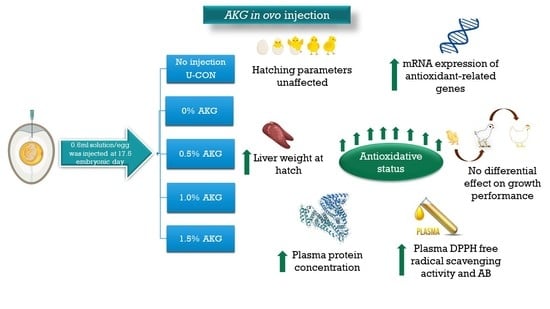
Effects of In Ovo Injection of α-Ketoglutaric Acid on Hatchability, Growth, Plasma Metabolites, and Antioxidant Status of Broilers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Egg Incubation and In Ovo Feeding
2.2. Rearing and Feeding of Birds
2.3. Sample Collection
2.4. Determination of Metabolite Concentrations and Antioxidant Capacity in Plasma
2.5. Real-Time PCR for mRNA Quantification
2.6. Statistical Analysis
3. Results
3.1. Hatchability Performance
3.2. Organ Weights and Length
3.3. Growth Performances
3.4. Plasma Metabolites
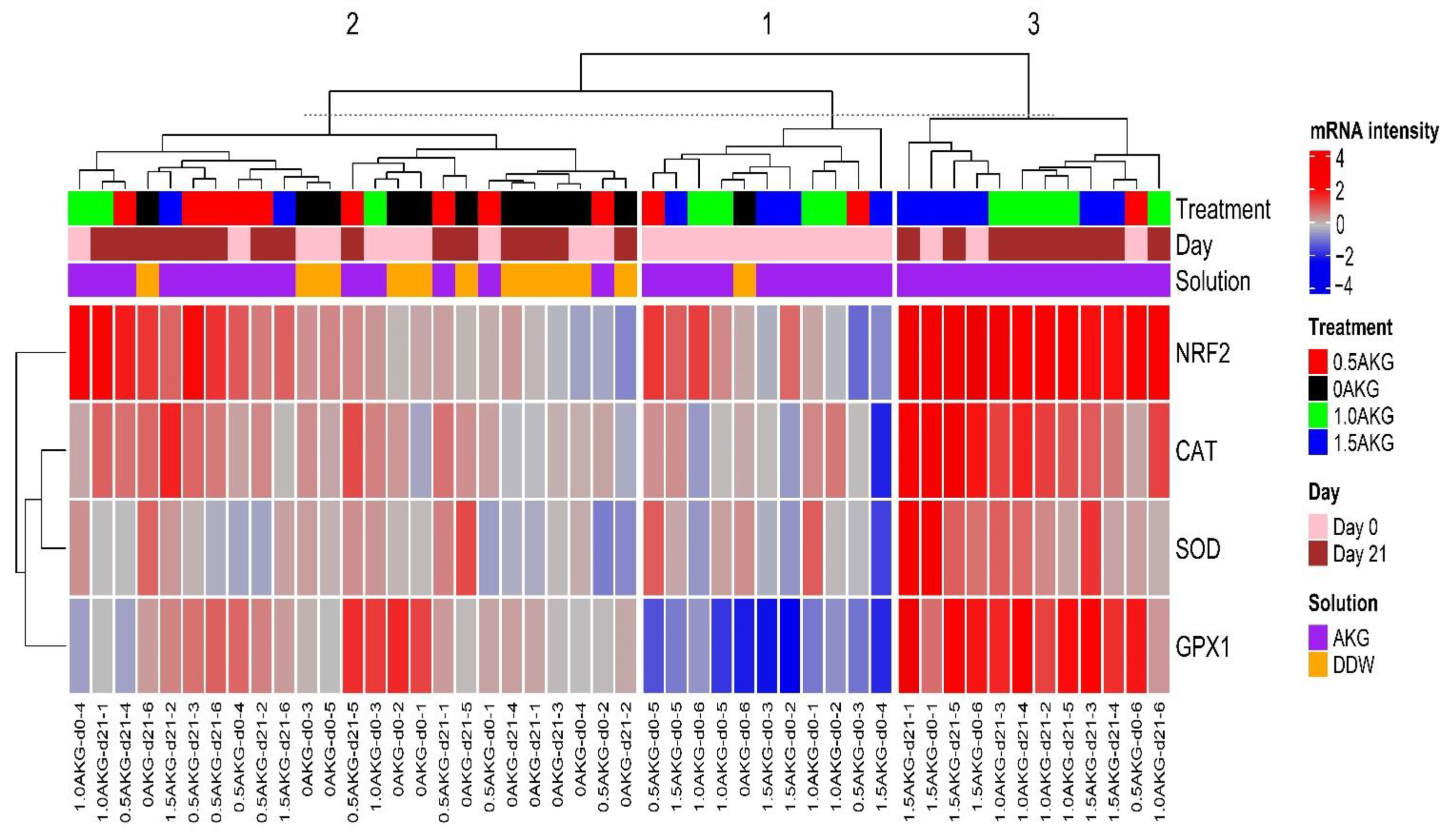
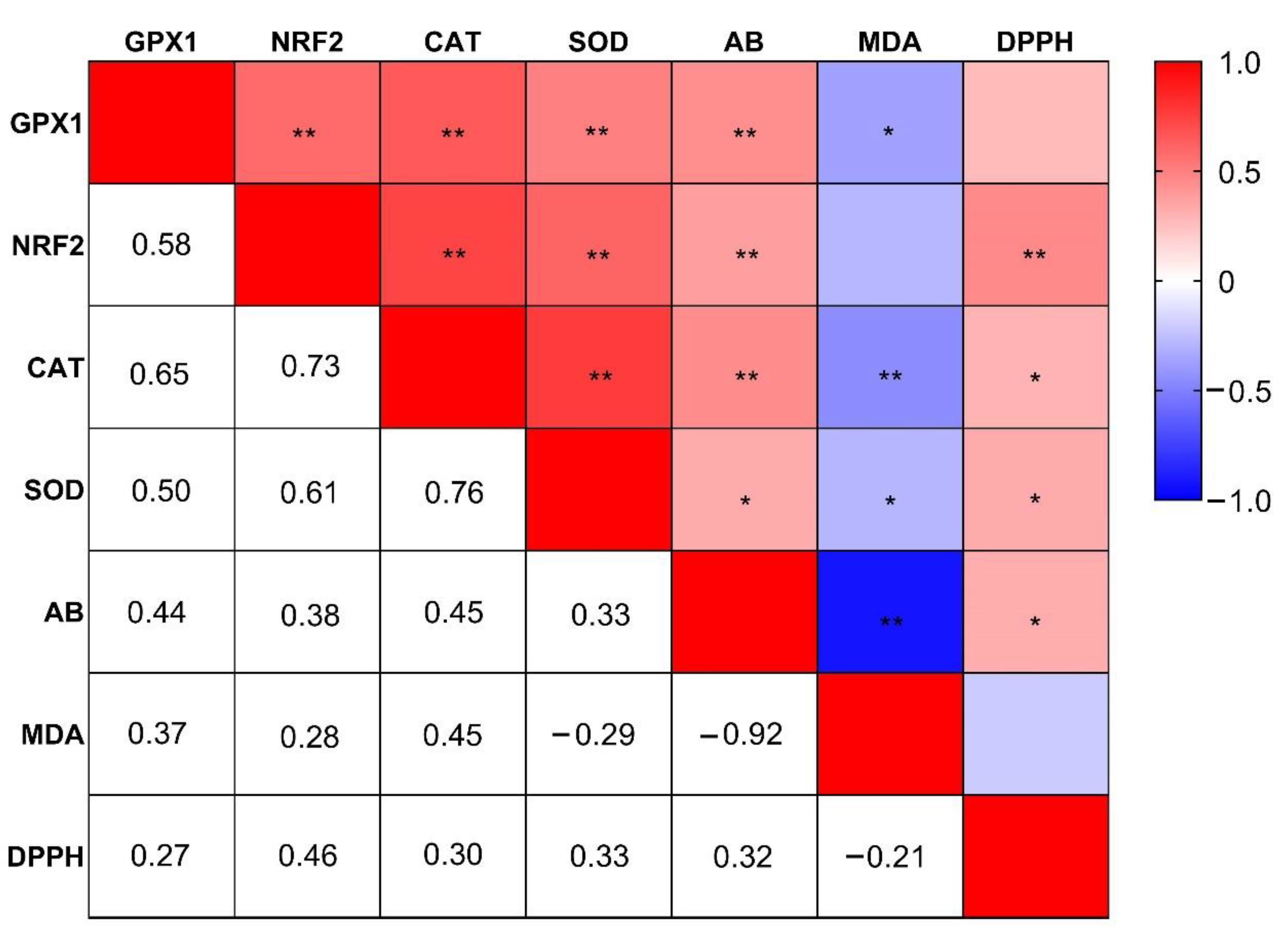
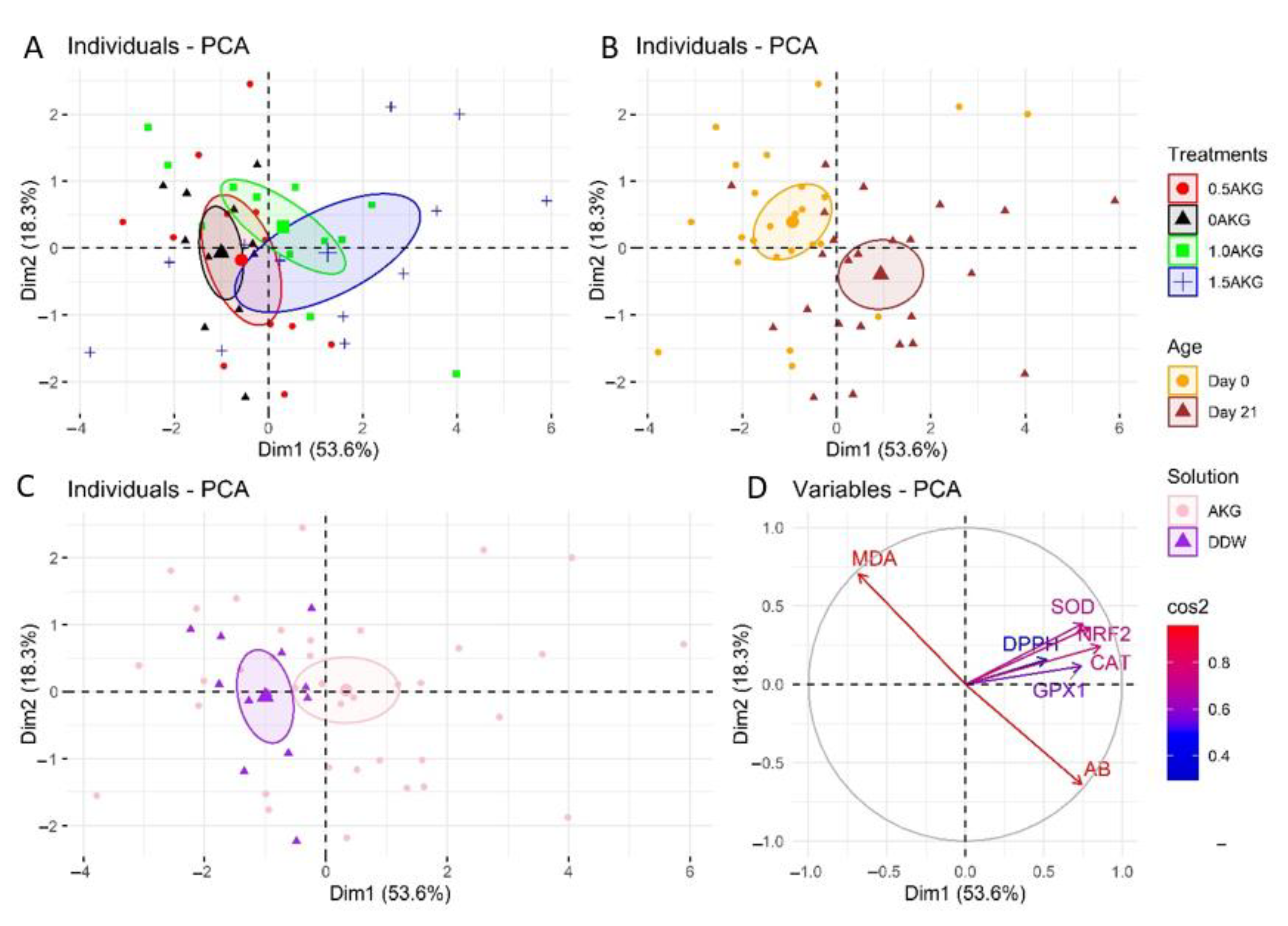
3.5. Plasma and Hepatic Oxidative Stress Markers
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zuidhof, M.J.; Schneider, B.L.; Carney, V.L.; Korver, D.R.; Robinson, F.E. Growth, efficiency, and yield of commercial broilers from 1957, 1978, and 2005. Poult. Sci. 2014, 93, 2970–2982. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, I. Climate change and the characterization, breeding and conservation of animal genetic resources. Anim. Genet. 2010, 41, 32–46. [Google Scholar] [CrossRef] [PubMed]
- Deeb, N.; Cahaner, A. Genotype-by-environment interaction with broiler genotypes differing in growth rate. 3. Growth rate and water consumption of broiler progeny from weight-selected versus nonselected parents under normal and high ambient temperatures. Poult. Sci. 2002, 81, 293–301. [Google Scholar] [CrossRef]
- Nawab, A.; Ibtisham, F.; Li, G.; Kieser, B.; Wu, J.; Liu, W.; Zhao, Y.; Nawab, Y.; Li, K.; Xiao, M. Heat stress in poultry production: Mitigation strategies to overcome the future challenges facing the global poultry industry. J. Therm. Biol. 2018, 78, 131–139. [Google Scholar] [CrossRef]
- Lara, L.J.; Rostagno, M.H. Impact of Heat Stress on Poultry Production. Animals 2013, 3, 356–369. [Google Scholar] [CrossRef] [Green Version]
- Esterbauer, H. Cytotoxicity and genotoxicity of lipid-oxidation products. Am. J. Clin. Nutr. 1993, 57, 779S–786S. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Marwan, A.-H.; Samaraweera, H.; Lee, E.J.; Ahn, D.U. Breast meat quality of broiler chickens can be affected by managing the level of nitric oxide. Poult. Sci. 2013, 92, 3044–3049. [Google Scholar] [CrossRef]
- Kirkman, H.N.; Gaetani, G.F. Catalase: A tetrameric enzyme with four tightly bound molecules of NADPH. Proc. Natl. Acad. Sci. USA 1984, 81, 4343–4347. [Google Scholar] [CrossRef] [Green Version]
- Fellenberg, M.; Speisky, H. Antioxidants: Their effects on broiler oxidative stress and its meat oxidative stability. World’s Poult. Sci. J. 2006, 62, 53–70. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, Q.; Ci, X.; Chen, S.; Xie, Z.; Li, H.; Zhang, H.; Chen, F.; Xie, Q. Evaluation of the efficacy of chlorogenic acid in reducing small intestine injury, oxidative stress, and inflammation in chickens challenged with Clostridium perfringens type A. Poult. Sci. 2020, 99, 6606–6618. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.; Moon, K.M.; Kim, C.Y. Tight junction in the intestinal epithelium: Its association with diseases and regulation by phytochemicals. J. Immunol. Res. 2018, 2018, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hohtola, E. Facultative and obligatory thermogenesis in young birds: A cautionary note. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2002, 131, 733–739. [Google Scholar] [CrossRef]
