1. Introduction
Obesity is a chronic relapsing disease, characterized by excessive body fat accumulation. It is associated with a series of disorders, which are threatening for the global public health and economy [
1]. During the last few decades, there has been a significant global increase in obesity rate. According to WHO, the prevalence of overweight and obesity cases in the adult population is 39% and 13%, respectively [
2]. In recent years, there has been a growing interest in metabolic disorders that represent a cluster of abnormalities, including abdominal adiposity, dyslipidemia, glucose intolerance, insulin resistance and hypertension [
3]. There are many health and economic consequences associated with obesity and metabolic disorders, such as type 2 diabetes mellitus (T2DM), cardiovascular disease (CVD), nonalcoholic fatty liver disease (NAFLD), certain types of cancer, obstructive sleep apnea and depression [
4,
5].
In order to address the obesity epidemic, a series of approaches have been proposed, including changes in diet. More specifically, there is an increasing interest in the consumption of functional foods which may improve cardiometabolic health [
6]. In this respect, edible mushrooms may be a great choice since they demonstrate various medicinal and functional properties [
7,
8]. Pleurotus species are among the most widely cultivated mushrooms and account for approximately 20% of the respective total global production [
9]; they are rich in proteins, fibers, essential amino acids, carbohydrates, water-soluble vitamins, and minerals [
10]. In particular, the fruitbodies of
Pleurotus eryngii (P. eryngii), also known as “King Oyster mushroom”, present a high content of bioactive compounds, such as ergosterol, beta-glucan, and ergothioneine [
11,
12]. In addition, they are a good source of vitamin D
2, which is produced by the conversion of ergosterol to ergocalciferol after exposure to UV light [
13]. As a result,
P. eryngii exhibits a variety of pharmacological activities and important nutritional value, and has gained a great deal of research attention in recent years due to its antioxidant, immunoregulatory, antihyperlipidemic, and other activities [
14].
Although there is a variety of literature on the cardioprotective activities of
P. eryngii extracts in vitro, as well as in animal studies [
15,
16,
17,
18,
19], no human clinical trials exist in this field. Our interest in the potential benefits of
P. eryngii on metabolic parameters was further reinforced by our recent study that showed that a meal with
P. eryngii can ameliorate postprandial glycemia and appetite, and can regulate ghrelin levels at the postprandial state [
20].
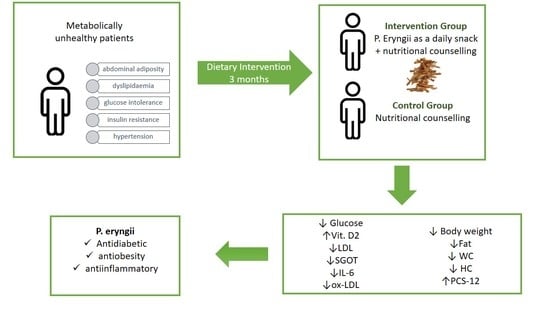
Thus, the aim of the present study was to investigate the effects of a P. eryngii mushroom snack administered daily for 3 months in metabolically unhealthy patients as regards the improvement in biochemical, inflammatory, oxidative stress, and lifestyle parameters.
2. Materials and Methods
2.1. Study Design
The protocol was reviewed and approved by the Ethics Committee of Harokopio University (ID protocol: 62/03-07-2018). The trial was conducted in accordance with the Helsinki Declaration and the Data Protection Act 1998 and was registered with
clinicaltrials.gov (ID Number: NCT04081818). Eligible subjects were enrolled in the study after being informed in detail about its nature and all procedures and having given their written consent for participation. The study took place in Harokopio University of Athens, Greece in 2020 and 2021.
