The Effects of Differentiated Organic Fertilization on Tomato Production and Phenolic Content in Traditional and High-Yielding Varieties
Abstract
1. Introduction
2. Materials and Methods
2.1. Field Experiment
2.2. Experimental Design
2.3. Leaf and Tomato Sampling and Analysis
2.4. Phenolic Extraction and Determination
2.5. Statistical Analysis
3. Results and Discussion
3.1. Effect of Fertilization on Tomato Yield
3.2. Productivity of the Different Varieties
3.3. Effect of Fertilization on Mineral Nutrients in Plant Leaves
3.4. Mineral Nutrient Status in Tomato Varieties and Its Effect on δ13C and Nitrate Values
3.5. Phenolic Content
3.6. Plant Nutritional Status, Water Stress Sensitivity, and Oxidative Stress Regulation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAOSTAT. Available online: http://www.fao.org/faostat/es/#data/QC (accessed on 10 December 2020).
- Vallverdú-Queralt, A.; Jáuregui, O.; Medina-Remón, A.; Lamuela-Raventós, R.M. Evaluation of a Method to Characterize the Phenolic Profile of Organic and Conventional Tomatoes. J. Agric. Food Chem. 2012, 60, 3373–3380. [Google Scholar] [CrossRef] [PubMed]
- Vallverdú-Queralt, A.; Regueiro, J.; Rinaldi De Alvarenga, J.; Torrado, X.; Lamuela-Raventos, R.M. Home cooking and phenolics: Effect of thermal treatment and addition of extra virgin olive oil on the phenolic profile of tomato sauces. J. Agr. Food Chem. 2014, 62, 3314–33209. [Google Scholar] [CrossRef] [PubMed]
- Vinha, A.F.; Alves, R.C.; Barreira, S.V.P.; Castro, A.; Costa, A.S.G.; Oliveira, M.B.P.P. Effect of Peel and Seed Removal on the Nutritional Value and Antioxidant Activity of Tomato (Lycopersicon esculentum L.) Fruits. LWT-Food Sci. Technol. 2014, 55, 197–202. [Google Scholar] [CrossRef]
- Canene-Adams, K.; Campbell, J.; Zaripheh, S.; Jeffery, E.; Erdman, J.W., Jr. The Tomato as a Functional Food. J. Nutr. 2005, 135, 1226–1230. [Google Scholar] [CrossRef] [PubMed]
- Bulluck, L.R.; Brosius, M.; Evanylo, G.K.; Ristaino, J.B. Organic and Synthetic Fertility Amendments Influence Soil Microbial, Physical and Chemical Properties on Organic and Conventional Farms. Appl. Soil Ecol. 2002, 19, 147–160. [Google Scholar] [CrossRef]
- Patel, K.F.; Smith, A.P.; Bond-Lamberty, B.; Fansler, S.J.; Tfaily, M.M.; Bramer, L.; Varga, T.; Bailey, V.L. Spatial Access and Resource Limitations Control Carbon Mineralization in Soils. Soil Biol. Biochem. 2021, 162, 108427. [Google Scholar] [CrossRef]
- Larkin, R.P. Soil Health Paradigms and Implications for Disease Management. Annu. Rev. Phytopathol. 2015, 53, 199–221. [Google Scholar] [CrossRef]
- Daly, A.B.; Jilling, A.; Bowles, T.M.; Buchkowski, R.W.; Frey, S.D.; Kallenbach, C.M.; Keiluweit, M.; Mooshammer, M.; Schimel, J.P.; Grandy, A.S. A Holistic Framework Integrating Plant-Microbe-Mineral Regulation of Soil Bioavailable Nitrogen. Biogeochemistry 2021, 154, 211–229. [Google Scholar] [CrossRef]
- Rabot, E.; Wiesmeier, M.; Schlüter, S.; Vogel, H.J. Soil Structure as an Indicator of Soil Functions: A Review. Geoderma 2018, 314, 122–137. [Google Scholar] [CrossRef]
- López-Yerena, A.; Lozano-Castellón, J.; Olmo-Cunillera, A.; Tresserra-Rimbau, A.; Quifer-Rada, P.; Jiménez, B.; Pérez, M.; Val-lverdú-Queralt, A. Effects of organic and conventional growing systems on the phenolic profile of extra-virgin olive oil. Molecules 2019, 24, 1986. [Google Scholar]
