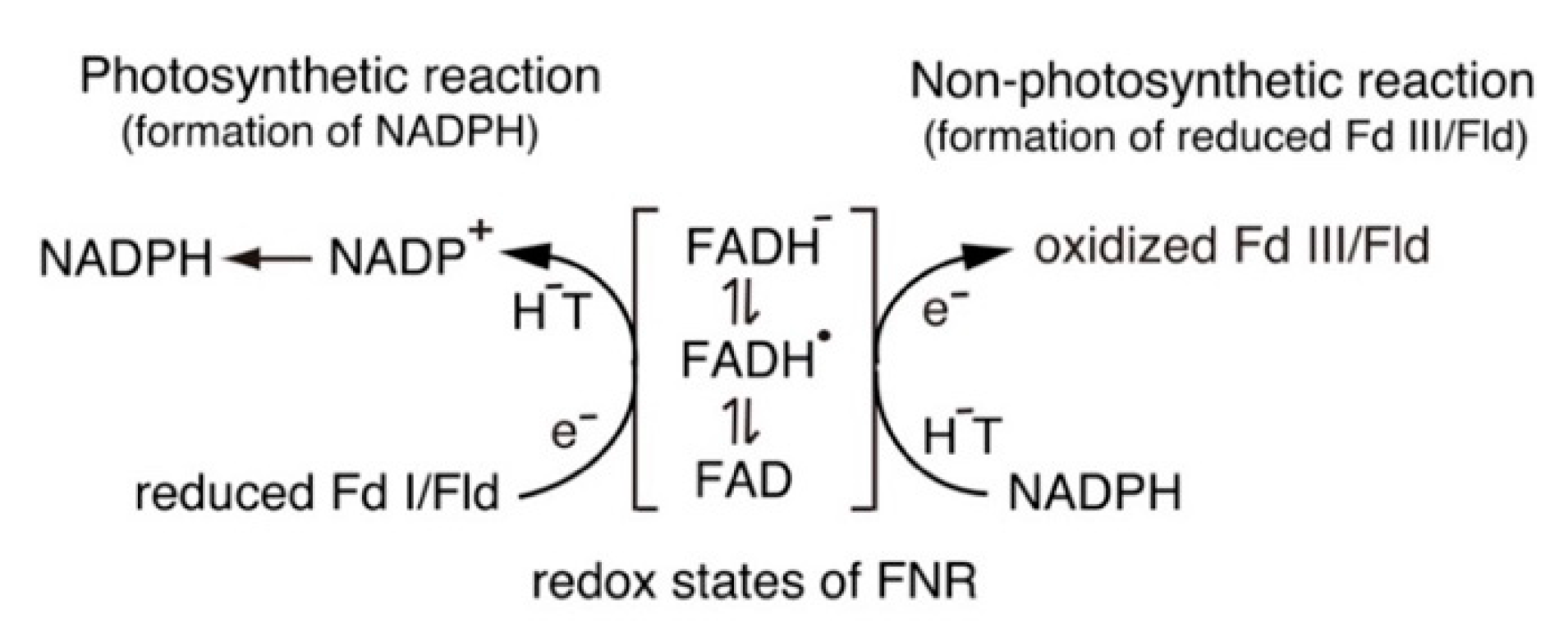
3.1. Catalytic Cycle of the FNR-Fd System
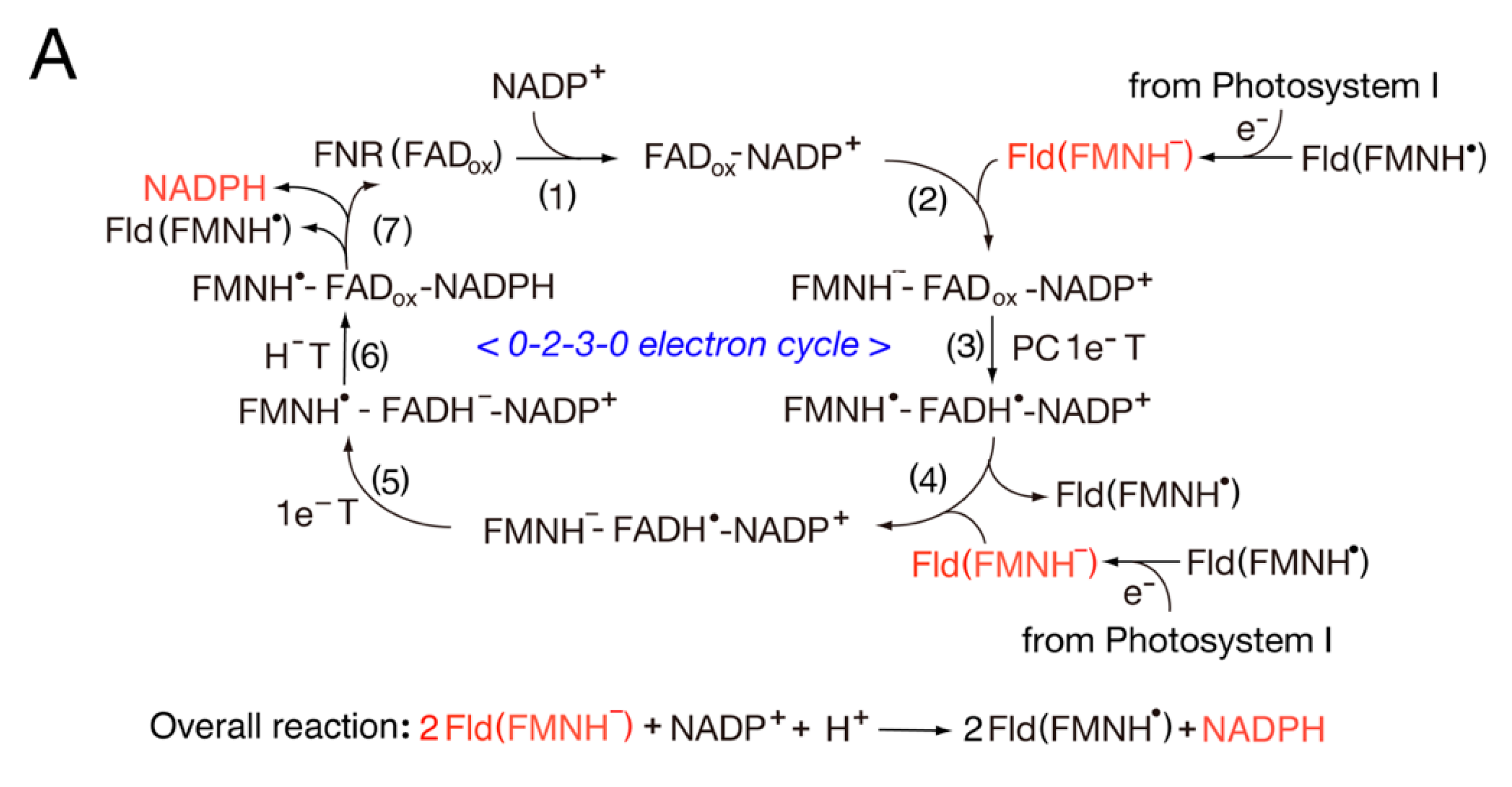
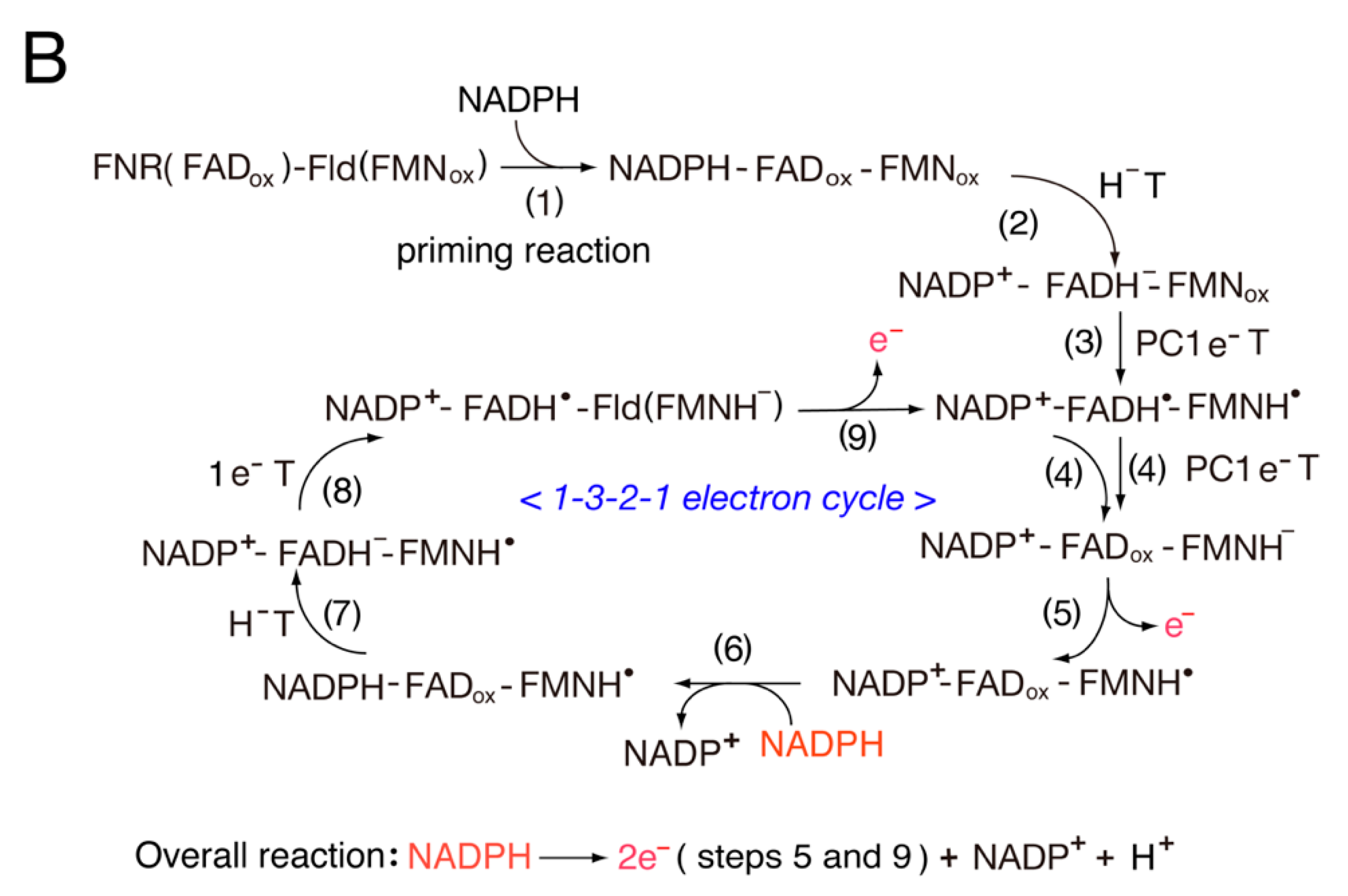
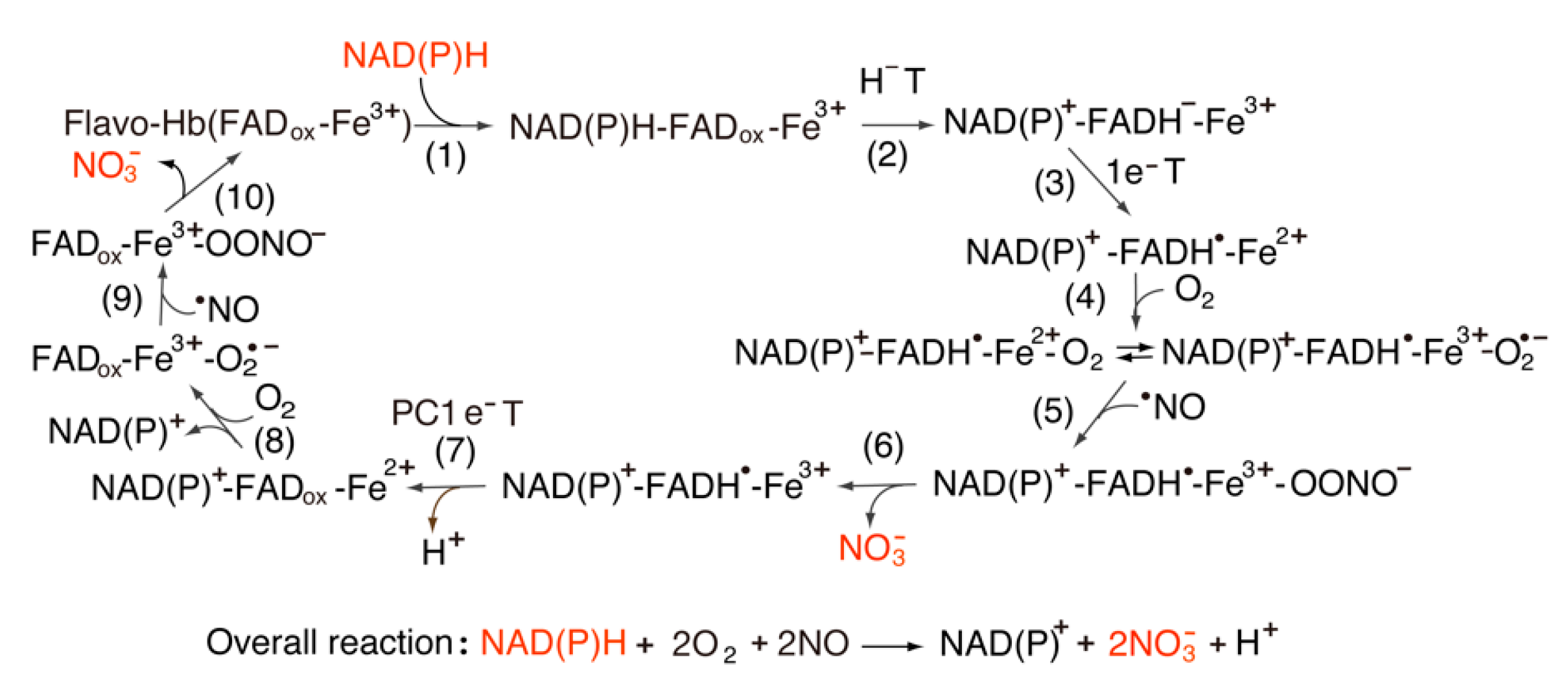
3.2. Catalytic Cycle of the FNR-Fld Systems
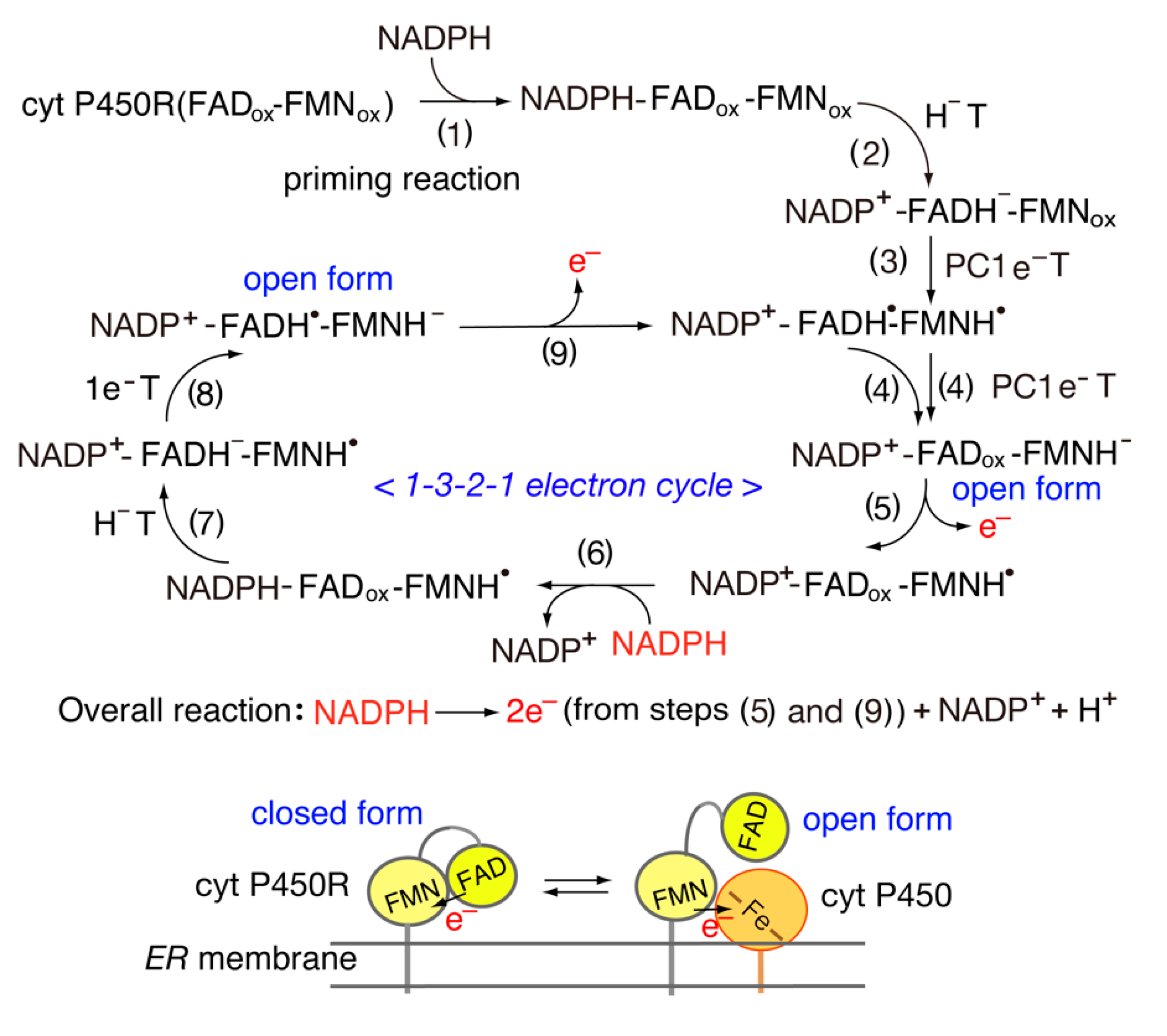
4. Structure and Properties of Diflavin Reductase Family
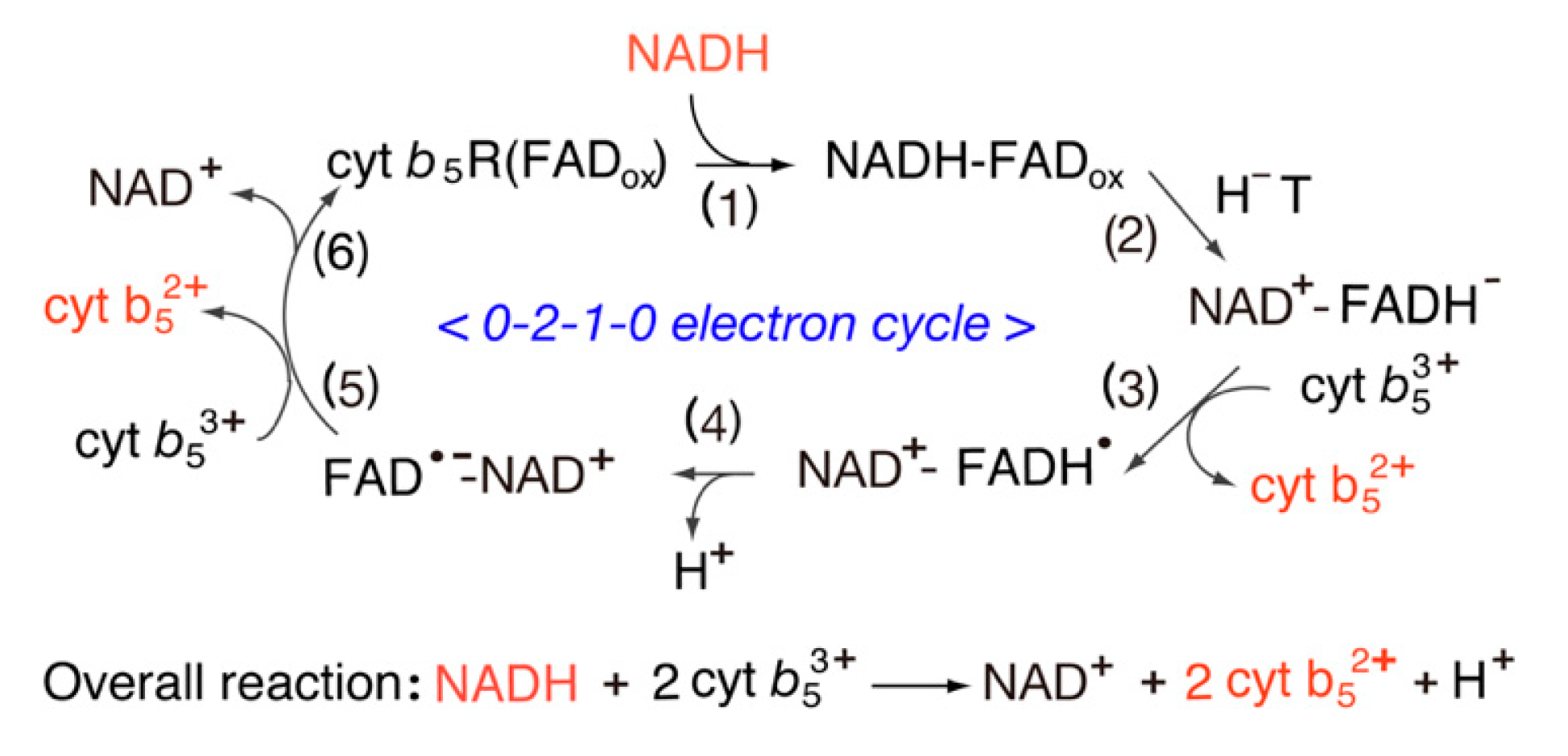
5. Catalytic Cycle of Cyt b5 Reductase
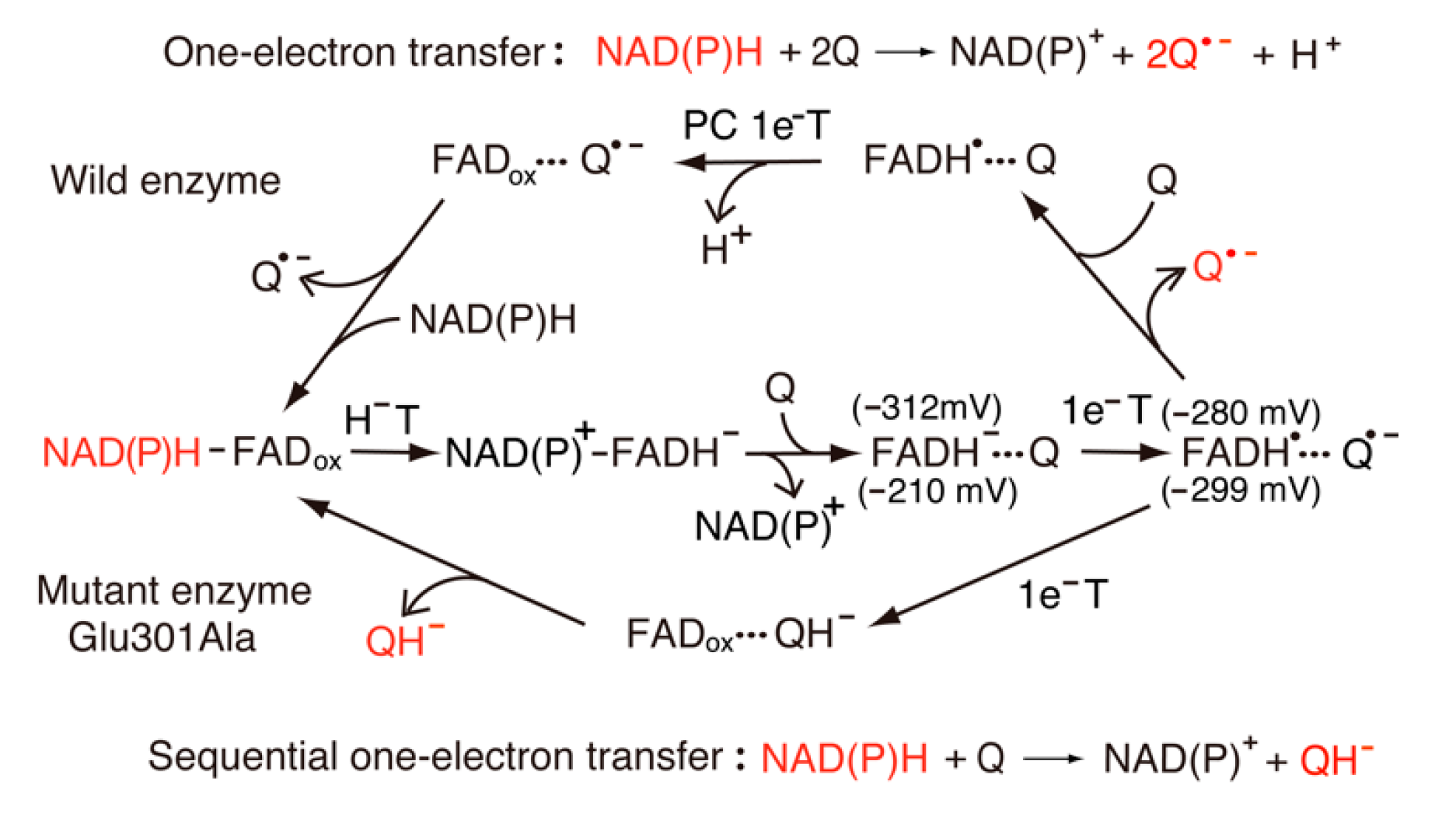
6. Single-Electron Reduction of Quinone Compounds
7. Antioxidant Enzymes: NAD(P)H-Quinone-Oxidoreductase (NQO1)
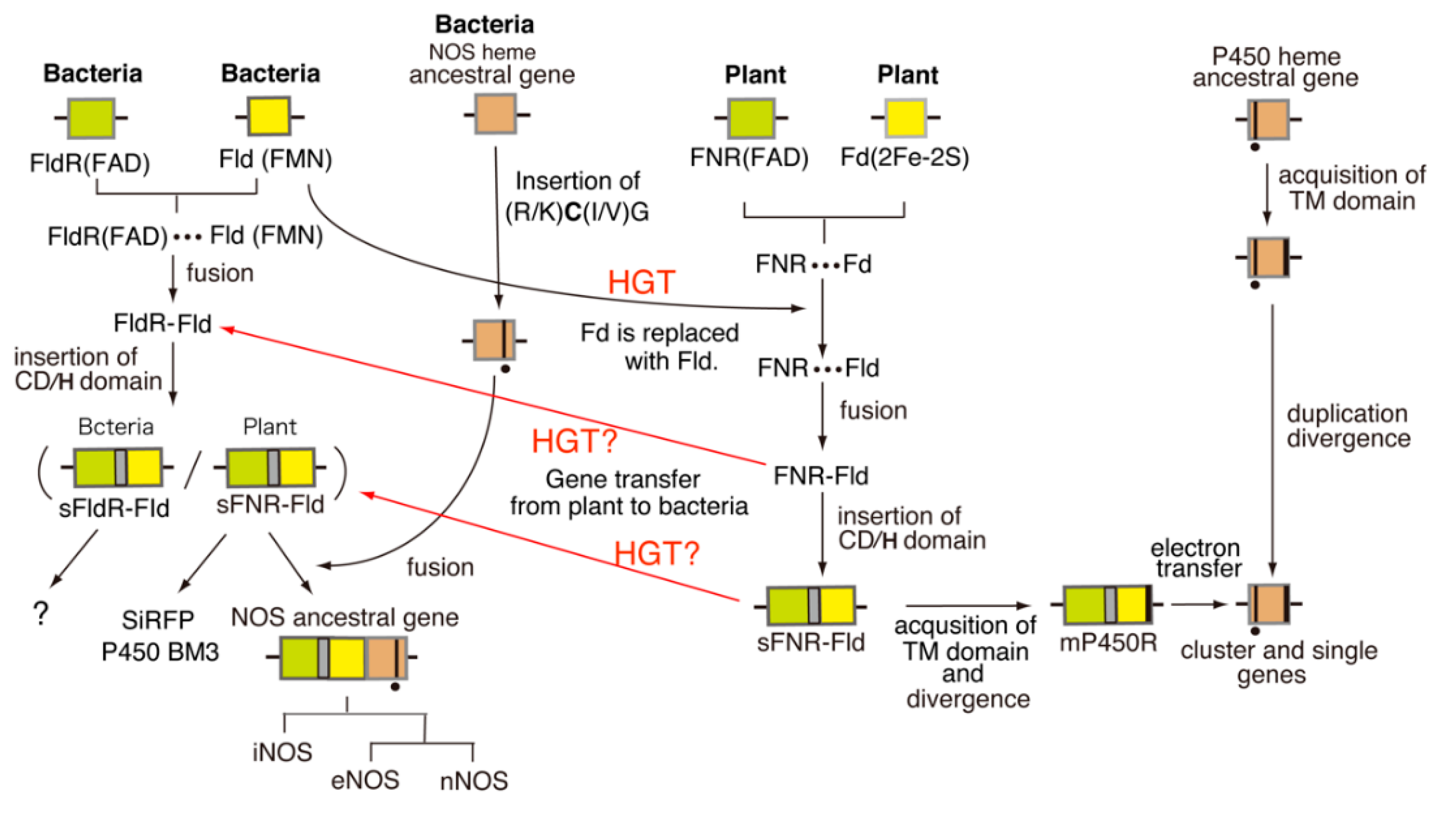
8. Evolutionary Aspects of FNR-Containing NAD(P)H-Dependent Enzymes
9. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Shinohara, F.; Kurisu, G.; Hanke, G.; Bowsher, C.; Hase, T.; Kimata-Ariga, Y. Structural basis for the isotype-specific interactions of ferredoxin and ferredoxin: NADP+ oxidoreductase: An evolutionary switch between photosynthetic and heterotrophic assimilation. Photosynth. Res. 2017, 134, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Hanke, G.T.; Kurisu, G.; Kusunoki, M.; Hase, T. Fd: FNR electron transfer complexes: Evolutionary refinement of structural interactions. Photosynth. Res. 2004, 81, 317–327. [Google Scholar] [CrossRef] [PubMed]
- Ceccarelli, E.A.; Arakaki, A.K.; Cortez, N.; Carrillo, N. Functional plasticity and catalytic efficiency in plant and bacterial ferredoxin-NADP (H) reductases. Biochim. Biophys. Acta Proteins Proteom. 2004, 1698, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Aliverti, A.; Pandini, V.; Pennati, A.; de Rosa, M.; Zanetti, G. Structural and functional diversity of ferredoxin-NADP+ reductases. Arch. Biochem. Biophys. 2008, 474, 283–291. [Google Scholar] [CrossRef] [PubMed]
- Karplus, P.A.; Daniels, M.J. Atomic structure of ferredoxin-NADP+ reductase: Prototype for a structurally novel flavoenzyme family. Science 1991, 251, 60–66. [Google Scholar] [CrossRef]
- Sánchez-Azqueta, A.; Catalano-Dupuy, D.L.; López-Rivero, A.; Tondo, M.L.; Orellano, E.G.; Ceccarelli, E.A.; Medina, M. Dynamics of the active site architecture in plant-type ferredoxin-NADP+ reductases catalytic complexes. Biochim. Biophys. Acta Bioenerg. 2014, 1837, 1730–1738. [Google Scholar] [CrossRef]
- Onda, Y.; Matsumura, T.; Kimata-Ariga, Y.; Sakakibara, H.; Sugiyama, T.; Hase, T. Differential interaction of maize root ferredoxin: NADP+ oxidoreductase with photosynthetic and non-photosynthetic ferredoxin isoproteins. Plant. Physiol. 2000, 123, 1037–1046. [Google Scholar] [CrossRef]
- Tognetti, V.B.; Zurbriggen, M.D.; Morandi, E.N.; Fillat, M.F.; Valle, E.M. Enhanced plant tolerance to iron starvation by functional substitution of chloroplast ferredoxin with a bacterial flavodoxin. Proc. Natl. Acad. Sci. USA 2007, 104, 11495–11500. [Google Scholar] [CrossRef]
- Porter, T.D.; Kasper, C.B. NADPH-cytochrome P-450 oxidoreductase: Flavin mononucleotide and flavin adenine dinucleotide domains evolved from different flavoproteins. Biochemistry 1986, 25, 1682–1687. [Google Scholar] [CrossRef]
- Haniu, M.; Iyanagi, T.; Miller, P.; Lee, T.D.; Shively, J.E. Complete amino acid sequence of NADPH-cytochrome P-450 reductase from porcine hepatic microsomes. Biochemistry 1986, 25, 7906–7911. [Google Scholar] [CrossRef]
- Bredt, D.S.; Hwang, P.M.; Glatt, C.E.; Lowenstein, C.; Reed, R.R.; Snyder, S.H. Cloned and expressed nitric oxide synthase structurally resembles cytochrome P-450 reductase. Nature 1991, 351, 714–718. [Google Scholar] [CrossRef] [PubMed]
- Iyanagi, T.; Xia, C.; Kim, J.J. NADPH-cytochrome P450 oxidoreductase: Prototypic member of the diflavin reductase family. Arch. Biochem. Biophys. 2012, 528, 72–89. [Google Scholar] [CrossRef] [PubMed]
- Ingelman, M.; Bianchi, V.; Eklund, H. The three-dimensional structure of flavodoxin reductase from Escherichia coli at 1.7 Å resolution. J. Mol. Biol. 1997, 268, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Iyanagi, T. Molecular mechanism of metabolic NAD(P)H-dependent systems: The role of redox cofactors. Biochim. Biophys. Acta Bioenerg. 2019, 1860, 233–258. [Google Scholar] [CrossRef]
- Iyanagi, T.; Yamazaki, I. One-electron-transfer reactions in biochemical systems III. One-electron reduction of quinones by microsomal flavin enzymes. Biochim. Biophys. Acta Bioenerg. 1969, 172, 370–381. [Google Scholar] [CrossRef]
- Iyanagi, T.; Yamazaki, I. One-electron-transfer reactions in biochemical systems V. Difference in the mechanism of quinone reduction by the NADH dehydrogenase and the NAD (P) H dehydrogenase (DT-diaphorase). Biochim. Biophys. Acta Bioenerg. 1970, 216, 282–294. [Google Scholar] [CrossRef]
- Čenas, N.; Anusevičius, Ž.; Bironaite, D.; Bachmanova, G.I.; Archakov, A.I.; Öllinger, K. The electron transfer reactions of NADPH: Cytochrome P450 reductase with nonphysiological oxidants. Arch. Biochem. Biophys. 1994, 315, 400–406. [Google Scholar] [CrossRef]