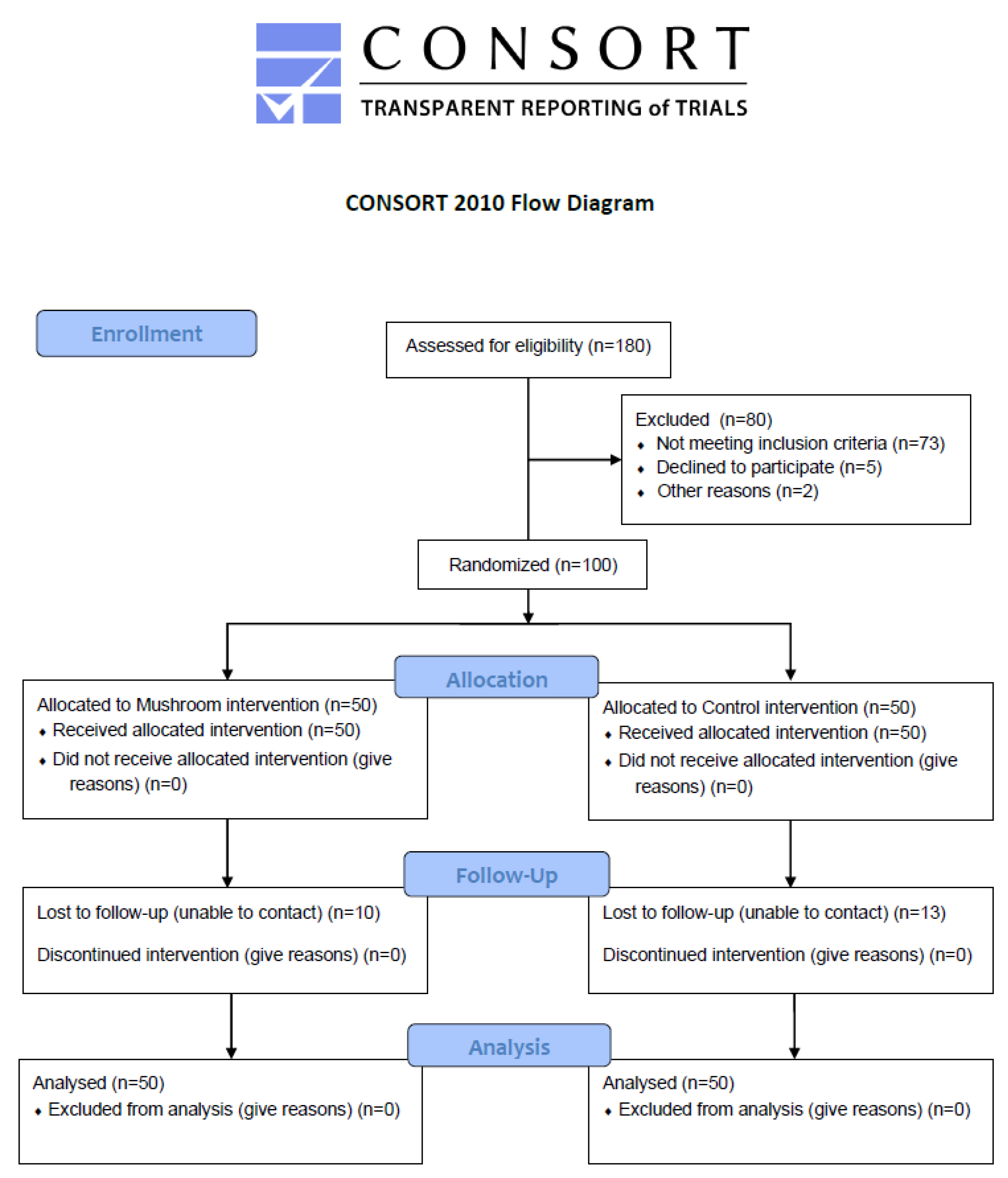
One hundred and eighty metabolically unhealthy subjects were recruited according to predetermined inclusion and exclusion criteria in a randomized controlled trial (
Figure 1). Eligible subjects were between the ages of 18 and 76 years and diagnosed with metabolic disorders. They also had a stable weight for at least 3 months before enrollment and a moderately active lifestyle. Exclusion criteria included pregnancy and lactation, untreated thyroid disease, any use of supplements within 3 months pre-intervention, a history of drug and/or alcohol abuse, and psychiatric or mental disorders.
After giving their consent, participants were randomized to the control or intervention group for 3 months. Both arms were given standard nutritional counseling for metabolic disorders throughout the 3-month trial, whereas the intervention group had also to consume the snack daily for 3 months. Randomization was carried out by an independent biostatistician, and compliance was monitored biweekly through phone calls. All baseline assessments were repeated at follow-up.