- Hurtado-Barroso, S.; Tresserra-Rimbau, A.; Vallverdú-Queralt, A.; Lamuela-Raventós, R.M. Organic food and the impact on human health. Crit. Rev. Food Sci. Nutr. 2019, 59, 704–714. [Google Scholar] [CrossRef] [PubMed]
- Buoso, S.; Zamboni, A.; Franco, A.; Commisso, M.; Guzzo, F.; Varanini, Z.; Pinton, R.; Tomasi, N.; Zanin, L. Nodulating White Lupins Take Advantage of the Reciprocal Interplay between N and P Nutritional Responses. Physiol. Plant. 2022, 174, e13607. [Google Scholar] [CrossRef] [PubMed]
- Termorshuizen, A.J.; van Rijn, E.; van der Gaag, D.J.; Alabouvette, C.; Chen, Y.; Lagerlöf, J.; Malandrakis, A.A.; Paplomatas, E.J.; Rämert, B.; Ryckeboer, J.; et al. Suppressiveness of 18 Composts against 7 Pathosystems: Variability in Pathogen Response. Soil Biol. Biochem. 2006, 38, 2461–2477. [Google Scholar] [CrossRef]
- Janvier, C.; Villeneuve, F.; Alabouvette, C.; Edel-Hermann, V.; Mateille, T.; Steinberg, C. Soil Health through Soil Disease Suppression: Which Strategy from Descriptors to Indicators? Soil Biol. Biochem. 2007, 39, 1–23. [Google Scholar] [CrossRef]
- Segarra, G.; Van Der Ent, S.; Trillas, I.; Pieterse, C.M.J. MYB72, a Node of Convergence in Induced Systemic Resistance Triggered by a Fungal and a Bacterial Beneficial Microbe. Plant Biol. 2009, 11, 90–96. [Google Scholar] [CrossRef]
- Segarra, G.; Elena, G.; Trillas, I. Systemic Resistance against Botrytis Cinerea in Arabidopsis Triggered by an Olive Marc Compost Substrate Requires Functional SA Signalling. Physiol. Mol. Plant Pathol. 2013, 82, 46–50. [Google Scholar] [CrossRef]
- Fenero, D.; Munné, G. Variabilitat Intravarietal per Caràcters Agromorfològics en Dos Varietats Tradicionals Catalanes: El Tomàquet de Montserrat/Pera de Girona i el Tomàquet de Penjar; Treball final de Carrera; Escola Superior d’Agricultura de Barcelona (ESAB-UPC): Castelldefels, Spain, 2009. [Google Scholar]
- Casals, J.; Pascual, L.; Cañizares, J.; Cebolla-Cornejo, J.; Casañas, F.; Nuez, F. The Risks of Success in Quality Vegetable Markets: Possible Genetic Erosion in Marmande Tomatoes (Solanum lycopersicum L.) and Consumer Dissatisfaction. Sci. Hortic. 2011, 130, 78–84. [Google Scholar] [CrossRef]
- Casals, J.; Casañas, F. Varietats Tradicionals de Tomàquet Catalanes: Caracterització Agromorfològica, Sensorial i Química de 13 Varietats; Fundació Miquel Agustí: Castelldefels, Spain, 2011. [Google Scholar]
- González Atero, R. Ús de Varietats Tradicionals de Tomàquet En Agricultura Ecològica; Universitat de Barcelona: Barcelona, Spain, 2011. [Google Scholar]
- Casals, J.; Pascual, L.; Cañizares, J.; Cebolla-Cornejo, J.; Casañas, F.; Nuez, F. Genetic Basis of Long Shelf Life and Variability into Penjar Tomato. Genet. Resour. Crop Evol. 2012, 59, 219–229. [Google Scholar] [CrossRef]
- Cataldo, D.A.; Maroon, M.; Schrader, L.E.; Youngs, V.L. Rapid Colorimetric Determination of Nitrate in Plant Tissue by Nitration of Salicylic Acid. Commun. Soil Sci. Plant Anal. 1975, 6, 71–80. [Google Scholar] [CrossRef]
- Zasoski, R.J.; Burau, R.G. A Rapid Nitric-Perchloric Acid Digestion Method for Multi-Element Tissue Analysis. Commun. Soil Sci. Plant Anal. 1977, 8, 425–436. [Google Scholar] [CrossRef]
- Chandrasekeran, A.; Mahalingam, P. Effect of Arbuscular Mycorrhizae Fungi on the Changes of Physicochemical Composition in Tomato (Lycopersicon esculentum) Fruit. EC Agric. 2015, 2, 317–324. [Google Scholar]