- Čenas, N.; Anusevičius, Ž.; Nivinskas, H.; Misevičienė, L.; Šarlauskas, J. Structure-Activity Relationships in Two-Electron Reduction of Quinones. Methods Enzym. B. 2004, 382, 258–277. [Google Scholar]
- Anusevičius, Ž.; Misevičienė, L.; Medina, M.; Martinez-Julvez, M.; Gomez- Moreno, C.; Čėnas, N. FAD semiquinone stability regulates single-and two-electron reduction of quinones by Anabaena PCC7119 ferredoxin: NADP+ reductase and its Glu301Ala mutant. Arch. Biochem. Biophys. 2005, 437, 144–150. [Google Scholar] [CrossRef]
- Bianchet, M.A.; Faig, M.; Amzel, L.M. Structure and mechanism of NAD [P] H: Quinone acceptor oxidoreductases (NQO). Methods Enzymol. 2004, 382, 144–174. [Google Scholar]
- Iyanagi, T. Molecular mechanism of phase I and phase II drug-metabolizing enzymes: Implications for detoxification. Int. Rev. Cytol. 2007, 260, 35–112. [Google Scholar] [PubMed]
- Martínez-Júlvez, M.; Medina, M.; Gómez-Moreno, C. Ferredoxin-NADP+ reductase uses the same site for the interaction with ferredoxin and flavodoxin. J. Biol. Chem. 1999, 4, 568–578. [Google Scholar]
- Medina, M. Structural and mechanistic aspects of flavoproteins: Photosynthetic electron transfer from photosystem I to NADP+. FEBS J. 2009, 276, 3942–3958. [Google Scholar] [CrossRef] [PubMed]
- Kurisu, G.; Kusunoki, M.; Katoh, E.; Yamazaki, T.; Teshima, K.; Onda, Y.; Kimata-Ariga, Y.; Hase, T. Structure of the electron transfer complex between ferredoxin and ferredoxin-NADP+ reductase. Nat. Struct. Biol. 2002, 8, 117–121. [Google Scholar] [CrossRef]
- Medina, M.; Abagyan, R.; Gómez-Moreno, C.; Fernandez-Recio, J. Docking analysis of transient complexes: Interaction of ferredoxin-NADP+ reductase with ferredoxin and flavodoxin. Proteins 2008, 72, 848–862. [Google Scholar] [CrossRef]
- Chikuma, Y.; Miyata, M.; Lee, Y.H.; Hase, T.; Kimata-Ariga, Y. Molecular mechanism of negative cooperativity of ferredoxin-NADP+ reductase by ferredoxin and NADP (H): Involvement of a salt bridge between Asp60 of ferredoxin and Lys33 of FNR. Biosci. Biotechnol. Biochem. 2021, 85, 860–865. [Google Scholar] [CrossRef] [PubMed]
- Buchert, F.; Hamon, M.; Gäbelein, P.; Scholz, M.; Hippler, M.; Wollman, F.A. The labile interactions of cyclic electron flow effector proteins. J. Biol. Chem. 2018, 293, 17559–17573. [Google Scholar] [CrossRef] [PubMed]
- Kean, K.M.; Carpenter, R.A.; Pandini, V.; Zanetti, G.; Hall, A.R.; Faber, R.; Aliverti, A.; Karplus, P.A. High resolution studies of hydride transfer in the ferredoxin: NADP+ reductase superfamily. FEBS J. 2017, 284, 3302–3319. [Google Scholar] [CrossRef]
- Nogués, I.; Tejero, J.; Hurley, J.K.; Paladini, D.; Frago, S.; Tollin, G.; Mayhew, S.G.; Gomez-Moreno, G.; Eduardo, A.; Ceccarelli, E.A.; et al. Role of the C-terminal tyrosine of ferredoxin-nicotinamide adenine dinucleotide phosphate reductase in the electron transfer processes with its protein partners ferredoxin and flavodoxin. Biochemistry 2004, 43, 6127–6137. [Google Scholar] [CrossRef]
- Dumit, V.I.; Essigke, T.; Cortez, N.; Ullmann, G.M. Mechanistic insights into ferredoxin–NADP(H) reductase catalysis involving the conserved glutamate in the active site. J. Mol. Biol. 2010, 397, 814–825. [Google Scholar] [CrossRef]
- Faro, M.; Gómez-Moreno, C.; Stankovich, M.; Medina, M. Role of critical charged residues in reduction potential modulation of ferredoxin-NADP+ reductase: Differential stabilization of FAD redox forms. Eur. J. Biochem. 2002, 269, 2656–2661. [Google Scholar] [CrossRef] [PubMed]
- Batie, C.J.; Kamin, H. Association of ferredoxin-NADP+ reductase with NADP(H) specificity and oxidation-reduction properties. J. Biol. Chem. 1986, 261, 11214–11223. [Google Scholar] [CrossRef]
- Aliverti, A.; Faber, R.; Finnerty, C.M.; Ferioli, C.; Pandini, V.; Negri, A.; Karplus, P.A.; Zanetti, G. Biochemical and crystallographic characterization of Ferredoxin− NADP+ Reductase from nonphotosynthetic tissues. Biochemistry 2001, 40, 14501–14508. [Google Scholar] [CrossRef] [PubMed]
- Corrado, M.E.; Aliverti, A.; Zanetti, G.; Mayhew, S.G. Analysis of the oxidation-reduction potentials of recombinant ferredoxin-NADP+ reductase from spinach chloroplasts. Eur. J. Biochem. 1996, 239, 662–667. [Google Scholar] [CrossRef] [PubMed]
- Druhan, L.J.; Swenson, R.P. Role of Methionine 56 in the Control of the Oxidation−Reduction Potentials of the Clostridium beijerinckii Flavodoxin: Effects of Substitutions by Aliphatic Amino Acids and Evidence for a Role of Sulfur−Flavin Interactions. Biochemistry 1998, 37, 9668–9678. [Google Scholar] [CrossRef]
- Ishikita, H. Influence of the protein environment on the redox potentials of flavodoxins from Clostridium beijerinckii. J. Biol. Chem. 2007, 282, 25240–25246. [Google Scholar] [CrossRef]
- Chang, C.W.; He, T.F.; Guo, L.; Stevens, J.A.; Li, T.; Wang, L.; Zhong, D. Mapping solvation dynamics at the function site of flavodoxin in three redox states. J. Am. Chem. Soc. 2010, 132, 12741–12747. [Google Scholar] [CrossRef]
- Rwere, F.; Im, S.; Waskell, L. The FMN “140s Loop” of Cytochrome P450 Reductase Controls Electron Transfer to Cytochrome P450. Int. J. Mol. Sci. 2021, 22, 10625. [Google Scholar] [CrossRef]