2.2. Snack Preparation
Following harvest, mushrooms were initially cut into 2 mm-thick slices; then, yeast extract (as a flavor enhancer) and garlic flavor powder were added at a ratio of 0.5% (
w/w), and the slices were baked at 120 °C for 20 min in a professional oven. After baking, the sliced mushrooms were subjected to UV-B irradiation (290–315 nm; 39 W lamps positioned at 20 cm from one another; mushrooms were placed at a distance of 20 cm from the light source, and were subjected to illumination for 120 min) to enhance vitamin D2 content. For the intervention, sachets made of aluminum foil were filled with the generated mushroom product (‘snack’, 6 g in each sachet) and were hermetically sealed. The nutritional composition and caloric content of the snack are presented in
Table 1, while the glucan content was ≈2.5 g [
12]. To ensure food safety, microbiological tests that are necessary for the food sector, including both control of safety and of hygiene criteria, as the applicable law requires them (Regulation 2073/2005), were carried out in an accredited laboratory. Finally, an experienced panel (Laboratory of Food Chemistry and Technology, School of Chemical Engineering, National Technical University of Athens) carried out the sensory testing of the mushroom snack (data not shown).
2.3. Outcomes
The primary outcome of the study was the changes in insulin sensitivity, and more specifically changes in fasting glucose levels after the 3 months’ intervention.
Secondary outcomes included changes in vitamin 25(OH)D2 levels, anthropometric measures, biochemical parameters, inflammatory and oxidative stress markers, as well as changes in quality of life.
2.4. Medical, Dietary, and Quality-of-Life Assessment
Detailed medical history was obtained including personal, family, medical history, and medication.
Dietary intake was assessed using a 24 h recall record (four non-consecutive days of the week) and was analyzed using Nutritionist Pro™ (Axxya Systems, Stafford, TX, USA) software (version 7.1.0).
Physical activity level was evaluated via the International Physical Activity Questionnaire Short Form (IPAQ-SF). This 7-day recall instrument measures frequency and duration of walking, moderate, and vigorous physical activity [
21].
Self-esteem was evaluated via the Rosenberg Self-Esteem scale. It includes a 10-item scale that estimates global self-worth by measuring both positive and negative feelings about the self. There is a 4-point rating scale (1 to 4) from strongly agree to strongly disagree. The total score ranges from 10 to 40 points. Higher scores indicate higher self-esteem [
22].
As obesity is considered a risk factor for depressive disorders, the 10-item questionnaire Center for Epidemiologic Studies Depression Scale Revised (CESD-R-10) was applied pre- and post-intervention. Subjects scoring ≥16 (range 0–60) are considered at risk for prevalent depression [
23].
Additionally, the insomnia level was evaluated via the Athens Insomnia Scale (AII) which records the assessment of any sleep difficulty. It consists of eight items that assess nocturnal sleep problems and daytime dysfunction. A higher score indicates greater severity of insomnia symptoms [
24].
Finally, the subjects completed questionnaires regarding their physical and mental health (Short Form-12 Physical Composite Score (PCS-12) and Mental Composite Score (MCS-12). The questionnaires include 12 questions with dichotomous responses (yes/no), ordinal (excellent to poor), or expressed by a frequency (always to never). The higher the score, the better the health status [
25].
2.5. Anthropometric Measurements
Body weight, body fat, fat free mass (FFM), total body water (TBW), and visceral fat rating were measured with bioelectrical impedance analysis (Tanita BC-418, Tokyo, Japan). Height was measured using a stadiometer (Seca Mode 220, Hamburg, Germany) with subjects not wearing shoes, their shoulders in a relaxed position, and their arms hanging freely. Waist circumference (WC) was determined at the midpoint between the lower margin of the last palpable rib and the top of the iliac crest in a standing position at the end of gentle expiration. Hip circumference (HP) measurement was taken around the widest portion of the buttocks. Body mass index (BMI) was computed as weight (kg)/height (m)2.
2.6. Blood Collection
Blood samples (20 mL) were collected after an overnight fast for biochemical and laboratory analyses. Blood samples were centrifuged at 3000 rpm for 10 min at 20 °C for plasma and serum isolation. EDTA was used as an anticoagulant for plasma isolation. All samples were stored at −80 °C until further laboratory analysis.
