Effects of Enzymatic- and Ultrasound-Assisted Extraction on Physicochemical and Antioxidant Properties of Collagen Hydrolysate Fractions from Alaska Pollack (Theragra chalcogramma) Skin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Collagen Hydrolysate
2.2. Yield of Collagen Hydrolysate
2.3. Antioxidant Properties
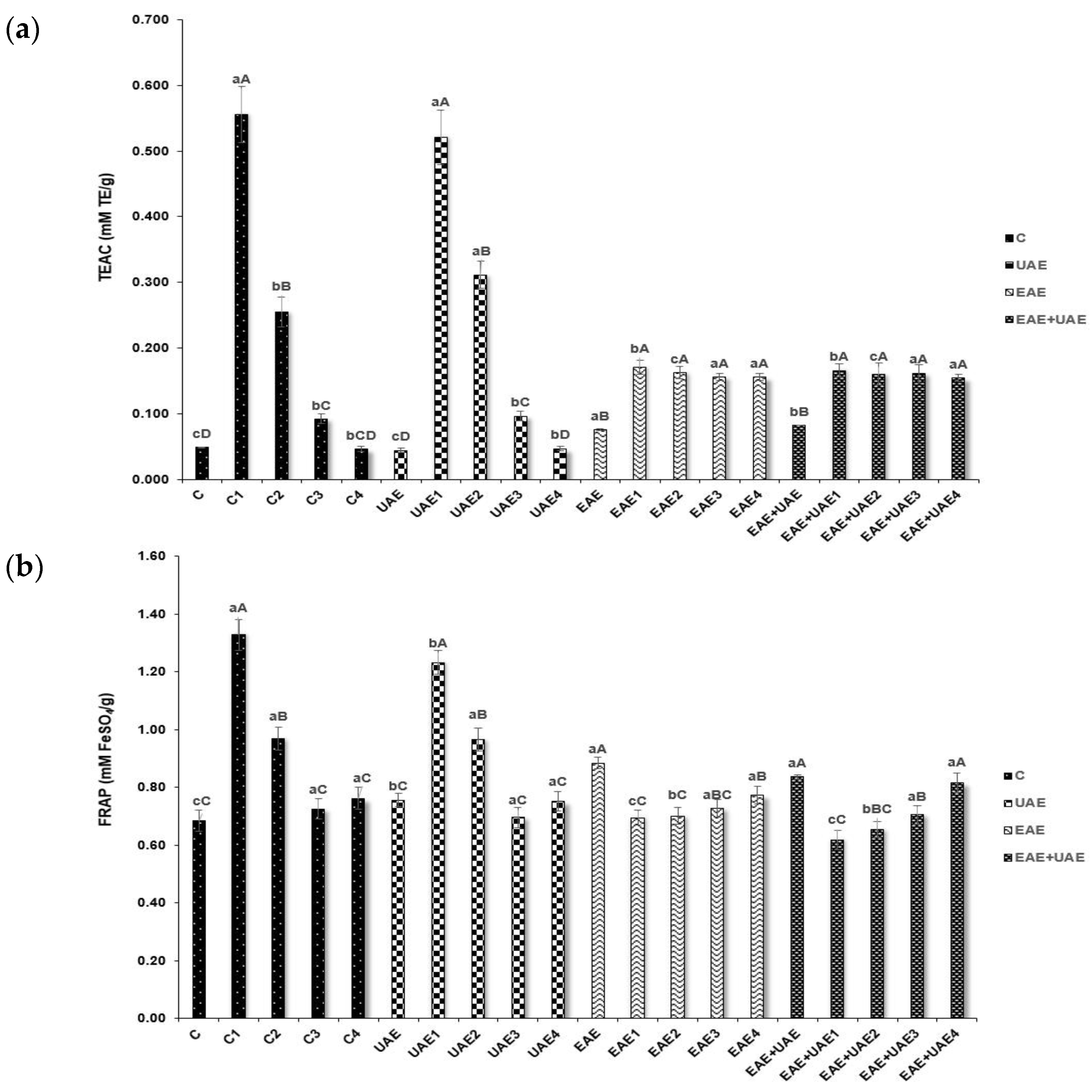
2.3.1. Trolox Equivalent Antioxidant Capacity (TEAC)
2.3.2. Ferric Ion Reducing Antioxidant Power (FRAP)
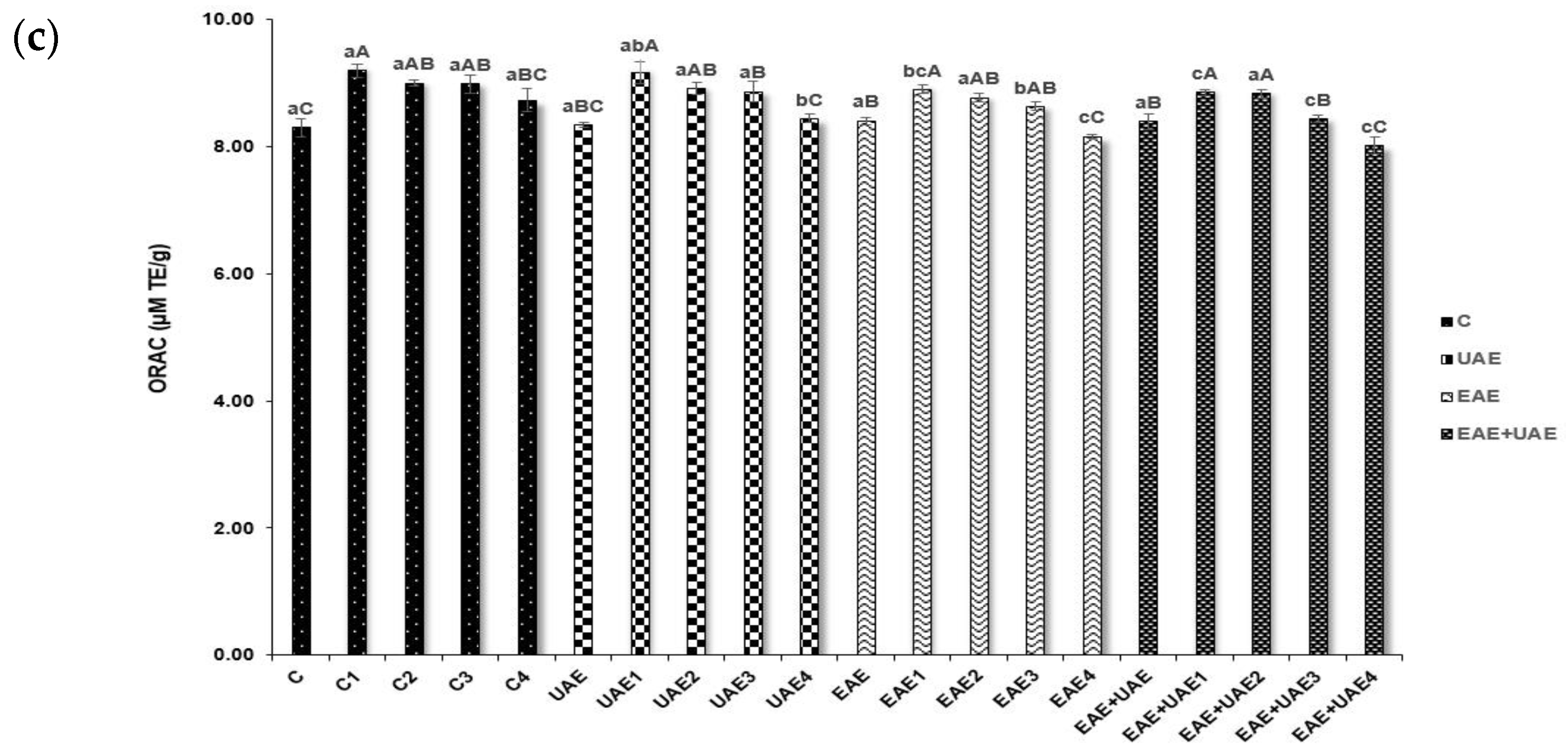
2.3.3. Oxygen Radical Absorbance Capacity (ORAC)
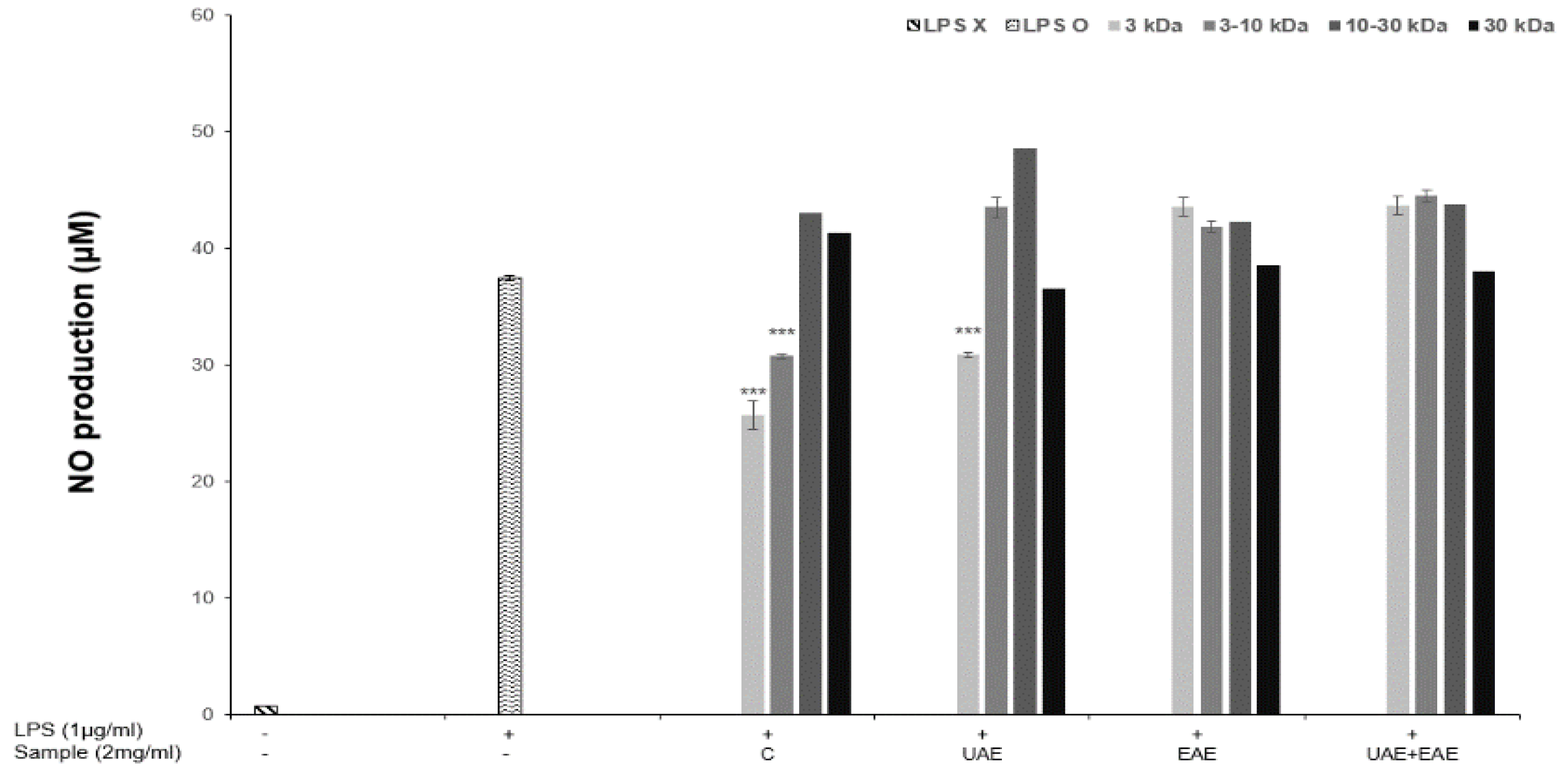
2.4. Nitrite Oxide Production