- Iyanagi, T.; Makino, N.; Mason, H.S. Redox properties of the reduced nicotinamide adenine dinucleotide phosphate-cytochrome P-450 and reduced nicotinamide adenine dinucleotide-cytochrome b5 reductases. Biochemistry 1974, 13, 1701–1710. [Google Scholar] [CrossRef]
- Munro, A.W.; Noble, M.A.; Robledo, L.; Daff, S.N.; Chapman, S.K. Determination of the redox properties of human NADPH-cytochrome P450 reductase. Biochemistry 2001, 40, 1956–1963. [Google Scholar] [CrossRef]
- Haque, M.M.; Tejero, J.; Bayachou, M.; Kenney, C.T.; Stuehr, D.J. A cross-domain charge interaction governs the activity of NO synthase. J. Biol. Chem. 2018, 293, 4545–4554. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.T.; Smith, S.M.; Weinberg, J.B.; Montgomery, H.J.; Newman, E.; Guillemette, J.G.; Ghosh, D.K.; Roman, L.J.; Martasek, P.; Salerno, J.C. Thermodynamics of oxidation-reduction reactions in mammalian nitric-oxide synthase isoforms. J. Biol. Chem. 2004, 279, 18759–18766. [Google Scholar] [CrossRef] [PubMed]
- Pueyo, J.J.; Gomez-Moreno, C.; Mayhew, S.G. Oxidation-reduction potentials of ferredoxin-NADP+ reductase and flavodoxin. from Anabaena PCC 7119 and their electrostatic and covalent complexes. Eur. J. Biochem. 1991, 202, 1065–1071. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Quintana, A.; Leibl, W.; Bottin, H.; Sétif, P. Electron transfer in photosystem I reaction centers follows a linear pathway in which iron-sulfur cluster FB is the immediate electron donor to soluble ferredoxin. Biochemistry 1998, 37, 3429–3439. [Google Scholar] [CrossRef] [PubMed]
- Batie, C.J.; Kamin, H. Electron transfer by ferredoxin: NADP+ reductase. Rapid -reaction evidence for participation of a ternary complex. J. Biol. Chem. 1984, 259, 11976–11985. [Google Scholar] [CrossRef]
- Walker, M.C.; Pueyo, J.; Navarro, J.; Gómez-Moreno, C.; Tollin, G. Laser flash photolysis studies of the kinetics of reduction of ferredoxins and ferredoxin-NADP+ reductases from Anabaena PCC 7119 and spinach: Electrostatic effects on intracomplex electron transfer. Arch. Biochem. Biophys. 1991, 287, 351–358. [Google Scholar] [CrossRef]
- Carrillo, N.; Ceccarelli, E.A. Open questions in ferredoxin-NADP+ reductase catalytic mechanism. Eur. J. Biochem. 2003, 270, 1900–1915. [Google Scholar] [CrossRef]
- Tejero, J.; Peregrina, J.R.; Martínez-Júlvez, M.; Gutiérrez, A.; Gomez-Moreno, C.; Scrutton, N.S.; Medina, M. Catalytic mechanism of hydride transfer between NADP+/H and ferredoxin-NADP+ reductase from Anabaena PCC 7119. Arch. Biochem. Biophys. 2007, 459, 79–90. [Google Scholar] [CrossRef]
- Mulo, P.; Medina, M. Interaction and electron transfer between ferredoxin–NADP+ oxidoreductase and its partners: Structural, functional, and physiological implications. Photosynth. Res. 2017, 134, 265–280. [Google Scholar] [CrossRef]
- Saen-Oon, S.; de Vaca, I.C.; Masone, D.; Medina, M.; Guallar, V. A theoretical multiscale treatment of protein–protein electron transfer: The ferredoxin/ ferredoxin-NADP+ reductase and flavodoxin/ferredoxin-NADP+ reductase systems. Biochim. Biophys. Acta Bioenerg. 2015, 1847, 1530–1538. [Google Scholar] [CrossRef]
- Nogués, I.; Martínez-Júlvez, M.; Navarro, J.A.; Hervás, M.; Armenteros, L.; de la Rosa, M.A.; Brodie, T.B.; Hurley, J.K.; Tollin, G.; Gomez-Moreno, C.; et al. Role of hydrophobic interactions in the flavodoxin mediated electron transfer from photosystem I to ferredoxin-NADP+ reductase in Anabaena PCC 7119. Biochemistry 2003, 42, 2036–2045. [Google Scholar] [CrossRef]
- Utschig, L.M.; Brahmachari, U.; Mulfort, K.L.; Niklas, J.; Poluektov, O.G. Biohybrid photosynthetic charge accumulation detected by flavin semiquinone formation in ferredoxin-NADP+ reductase. Chem. Sci. 2022, 12, 6502–6511. [Google Scholar] [CrossRef] [PubMed]
- Iyanagi, T.; Mason, H.S. Properties of hepatic reduced nicotinamide adenine dinucleotide phosphate-cytochrome c reductase. Biochemistry 1973, 12, 2297–2308. [Google Scholar] [CrossRef] [PubMed]
- Leclerc, D.; Wilson, A.; Dumas, R.; Gafuik, C.; Song, D.; Watkins, D.; Heng, H.H.; Rommens, J.M.; Scherer, S.W.; Rosenblatt, D.S.; et al. Cloning and mapping of a cDNA for methionine synthase reductase, a flavoprotein defective in patients with homocystinuria. Proc. Natl. Acad. Sci. USA 1998, 95, 3059–3064. [Google Scholar] [CrossRef]
- Wolthers, K.R.; Scrutton, N.S. Cobalamin uptake and reactivation occurs through specific protein interactions in the methionine synthase–methionine synthase reductase complex. FEBS J. 2009, 276, 1942–1951. [Google Scholar] [CrossRef]
- Paine, M.J.; Garner, A.P.; Powell, D.; Sibbald, J.; Sales, M.; Pratt, N.; Smith, T.; Tew, D.G.; Wolf, C.R. Cloning and characterization of a novel human dual flavin reductase. J. Biol. Chem. 2000, 275, 1471–1478. [Google Scholar] [CrossRef]