2.7. Laboratory Analyses
2.7.1. Biochemical Analyses
Serum glucose, insulin, urea, uric acid, creatinine, total cholesterol (TC), HDL-C, low-density lipoprotein cholesterol (LDL-C), triglycerides (TG), alanine aminotransferase (ALT), aspartate aminotransferase (AST), γ-glutamyl transferase (γ-GT), alkaline phosphatase (ALP), uric acid, lactate dehydrogenase (LDH), iron (Fe), ferritin, albumin, C-reactive protein (CRP) were measured in serum with an automatic biochemical analyser (Cobas 8000 analyser, Roche Diagnostics GmbH, Mannheim, Germany).
2.7.2. Evaluation of Inflammation and Oxidative Stress
Interleukin-6 (IL-6), tumor necrosis factor α (TNFα), leptin, adiponectin (R&D Systems, Inc., Minneapolis, MN, USA), MPO (Thermo Fisher Scientific Inc., Waltham, MA, USA), oxLDL (Mercodia, AB, Uppsala, Sweden) and 8-isoprostanes (Abcam, Cambridge, UK) were measured applying ELISA as indicators of chronic inflammatory grade and oxidative stress. All ELISA measurements were conducted in duplicate.
2.7.3. 25(OH)D2 and 25(OH)D3 Using Liquid Chromatography–Tandem Mass Spectrometry (LC-MS/MS)
Reagents and Standards
Standards of 25-Hydroxy vitamin D2, 25-Hydroxy vitamin D3 as well as vitamin D2 deuterated (25-Hydroxy Vitamin D2-d6), used as internal standard (IS) were purchased from Santa Cruz Biotechnology (Dallas, TX, USA), while D3 deuterated (25-Hydroxy Vitamin D3-d6, IS) was acquired from Glentham Life Sciences (Leafield, UK). All standard stock solutions were prepared in methanol and stored at −18 °C. All solvents were of a liquid chromatography–mass spectrometry (LC-MS) grade. More specifically, acetonitrile and formic acid were provided from Carlo Erba (Reuil, France), whereas water, hexane and methanol were purchased from Fischer Scientific (Hampton, VA, USA) and Sharlau (Barcelona, Spain), respectively.
25(OH)D2 and 25(OH)D3 Extraction Procedure
The extraction of vitamins 25(OH)D2 and 25(OH)D3 was performed as described in a previously published work with slight modifications [
26,
27,
28]. Briefly, frozen serum samples (280 μL) were thawed, mixed gently (multi vortex V-32, BioSan, Riga, Latvia) with another 280 μL acetonitrile containing 0.1% formic acid and finally spiked with 40 μL of a combined mixture of the internal standards’ solution at concentrations 0.5 and 4.0 μg mL
−1 of 25(OH)D2-d6 and 25(OH)D3-d6, respectively. The mixture was incubated for 30 min in 7 °C in order to induce protein precipitation. Then, 1200 μL of hexane was added to the above solution followed by a 5 min vortex. The new solution was incubated for another 25 min at 7 °C and then centrifuged at 10 °C and 12,000 rpm (Centrifuge Z32 HK, Hermle, Wehingen, Germany). After centrifugation, 900 μL of the upper organic phase was transferred to a new Eppendorf tube. Next, 1000 μL of hexane was added to the remaining solution and treated as previously described (vortex, incubation, centrifugation and collection of 1000 μL of the supernatant). The merged organic layers (1900 μL total) were centrifuged for 15 min at 10 °C and 1700 μL of the supernatant was collected in order to remove the solvent by using a nitrogen pump. Prior to analysis, the dry residue was reconstituted using a mixture of 2-propanol (35 μL) and methanol (50 μL), followed by a 10 min centrifugation (12,000 rpm at 10 °C). Finally, the supernatant was inserted in Liquid chromatography–mass spectrometry (LC-MS) vials.