2.5. Scanning Electron Microscopy
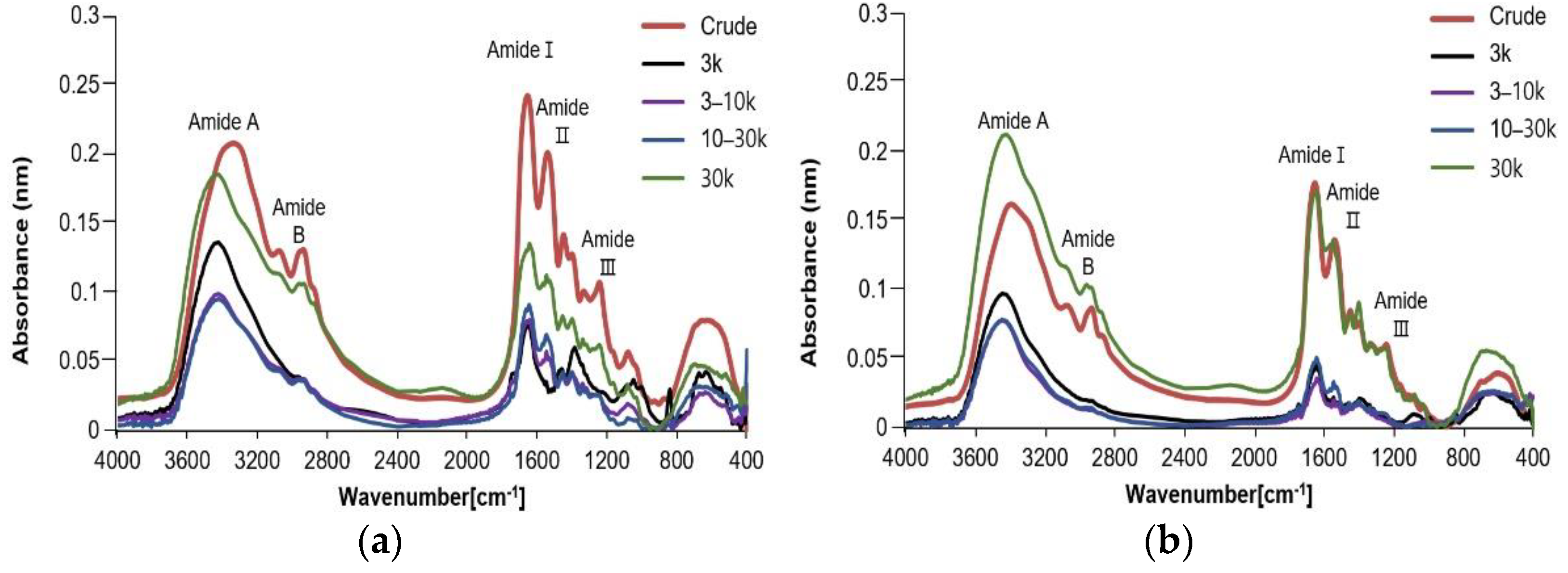
2.6. Fourier Transforms Infrared Spectroscopy (FTIR)
2.7. Sodium Dodecyl Sulphate-Polyacrylamide Gel Electrophoresis (SDS-PAGE)
2.8. Statistical Analysis
3. Results and Discussion
3.1. Yield of Collagen Hydrolysate