- Surai, P. Tissue-specific changes in the activities of antioxidant enzymes during the development of the chicken embryo. Br. Poult. Sci. 1999, 40, 397–405. [Google Scholar] [CrossRef] [PubMed]
- Speake, B.K.; Noble, R.C.; Murray, A.M. The utilization of yolk lipids by the chick embryo. World’s Poult. Sci. J. 1998, 54, 319–334. [Google Scholar] [CrossRef]
- Surai, P.F.; Fisinin, V.I.; Karadas, F. Antioxidant systems in chick embryo development. Part 1. Vitamin E, carotenoids and selenium. Anim. Nutr. 2016, 2, 1–11. [Google Scholar] [CrossRef]
- Jha, R.; Singh, A.K.; Yadav, S.; Berrocoso, J.F.D.; Mishra, B. Early nutrition programming (in ovo and post-hatch feeding) as a strategy to modulate gut health of poultry. Front. Vet. Sci. 2019, 6, 82. [Google Scholar] [CrossRef]
- Sharma, J.; Burmester, B. Resistance of Marek’s disease at hatching in chickens vaccinated as embryos with the turkey herpesvirus. Avian Dis. 1982, 26, 134–149. [Google Scholar] [CrossRef]
- Lu, P.; Morawong, T.; Molee, A.; Molee, W. Influences of L-Arginine In Ovo Feeding on the Hatchability, Growth Performance, Antioxidant Capacity, and Meat Quality of Slow-Growing Chickens. Animals 2022, 12, 392. [Google Scholar] [CrossRef]
- Dang, D.X.; Zhou, H.; Lou, Y.; Li, D. Effects of in ovo feeding of disaccharide and/or methionine on hatchability, growth performance, blood hematology, and serum antioxidant parameters in geese. J. Anim. Sci. 2022, 100, skac014. [Google Scholar] [CrossRef]
- Kadam, M.; Bhanja, S.; Mandal, A.; Thakur, R.; Vasan, P.; Bhattacharyya, A.; Tyagi, J. Effect of in ovo threonine supplementation on early growth, immunological responses and digestive enzyme activities in broiler chickens. Br. Poult. Sci. 2008, 49, 736–741. [Google Scholar] [CrossRef]
- Wu, N.; Yang, M.; Gaur, U.; Xu, H.; Yao, Y.; Li, D. Alpha-ketoglutarate: Physiological functions and applications. Biomol. Ther. 2016, 24, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harrison, A.; Pierzynowski, S. Biological effects of 2-oxoglutarate with particular emphasis on the regulation of protein, mineral and lipid absorption/metabolism, muscle performance, kidney function, bone formation and cancerogenesis, all viewed from a healthy ageing perspective state of the art--review article. J. Physiol. Pharmacol. Off. J. Pol. Physiol. Soc. 2008, 59, 91–106. [Google Scholar]
- Pierzynowski, S.; Sjodin, A. Perspectives of glutamine and its derivatives as feed additives for farm animals. J. Anim. Feed Sci. 1998, 7, 79–91. [Google Scholar] [CrossRef] [Green Version]
- Tatara, M.R.; Śliwa, E.; Krupski, W.; Brodzki, A.; Pasternak, K. Ornithine alpha-ketoglutarate increases mineralization and mechanical properties of tibia in turkeys. Bone 2006, 39, 100–105. [Google Scholar] [CrossRef]
- Guo, S.; Duan, R.; Wang, L.; Hou, Y.; Tan, L.; Cheng, Q.; Liao, M.; Ding, B. Dietary α-ketoglutarate supplementation improves hepatic and intestinal energy status and anti-oxidative capacity of Cherry Valley ducks. Anim. Sci. J. 2017, 88, 1753–1762. [Google Scholar] [CrossRef]
- Tomaszewska, E.; Świątkiewicz, S.; Arczewska-Włosek, A.; Wojtysiak, D.; Dobrowolski, P.; Domaradzki, P.; Świetlicka, I.; Donaldson, J.; Hułas-Stasiak, M.; Muszyński, S. Alpha-Ketoglutarate: An Effective Feed Supplement in Improving Bone Metabolism and Muscle Quality of Laying Hens: A Preliminary Study. Animals 2020, 10, 2420. [Google Scholar] [CrossRef]