25(OH)D2 and 25(OH)D3 Analysis
LC-MS analysis was used for the identification and quantification of 25(OH)D2 and 25(OH)D3. LC-MS included an API 3200 QTrap triple quadrupole/Linear ion trap mass spectrometer (AB Sciex, Foster City, CA, USA) coupled to an Agilent 1200 HPLC system (Agilent, Waldbronn, Germany). All spectra were processed by the Analyst software (version 1.4.2, AB Sciex, Foster City, CA, USA). In addition, for the separation of the four analytes, a Poroshell HPH-C18 column was used (2.1 particle size, 50 mm i.d., 2.7 μm). The elution of the analytes was performed by using a gradient system with two solvents (Solvent A, water with 0.1% formic acid and Solvent B: methanol with 0.1% formic acid) at flow rate 0.150 mL min−1. More specifically, the gradient started with 23% of solvent A, reduced to 0% over 8 min of analysis, and, in 23 min, percentage of solvent A ramped to initial conditions (23%), which remained till the end of the analysis. The injection volume was set at 5 μL. The MS/MS parameters for all the analytes were optimized with the direct infusion of a mixed standard methanol solution (1 mg mL−1) of the analytes. Tandem mass spectrometry analysis of all samples was performed in a positive mode using an electrospray chemical ionization (ESI) source.
The identification and quantification of the two forms of vitamin D were based on the fragmentation of the precursor ions into the respective product ions using a multiple monitoring reaction (MRM) technique. More specifically, product fragments of deuterated 25-Hydroxy vitamin D2 (m/z 419.3 > 355.4) were observed at a retention time (RT) of 12. 86 min, while product fragments of 25-Hydroxy vitamin D2 (m/z 413.4 > 355.4) were identified at a retention time of 12.91 min. Similarly, a product ion of 25-Hydroxy D3 (m/z 401.4 > 365.3) was detected at a retention time of 12.63 min, while a product ion of deuterated 25-Hydroxy D3 (m/z 407.5 > 371.4) was identified at a retention time of 12.68 min.
A mixture of plasma samples (pooled), which contained traces of the investigated analytes and 20 uL of each internal standard (25(OH)D2-d6 and 25(OH)D3 -d6), was used for the construction of calibration curves. The developed method exhibited good linearity (Peak area = 5.2456(±0.1362) × C 25(OH)D2 + 0.0004(±0.0130), R2 = 0.995 and Peak area = 8.2914(± 0.3029) × C 25(OH)D3–0.028(± 0.031), R2 = 0.993, respectively) within a wide range of concentrations (0.0001 to 0.25 μg mL
−1, n = 10). The method showed also good (a) intra-day (repeatability) (4.28% and 4.0%, for 25(OH)D2 and 25(OH)D3, respectively) and inter-day precision (reproducibility) (10.28% and 9.51%, for 25(OH)D2 and 25(OH)D3, respectively), (b) accuracy (115.1% and 86.42%, for 25(OH)D2 and 25(OH)D3, respectively) and (c) recovery (95.38% and 73.43%, for 25(OH)D2 and 25(OH)D3, respectively), as calculated by the
Official Journal of the European Communities guidelines [
29]. The limit of detection (LOD) was determined at 0.29 ng mL
−1 for 25(OH)D2 and at 0.48 ng mL
−1 for 25(OH)D3, whilst the limit of quantification (LOQ) was 0.00095 μg mL
−1 and 0.00158 μg mL
−1 for 25(OH)D2 and 25(OH)D3, respectively.
2.8. Sample Size Determination and Statistical Analysis
Continuous variables are presented with mean and standard deviation (SD). Quantitative variables are presented with absolute and relative frequencies. All analyses were conducted on an intention-to-treat basis. For the comparison of proportions, chi-square and Fisher’s exact tests were used. For the comparison of means between the control and intervention group, Student’s t-test was computed. To reduce the bias implicit in utilizing only complete cases, multiple imputation procedures for all data were implemented. Differences in changes of study variables during the follow-up period between the two study groups were evaluated using repeated measurements analysis of variance (ANOVA). All p values reported are two-tailed. Statistical significance was set at 0.05 and analyses were conducted using SPSS statistical software (version 24.0, IBM, New York, NY, USA).
A repeated-measures power analysis was conducted for a single within-subjects factor assessed over two time points. For this design, 50 participants per group achieve a power of 0.94 for the between-subjects main effect at an effect size of 0.30; a power of 0.95 for the within-subjects main effect at an effect size of 0.20; and a power of 0.95 for the interaction effect at an effect size of 0.20.