- Rinaldi de Alvarenga, J.F.; Quifer-Rada, P.; Juliano, F.F.; Hurtado-Barroso, S.; Illan, M.; Torrado-Prat, X.; Lamuela-Raventós, R.M. Using Extra Virgin Olive Oil to Cook Vegetables Enhances Polyphenol and Carotenoid Extractability: A Study Applying the Sofrito Technique. Molecules 2019, 24, 1555. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Davidson, E.A.; Zou, T.; Lassaletta, L.; Quan, Z.; Li, T.; Zhang, W. Quantifying Nutrient Budgets for Sustainable Nutrient Management. Glob. Biogeochem. Cycles 2020, 34, e2018GB006060. [Google Scholar] [CrossRef]
- Carricondo-Martínez, I.; Berti, F.; Salas-Sanjuán, M.D.C. Different Organic Fertilisation Systems Modify Tomato Quality: An Opportunity for Circular Fertilisation in Intensive Horticulture. Agronomy 2022, 12, 174. [Google Scholar] [CrossRef]
- Gatsios, A.; Ntatsi, G.; Celi, L.; Said-Pullicino, D.; Tampakaki, A.; Savvas, D. Legume-Based Mobile Green Manure Can Increase Soil Nitrogen Availability and Yield of Organic Greenhouse Tomatoes. Plants 2021, 10, 2419. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Ortiz, J.C.; Díaz-Flores, P.E.; Zavala-Sierra, D.; Preciado-Rangel, P.; Rodríguez-Fuentes, H.; Estrada-González, A.J.; Carballo-Méndez, F.J. Organic vs. Conventional Fertilization: Soil Nutrient Availability, Production, and Quality of Tomato Fruit. Water Air Soil Pollut. 2022, 233, 87. [Google Scholar] [CrossRef]
- Bénard, C.; Gautier, H.; Bourgaud, F.; Grasselly, D.; Navez, B.; Caris-Veyrat, C.; Weiss, M.; Génard, M. Effects of Low Nitrogen Supply on Tomato (Solanum lycopersicum) Fruit Yield and Quality with Special Emphasis on Sugars, Acids, Ascorbate, Carotenoids, and Phenolic Compounds. J. Agric. Food Chem. 2009, 57, 4112–4123. [Google Scholar] [CrossRef] [PubMed]
- Duma, M.; Alsina, I.; Dubova, L.; Erdberga, I. Chemical Composition of Tomatoes Depending on the Stage of Ripening. Chem. Technol. 2015, 66, 24–28. [Google Scholar] [CrossRef]
- Benbrook, C.; Kegley, S.; Baker, B. Organic Farming Lessens Reliance on Pesticides and Promotes Public Health by Lowering Dietary Risks. Agronomy 2021, 11, 1266. [Google Scholar] [CrossRef]
- Villar, P.; Villar, J. Guia de la Fertilitat Dels Sòls I la Nutrició Vegetal en Producció Integrada; Consell Ca. Lleida: Lleida, Spain; Generalitat de Catalunya: Catalunya, Spain, 2016. [Google Scholar]
- Hernández, V.; Hellín, P.; Fenoll, J.; Flores, P. Impact of Nitrogen Supply Limitation on Tomato Fruit Composition. Sci. Hortic. 2020, 264, 109173. [Google Scholar] [CrossRef]
- Cvijanović, V.; Sarić, B.; Dramićanin, A.; Kodranov, I.; Manojlović, D.; Momirović, N.; Momirović, N.; Milojković-Opsenica, D. Content and Distribution of Macroelements, Microelements, and Rare-Earth Elements in Different Tomato Varieties as a Promising Tool for Monitoring the Distinction between the Integral and Organic Systems of Production in Zeleni Hit—Official Enza and Vitalis Trial and Breeding Station. Agriculture 2021, 11, 1009. [Google Scholar] [CrossRef]
- Kaiser, B.N.; Gridley, K.L.; Brady, J.N.; Phillips, T.; Tyerman, S.D. The Role of Molybdenum in Agricultural Plant Production. Ann. Bot. 2005, 96, 745–754. [Google Scholar] [CrossRef] [PubMed]
- Iatrou, M.; Papadopoulos, A. Influence of Nitrogen Nutrition on Nitrate Levels of Strawberry Leaf Blades and Petioles. J. Plant Nutr. 2016, 39, 1131–1136. [Google Scholar] [CrossRef]