- Bayliak, M.; Burdyliuk, N.; Lushchak, V. Growth on alpha-ketoglutarate increases oxidative stress resistance in the yeast Saccharomyces cerevisiae. Int. J. Microbiol. 2017, 2017, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Lin, X.; Jin, B.; Wang, H.; Zhao, Y. Effects of diet α-ketoglutarate (AKG) supplementation on the growth performance, antioxidant defense system, intestinal digestive enzymes, and immune response of grass carp (Ctenopharyngodon idellus). Aquac. Int. 2020, 28, 511–524. [Google Scholar] [CrossRef]
- Ali, R.; Mittal, G.; Sultana, S.; Bhatnagar, A. Ameliorative potential of alpha-ketoglutaric acid (AKG) on acute lung injuries induced by ammonia inhalation in rats. Exp. Lung Res. 2012, 38, 435–444. [Google Scholar] [CrossRef]
- Mailloux, R.J.; Singh, R.; Brewer, G.; Auger, C.; Lemire, J.; Appanna, V.D. α-ketoglutarate dehydrogenase and glutamate dehydrogenase work in tandem to modulate the antioxidant α-ketoglutarate during oxidative stress in Pseudomonas fluorescens. J. Bacteriol. 2009, 191, 3804–3810. [Google Scholar] [CrossRef] [Green Version]
- Tulsawani, R.; Debnath, M.; Pant, S.; Kumar, O.; Prakash, A.; Vijayaraghavan, R.; Bhattacharya, R. Effect of sub-acute oral cyanide administration in rats: Protective efficacy of alpha-ketoglutarate and sodium thiosulfate. Chem. Biol. Interact. 2005, 156, 1–12. [Google Scholar] [CrossRef]
- Velvizhi, S.; Dakshayani, K.B.; Subramanian, P. Effects of α-ketoglutarate on antioxidants and lipid peroxidation products in rats treated with ammonium acetate. Nutrition 2002, 18, 747–750. [Google Scholar] [CrossRef]
- Velvizhi, S.; Nagalashmi, T.; Essa, M.M.; Dakshayani, K.B.; Subramanian, P. Effects of alpha-ketoglutarate on lipid peroxidation and antioxidant status during chronic ethanol administration in Wistar rats. Pol. J. Pharmacol. 2002, 54, 231–236. [Google Scholar] [PubMed]
- Shuhui, Y.; Linglin, T.; Man, L.; Binying, D.; Dan, Y.; Lei, W. Effects of Alpha-ketoglutarate on growth performance and meat quality of broilers under heat stress. Feed Ind. 2014, 35, 11–16. [Google Scholar]
- Tatara, M.; Brodzki, A.; Krupski, W.; Silmanowicz, P.; Majcher, P.; Pierzynowski, S.; Studziński, T. Effects of alpha-ketoglutarate on bone homeostasis and plasma amino acids in turkeys. Poult. Sci. 2005, 84, 1604–1609. [Google Scholar] [CrossRef]
- Ncho, C.-M.; Goel, A.; Jeong, C.-M.; Youssouf, M.; Choi, Y.-H. In Ovo Injection of GABA Can Help Body Weight Gain at Hatch, Increase Chick Weight to Egg Weight Ratio, and Improve Broiler Heat Resistance. Animals 2021, 11, 1364. [Google Scholar] [CrossRef]
- Ncho, C.-M.; Goel, A.; Jeong, C.-M.; Gupta, V.; Choi, Y.-H. Effects of In Ovo Feeding of γ-Aminobutyric Acid on Growth Performances, Plasma Metabolites, and Antioxidant Status in Broilers Exposed to Cyclic Heat Stress. Sustainability 2021, 13, 11032. [Google Scholar] [CrossRef]
- Ncho, C.M.; Goel, A.; Gupta, V.; Jeong, C.-M.; Choi, Y.-H. Embryonic manipulations modulate differential expressions of heat shock protein, fatty acid metabolism, and antioxidant-related genes in the liver of heat-stressed broilers. PloS ONE 2022, 17, e0269748. [Google Scholar] [CrossRef]
- Gerasopoulos, K.; Stagos, D.; Kokkas, S.; Petrotos, K.; Kantas, D.; Goulas, P.; Kouretas, D. Feed supplemented with byproducts from olive oil mill wastewater processing increases antioxidant capacity in broiler chickens. Food Chem. Toxicol. 2015, 82, 42–49. [Google Scholar] [CrossRef]