3.2. Antioxidant Properties and NO Production
3.3. Morphology
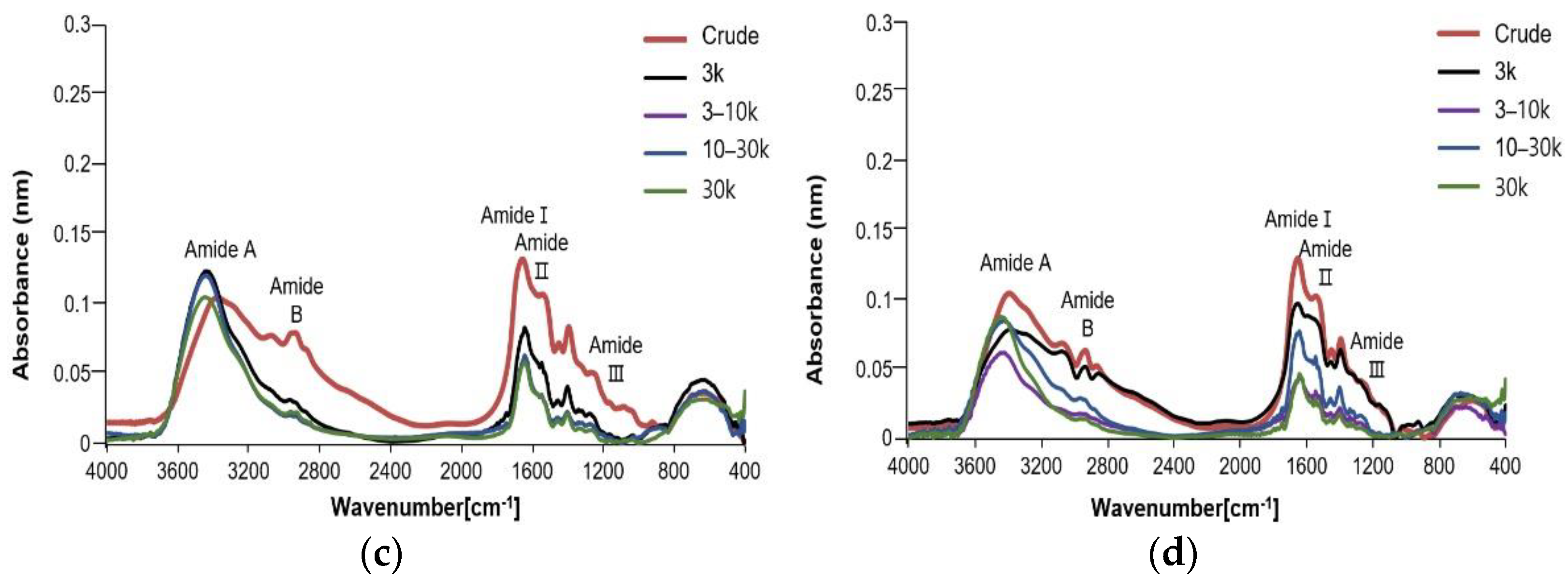
3.4. Fourier Transforms Infrared Spectroscopy (FTIR)
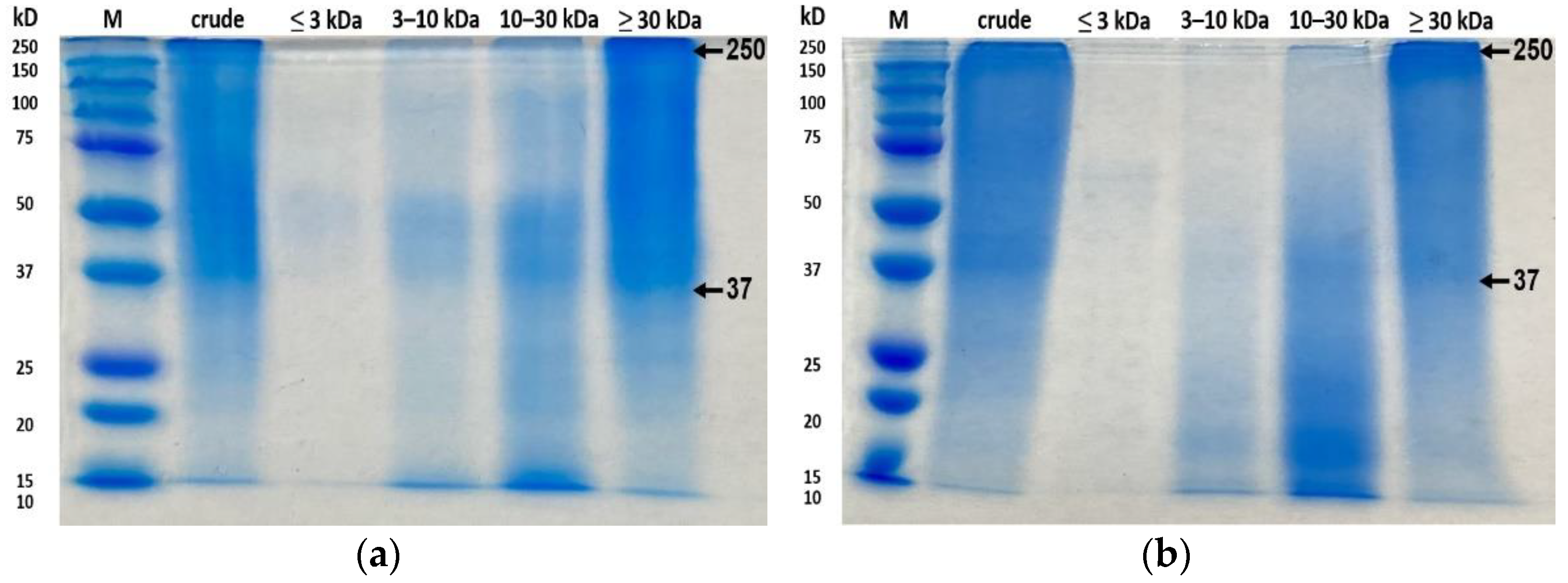
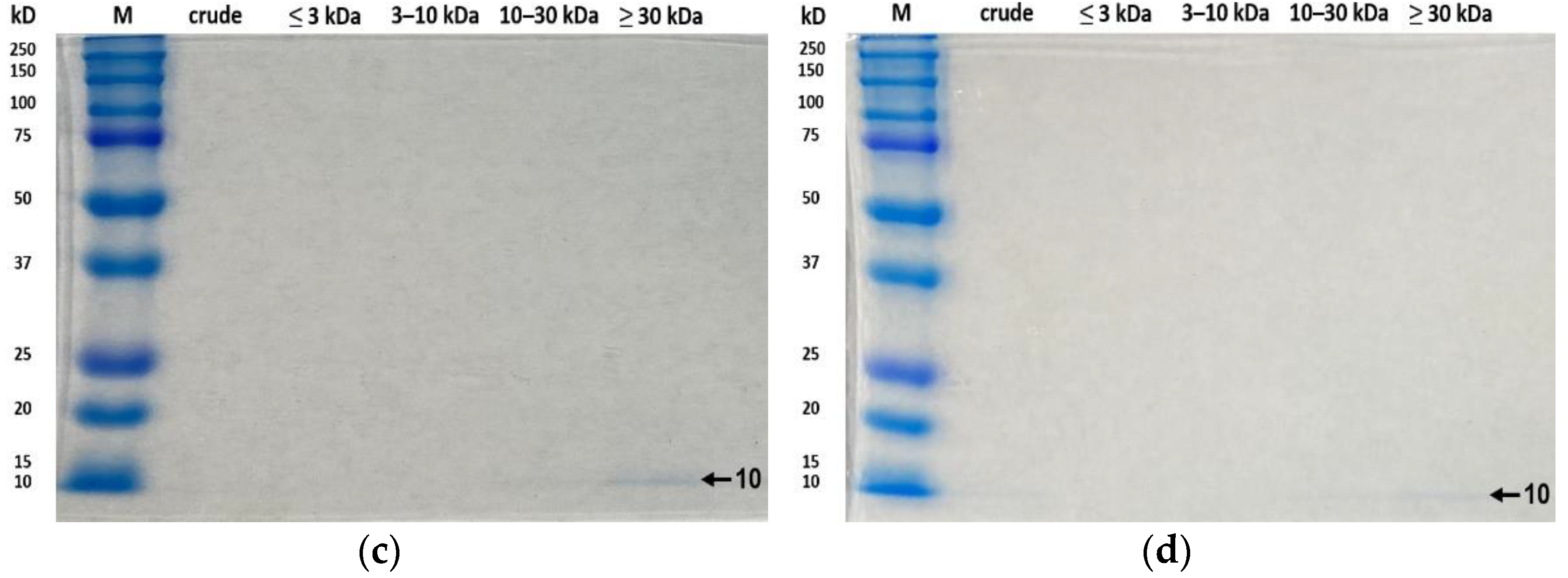
3.5. SDS-PAGE Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stankus, A. State of world aquaculture 2020 and regional reviews: FAO webinar series. FAO Aquac. Newsl. 2021, 63, 17–18. [Google Scholar]
- FAO. The State of World Fisheries and Aquaculture. Sustain. Action 2020, 62–83. Available online: https://www.fao.org/documents/card/en/c/ca9229en/ (accessed on 2 October 2022).