- Ostrowski, J.; Barber, M.J.; Rueger, D.C.; Miller, B.E.; Siegel, L.M.; Kredich, N.M. Characterization of the flavoprotein moieties of NADPH-sulfite reductase from Salmonella typhimurium and Escherichia coli. Physicochemical and catalytic properties, amino acid sequence deduced from DNA sequence of cysJ, and comparison with NADPH-cytochrome P-450 reductase. J. Biol. Chem. 1989, 264, 15796–15808. [Google Scholar]
- Narhi, L.O.; Fulco, A.J. Characterization of a catalytically self-sufficient 119,000-dalton cytochrome P-450 monooxygenase induced by barbiturates in Bacillus megaterium. J. Biol. Chem. 1986, 261, 7160–7169. [Google Scholar] [CrossRef]
- Wang, M.; Roberts, D.L.; Paschke, R.; Shea, T.M.; Masters, B.S.S.; Kim, J.J.P. Three-dimensional structure of NADPH–cytochrome P450 reductase: Prototype for FMN-and FAD-containing enzymes. Proc. Natl. Acad. Sci. USA 1997, 94, 8411–8416. [Google Scholar] [CrossRef]
- Xia, C.; Misra, I.; Iyanagi, T.; Kim, J.J.P. Regulation of interdomain interactions by calmodulin in inducible nitric-oxide synthase. J. Biol. Chem. 2009, 284, 30708–30717. [Google Scholar] [CrossRef]
- Takaba, K.; Takeda, K.; Kosugi, M.; Tamada, T.; Miki, K. Distribution of valence electrons of the flavin cofactor in NADH-cytochrome b5 reductase. Sci. Rep. 2017, 7, 43162. [Google Scholar] [CrossRef]
- Samhan-Arias, A.K.; Almeida, R.M.; Ramos, S.; Cordas, C.M.; Moura, I.; Gutierrez-Merino, C.; Moura, J.J. Topography of human cytochrome b5/cytochrome b5 reductase interacting domain and redox alterations upon complex formation. Biochim. Biophys. Acta Bioenerg. 2018, 1859, 78–87. [Google Scholar] [CrossRef]
- Iyanagi, T.; Watanabe, S.; Anan, K.F. One-electron oxidation-reduction properties of hepatic NADH-cytochrome b5 reductase. Biochemistry 1984, 23, 1418–1425. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Merino, C.; Martínez-Costa, O.H.; Monsalve, M.; Samhan-Arias, A.K. Structural Features of Cytochrome b5–Cytochrome b5 Reductase Complex Formation and Implications for the Intramolecular Dynamics of Cytochrome b5 Reductase. Int. J. Mol. Sci. 2022, 23, 118. [Google Scholar] [CrossRef] [PubMed]
- Kimura, S.; Kawamura, M.; Iyanagi, T. Role of Thr (66) in porcine NADH-cytochrome b5 reductase in catalysis and control of the rate-limiting step in electron transfer. J. Biol. Chem. 2003, 278, 3580–3589. [Google Scholar] [CrossRef] [PubMed]
- Guan, Z.W.; Kamatani, D.; Kimura, S.; Iyanagi, T. Mechanistic studies on the intramolecular one-electron transfer between the two flavins in the human neuronal nitric-oxide synthase and inducible nitric-oxide synthase flavin domains. J. Biol. Chem. 2003, 278, 30859–30868. [Google Scholar] [CrossRef] [PubMed]
- Nishino, Y.; Yamamoto, K.; Kimura, S.; Kikuchi, A.; Shiro, Y.; Iyanagi, T. Mechanistic studies on the intramolecular one-electron transfer between the two flavins in the human endothelial NOS reductase domain. Arch. Biochem. Biophys. 2007, 465, 254–265. [Google Scholar] [CrossRef]
- Fukuchi-Mizutani, M.; Mizutani, M.; Tanaka, Y.; Kusumi, T.; Ohta, D. Microsomal Electron Transfer in Higher Plants: Cloning and Heterologous Expression of NADH-Cytochrome b5 Reductase from Arabidopsis. Plant. Physiol. 1999, 119, 353–362. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, T.; Das, D. Biochemical Investigation of Membrane-Bound Cytochrome b5 and the Catalytic Domain of Cytochrome b5 Reductase from Arabidopsis thaliana. Biochemistry 2022, 61, 909–921. [Google Scholar] [CrossRef]
- Deng, B.; Parthasarathy, S.; Wang, W.-F.; Gibney, B.R.; Battaile, K.P.; Lovell, S.; Benson, D.R.; Zhu, H. Study of the individual cytochrome b5 and cytochrome b5 reductase domains of Ncb5or reveals a unique heme pocket and a possible role of the CS domain. J. Biol. Chem. 2010, 285, 30181–30191. [Google Scholar] [CrossRef]
- Bando, S.; Takano, T.; Yubisui, T.; Shirabe, K.; Takeshita, M.; Nakagawa, A. Structure of human erythrocyte NADH-cytochrome b5 reductase. Acta Crystallogr. D Biol. Crystallogr. 2004, 60, 1929–1934. [Google Scholar] [CrossRef] [PubMed]
- Abe, K.; Sugita, Y. Properties of cytochrome b5, and methemoglobin reduction in human erythrocytes. Eur. J. Biochem. 1979, 101, 423–428. [Google Scholar] [CrossRef] [PubMed]
- Amdahl, M.B.; Sparacino-Watkins, C.E.; Corti, P.; Gladwin, M.T.; Tejero, J. Efficient reduction of vertebrate cytoglobins by the cytochrome b5/cytochrome b5 reductase/NADH system. Biochemistry 2017, 56, 3993–4004. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, L.C.; Chien, J.C. Comparative biological chemistry of cobalt hemoglobin. J. Biol. Chem. 1973, 248, 5005–5011. [Google Scholar] [CrossRef]
- Liu, X.; El-Mahdy, M.A.; Boslett, J.; Varadharaj, S.; Hemann, C.; Abdelghany, T.M.; Ismail, R.S.; Little, S.C.; Zhou, D.; Thuy, L.; et al. Cytoglobin regulates blood pressure and vascular tone through nitric oxide metabolism in the vascular wall. Nat. Ccommun. 2017, 8, 14807. [Google Scholar] [CrossRef]
- Ermler, U.; Siddiqui, R.A.; Cramm, R.; Friedrich, B. Crystal structure of the flavohemoglobin from Alcaligenes eutrophus at 1.75 A resolution. EMBO J. 1995, 14, 6067–6077. [Google Scholar] [CrossRef]