- Farquhar, G.; Ehleringer, J.; Hubick, K. Carbon Isotope Discrimination and Photosynthesis. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1989, 40, 503–537. [Google Scholar] [CrossRef]
- McNulty, S.G.; Swank, W.T. Wood D13C as a Measure of Annual Basal Area Growth and Soil Water Stress in a Pinus Strobus Forest. Ecology 1995, 76, 1581–1586. [Google Scholar] [CrossRef]
- Martin-StPaul, N.; Delzon, S.; Cochard, H. Plant Resistance to Drought Depends on Timely Stomatal Closure. Ecol. Lett. 2017, 20, 1437–1447. [Google Scholar] [CrossRef] [PubMed]
- Slimestad, R.; Verheul, M.J. Seasonal Variations in the Level of Plant Constituents in Greenhouse Production of Cherry Tomatoes. J. Agric. Food Chem. 2005, 53, 3114–3119. [Google Scholar] [CrossRef]
- Barros, L.; Dueñas, M.; Pinela, J.; Carvalho, A.M.; Buelga, C.S.; Ferreira, I.C.F.R. Characterization and Quantification of Phenolic Compounds in Four Tomato (Lycopersicon esculentum L.) Farmers’ Varieties in Northeastern Portugal Homegardens. Plant Foods Hum. Nutr. 2012, 67, 229–234. [Google Scholar] [CrossRef]
- Slimestad, R.; Fossen, T.; Verheul, M.J. The Flavonoids of Tomatoes. J. Agric. Food Chem. 2008, 56, 2436–2441. [Google Scholar] [CrossRef]
- Cruz-Carrión, Á.; Calani, L.; de Azua, M.J.R.; Mena, P.; del Rio, D.; Arola-Arnal, A.; Suárez, M. Impact of Seasonal Consumption of Local Tomatoes on the Metabolism and Absorption of (Poly)Phenols in Fischer Rats. Nutrients 2022, 14, 2047. [Google Scholar] [CrossRef]
- Gómez-Romero, M.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Metabolite Profiling and Quantification of Phenolic Compounds in Methanol Extracts of Tomato Fruit. Phytochemistry 2010, 71, 1848–1864. [Google Scholar] [CrossRef] [PubMed]
- Stewart, A.J.; Chapman, W.; Jenkins, G.I.; Graham, I.; Martin, T.; Crozier, A. The Effect of Nitrogen and Phosphorus Deficiency on Flavonol Accumulation in Plan Tissues. Plant Cell Environ. 2001, 24, 1189–1197. [Google Scholar] [CrossRef]
- Bénard, C.; Bourgaud, F.; Gautier, H. Impact of Temporary Nitrogen Deprivation on Tomato Leaf Phenolics. Int. J. Mol. Sci. 2011, 12, 7971–7981. [Google Scholar] [CrossRef]
- De Pascale, S.; Maggio, A.; Orsini, F.; Barbieri, G. Cultivar, Soil Type, Nitrogen Source and Irrigation Regime as Quality Determinants of Organically Grown Tomatoes. Sci. Hortic. 2016, 199, 88–94. [Google Scholar] [CrossRef]
- Tattini, M.; Loreto, F.; Fini, A.; Guidi, L.; Brunetti, C.; Velikova, V.; Gori, A.; Ferrini, F. Isoprenoids and Phenylpropanoids Are Part of the Antioxidant Defense Orchestrated Daily by Drought-Stressed Platanus Acerifolia Plants during Mediterranean Summers. New Phytol. 2015, 207, 613–626. [Google Scholar] [CrossRef] [PubMed]
- Barbagallo, R.N.; Di Silvestro, I.; Patanè, C. Yield, Physicochemical Traits, Antioxidant Pattern, Polyphenol Oxidase Activity and Total Visual Quality of Field-Grown Processing Tomato Cv. Brigade as Affected by Water Stress in Mediterranean Climate. J. Sci. Food Agric. 2013, 93, 1449–1457. [Google Scholar] [CrossRef]
- Larbat, R.; Paris, C.; Le Bot, J.; Adamowicz, S. Phenolic Characterization and Variability in Leaves, Stems and Roots of Micro-Tom and Patio Tomatoes, in Response to Nitrogen Limitation. Plant Sci. 2014, 224, 62–73. [Google Scholar] [CrossRef]
- Conesa, M.; Muir, C.D.; Roldán, E.J.; Molins, A.; Perdomo, J.A.; Galmés, J. Growth Capacity in Wild Tomatoes and Relatives Correlates with Original Climate in Arid and Semi-Arid Species. Environ. Exp. Bot. 2017, 141, 181–190. [Google Scholar] [CrossRef]