- Vázquez, J.A.; Rodríguez-Amado, I.; Sotelo, C.G.; Sanz, N.; Pérez-Martín, R.I.; Valcárcel, J. Production, characterization, and bioactivity of fish protein hydrolysates from aquaculture turbot (Scophthalmus maximus) wastes. Biomolecules 2020, 10, 310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qiu, Y.-T.; Wang, Y.-M.; Yang, X.-R.; Zhao, Y.-Q.; Chi, C.-F.; Wang, B. Gelatin and antioxidant peptides from gelatin hydrolysate of skipjack tuna (Katsuwonus pelamis) scales: Preparation, identification and activity evaluation. Mar. Drugs 2019, 17, 565. [Google Scholar] [CrossRef] [Green Version]
- Hong, H.; Fan, H.; Chalamaiah, M.; Wu, J. Preparation of low-molecular-weight, collagen hydrolysates (peptides): Current progress, challenges, and future perspectives. Food Chem. 2019, 301, 125222. [Google Scholar] [CrossRef] [PubMed]
- Nurilmala, M.; Hizbullah, H.H.; Karnia, E.; Kusumaningtyas, E.; Ochiai, Y. Characterization and antioxidant activity of collagen, gelatin, and the derived peptides from yellowfin tuna (Thunnus albacares) skin. Mar. Drugs 2020, 18, 98. [Google Scholar] [CrossRef] [Green Version]
- Hong, G.-P.; Min, S.-G.; Jo, Y.-J. Anti-oxidative and anti-aging activities of porcine by-product collagen hydrolysates produced by commercial proteases: Effect of hydrolysis and ultrafiltration. Molecules 2019, 24, 1104. [Google Scholar] [CrossRef] [Green Version]
- Zague, V.; de Freitas, V.; Rosa, M.d.C.; de Castro, G.A.; Jaeger, R.G.; Machado-Santelli, G.M. Collagen hydrolysate intake increases skin collagen expression and suppresses matrix metalloproteinase 2 activity. J. Med. Food 2011, 14, 618–624. [Google Scholar] [CrossRef]
- Saiga, A.; Iwai, K.; Hayakawa, T.; Takahata, Y.; Kitamura, S.; Nishimura, T.; Morimatsu, F. Angiotensin I-converting enzyme-inhibitory peptides obtained from chicken collagen hydrolysate. J. Agric. Food Chem. 2008, 56, 9586–9591. [Google Scholar] [CrossRef]
- Hajfathalian, M.; Ghelichi, S.; García-Moreno, P.J.; Moltke Sørensen, A.-D.; Jacobsen, C. Peptides: Production, bioactivity, functionality, and applications. Crit. Rev. Food Sci. Nutr. 2018, 58, 3097–3129. [Google Scholar] [CrossRef] [Green Version]
- Zamorano-Apodaca, J.C.; García-Sifuentes, C.O.; Carvajal-Millán, E.; Vallejo-Galland, B.; Scheuren-Acevedo, S.M.; Lugo-Sánchez, M.E. Biological and functional properties of peptide fractions obtained from collagen hydrolysate derived from mixed by-products of different fish species. Food Chem. 2020, 331, 127350. [Google Scholar] [CrossRef] [PubMed]
- León-López, A.; Fuentes-Jiménez, L.; Hernández-Fuentes, A.D.; Campos-Montiel, R.G.; Aguirre-Álvarez, G. Hydrolysed collagen from sheepskins as a source of functional peptides with antioxidant activity. Int. J. Mol. Sci. 2019, 20, 3931. [Google Scholar] [CrossRef] [PubMed]
- Mirzapour-Kouhdasht, A.; Moosavi-Nasab, M.; Kim, Y.-M.; Eun, J.-B. Antioxidant mechanism, antibacterial activity, and functional characterization of peptide fractions obtained from barred mackerel gelatin with a focus on application in carbonated beverages. Food Chem. 2021, 342, 128339. [Google Scholar] [CrossRef]
- Zhang, Y.; Olsen, K.; Grossi, A.; Otte, J. Effect of pretreatment on enzymatic hydrolysis of bovine collagen and formation of ACE-inhibitory peptides. Food Chem. 2013, 141, 2343–2354. [Google Scholar] [CrossRef] [PubMed]