- Cooper, C.E.; Ioannidis, N.; D’mello, R.; Poole, R.K. Haem, flavin and oxygen interactions in Hmp, a flavohaemoglobin from Escherichia coli. Biochem. Soc. Trans. 1994, 22, 709–713. [Google Scholar] [CrossRef]
- Gardner, P.R.; Gardner, A.M.; Martin, L.A.; Salzman, A.L. Nitric oxide dioxygenase: An enzymic function for flavohemoglobin. Proc. Natl. Acad. Sci. USA 1998, 95, 10378–10383. [Google Scholar] [CrossRef]
- Frey, A.D.; Kallio, P.T. Bacterial hemoglobins and flavohemoglobins: Versatile proteins and their impact on microbiology and biotechnology. FEMS Microbiol. Rev. 2003, 27, 525–545. [Google Scholar] [CrossRef]
- Sumimoto, H. Structure, regulation and evolution of Nox-family NADPH oxidases that produce reactive oxygen species. FEBS J. 2008, 275, 3249–3277. [Google Scholar] [CrossRef]
- Magnani, F.; Mattevi, A. Structure and mechanisms of ROS generation by NADPH oxidases. Curr. Opin. Struct. Biol. 2019, 59, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Isogai, Y.; Iizuka, T.; Makino, R.; Iyanagi, T.; Orii, Y. Superoxide-producing cytochrome b. Enzymatic and electron paramagnetic resonance properties of cytochrome b558 purified from neutrophils. J. Biol. Chem. 1993, 268, 4025–4031. [Google Scholar] [CrossRef]
- Marcus, R.A.; Sutin, N. Electron transfers in chemistry and biology. Biochim. Biophys. Acta Bioenerg. 1985, 811, 265–322. [Google Scholar] [CrossRef]
- Lesanavičius, M.; Aliverti, A.; Šarlauskas, J.; Čėnas, N. Reactions of Plasmodium falciparum ferredoxin: NADP+ oxidoreductase with redox cycling xenobiotics: A mechanistic study. Int. J. Mol. Sci. 2020, 21, 3234. [Google Scholar] [CrossRef]
- Iyanagi, T. On the mechanisms of one- and two-electron transfer by flavin enzymes. Chemica Scripta 1987, 27A, 31–36. [Google Scholar]
- Yamamoto, K.; Kimura, S.; Shiro, Y.; Iyanagi, T. Interflavin one-electron transfer in the inducible nitric oxide synthase reductase domain and NADPH-cytochrome P450 reductase. Arch. Biochem. Biophys. 2005, 440, 65–78. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, H.; Kimura, S.; Iyanagi, T. One-electron reduction of quinones by the neuronal nitric oxide synthase reductase domain. Biochim. Biophys. Acta Gen. 2000, 1459, 106–116. [Google Scholar] [CrossRef]
- Fu, J.; Yamamoto, K.; Guan, Z.W.; Kimura, S.; Iyanagi, T. Human neuronal nitric oxide synthase can catalyze one-electron reduction of adriamycin: Role of flavin domain. Arch. Biochem. Biophys. 2004, 427, 180–187. [Google Scholar] [CrossRef]
- Anusevičius, Z.; Nivinskas, H.; Sarlauskas, J.; Sari, M.A.; Boucher, J.L.; Čėnas, N. Single-electron reduction of quinone and nitroaromatic xenobiotics by recombinant rat neuronal nitric oxide synthase. Acta Biochim. Pol. 2013, 60, 217–222. [Google Scholar] [CrossRef]
- Lesanavičius, M.; Boucher, J.L.; Čėnas, N. Reactions of Recombinant Neuronal Nitric Oxide Synthase with Redox Cycling Xenobiotics: A Mechanistic Study. Int. J. Mol. Sci. 2022, 23, 980. [Google Scholar] [CrossRef]
- Szilagyi, J.T.; Fussell, K.C.; Wang, Y.; Jan, Y.H.; Mishin, V.; Richardson, J.R.; Heck, D.E.; Yang, S.; Aleksunes, L.M.; Laskin, D.L.; et al. Quinone and nitrofurantoin redox cycling by recombinant cytochrome b5 reductase. Toxicol. Appl. Pharmacol. 2018, 359, 102–107. [Google Scholar] [CrossRef] [PubMed]
- Iyanagi, T. On the mechanism of one-electron reduction of quinones by microsomal flavin enzymes: The kinetic analysis between cytochrome B5 and menadione. Free Radic. Res. Commun. 1990, 8, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Dinkova-Kostova, A.T.; Talalay, P. NAD (P) H: Quinone acceptor oxidoreductase 1 (NQO1), a multifunctional antioxidant enzyme and exceptionally versatile cytoprotector. Arch. Biochem. Biophys. 2010, 501, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Čėnas, N.; Nemeikaitė-Čėnienė, A.; Kosychova, L. Single-and two-electron reduction of nitroaromatic compounds by flavoenzymes: Mechanisms and implications for cytotoxicity. Int. J. Mol. Sci. 2021, 22, 8534. [Google Scholar] [CrossRef]
- Li, R.; Bianchet, M.A.; Talalay, P.; Amzel, L.M. The three-dimensional structure of NAD (P) H: Quinone reductase, a flavoprotein involved in cancer chemoprotection and chemotherapy: Mechanism of the two-electron reduction. Proc. Natl. Acad. Sci. USA 1995, 92, 8846–8850. [Google Scholar] [CrossRef]
- Ryan, A.; Kaplan, E.; Nebel, J.C.; Polycarpou, E.; Crescente, V.; Lowe, E.; Preston, G.M.; Sim, E. Identification of NAD (P) H quinone oxidoreductase activity in azoreductases from P. aeruginosa: Azoreductases and NAD (P) H quinone oxidoreductases belong to the same FMN-dependent superfamily of enzymes. PLoS ONE 2014, 9, e98551. [Google Scholar] [CrossRef] [PubMed]
- Mendoza, M.F.; Hollabaugh, N.M.; Hettiarachchi, S.U.; McCarley, R.L. Human NAD (P) H: Quinone oxidoreductase type I (hNQO1) activation of quinone propionic acid trigger groups. Biochemistry 2012, 51, 8014–8026. [Google Scholar] [CrossRef]
- Anoz-Carbonell, E.; Timson, D.J.; Pey, A.L.; Medina, M. The catalytic cycle of the antioxidant and cancer-associated human NQO1 enzyme: Hydride transfer, conformational dynamics and functional cooperativity. Antioxidants 2020, 9, 772. [Google Scholar] [CrossRef]