- Chea, L.; Erika, C.; Naumann, M.; Smit, I.; Horneburg, B.; Pawelzik, E. Morphological, Leaf Nutrient, and Fruit Quality Characteristics of Diverse Tomato Cultivars under Organic Low-input Management. Sustainability 2021, 13, 2326. [Google Scholar] [CrossRef]
- Tahiri, A.-I.; Meddich, A.; Raklami, A.; Alahmad, A.; Bechtaoui, N.; Anli, M.; Göttfert, M.; Heulin, T.; Achouak, W.; Oufdou, K. Assessing the Potential Role of Compost, PGPR, and AMF in Improving Tomato Plant Growth, Yield, Fruit Quality, and Water Stress Tolerance. J. Soil Sci. Plant Nutr. 2022, 22, 743–764. [Google Scholar] [CrossRef]
- Sánchez-Rodríguez, E.; Moreno, D.A.; Ferreres, F.; Rubio-Wilhelmi, M.D.M.; Ruiz, J.M. Differential Responses of Five Cherry Tomato Varieties to Water Stress: Changes on Phenolic Metabolites and Related Enzymes. Phytochemistry 2011, 72, 723–729. [Google Scholar] [CrossRef] [PubMed]
- Chacón-Vozmediano, J.L.; Martínez-Gascueña, J.; García-Romero, E.; Gómez-Alonso, S.; García-Navarro, F.J.; Jiménez-Ballesta, R. Effects of Water Stress on the Phenolic Compounds of ‘Merlot’ Grapes in a Semi-Arid Mediterranean Climate. Horticulturae 2021, 7, 161. [Google Scholar] [CrossRef]
- Hadacek, F.; Bachmann, G.; Engelmeier, D.; Chobot, V. Hormesis and a Chemical Raison D’être for Secondary Plant Metabolites. Dose-Response 2011, 9, 79–116. [Google Scholar] [CrossRef] [PubMed]
- Hartley, S.E.; Nelson, K.; Gorman, M. The Effect of Fertiliser and Shading on Plant Chemical Composition and Palatability to Orkney Voles, Microtus Arvalis Orcadensis. Oikos 1995, 72, 79–87. [Google Scholar] [CrossRef]
- Garza-Alonso, C.A.; Niño-Medina, G.; Gutiérrez-Díez, A.; García-López, J.I.; Vázquez-Alvarado, R.E.; López-Jiménez, A.; Olivares-Sáenz, E. Physicochemical Characteristics, Minerals, Phenolic Compounds, and Antioxidant Capacity in Fig Tree Fruits with Macronutrient Deficiencies. Not. Bot. Horti Agrobot. Cluj-Napoca 2020, 48, 1585–1599. [Google Scholar] [CrossRef]


| Varietal Group | Variety | Type | |
|---|---|---|---|
| 1 | Pebroter | Cornabel | High-yielding |
| 2 | Pebroter | Cuban Pepper | Traditional |
| 3 | Pebroter | Corno Andino | Traditional |
| 4 | Montserrat | Montserrat Fitó | High-yielding |
| 5 | Montserrat | Can Duran | Traditional |
| 6 | Montserrat | Montserrat Ple | Traditional |
| N-rich Fertilizer C/N 4 | N-rich+C Fertilizer C/N 10 | N-poor+C Fertilizer C/N 20 | |
|---|---|---|---|
| Dry weight (%) | - | 53.1 | 58.7 |
| C (%) | 40 | 29.5 | 14.4 |
| N (%) | 10 | 2.7 | 0.83 |
| C/N | 4 | 10.9 | 17.3 |
| N-NH4+ (%) | - | 0.5 | 0.1 |
| pH | - | 7.8 | 8.94 |
| CE (µS/cm) | - | 6010 | 770 |
| C (kg/ha) | 1000 | 2741 | 1775 |
| N (kg/ha) | 250 | 251 | 102 |
| N-NH4 (kg/ha) | - | 46 | 12 |
| P (kg/ha) | - | 84 | 37 |
| K (kg/ha) | 750 | 158 | 136 |
| Mg (kg/ha) | 180 | 74 | 111 |
| S (kg/ha) | 510 | 12 | 12 |
| Dose (kg/m2) | 0.25/0.3 | 1.7 | 2.1 |
| N-rich Fertilizer C/N 4 | N-rich+C Fertilizer C/N 10 | N-poor+C Fertilizer C/N 20 | |
|---|---|---|---|
| Production (Tn/ha) | 22.05 ± 7.76 | 23.59 ± 10.59 | 18.37 ± 7.58 |
| Varietal Group | Variety | Production (Tn/ha) | |
|---|---|---|---|
| 1 | Pebroter | Cornabel | 32.24 ± 11.57 a |
| 2 | Pebroter | Cuban Pepper | 20.54 ± 5.51 b |
| 3 | Pebroter | Corno Andino | 19.04 ± 5.65 b |
| 4 | Montserrat | Montserrat Fitó | 18.06 ± 5.69 b |
| 5 | Montserrat | Can Duran | 19.40 ± 8.20 b |