- Nadzri, F.A.; Tawalbeh, D.; Sarbon, N. Physicochemical properties and antioxidant activity of enzymatic hydrolysed chickpea (Cicer arietinum L.) protein as influence by alcalase and papain enzyme. Biocatal. Agric. Biotechnol. 2021, 36, 102131. [Google Scholar] [CrossRef]
- Ali, A.M.M.; Kishimura, H.; Benjakul, S. Extraction efficiency and characteristics of acid and pepsin soluble collagens from the skin of golden carp (Probarbus Jullieni) as affected by ultrasonication. Process Biochem. 2018, 66, 237–244. [Google Scholar] [CrossRef]
- Duangjai, A.; Trisat, K.; Saokaew, S. Effect of roasting degree, extraction time, and temperature of coffee beans on anti-hyperglycemic and anti-hyperlipidemic activities using ultrasound-assisted extraction. Prev. Nutr. Food Sci. 2021, 26, 338. [Google Scholar] [CrossRef]
- Ong, T.; Shaik, M.; Sarbon, N. Isolation and characterization of acid and pepsin soluble collagen extracted from sharpnose stingray (Dasyatis zugei) skin. Food Res. 2021, 5, 214–224. [Google Scholar] [CrossRef]
- VIDAL, A.R.; Ferreira, T.E.; Mello, R.d.O.; SCHMIDT, M.M.; Kubota, E.H.; Demiate, I.M.; ZIELINSKI, A.A.F.; DORNELLES, R.C.P. Effects of enzymatic hydrolysis (Flavourzyme®) assisted by ultrasound in the structural and functional properties of hydrolyzates from different bovine collagens. Food Sci. Technol. 2018, 38, 103–108. [Google Scholar] [CrossRef] [Green Version]
- Li, D.; Mu, C.; Cai, S.; Lin, W. Ultrasonic irradiation in the enzymatic extraction of collagen. Ultrason. Sonochem. 2009, 16, 605–609. [Google Scholar] [CrossRef]
- Yang, S.J.; Hong, J.-H. Extraction and physicochemical properties of collagen from squid (Todarodes pacificus) skin and alaska pollack (Theragra chalcogramma) skin. Korean J. Food Cook. Sci. 2012, 28, 711–719. [Google Scholar] [CrossRef] [Green Version]
- Ali, A.M.M.; Kishimura, H.; Benjakul, S. Physicochemical and molecular properties of gelatin from skin of golden carp (Probarbus Jullieni) as influenced by acid pretreatment and prior-ultrasonication. Food Hydrocoll. 2018, 82, 164–172. [Google Scholar] [CrossRef]
- Li, H.-B.; Cheng, K.-W.; Wong, C.-C.; Fan, K.-W.; Chen, F.; Jiang, Y. Evaluation of antioxidant capacity and total phenolic content of different fractions of selected microalgae. Food Chem. 2007, 102, 771–776. [Google Scholar] [CrossRef]
- Chen, Y.; Huang, F.; Xie, B.; Sun, Z.; McClements, D.J.; Deng, Q. Fabrication and characterization of whey protein isolates-lotus seedpod proanthocyanin conjugate: Its potential application in oxidizable emulsions. Food Chem. 2021, 346, 128680. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Kim, S.S. Utilisation of immature wheat flour as an alternative flour with antioxidant activity and consumer perception on its baked product. Food Chem. 2017, 232, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Li, T.; Cheng, X.; Ji, X.; Gao, D.; Du, M.; Jiang, N.; Liu, X.; Mao, X. Sea cucumber peptides exert anti-inflammatory activity through suppressing NF-κB and MAPK and inducing HO-1 in RAW264. 7 macrophages. Food Funct. 2016, 7, 2773–2779. [Google Scholar] [CrossRef]
- Sun, L.; Li, B.; Song, W.; Si, L.; Hou, H. Characterization of Pacific cod (Gadus macrocephalus) skin collagen and fabrication of collagen sponge as a good biocompatible biomedical material. Process Biochem. 2017, 63, 229–235. [Google Scholar] [CrossRef]