- Jyothi, P.; Riyaz, N.; Nandakumar, G.; Binitha, M. A study of oxidative stress in paucibacillary and multibacillary leprosy. Indian J. Dermatol. Venereol. Leprol. 2008, 74, 80. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Pietrzak, E.; Dunislawska, A.; Siwek, M.; Zampiga, M.; Sirri, F.; Meluzzi, A.; Tavaniello, S.; Maiorano, G.; Slawinska, A. Splenic Gene Expression Signatures in Slow-Growing Chickens Stimulated in Ovo with Galactooligosaccharides and Challenged with Heat. Animals 2020, 10, 474. [Google Scholar] [CrossRef]
- Goel, A.; Ncho, C.M.; Jeong, C.-M.; Choi, Y.-H. Embryonic thermal manipulation and in ovo gamma-aminobutyric acid supplementation regulating the chick weight and stress-related genes at hatch. Front. Vet. Sci. 2022, 8, 807450. [Google Scholar] [CrossRef]
- Zhang, J.; Bai, K.; Su, W.; Wang, A.; Zhang, L.; Huang, K.; Wang, T. Curcumin attenuates heat-stress-induced oxidant damage by simultaneous activation of GSH-related antioxidant enzymes and Nrf2-mediated phase II detoxifying enzyme systems in broiler chickens. Poult. Sci. 2018, 97, 1209–1219. [Google Scholar] [CrossRef]
- Slawinska, A.; Dunislawska, A.; Plowiec, A.; Gonçalves, J.; Siwek, M. TLR-Mediated Cytokine Gene Expression in Chicken Peripheral Blood Mononuclear Cells as a Measure to Characterize Immunobiotics. Genes 2021, 12, 195. [Google Scholar] [CrossRef]
- Shafey, T.; Sami, A.; Abouheif, M. Effects of in ovo feeding of L-glutamine on hatchability performance and hatching time of meat-type breeder eggs. J. Anim. Vet. Adv. 2013, 12, 135–139. [Google Scholar]
- Feng, H.; Li, Z.; Qi, C.; Wang, X.; Qiao, B.; Wang, K.; Tang, X.; Liu, A. Effect of In Ovo Ornithine-á-Ketoglutarate Feeding on Early Body Weight and Pectoral Muscle Development of Chicks. Indian J. Anim. Res. 2019, 53, 1679–1683. [Google Scholar] [CrossRef]
- Zhong, Z.; Yu, Y.; Jin, S.; Pan, J. Effects of mixing eggs of different initial incubation time on the hatching pattern, chick embryonic development and post-hatch performance. PeerJ 2018, 6, e4634. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; He, L.; Yao, K. The antioxidative function of alpha-ketoglutarate and its applications. BioMed Res. Int. 2018, 2018, 3408467. [Google Scholar] [CrossRef]
- Janaszewska, A.; Bartosz, G. Assay of total antioxidant capacity: Comparison of four methods as applied to human blood plasma. Scand. J. Clin. Lab. Investig. 2002, 62, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Bayliak, M.M.; Lylyk, M.P.; Vytvytska, O.M.; Lushchak, V.I. Assessment of antioxidant properties of alpha-keto acids in vitro and in vivo. Eur. Food Res. Technol. 2016, 242, 179–188. [Google Scholar] [CrossRef]
- Long, L.H.; Halliwell, B. Artefacts in cell culture: α-Ketoglutarate can scavenge hydrogen peroxide generated by ascorbate and epigallocatechin gallate in cell culture media. Biochem. Biophys. Res. Commun. 2011, 406, 20–24. [Google Scholar] [CrossRef]
- Zdzisińska, B.; Żurek, A.; Kandefer-Szerszeń, M. Alpha-ketoglutarate as a molecule with pleiotropic activity: Well-known and novel possibilities of therapeutic use. Arch. Immunol. Et Ther. Exp. 2017, 65, 21–36. [Google Scholar] [CrossRef] [PubMed]
- Niemiec, T.; Sikorska, J.; Harrison, A.; Szmidt, M.; Sawosz, E.; Wirth-Dzieciolowska, E.; Wilczak, J.; Pierzynowski, S. Alpha-ketoglutarate stabilizes redox homeostasis and improves arterial elasticity in aged mice. J. Physiol. Pharmacol. 2011, 62, 37. [Google Scholar] [PubMed]