- Deller, S.; Macheroux, P.; Sollner, S. Flavin-dependent quinone reductases. Cell Mol. Life Sci. 2008, 65, 141–160. [Google Scholar] [CrossRef]
- Tedeschi, G.; Chen, S.; Massey, V. DT-diaphorase. Redox potential, steady-state, and rapid reaction studies. J. Biol. Chem. 1995, 270, 1198–1204. [Google Scholar] [CrossRef]
- Tedeschi, G.; Chen, S.; Massey, V. Active site studies of DT-diaphorase employing artificial flavins. J. Biol. Chem. 1995, 270, 2512–2516. [Google Scholar] [CrossRef]
- Sarla, F.; Tedeschi, G.; Pupillo, P.; Trost, P. Cloning and heterologous expression of NAD (P) H: Quinone reductase of Arabidopsis thaliana, a functional homologue of animal DT-diaphorase. FEBS Lett. 1999, 463, 382–386. [Google Scholar] [CrossRef]
- Adams, M.A.; Jia, Z. Modulator of drug activity B from Escherichia coli: Crystal structure of a prokaryotic homologue of DT-diaphorase. J. Mol. Biol. 2006, 359, 455–465. [Google Scholar] [CrossRef]
- Ball, J.; Salvi, F.; Gadda, G. Functional annotation of a presumed nitronate monoxygenase reveals a new class of NADH: Quinone reductases. J. Biol. Chem. 2016, 291, 21160–21170. [Google Scholar] [CrossRef]
- Ross, D.; Siegel, D. The diverse functionality of NQO1 and its roles in redox control. Redox Biol. 2021, 41, 101950. [Google Scholar] [CrossRef] [PubMed]
- Zurbriggen, M.D.; Tognetti, V.B.; Carrillo, N. Stress-inducible flavodoxin from photosynthetic microorganisms. The mystery of flavodoxin loss from the plant genome. IUBMB Life 2007, 59, 355–360. [Google Scholar] [CrossRef] [PubMed]
- Pierella Karlusich, J.J.; Ceccoli, R.D.; Graña, M.; Romero, H.; Carrillo, N. Environmental selection pressures related to iron utilization are involved in the loss of the flavodoxin gene from the plant genome. Genome Biol. Evol. 2015, 7, 750–767. [Google Scholar] [CrossRef] [PubMed]
- Zeghouf, M.; Fontecave, M.; Macherel, D.; Covès, J. The flavoprotein component of the Escherichia coli sulfite reductase: Expression, purification, and spectral and catalytic properties of a monomeric form containing both the flavin adenine dinucleotide and the flavin mononucleotide cofactors. Biochemistry 1998, 37, 6114–6123. [Google Scholar] [CrossRef]
- Tavolieri, A.M.; Murray, D.T.; Askenasy, I.; Pennington, J.M.; McGarry, L.; Stanley, C.B.; Stroupe, M.E. NADPH-dependent sulfite reductase flavoprotein adopts an extended conformation unique to this diflavin reductase. J. Struct. Biol. 2019, 205, 170–179. [Google Scholar] [CrossRef]
- Shet, M.S.; Sathasivan, K.; Arlotto, M.A.; Mehdy, M.C.; Estabrook, R.W. Purification, characterization, and cDNA cloning of an NADPH-cytochrome P450 reductase from mung bean. Proc. Natl. Acad. Sci. USA 1993, 90, 2890–2894. [Google Scholar] [CrossRef]
- Hubbard, P.A.; Shen, A.L.; Paschke, R.; Kasper, C.B.; Kim, J.J.P. NADPH-cytochrome P450 oxidoreductase: Structural basis for hydride and electron transfer. J. Biol. Chem. 2001, 276, 29163–29170. [Google Scholar] [CrossRef]
- Zhang, J.; Martàsek, P.; Paschke, R.; Shea, T.; Masters, B.S.S.; Kim, J.J.P. Crystal structure of the FAD/NADPH-binding domain of rat neuronal nitric-oxide synthase: Comparisons with NADPH-cytochrome P450 oxidoreductase. J. Biol. Chem. 2001, 276, 37506–37513. [Google Scholar] [CrossRef]
- Rwere, F.; Xia, C.; Im, S.; Haque, M.M.; Stuehr, D.J.; Waskell, L.; Kim, J.J.P. Mutants of cytochrome P450 reductase lacking either Gly-141 or Gly-143 destabilize its FMN semiquinone. J. Biol. Chem. 2016, 291, 14639–14661. [Google Scholar] [CrossRef] [PubMed]
- McIver, L.; Leadbeater, C.; Campopiano, D.J.; Baxter, R.L.; Daff, S.N.; Chapman, S.K.; Munro, A.W. Characterisation of flavodoxin NADP+ oxidoreductase and flavodoxin; key components of electron transfer in Escherichia coli. Eur. J. Biochem. 1998, 257, 577–585. [Google Scholar] [CrossRef] [PubMed]
- Wolthers, K.R.; Basran, J.; Munro, A.W.; Scrutton, N.S. Molecular dissection of human methionine synthase reductase: Determination of the flavin redox potentials in full-length enzyme and isolated flavin-binding domains. Biochemistry 2003, 42, 3911–3920. [Google Scholar] [CrossRef] [PubMed]
- Dunford, A.J.; Rigby, S.E.; Hay, S.; Munro, A.W.; Scrutton, N.S. Conformational and thermodynamic control of electron transfer in neuronal nitric oxide synthase. Biochemistry 2007, 46, 5018–5029. [Google Scholar] [CrossRef]
- Ruettinger, R.T.; Wen, L.P.; Fulco, A.J. Coding nucleotide, 5′ regulatory, and deduced amino acid sequences of P-450BM-3, a single peptide cytochrome P-450: NADPH-P-450 reductase from Bacillus megaterium. J. Biol. Chem. 1989, 264, 10987–10995. [Google Scholar] [CrossRef]
- Zhang, H.; Yokom, A.L.; Cheng, S.; Su, M.; Hollenberg, P.F.; Southworth, D.R.; Osawa, Y. The full-length cytochrome P450 enzyme CYP102A1 dimerizes at its reductase domains and has flexible heme domains for efficient catalysis. J. Biol. Chem. 2018, 293, 7727–7736. [Google Scholar] [CrossRef]