- LAEMMLI, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Tacias-Pascacio, V.G.; Morellon-Sterling, R.; Siar, E.-H.; Tavano, O.; Berenguer-Murcia, Á.; Fernandez-Lafuente, R. Use of Alcalase in the production of bioactive peptides: A review. Int. J. Biol. Macromol. 2020, 165, 2143–2196. [Google Scholar] [CrossRef]
- Kadam, S.U.; Tiwari, B.K.; Álvarez, C.; O’Donnell, C.P. Ultrasound applications for the extraction, identification and delivery of food proteins and bioactive peptides. Trends Food Sci. Technol. 2015, 46, 60–67. [Google Scholar] [CrossRef]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef]
- Dara, P.K.; Elavarasan, K.; Shamasundar, B.A. Improved utilization of croaker skin waste and freshwater carps visceral waste: Conversion of waste to health benefitting peptides. Int. J. Pept. Res. Ther. 2020, 26, 2641–2651. [Google Scholar] [CrossRef]
- Bryan, M.A.; Brauner, J.W.; Anderle, G.; Flach, C.R.; Brodsky, B.; Mendelsohn, R. FTIR studies of collagen model peptides: Complementary experimental and simulation approaches to conformation and unfolding. J. Am. Chem. Soc. 2007, 129, 7877–7884. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, D.; Wei, G.; Li, T.; Hu, J.; Lu, N.; Regenstein, J.M.; Zhou, P. Effects of alkaline pretreatments and acid extraction conditions on the acid-soluble collagen from grass carp (Ctenopharyngodon idella) skin. Food Chem. 2015, 172, 836–843. [Google Scholar] [CrossRef] [PubMed]

| Sample (1),(2) | Yield *** (%) |
|---|---|
| C0 | 31.00 ± 2.68 dA |
| C1 | 0.18 ± 0.14 cC |
| C2 | 0.57 ± 0.23 bC |
| C3 | 2.17 ± 0.93 bC |
| C4 | 21.16 ± 1.63 aB |
| UAE0 | 40.22 ± 1.29 cA |
| UAE1 | 0.39 ± 0.2 cC |
| UAE2 | 0.55 ± 0.27 bC |
| UAE3 | 3.16 ± 1.4 bC |
| UAE4 | 25.99 ± 0.69 aB |
| EAE0 | 62.66 ± 1.36 bA |
| EAE1 | 4.76 ± 0.65 bD |
| EAE2 | 11.62 ± 1.69 aC |
| EAE3 | 15.95 ± 1.64 aB |
| EAE4 | 14.74 ± 1.48 bBC |
| EAE+UAE0 | 65.33 ± 0.98 aA |
| EAE+UAE1 | 6.46 ± 1.17 aD |
| EAE+UAE2 | 12.90 ± 0.98 aC |
| EAE+UAE3 | 17.84 ± 0.34 aB |
| EAE+UAE4 | 11.73 ± 3.03 bC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.E.; Noh, S.-K.; Kim, M.J. Effects of Enzymatic- and Ultrasound-Assisted Extraction on Physicochemical and Antioxidant Properties of Collagen Hydrolysate Fractions from Alaska Pollack (Theragra chalcogramma) Skin. Antioxidants 2022, 11, 2112. https://doi.org/10.3390/antiox11112112
Lee JE, Noh S-K, Kim MJ. Effects of Enzymatic- and Ultrasound-Assisted Extraction on Physicochemical and Antioxidant Properties of Collagen Hydrolysate Fractions from Alaska Pollack (Theragra chalcogramma) Skin. Antioxidants. 2022; 11(11):2112. https://doi.org/10.3390/antiox11112112
Chicago/Turabian StyleLee, Ju Eun, Sang-Kyu Noh, and Mi Jeong Kim. 2022. "Effects of Enzymatic- and Ultrasound-Assisted Extraction on Physicochemical and Antioxidant Properties of Collagen Hydrolysate Fractions from Alaska Pollack (Theragra chalcogramma) Skin" Antioxidants 11, no. 11: 2112. https://doi.org/10.3390/antiox11112112
APA StyleLee, J. E., Noh, S.-K., & Kim, M. J. (2022). Effects of Enzymatic- and Ultrasound-Assisted Extraction on Physicochemical and Antioxidant Properties of Collagen Hydrolysate Fractions from Alaska Pollack (Theragra chalcogramma) Skin. Antioxidants, 11(11), 2112. https://doi.org/10.3390/antiox11112112