- Kang, M.J.; Kim, J.I. Protective effect of Hedyotis diffusa on lipopolysaccharide (LPS)-induced liver damage. Physiology 2013, 27, 1155-5. [Google Scholar] [CrossRef]
- Mehta, S.K.; Gowder, S.J.T. Members of antioxidant machinery and their functions. Basic Princ. Clin. Significance Oxidative Stress 2015, 11, 59–85. [Google Scholar]
- Ma, Q. Role of nrf2 in oxidative stress and toxicity. Annu. Rev. Pharmacol. Toxicol. 2013, 53, 401–426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Surai, P.F. Selenium in Poultry Nutrition and Health; Wageningen Academic Publishers: Wageningen, The Netherlands, 2018. [Google Scholar]
- Eid, Y.; Ebeid, T.; Younis, H. Vitamin E supplementation reduces dexamethasone-induced oxidative stress in chicken semen. Br. Poult. Sci. 2006, 47, 350–356. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-F.; Chang, S.-J.; Yang, J.; Lee, Y.; Hsu, A. Effects of supplemental vitamin E during the mature period on the reproduction performance of Taiwan Native Chicken cockerels. Br. Poult. Sci. 2005, 46, 366–373. [Google Scholar] [CrossRef] [PubMed]
- Aluwong, T.; Kawu, M.; Raji, M.; Dzenda, T.; Govwang, F.; Sinkalu, V.; Ayo, J. Effect of Yeast Probiotic on Growth, Antioxidant Enzyme Activities and Malondialdehyde Concentration of Broiler Chickens. Antioxidants 2013, 2, 326–339. [Google Scholar] [CrossRef] [Green Version]
- Xia, E.; Rao, G.; Van Remmen, H.; Heydari, A.R.; Richardson, A. Activities of antioxidant enzymes in various tissues of male Fischer 344 rats are altered by food restriction. J. Nutr. 1995, 125, 195–201. [Google Scholar]
- Chen, L.H.; Snyder, D.L. Effects of age, dietary restriction and germ-free environment on glutathione-related enzymes in Lobund—Wistar rats. Arch. Gerontol. Geriatr. 1992, 14, 17–26. [Google Scholar] [CrossRef]
- Pieri, C.; Falasca, M.; Marcheselli, F.; Moroni, F.; Recchioni, R.; Marmocchi, F.; Lupidi, G. Food restriction in female Wistar rats: V. Lipid peroxidation and antioxidant enzymes in the liver. Arch. Gerontol. Geriatr. 1992, 14, 93–99. [Google Scholar] [CrossRef]
- Han, G.; Ouchi, Y.; Hirota, T.; Haraguchi, S.; Miyazaki, T.; Arakawa, T.; Masuhara, N.; Mizunoya, W.; Tatsumi, R.; Tashiro, K. Effects of l-leucine in ovo feeding on thermotolerance, growth and amino acid metabolism under heat stress in broilers. Animal 2020, 14, 1701–1709. [Google Scholar] [CrossRef]
- Tavaniello, S.; Slawinska, A.; Prioriello, D.; Petrecca, V.; Bertocchi, M.; Zampiga, M.; Salvatori, G.; Maiorano, G. Effect of galactooligosaccharides delivered in ovo on meat quality traits of broiler chickens exposed to heat stress. Poult. Sci. 2020, 99, 612–619. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Xu, Q.; Wang, C.a.; Li, J.; Chen, D.; Zhao, Z.; Luo, L.; Du, X. Effects of dietary α-ketoglutarate supplementation on the antioxidant defense system and HSP 70 and HSP 90 gene expression of hybrid sturgeon A cipenser schrenckii♀× A. baerii♂ exposed to ammonia-N stress. Aquac. Res. 2017, 48, 2266–2277. [Google Scholar] [CrossRef]
- Luo, M.; Bazargan, N.; Griffith, D.P.; Estívariz, C.F.; Leader, L.M.; Easley, K.A.; Daignault, N.M.; Hao, L.; Meddings, J.B.; Galloway, J.R. Metabolic effects of enteral versus parenteral alanyl-glutamine dipeptide administration in critically ill patients receiving enteral feeding: A pilot study. Clin. Nutr. 2008, 27, 297–306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, A.-L.; Niu, Q.; Shi, N.; Wang, J.; Jia, X.-F.; Lian, H.-F.; Liu, Z.; Liu, C.-X. Glutamine ameliorates intestinal ischemia-reperfusion Injury in rats by activating the Nrf2/Are signaling pathway. Int. J. Clin. Exp. Pathol. 2015, 8, 7896. [Google Scholar]