- Su, M.; Chakraborty, S.; Osawa, Y.; Zhang, H. Cryo-EM reveals the architecture of the dimeric cytochrome P450 CYP102A1 enzyme and conformational changes required for redox partner recognition. J. Biol. Chem. 2020, 295, 1637–1645. [Google Scholar] [CrossRef]
- Chen, H.C.; Swenson, R.P. Effect of the insertion of a glycine residue into the loop spanning residues 536–541 on the semiquinone state and redox properties of the flavin mononucleotide-binding domain of flavocytochrome P450BM-3 from Bacillus megaterium. Biochemistry 2008, 47, 13788–13799. [Google Scholar] [CrossRef]
- Hanley, S.C.; Ost, T.W.; Daff, S. The unusual redox properties of flavorcytochrome P450 BM3 flavodoxin domain. Biochem. Biophys. Res. Commun. 2004, 325, 1418–1423. [Google Scholar] [CrossRef] [PubMed]
- Murataliev, M.B.; Klein, M.; Fulco, A.; Feyereisen, R. Functional interactions in cytochrome P450BM3: Flavin semiquinone intermediates, role of NADP (H), and mechanism of electron transfer by the flavoprotein domain. Biochemistry 1997, 36, 8401–8412. [Google Scholar] [CrossRef] [PubMed]
- Prakash, D.; Iyer, P.R.; Suharti, S.; Walters, K.A.; Santiago-Martinez, M.G.; Golbeck, J.H.; Murakami, K.S.; Ferry, J.G. Structure and function of an unusual flavodoxin from the domain Archaea. Proc. Natl. Acad. Sci. USA 2019, 116, 25917–25922. [Google Scholar] [CrossRef]
- Peterson, J.A.; Lorence, M.C.; Amarneh, B. Putidaredoxin reductase and putidaredoxin. Cloning, sequence determination, and heterologous expression of the proteins. J. Biol. Chem. 1990, 265, 6066–6073. [Google Scholar] [CrossRef]
- Sevrioukova, I.F.; Poulos, T.L.; Churbanova, I.Y. Crystal structure of the putidaredoxin reductase putidaredoxin electron transfer complex. J. Biol. Chem. 2010, 285, 13616–13620. [Google Scholar] [CrossRef] [PubMed]
- Kimmich, N.; Das, A.; Sevrioukova, I.; Meharenna, Y.; Sligar, S.G.; Poulos, T.L. Electron transfer between cytochrome P450cin and its FMN-containing redox partner, cindoxin. J. Biol. Chem. 2007, 282, 27006–27011. [Google Scholar] [CrossRef]
- Madrona, Y.; Hollingsworth, S.A.; Tripathi, S.; Fields, J.B.; Rwigema, J.C.N.; Tobias, D.J.; Poulos, T.L. Crystal structure of cindoxin, the P450cin redox partner. Biochemistry 2014, 53, 1435–1446. [Google Scholar] [CrossRef]
- Klenk, J.M.; Fischer, M.P.; Dubiel, P.; Sharma, M.; Rowlinson, B.; Grogan, G.; Hauer, B. Identification and characterization of cytochrome P450 1232A24 and 1232F1 from Arthrobacter sp. and their role in the metabolic pathway of papaverine. J. Biol. Chem. 2019, 166, 51–66. [Google Scholar] [CrossRef]
- Zhang, L.; Xie, Z.; Liu, Z.; Zhou, S.; Ma, L.X.; Liu, W.; Huang, J.W.; Ko, T.P.; Li, X.Q.; Hu, Y.; et al. Structural insight into the electron transfer pathway of a self-sufficient P450 monooxygenase. Nat. Commun. 2020, 11, 2676. [Google Scholar] [CrossRef]
- Wang, Z.; Shaik, S.; Wang, B. Conformational motion of ferredoxin enables efficient electron transfer to heme in the full-length P450TT. J. Am. Chem. Soc. 2021, 143, 1005–1016. [Google Scholar] [CrossRef]
- Yamamoto, T.; Hasegawa, Y.; Iwaki, H. Identification and characterization of a novel class of self-sufficient cytochrome P450 hydroxylase involved in cyclohexanecarboxylate degradation in Paraburkholderia terrae strain KU-64. Biosci. Biotechnol. Biochem. 2022, 86, 199–208. [Google Scholar] [CrossRef]
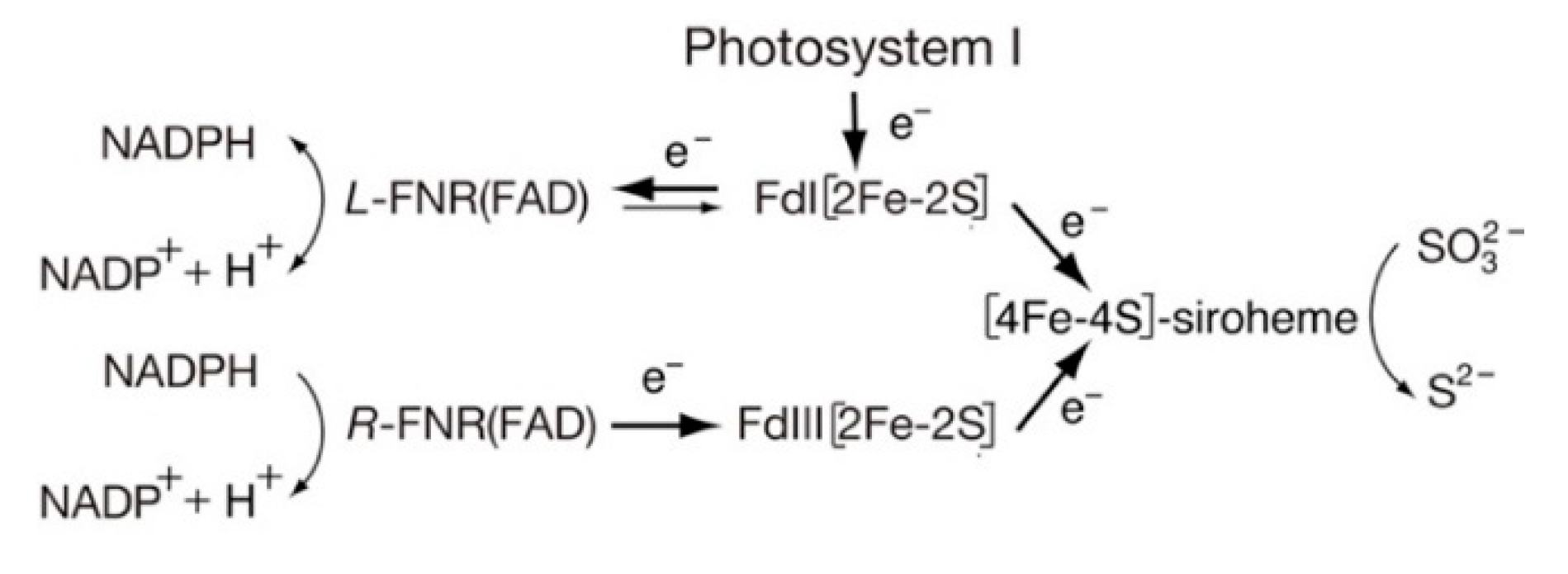
- Yonekura-Sakakibara, K.; Onda, Y.; Ashikari, T.; Tanaka, Y.; Kusumi, T.; Hase, T. Analysis of reductant supply systems for ferredoxin-dependent sulfite reductase in photosynthetic and nonphotosynthetic organs of maize. Plant. Physiol. 2000, 122, 887–894. [Google Scholar] [CrossRef]
- Hase, T.; Hanke, G.T.; Ariga-Kimata, Y.; Ping, G.; Okutani, S.; Kusunoki, M.; Kurisu, G. Structure and Function of the Electron Transfer Complex of Ferredoxin and Ferredoxin: NAD(P)H Oxidoreductase; Nishino, T., Miura, R., Tanokura, M., Fukui, K., Eds.; Flavins and Flavoproteins; ARchiTect Inc. Publishing: Tokyo, Japan, 2005; pp. 503–508. [Google Scholar]
- Murray, D.T.; Weiss, K.L.; Stanley, C.B.; Nagy, G.; Stroupe, M.E. Small-angle neutron scattering solution structures of NADPH-dependent sulfite reductase. J. Struct. Biol. 2021, 213, 107724. [Google Scholar] [CrossRef]
- Adak, S.; Aulak, K.S.; Stuehr, D.J. Direct evidence for nitric oxide production by a nitric-oxide synthase-like protein from Bacillus subtilis. J. Biol. Chem. 2002, 277, 16167–16171. [Google Scholar] [CrossRef]
- Wang, Z.Q.; Lawson, R.J.; Buddha, M.R.; Wei, C.C.; Crane, B.R.; Munro, A.W.; Stuehr, D.J. Bacterial flavodoxins support nitric oxide production by Bacillus subtilis nitric-oxide synthase. J. Biol. Chem. 2007, 282, 2196–2202. [Google Scholar] [CrossRef] [PubMed]