| 6 | Montserrat | Montserrat Ple | 18.73 ± 7.80 b |
| Parameter | Optimal Level | N-rich Fertilizer C/N 4 | N-rich+C Fertilizer C/N 10 | N-poor+C Fertilizer C/N 20 |
|---|---|---|---|---|
| N (%) | 2.9–4 | 3.77 ± 0.28 | 3.69 ± 0.42 | 3.73 ± 0.56 |
| P (%) | 0.3–0.75 | 0.22 ± 0.05 | 0.19 ± 0.07 | 0.22 ± 0.08 |
| K (%) | 2.1–4.7 | 1.42 ± 0.74 b | 0.91 ± 0.47 a | 1.01 ± 0.49 ab |
| Ca (%) | 2.6–7.0 | 3.76 ± 1.28 | 3.06 ± 1.18 | 3.84 ± 1.39 |
| Mg (%) | 0.3–0.9 | 0.84 ± 0.23 a | 0.60 ± 0.19 b | 0.66 ± 0.20 ab |
| Na (mg/Kg) | 744.65 ± 597.28 ab | 807.68 ± 412.68 a | 492.51 ± 195.72 b | |
| Zn (mg/Kg) | 22.61 ± 9.97 a | 16.30 ±7.47 b | 17.16 ± 6.60 ab | |
| S (%) | 1.38 ± 0.57 | 1.13 ± 0.48 | 1.19 ± 0.52 | |
| Fe (%) | 0.03 ± 0.008 | 0.03 ± 0.010 | 0.03 ± 0.008 | |
| Mo (mg/kg) | 6.05 ± 11.56 | 6.56 ± 5.71 | 8.0 ± 6.06 | |
| C (%) | 37.12 ± 2.13 | 37.11 ± 2.88 | 37.65 ± 2.01 |
| Parameter | Cornabel | Cuban Pepper | Corno Andino | Montserrat Fitó | Can Duran | Montserrat Ple |
|---|---|---|---|---|---|---|
| N (%) | 3.59 ± 0.63 | 4.01 ± 0.39 | 3.72 ± 0.32 | 3.74 ± 0.34 | 3.67 ± 0.33 | 3.66 ± 0.48 |
| P (%) | 0.21 ± 0.09 | 0.23 ± 0.06 | 0.23 ± 0.07 | 0.22 ± 0.06 | 0.20 ± 0.08 | 0.19 ± 0.03 |
| K (%) | 1.04 ± 0.64 | 1.22 ± 0.62 | 1.18 ± 0.76 | 1.13 ± 0.63 | 0.89 ± 0.49 | 1.22 ± 0.64 |
| Na (mg/Kg) | 583.79 ± 483.2 | 540.79 ± 175.07 | 783.13 ± 295.98 | 751.18 ± 455.44 | 501.24 ± 298.83 | 929.53 ± 717.34 |
| Mo (mg/kg) | 4.66 ± 3.13 | 5.72 ± 6.52 | 4.84 ± 1.91 | 8.23 ± 7.09 | 5.84 ± 4.84 | 11.96 ± 16.20 |
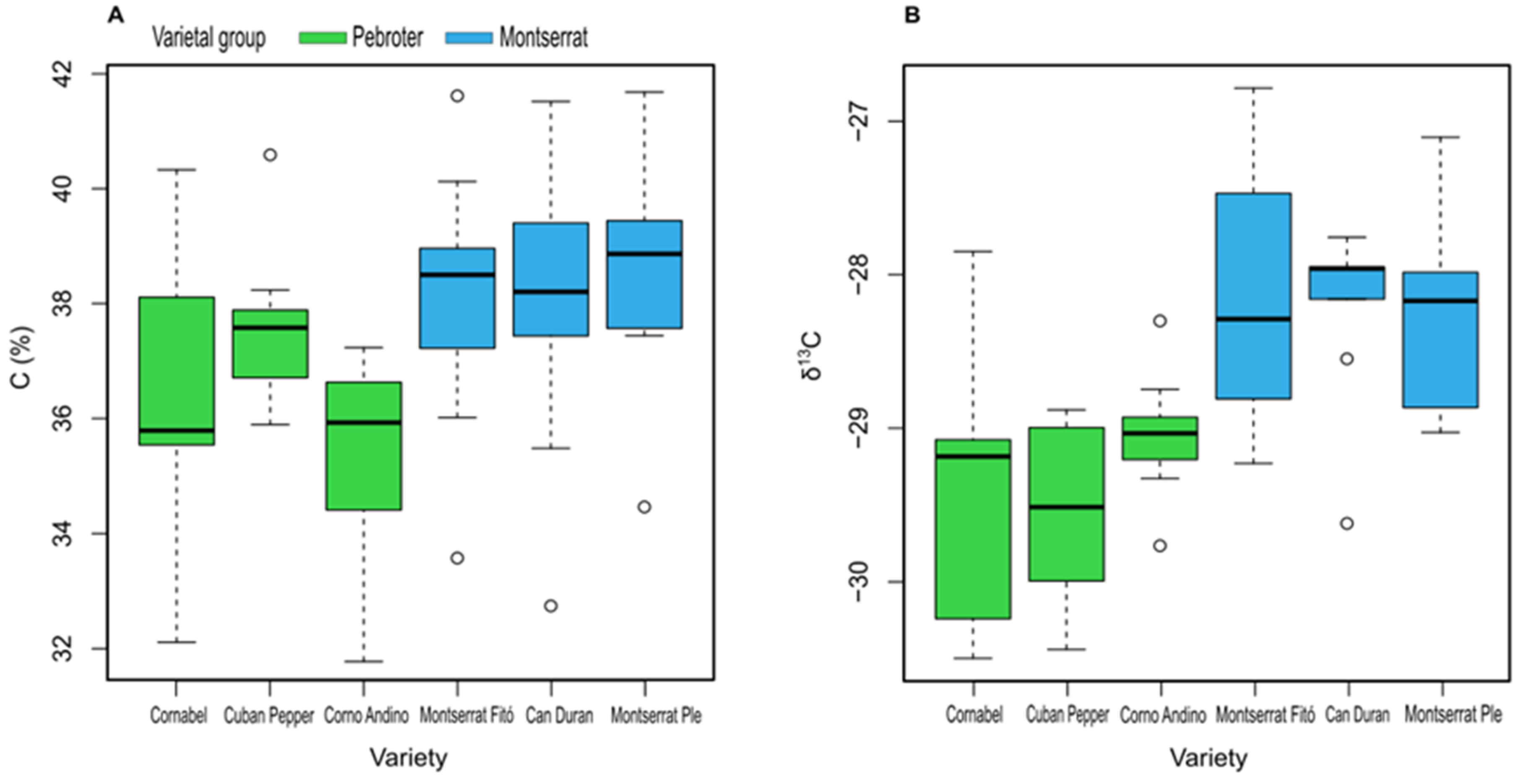
| C (%) | 36.50 ± 2.52 ab | 37.55 ± 1.41 b | 35.28 ± 2.0 a | 38.01 ± 2.32 b | 37.98 ± 2.57 b | 38.44 ± 1.99 b |
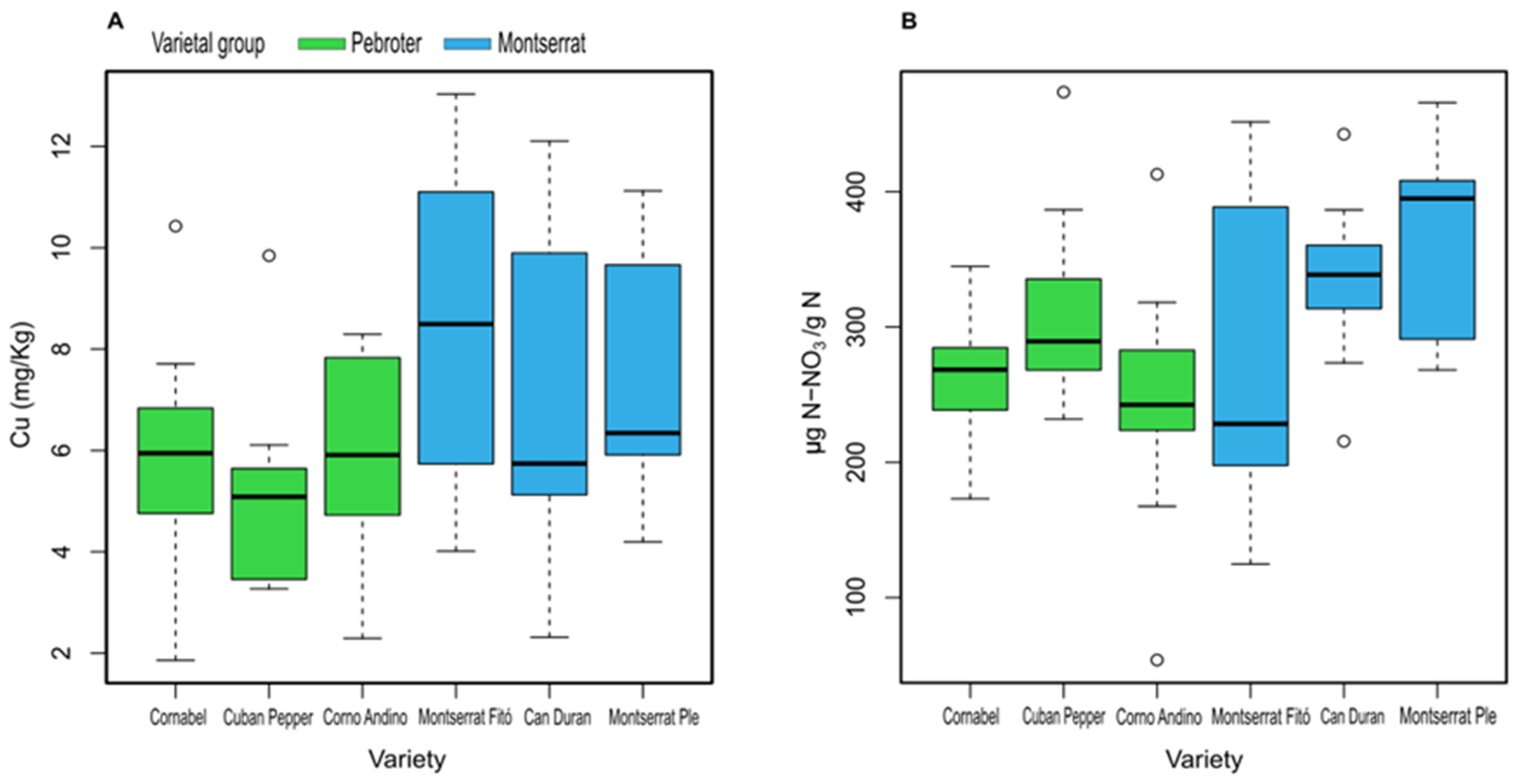
| Cu (mg/Kg) | 5.89 ± 2.38 ab | 5.17 ± 2.04 a | 6.03 ± 2.02 ab | 8.66 ± 3.03 b | 7.20 ± 3.38 ab | 7.42 ± 2.37 ab |
| N-NO3 (µg/g) | 262.06 ± 56.92 ab | 309.91 ± 78.53 ab | 243.17 ±98.74 a | 268.46 ± 118.72 ab | 332.67 ± 65.22 ab | 364.82 ± 70.07 b |
| Phenolic Compound | Cornabel | Cuban Pepper | Corno Andino | ||||||
|---|---|---|---|---|---|---|---|---|---|
| N-rich | N-rich+C | N-poor+C | N-rich | N-rich+C | N-poor+C | N-rich | N-rich+C | N-poor+C | |
| 4-Hydroxybenzoic acid | 0.41 ± 0.11 | 0.37 ± 0.03 | 0.56 ± 0.07 | 0.46 ± 0.03 | 0.59 ± 0.12 | 0.61 ± 0.09 | 0.52 ± 0.02 | 0.46 ± 0.06 | 0.57 ± 0.16 |
| Gallic acid | 12.08 ± 0.62 | 12.87 ± 0.67 | 12.61 ± 0.91 | 12.59 ± 0.63 | 12.65 ± 0.91 | 12.57 ± 0.65 | 12.24 ± 0.44 | 12.24 ± 0.91 | 12.63 ± 0.58 |
| Caffeic acid | 0.71 ± 0.63 | 1.70 ± 0.32 | 1.71 ± 0.50 | 1.48 ± 0.27 | 2.16 ± 0.48 | 1.21 ± 0.82 | 2.54 ± 0.24 | 2.76 ± 0.86 | 2.67 ± 1.33 |
| Caffeic acid hexoside | 3.66 ± 1.37 | 6.11 ± 2.43 | 6.55 ± 2.62 | 4.03 ± 1.03 | 5.38 ± 1.01 | 6.10 ± 2.60 | 5.86 ± 2.63 | 5.87 ± 1.17 | 5.68 ± 1.91 |
| Chlorogenic acid | 5.47 ± 4.57 | 14.08 ± 4.10 | 22.38 ± 6.41 | 13.99 ± 3.07 | 13.36 ± 3.96 | 19.07 ± 12.60 | 10.85 ± 5.09 | 13.38 ± 3.45 | 19.01 ± 2.61 |
| Neochlorogenic acid | 0.05 ± 0.00 | 0.09 ± 0.02 | 0.11 ± 0.03 | 0.09 ± 0.02 | 0.09 ± 0.01 | 0.11 ± 0.02 | 0.09 ± 0.02 | 0.12 ± 0.04 | 0.07 ± 0.04 |
| Cryptochlorogenic acid | 1.20 ± 0.12 | 1.40 ± 0.31 | 1.75 ± 0.41 | 1.18 ± 0.20 | 1.36 ± 0.53 | 1.21 ± 0.65 | 1.49 ± 0.74 | 1.85 ± 0.46 | 1.83 ± 0.39 |
| Protocatechuic acid | 1.37 ± 1.00 | 0.68 ± 0.29 | 1.27 ± 0.44 | 0.64 ± 0.23 | 0.70 ± 0.14 | 0.49 ± 0.14 | 1.17 ± 0.25 | 1.07 ± 0.49 | 1.14 ± 0.30 |