- Amores-Sánchez, M.a.I.; Medina, M.Á. Glutamine, as a precursor of glutathione, and oxidative stress. Mol. Genet. Metab. 1999, 67, 100–105. [Google Scholar] [CrossRef] [Green Version]
- Venoji, R.; Amirtharaj, G.J.; Kini, A.; Vanaparthi, S.; Venkatraman, A.; Ramachandran, A. Enteral glutamine differentially regulates Nrf 2 along the villus-crypt axis of the intestine to enhance glutathione levels. J. Gastroenterol. Hepatol. 2015, 30, 1740–1747. [Google Scholar] [CrossRef]
- Chen, S.; Zou, L.; Li, L.; Wu, T. The protective effect of glycyrrhetinic acid on carbon tetrachloride-induced chronic liver fibrosis in mice via upregulation of Nrf2. PloS ONE 2013, 8, e53662. [Google Scholar] [CrossRef] [Green Version]
- Zaefarian, F.; Abdollahi, M.R.; Cowieson, A.; Ravindran, V. Avian liver: The forgotten organ. Animals 2019, 9, 63. [Google Scholar] [CrossRef] [Green Version]
- Dąbek, M.; Kruszewska, D.; Filip, R.; Hotowy, A.; Pierzynowski, Ł.; Wojtasz-Pająk, A.; Szymanczyk, S.; Valverde Piedra, J.; Werpachowska, E.; Pierzynowski, S. α-Ketoglutarate (AKG) absorption from pig intestine and plasma pharmacokinetics. J. Anim. Physiol. Anim. Nutr. 2005, 89, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Sasse, D. Dynamics of liver glycogen. Histochemistry 1975, 45, 237–254. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, J.; Uni, Z.; Ferket, P. Important metabolic pathways in poultry embryos prior to hatch. World’s Poult. Sci. J. 2008, 64, 488–499. [Google Scholar] [CrossRef] [Green Version]
- Soltan, M. Influence of dietary glutamine supplementation on growth performance, small intestinal morphology, immune response and some blood parameters of broiler chickens. Int. J. Poult. Sci. 2009, 8, 60–68. [Google Scholar] [CrossRef]
- Sakamoto, M.; Murakami, A.; Silveira, T.; Fernandes, J.; De Oliveira, C. Influence of glutamine and vitamin E on the performance and the immune responses of broiler chickens. Braz. J. Poult. Sci. 2006, 8, 243–249. [Google Scholar] [CrossRef] [Green Version]
- Maiorka, A.; Silva, A.; Santin, E.; Borges, S.; Boleli, I.; Macari, M. Influence of glutamine supplementation on performance and intestinal villous and crypt development in broiler chickens. Arq. Bras. De Med. Vet. E Zootec. 2000, 52, 487–490. [Google Scholar] [CrossRef]
- Rotava, R.; Zanella, I.; Karkow, A.K.; Dullius, A.P.; da da Silva, L.P.; Denardin, C.C. Bioquímica sanguínea de frangos de corte alimentados com subprodutos da uva. Agrarian 2008, 1, 91–104. [Google Scholar]
- Sorapukdee, S.; Narunatsopanon, S. Comparative study on compositions and functional properties of porcine, chicken and duck blood. Korean J. Food Sci. Anim. Resour. 2017, 37, 228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lambert, B.D.; Filip, R.; Stoll, B.; Junghans, P.; Derno, M.; Hennig, U.; Souffrant, W.B.; Pierzynowski, S.; Burrin, D.G. First-pass metabolism limits the intestinal absorption of enteral α-ketoglutarate in young pigs. J. Nutr. 2006, 136, 2779–2784. [Google Scholar] [CrossRef] [PubMed]

| S. No. | Gene | Sequence | Accession Number | Reference |
|---|---|---|---|---|
| 1. | GAPDH | F: TTGGCATTGTGGAGGGTCTTA R: GTGGACGCTGGGATGATGTT | NM_204305.1 | [43] |