- Nishida, C.R.; de Montellano, P.R.O. Electron transfer and catalytic activity of nitric oxide synthases: Chimeric constructs of the neuronal, inducible, and endothelial isoforms. J. Biol. Chem. 1998, 273, 5566–5571. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Wang, B.; Zheng, B.; Ma, P.; Gou, R.; Guo, Y.; Chen, F.; Li, H.; Wang, Y.; Pu, J.; et al. The FNR modules contribute to control nitric oxide synthase catalysis revealed by chimera enzymes. Mol. Med. Rep. 2017, 16, 9263–9269. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mizutani, M.; Ohta, D. Two isoforms of NADPH: Cytochrome P450 reductase in Arabidopsis thaliana: Gene structure, heterologous expression in insect cells, and differential regulation. Plant. Physiol. 1998, 116, 357–367. [Google Scholar] [CrossRef]
- Jensen, K.; Møller, B.L. Plant NADPH-cytochrome P450 oxidoreductases. Phytochemistry 2010, 71, 132–141. [Google Scholar] [CrossRef]
- Parween, S.; Rojas Velazquez, M.N.; Udhane, S.S.; Kagawa, N.; Pandey, A.V. Variability in loss of multiple enzyme activities due to the human genetic variation P284T located in the flexible hinge region of NADPH cytochrome P450 oxidoreductase. Front. Pharmacol. 2019, 10, 1187. [Google Scholar] [CrossRef] [PubMed]
- Sugishima, M.; Sato, H.; Higashimoto, Y.; Harada, J.; Wada, K.; Fukuyama, K.; Noguchi, M. Structural basis for the electron transfer from an open form of NADPH- cytochrome P450 oxidoreductase to heme oxygenase. Proc. Natl. Acad. Sci. USA 2014, 111, 2524–2529. [Google Scholar] [CrossRef] [PubMed]
- Sugishima, M.; Taira, J.; Sagara, T.; Nakao, R.; Sato, H.; Noguchi, M.; Fukuyama, K.; Yamamoto, K.; Yasunaga, T.; Sakamoto, H. Conformational equilibrium of NADPH–cytochrome P450 oxidoreductase is essential for heme oxygenase reaction. Antioxidants 2020, 9, 673. [Google Scholar] [CrossRef] [PubMed]
- Hedison, T.M.; Scrutton, N.S. Tripping the light fantastic in membrane redox biology: Linking dynamic structures to function in ER electron transfer chains. FEBS J. 2019, 286, 2004–2017. [Google Scholar] [CrossRef] [PubMed]
- Crawford, N.M.; Smith, M.; Bellissimo, D.; Davis, R.W. Sequence and nitrate regulation of the Arabidopsis thaliana mRNA encoding nitrate reductase, a metalloflavoprotein with three functional domains. Proc. Natl. Acad. Sci. USA 1988, 85, 5006–5010. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Vivián, C.; Flores, E. Nitrate assimilation in bacteria. In Biology of the Nitrogen Cycle; Elsevier: Amsterdam, The Netherlands, 2007; pp. 263–282. [Google Scholar]
- Tan, W.; Liao, T.H.; Wang, J.; Ye, Y.; Wei, Y.C.; Zhou, H.K.; Xiao, Y.; Zhi, X.Y.; Shao, Z.H.; Lyu, L.D.; et al. A recently evolved diflavin-containing monomeric nitrate reductase is responsible for highly efficient bacterial nitrate assimilation. J. Biol. Chem. 2020, 295, 5051–5066. [Google Scholar] [CrossRef] [PubMed]
- Bock, R. The give-and-take of DNA: Horizontal gene transfer in plants. Trends Plant Sci. 2010, 15, 11–22. [Google Scholar] [CrossRef]
- Nielsen, K.M.; Bones, A.M.; Smalla, K.; van Elsas, J.D. Horizontal gene transfer from transgenic plants to terrestrial bacteria–a rare event? FEMS Microbiol. Rev. 1998, 22, 79–103. [Google Scholar] [CrossRef]
- Thomas, C.M.; Nielsen, K.M. Mechanisms of, and barriers to, horizontal gene transfer between bacteria. Nat. Rev. Microbiol. 2005, 3, 711–721. [Google Scholar] [CrossRef]
- Campbell, I.J.; Bennett, G.N.; Silberg, J.J. Evolutionary relationships between low potential ferredoxin and flavodoxin electron carriers. Front. Energy Res. 2019, 7, 79. [Google Scholar] [CrossRef]




| Enzyme Specicies | Eox/sq (mV) | Esq/red (mV) | pH |
|---|---|---|---|
| FNR (FAD) | |||
| Spinach (FAD) | −350 | −335 | 7 |
| −402 | −358 | 8 | |
| Spinach (FAD) | −306 | −386 | 8 |
| (in the presence of NADP+) | |||
| Anabaena (FAD) | −385 | −371 | 8 |
| Anabaena Flavodoxin (FMN) | −212 | −436 | 7 |
| Cyt P450 reductase (FAD-FMN) | |||
| Rabbit (FAD) | −290 | −365 | 7 |
| Rabbit (FMN) | −110 | −270 | 7 |
| Human (FAD) | −283 | −382 | 7 |
| Human (FMN) | −56 | −274 | 7 |
| nNOS reductase domain (FAD-FMN) | |||
| Rat (FAD) | −260 | −280 | 7.6 |
| Rat (FMN) | −50 | −276 | 7.6 |
| iNOSreductase domain (FAD-FMN) | |||
| Human (FAD) | −240 | −270 | 7 |
| Human (FMN) | −105 | −245 | 7 |
| FNR Species | (µM) | (µM s−1) | (µM) | (µM s−1) | ||
|---|---|---|---|---|---|---|
| Pea FNR | 139 | 6.5 | 21.3 | 30.6 | 16.7 | 1.8 |
| Anabaena FNR | 200 | 11 | 18.2 | 23.3 | 33 | 0.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iyanagi, T. Roles of Ferredoxin-NADP+ Oxidoreductase and Flavodoxin in NAD(P)H-Dependent Electron Transfer Systems. Antioxidants 2022, 11, 2143. https://doi.org/10.3390/antiox11112143
Iyanagi T. Roles of Ferredoxin-NADP+ Oxidoreductase and Flavodoxin in NAD(P)H-Dependent Electron Transfer Systems. Antioxidants. 2022; 11(11):2143. https://doi.org/10.3390/antiox11112143
Chicago/Turabian StyleIyanagi, Takashi. 2022. "Roles of Ferredoxin-NADP+ Oxidoreductase and Flavodoxin in NAD(P)H-Dependent Electron Transfer Systems" Antioxidants 11, no. 11: 2143. https://doi.org/10.3390/antiox11112143
APA StyleIyanagi, T. (2022). Roles of Ferredoxin-NADP+ Oxidoreductase and Flavodoxin in NAD(P)H-Dependent Electron Transfer Systems. Antioxidants, 11(11), 2143. https://doi.org/10.3390/antiox11112143