| Dicaffeyolquinic acid | 0.32 ± 0.24 | 0.91 ± 0.42 | 0.91 ± 0.23 | 1.10 ± 0.26 | 0.89 ± 0.38 | 1.05 ± 0.71 | 1.01 ± 0.49 | 1.12 ± 0.11 | 1.07 ± 0.08 |
| m-Coumaric acid | 2.53 ± 0.63 | 3.78 ± 1.02 | 5.38 ± 0.39 | 6.83 ± 1.86 | 7.05 ± 4.00 | 6.65 ± 1.20 | 2.87 ± 0.13 | 3.79 ± 1.24 | 5.96 ± 1.43 |
| o-Coumaric acid | 3.36 ± 1.47 | 4.48 ± 1.30 | 5.24 ± 3.01 | 3.54 ± 0.88 | 4.38 ± 0.79 | 7.58 ± 2.79 | 5.26 ± 0.62 | 6.34 ± 2.89 | 10.78 ± 3.94 |
| p-Coumaric acid | 0.71 ± 0.17 | 0.48 ± 0.11 | 0.94 ± 0.23 | 1.08 ± 0.21 | 0.99 ± 0.17 | 1.23 ± 0.17 | 1.15 ± 0.44 | 1.26 ± 0.75 | 2.59 ± 1.36 |
| Coumaric acid glucoside | 9.03 ± 2.84 | 12.82 ± 2.91 | 16.98 ± 3.66 | 21.73 ± 5.72 | 22.05 ± 9.69 | 21.80 ± 1.75 | 11.50 ± 1.16 | 15.33 ± 1.19 | 23.97 ± 6.39 |
| Homovanillic acid glucoside | 0.25 ± 0.03 | nd | 0.28 ± 0.02 | 0.23 ± 0.01 | 0.27 ± 0.04 | 0.29 ± 0.05 | 0.24 ± 0.03 | 0.24 ± 0.00 | nd |
| Ferulic acid glucoside | 0.35 ± 0.06 | 0.49 ± 0.16 | 0.70 ± 0.09 | 0.47 ±0.04 | 0.55 ± 0.16 | 0.65 ± 0.27 | 0.48 ± 0.19 | 0.51 ± 0.06 | 0.73 ± 0.05 |
| Rutin | 14.05 ± 5.37 | 14.95 ± 2.81 | 28.53 ± 7.70 | 24.56 ± 3.29 | 29.29 ± 4.72 | 20.85 ± 3.74 | 18.98 ± 10.11 | 20.71 ± 5.31 | 15.80 ± 7.87 |
| Quercetin | 1.56 ± 0.12 | 1.58 ± 0.13 | 1.62 ± 0.15 | 1.69 ± 0.14 | 1.97 ± 0.32 | 1.59 ± 0.13 | 1.82 ± 0.41 | 1.76 ± 0.31 | 2.46 ± 1.05 |
| Naringenin | 20.34 ± 16.51 | 8.79 ± 9.01 | 10.94 ± 9.44 | 41.83 ± 41.73 | 23.84 ± 19.80 | 59.08 ± 44.40 | 31.16 ± 24.84 | 23.99 ± 14.96 | 36.19 ± 24.14 |
| Naringenin glucoside | 11.93 ± 7.35 | 5.93 ± 4.38 | 9.45 ± 2.05 | 6.78 ± 1.48 | 11.99 ± 6.99 | 7.22 ± 4.03 | 13.82 ± 9.56 | 11.91 ± 4.99 | 17.33 ± 12.81 |
| Apigenin glucoside | 1.34 ± 0.32 | 1.70 ± 0.48 | 1.53 ± 0.39 | 1.60 ± 0.37 | 1.63 ± 0.14 | 2.05 ± 0.25 | 1.73 ± 0.29 | 2.28 ± 0.40 | 2.21 ± 0.67 |
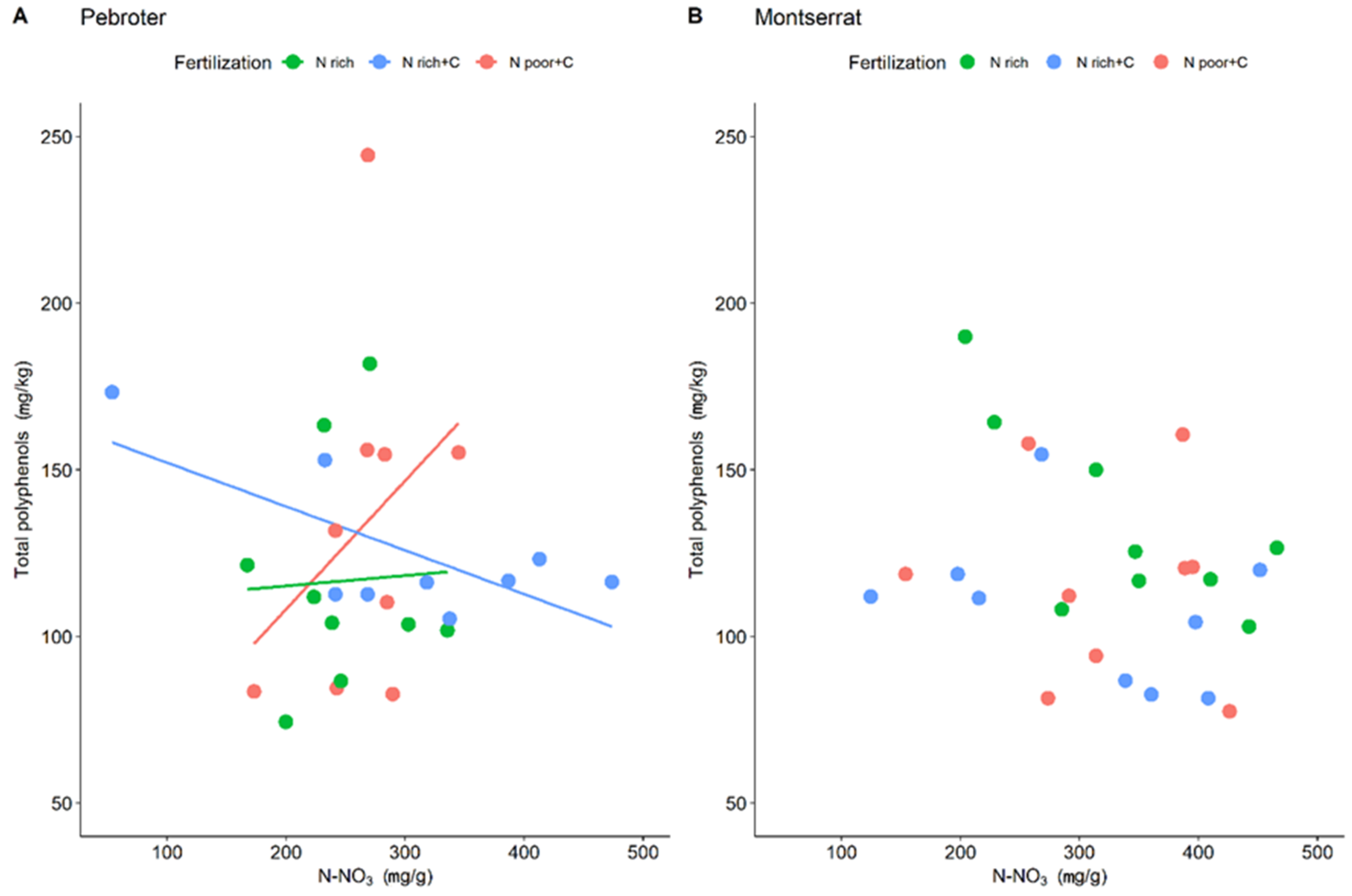
| Total phenolics | 90.72 ± 15.54 | 93.21 ± 16.01 | 129.44 ± 23.21 | 145.9 ± 22.05 | 141.19 ± 19.00 | 171.41 ± 65.33 | 124.78 ± 55.78 | 126.99 ± 23.10 | 162.78 ± 30.12 |
| Phenolic Compound | Montserrrat Fitó | Can Duran | Montserrat Ple | ||||||
|---|---|---|---|---|---|---|---|---|---|
| N-rich | N-rich+C | N-poor+C | N-rich | N-rich+C | N-poor+C | N-rich | N-rich+C | N-poor+C | |
| 4-Hydroxybenzoic acid | 0.44 ± 0.10 | 0.56 ± 0.13 | 0.62 ± 0.14 | 0.63 ± 0.09 | 0.69 ± 0.23 | 0.60 ± 0.21 | 0.68 ± 0.11 | 0.55 ± 0.15 | 0.99 ± 0.23 |
| Gallic acid | 12.40 ± 1.56 | 12.68 ± 0.76 | 12.47 ± 0.58 | 12.31 ± 0.53 | 12.92 ± 0.72 | 12.82 ± 1.05 | 12.13 ± 0.83 | 12.91 ± 0.43 | 12.88 ± 0.84 |
| Caffeic acid | 2.29 ± 0.74 | 2.28 ± 0.51 | 3.38 ± 1.83 | 2.26 ± 0.42 | 2.06 ± 0.40 | 1.71 ± 0.81 | 1.59 ± 0.95 | 2.56 ± 0.75 | 1.63 ± 0.93 |
| Caffeic acid hexoside | 10.40 ± 6.62 | 11.12 ± 1.42 | 13.11 ± 1.25 | 11.95 ± 1.40 | 15.05 ± 5.87 | 7.78 ± 2.36 | 9.94 ± 2.09 | 10.80 ± 2.92 | 14.98 ± 3.86 |
| Chlorogenic acid | 22.09 ± 6.34 | 17.62 ± 2.46 | 20.45 ± 7.86 | 20.94 ± 3.32 | 18.26 ± 1.47 | 14.57 ± 4.01 | 15.47 ± 3.10 | 22.55 ± 15.37 | 14.01 ± 9.33 |
| Neochlorogenic acid | 0.12 ± 0.04 | 0.13 ± 0.06 | 0.12 ± 0.05 | 0.13 ± 0.03 | 0.12 ± 0.04 | 0.10 ± 0.06 | 0.14 ± 0.06 | 0.13 ± 0.05 | 0.19 ± 0.16 |
| Cryptochlorogenic acid | 1.68 ± 1.18 | 1.47 ± 0.74 | 2.19 ± 0.65 | 2.40 ± 0.42 | 1.98 ± 0.27 | 1.84 ± 0.39 | 1.74 ± 0.37 | 1.36 ± 0.34 | 1.40 ± 0.83 |
| Protocatechuic acid | 0.62 ± 0.18 | 1.05 ± 0.48 | 0.94 ± 0.24 | 0.64 ± 0.17 | 0.67 ± 0.12 | 0.73 ± 0.38 | 0.74 ± 0.30 | 0.84 ± 0.40 | 0.92 ± 0.28 |