| 2. | β-actin | F: ACCGGACTGTTACCAACA R: GACTGCTGCTGACACCTT | NM_205518.1 | [44] |
| 3. | NRF2 | F: CAGAAGCTTTCCCGTTCATAGA R: GACATTGGAGGGATGGCTTAT | NM_205117 | [44] |
| 4. | CAT | F: ACCAAGTACTGCAAGGCGAA R: TGAGGGTTCCTCTTCTGGCT | NM_001031215.1 | [43] |
| 5. | SOD | F: AGGGGGTCATCCACTTCC R: CCCATTTGTGTTGTCTCCAA | NM_205064.1 | [43] |
| 6. | GPX1 | F: AACCAATTCGGGCACCAG R: CCGTTCACCTCGCACTTCTC | NM_001277853.2 | [36] |
| Parameters | Treatments | Pooled SEM | p-Value | ||||||
|---|---|---|---|---|---|---|---|---|---|
| U-CON | 0 AKG | 0.5 AKG | 1.0 AKG | 1.5 AKG | ANOVA 1 | Lin 2 | Quad 2 | ||
| Hatchability (%) # | 88.71 | 85.48 | 88.89 | 80 | 79.31 | NA | NA | NA | NA |
| Late EM (%) # | 6.45 | 11.29 | 9.52 | 7.27 | 6.9 | NA | NA | NA | NA |
| Live pipping (%) # | 1.61 | 3.23 | 1.59 | 9.09 | 6.9 | NA | NA | NA | NA |
| Dead pipping (%) # | 3.23 | 0 | 0 | 3.64 | 6.9 | NA | NA | NA | NA |
| Egg weight (g) | 54.2 | 54.3 | 54.2 | 54.2 | 54.3 | NA | NA | NA | NA |
| BW at hatch | 36.8 | 36.8 | 36.7 | 36.6 | 36.9 | 0.121 | 0.978 | 0.961 | 0.274 |
| CWEWR (%) | 69.1 | 70.0 | 69.4 | 69.7 | 68.8 | 0.368 | 0.883 | 0.42 | 0.922 |
| Parameters | Treatments | Pooled SEM | p-Value | |||||
|---|---|---|---|---|---|---|---|---|
| 0 AKG | 0.5 AKG | 1.0 AKG | 1.5 AKG | ANOVA | Lin | Quad | ||
| Absolute organ weight (g) | ||||||||
| Liver | 0.70 a | 0.90 b | 0.80 ab | 0.88 b | 0.022 | 0.002 | 0.04 | 0.178 |
| Yolk sac | 4.47 | 3.68 | 3.49 | 4.38 | 0.172 | 0.091 | 0.767 | 0.039 |
| Gizzard | 1.68 | 1.86 | 1.82 | 1.82 | 0.047 | 0.609 | 0.377 | 0.377 |
| Proventriculus | 0.34 | 0.31 | 0.32 | 0.29 | 0.015 | 0.687 | 0.331 | 0.917 |
| Heart | 0.22 a | 0.29 b | 0.23 a | 0.27 ab | 0.008 | 0.006 | 0.32 | 0.436 |
| Relative organ weight (g/g BW) | ||||||||
| Liver | 1.97 a | 2.47 b | 2.24 ab | 2.43 b | 0.062 | 0.008 | 0.035 | 0.185 |
| Yolk sac | 12.52 | 10.06 | 9.83 | 12.07 | 0.461 | 0.072 | 0.715 | 0.009 |
| Gizzard | 4.72 | 5.09 | 5.14 | 5.04 | 0.136 | 0.716 | 0.414 | 0.401 |
| Proventriculus | 0.94 | 0.84 | 0.91 | 0.79 | 0.043 | 0.582 | 0.316 | 0.914 |
| Heart | 0.62 a | 0.79 b | 0.64 a | 0.74 ab | 0.021 | 0.006 | 0.277 | 0.387 |
| Parameters | Treatments | Pooled SEM | p-Value | |||||
|---|---|---|---|---|---|---|---|---|
| 0 AKG | 0.5 AKG | 1.0 AKG | 1.5 AKG | ANOVA | Lin | Quad | ||
| BW (g/bird) | ||||||||
| Day 0 | 36.8 | 36.7 | 36.6 | 36.9 | 0.073 | 0.669 | 0.961 | 0.274 |
| Day 7 | 144.2 | 134.0 | 139.0 | 144.5 | 2.000 | 0.199 | 0.744 | 0.051 |
| Day 21 | 836.4 | 764.3 | 795.3 | 838.4 | 12.525 | 0.100 | 0.749 | 0.020 |
| ADG (g/bird) | ||||||||
| 0 to 7 days | 15.3 | 13.9 | 14.6 | 15.4 | 0.290 | 0.218 | 0.746 | 0.057 |
| 8 to 21 days | 49.5 | 45.0 | 46.9 | 49.6 | 0.801 | 0.123 | 0.761 | 0.025 |
| ADFI (g/bird) | ||||||||
| 0 to 7 days | 15.4 | 14.5 | 15.1 | 15.5 | 0.264 | 0.554 | 0.696 | 0.228 |
| 8 to 21 days | 67.5 ab | 63.6 a | 67.2 ab | 70.3 b | 0.877 | 0.048 | 0.125 | 0.036 |
| FCR | ||||||||
| 0 to 7 days | 1.00 | 1.05 | 1.03 | 1.01 | 0.008 | 0.148 | 0.924 | 0.028 |
| 8 to 21 days | 1.37 | 1.42 | 1.44 | 1.42 | 0.012 | 0.189 | 0.084 | 0.167 |
| Treatments | Glucose | Total Protein | Triglycerides | Cholesterol | Calcium | |
|---|---|---|---|---|---|---|
| AKG effect | ||||||
| 0 AKG | 260.8 ± 14.4 | 2.5 ± 0.2 a | 21.2 ± 6.1 | 261.8 ± 37.8 | 10.1 ± 0.4 | |
| 0.5 AKG | 252.3 ± 8.9 | 2.9 ± 0.2 b | 57.8 ± 4.4 | 255.8 ± 37.4 | 10.6 ± 0.3 | |
| 1.0 AKG | 254.3 ± 13.3 | 2.8 ± 0.2 ab | 43.3 ± 5.4 | 248.0 ± 38.6 | 10.9 ± 0.3 | |
| 1.5 AKG | 248.7 ± 12.3 | 2.8 ± 0.2 ab | 42.8 ± 4.1 | 238.2 ± 32.5 | 10.4 ± 0.3 | |
| p-value | 0.073 | 0.025 | 0.124 | 0.493 | 0.066 | |
| Day effect | ||||||
| Day 0 | 223.6 ± 4.1 | 2.2 ± 0.1 | 51.0 ± 2.7 | 366.8 ± 10.7 | 9.7 ± 0.2 | |
| Day 21 | 284.3 ± 7.2 | 3.3 ± 0.1 | 43.2 ± 4.4 | 135.0 ± 3.2 | 11.3 ± 0.2 | |
| p-value | 0.001 | 0.001 | 0.130 | 0.001 | 0.001 | |
| AKG * Day effect | ||||||
| Day 0 | 0 AKG | 232.7 ± 13.4 | 1.9 ± 0.1 | 50.3 ± 4.9 | 381.5 ± 22.2 | 8.9 ± 0.2 |
| 0.5 AKG | 227.0 ± 2.6 | 2.3 ± 0.1 | 55.3 ± 6.4 | 375.8 ± 19.2 | 9.9 ± 0.2 | |
| 1.0 AKG | 222.0 ± 4.6 | 2.2 ± 0.1 | 49.7 ± 6.6 | 370.2 ± 23.5 | 10.2 ± 0.4 | |
| 1.5 AKG | 213.0 ± 8.0 | 2.3 ± 0.1 | 48.7 ± 5.0 | 339.8 ± 21.9 | 9.7 ± 0.2 | |
| Day 21 | 0 AKG | 289.0 ± 20.5 | 3.0 ± 0.1 | 38.7 ± 11.3 | 142.0 ± 7.2 | 11.3 ± 0.4 |
| 0.5 AKG | 277.5 ± 9.3 | 3.4 ± 0.2 | 60.3 ± 6.4 | 135.8 ± 6.2 | 11.3 ± 0.2 | |
| 1.0 AKG | 286.5 ± 18.5 | 3.4 ± 0.2 | 36.8 ± 8.3 | 125.8 ± 6.7 | 11.5 ± 0.4 | |
| 1.5 AKG | 284.3 ± 9.5 | 3.3 ± 0.2 | 37.0 ± 6.1 | 136.5 ± 4.7 | 11.2 ± 0.2 | |
| p-value | 0.842 | 0.816 | 0.546 | 0.550 | 0.230 | |
| Treatments | DPPH-RSA (%) | MDA (nmol/mL) | Antioxidant Balance (U) | |
|---|---|---|---|---|
| AKG effect | ||||
| 0 AKG | 27.3 ± 1.0 a | 1.2 ± 0.1 | 21.4 ± 1.5 | |
| 0.5 AKG | 28.4 ± 1.4 b | 1.1 ± 0.1 | 23.6 ± 2.4 | |
| 1.0 AKG | 28.3 ± 1.2 b | 1.1 ± 0.1 | 24.7 ± 3.0 | |
| 1.5 AKG | 28.8 ± 1.1 b | 0.9 ± 0.1 | 29.0 ± 2.1 | |
| p-value | ANOVA | 0.001 | 0.080 | 0.053 |
| Linear | 0.381 | 0.066 | 0.024 | |
| Quadratic | 0.797 | 0.390 | 0.644 | |
| Day effect | ||||
| Day 0 | 32.1 ± 0.3 | 1.3 ± 0.1 | 20.2 ± 1.0 | |
| Day 21 | 24.3 ± 0.2 | 0.9 ± 0.1 | 29.2 ± 1.7 | |
| p-value | 0.001 | 0.001 | 0.001 | |
| AKG * Day effect | ||||
| Day 0 | 0 AKG | 30.5 ± 0.6 y | 1.2 ± 0.1 | 19.4 ± 0.9 |
| 0.5 AKG | 33.1 ± 0.3 z | 1.4 ± 0.1 | 18.1 ± 2.2 | |
| 1.0 AKG | 32.2 ± 0.5 z | 1.4 ± 0.2 | 19.5 ± 2.6 | |
| 1.5 AKG | 32.6 ± 0.1 z | 1.1 ± 0.1 | 23.6 ± 1.6 | |
| Day 21 | 0 AKG | 24.0 ± 0.2 x | 1.1 ± 0.1 | 23.4 ± 2.7 |
| 0.5 AKG | 23.7 ± 0.4 x | 0.9 ± 0.1 | 29.1 ± 2.9 | |
| 1.0 AKG | 24.5 ± 0.1 x | 0.9 ± 0.1 | 29.9 ± 4.7 | |
| 1.5 AKG | 25.1 ± 0.3 x | 0.7 ± 0.1 | 34.4 ± 2.2 | |
| p-value | 0.003 | 0.285 | 0.503 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gupta, V.; Ncho, C.M.; Goel, A.; Jeong, C.-M.; Choi, Y.-H. Effects of In Ovo Injection of α-Ketoglutaric Acid on Hatchability, Growth, Plasma Metabolites, and Antioxidant Status of Broilers. Antioxidants 2022, 11, 2102. https://doi.org/10.3390/antiox11112102
Gupta V, Ncho CM, Goel A, Jeong C-M, Choi Y-H. Effects of In Ovo Injection of α-Ketoglutaric Acid on Hatchability, Growth, Plasma Metabolites, and Antioxidant Status of Broilers. Antioxidants. 2022; 11(11):2102. https://doi.org/10.3390/antiox11112102
Chicago/Turabian StyleGupta, Vaishali, Chris Major Ncho, Akshat Goel, Chae-Mi Jeong, and Yang-Ho Choi. 2022. "Effects of In Ovo Injection of α-Ketoglutaric Acid on Hatchability, Growth, Plasma Metabolites, and Antioxidant Status of Broilers" Antioxidants 11, no. 11: 2102. https://doi.org/10.3390/antiox11112102
APA StyleGupta, V., Ncho, C. M., Goel, A., Jeong, C.-M., & Choi, Y.-H. (2022). Effects of In Ovo Injection of α-Ketoglutaric Acid on Hatchability, Growth, Plasma Metabolites, and Antioxidant Status of Broilers. Antioxidants, 11(11), 2102. https://doi.org/10.3390/antiox11112102