| Dicaffeyolquinic acid | 1.30 ± 0.51 | 1.09 ± 0.74 | 1.10 ± 0.42 | 1.09 ± 0.71 | 0.90 ± 0.32 | 0.60 ± 0.14 | 0.82 ± 0.45 | 1.25 ± 0.69 | 1.07 ± 0.68 |
| m-Coumaric acid | 5.92 ± 1.71 | 5.36 ± 0.83 | 7.82 ± 2.12 | 5.97 ± 0.39 | 4.89 ± 1.88 | 5.95 ± 0.82 | 3.99 ± 0.92 | 3.90 ± 1.65 | 5.21 ± 1.78 |
| o-Coumaric acid | 11.44 ± 4.16 | 15.98 ± 0.89 | 16.80 ± 4.92 | 12.44 ± 1.45 | 17.55 ± 7.23 | 11.86 ± 2.40 | 9.97 ± 2.80 | 13.07 ± 1.72 | 14.44 ± 2.41 |
| p-Coumaric acid | 1.39 ± 0.68 | 2.23 ± 1.19 | 2.46 ± 1.86 | 1.63 ± 0.41 | 1.61 ± 0.22 | 1.79 ± 0.58 | 1.18 ± 0.11 | 1.57 ± 0.34 | 1.02 ± 0.18 |
| Coumaric acid glucoside | 26.21 ± 8.06 | 27.67 ± 2.58 | 32.66 ± 5.05 | 26.60 ± 1.98 | 27.80 ± 10.29 | 22.59 ± 4.01 | 18.71 ± 5.08 | 20.77 ± 6.20 | 23.82 ± 4.63 |
| Homovanillic acid glucoside | 0.43 ± 0.10 | 0.52 ± 0.13 | 0.40 ± 0.11 | 0.53 ± 0.06 | 0.57 ± 0.22 | 0.32 ± 0.07 | 0.44 ± 0.04 | 0.41 ± 0.08 | 0.60 ± 0.18 |
| Ferulic acid glucoside | 0.89 ± 0.56 | 1.02 ± 0.48 | 1.39 ± 0.64 | 1.23 ± 0.31 | 1.48 ± 0.38 | 1.37 ± 0.39 | 0.86 ± 0.20 | 0.89 ± 0.25 | 0.74 ± 0.20 |
| Rutin | 9.63 ± 7.17 | 9.46 ± 6.75 | 0.75 ± 0.29 | 3.47 ± 4.69 | 0.58 ± 0.12 | 2.87 ± 5.98 | 12.23 ± 6.91 | 13.45 ± 10.51 | 13.64 ± 2.51 |
| Quercetin | nd | nd | nd | nd | nd | nd | nd | nd | nd |
| Naringenin | nd | nd | nd | nd | nd | nd | nd | nd | nd |
| Naringenin glucoside | nd | nd | nd | nd | nd | nd | nd | nd | nd |
| Apigenin glucoside | 3.18 ± 0.75 | 4.92 ± 1.10 | 4.07 ± 0.53 | 2.22 ± 0.90 | 3.76 ± 1.00 | 2.01 ± 0.43 | 3.36 ± 0.83 | 3.12 ± 1.08 | 5.60 ± 1.21 |
| Total phenolics | 110.43 ± 9.59 | 115.16 ± 3.52 | 120.73 ± 4.05 | 123.31 ± 34.86 | 114.37 ± 34.54 | 96.36 ± 10.85 | 94.95 ± 21.83 | 110.08 ± 39.44 | 112.54 ± 10.73 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Coria, J.; Lozano-Castellón, J.; Jaime-Rodríguez, C.; Olmo-Cunillera, A.; Laveriano-Santos, E.P.; Pérez, M.; Lamuela-Raventós, R.M.; Puig, J.; Vallverdú-Queralt, A.; Romanyà, J. The Effects of Differentiated Organic Fertilization on Tomato Production and Phenolic Content in Traditional and High-Yielding Varieties. Antioxidants 2022, 11, 2127. https://doi.org/10.3390/antiox11112127
González-Coria J, Lozano-Castellón J, Jaime-Rodríguez C, Olmo-Cunillera A, Laveriano-Santos EP, Pérez M, Lamuela-Raventós RM, Puig J, Vallverdú-Queralt A, Romanyà J. The Effects of Differentiated Organic Fertilization on Tomato Production and Phenolic Content in Traditional and High-Yielding Varieties. Antioxidants. 2022; 11(11):2127. https://doi.org/10.3390/antiox11112127
Chicago/Turabian StyleGonzález-Coria, Johana, Julián Lozano-Castellón, Carolina Jaime-Rodríguez, Alexandra Olmo-Cunillera, Emily P. Laveriano-Santos, Maria Pérez, Rosa Mª Lamuela-Raventós, Jordi Puig, Anna Vallverdú-Queralt, and Joan Romanyà. 2022. "The Effects of Differentiated Organic Fertilization on Tomato Production and Phenolic Content in Traditional and High-Yielding Varieties" Antioxidants 11, no. 11: 2127. https://doi.org/10.3390/antiox11112127
APA StyleGonzález-Coria, J., Lozano-Castellón, J., Jaime-Rodríguez, C., Olmo-Cunillera, A., Laveriano-Santos, E. P., Pérez, M., Lamuela-Raventós, R. M., Puig, J., Vallverdú-Queralt, A., & Romanyà, J. (2022). The Effects of Differentiated Organic Fertilization on Tomato Production and Phenolic Content in Traditional and High-Yielding Varieties. Antioxidants, 11(11), 2127. https://doi.org/10.3390/antiox11112127