4. Discussion
During the last few decades, functional ingredients of foods have attracted research interest in the prevention and management of obesity and related metabolic disorders. Several bioactive compounds of mushrooms have been documented to have beneficial impacts on various metabolic markers.The consumption of mushrooms has been associated with cardioprotective effects, such as hypocholesterolemic, antihyperglycemic, antihypertensive, anti-inflammatory and antioxidant properties [
30]. To the best of our knowledge, this is the first randomized controlled clinical trial exploring the effect of vitamin D2-enhanced
P. eryngii snack on parameters related to metabolic disorders.
We succeeded in proving our primary hypothesis that the daily consumption of the snack regulates glucose levels compared with the control group. Our findings are in accordance with the existing literature, as it has been shown that mushrooms possess an antidiabetic effect mainly due to their polysaccharide content [
31] and by increasing glucokinase activity [
32].
P. ostreatus exhibits similar effects, with a 7-day consumption of a cooked mushroom meal in exchange for vegetables to hospitalized patients with insulin resistance, resulting in a 22% reduction in fasting glucose [
33].
During follow-up, 25(OH)D2 levels increased significantly only in the intervention group with the degree of change being significantly different between the intervention and the control group. No significant differences were found in 25(OH)D3. Recently, Hu et al. showed that UV irradiation increases vitamin D2 concentration in
P. ostreatus mushrooms in ethanol suspension, thus enhancing their nutritional value [
34]. Several studies have demonstrated that UV irradiation of edible mushrooms results in a high rate of ergosterol conversion to vitamin D2; in addition, serum 25(OH)D levels are increased as shown in some clinical trials [
35]. Other clinical trials showed that serum 25(OH)D did not significantly increase by consumption of UV-exposed mushrooms [
36]. Although most studies have investigated D3 supplementation and its benefits on human health, D2 has also been shown to exhibit beneficial effects. Hence, D2 improves the quality of life in osteoarthritis subjects [
37], and it regulates endothelial function [
38] and arterial stiffness [
39]. Endothelial dysfunction is not only a consequence of insulin resistance but also impairs insulin signaling to further reduce insulin sensitivity, thereby resulting in a destructive cycle in metabolic disorders and diabetes [
40].
Additionally, participants of the intervention group exhibited a reduction in body weight, fat, waist, and hip circumference, whereas no significant alterations appeared in the control group, with the mean changes being different between the two intervention groups. This may be due to better appetite regulation as also shown in our previous study where
P. eryngii mushrooms ameliorate appetite and suppress ghrelin levels postprandially owed to their beta-glucan content [
20]. Moreover, it has been shown that a meal enriched with powder from dried oyster mushrooms can increase GLP-1 postprandially and decrease hunger rate for the same reasons [
41]. In a long-term (1 year) clinical trial with obese patients that substituted mushrooms for red meat lower BMI and waist circumference were reported [
42].
The results of the present investigation suggest that
P. eryngii might improve lipid profile. LDL levels decreased only in the intervention group although mean changes were not different between the intervention and the control group. Polysaccharides in mushrooms, including chitosans and glucans can reduce LDL levels [
43,
44]. However, modest weight loss in obese individuals may also provide lower fasting glucose and LDL levels [
45]. Our findings are in consistency with those of Choudhury et al. [
46] who showed a reduction in TC and LDL-C levels of obese hypertensive non-diabetic males administered with 3 g of
P. ostreatus powder in capsule form daily for 3 months. Similar results were also found in another study where lipid levels, including TC and LDL-C were significantly different after the consumption of
Agaricus bisporus cooked with olive oil [
47]. In contrast, other studies reported that LDL-C remains unchanged [
48,
49].
As for SGOT/AST levels, consumption of several mushroom species, including those of the genus Pleurotus, can reduce AST levels according to results obtained from animal models [
50]. Pleurotus species can generate the paths for diffraction of different liver enzymes and reduce the levels of serum enzyme activities [
51].
As obesity is associated with chronic low-grade inflammation and oxidative stress, studying them in clinical trials of obese and metabolic patients is essential. Insulin signaling is impaired and chronic inflammation is induced by such markers [
52]. In the present, a decrease in IL-6 and ox-LDL was found in the mushroom group, which did not appear in the control, the mean changes being significantly different between groups. To the best of our knowledge, this study is the first to demonstrate that
P. eryngii mushrooms regulate inflammation and oxidative stress in humans. The CRP alterations after mushroom intake were not accompanied by IL-6 and oxLDL differences when compared with the control [
53]. Similarly, other trials failed to report any significant effects on inflammatory markers [
54,
55]. The presence of lovastatin, a member of the statins family that lower TC and LDL levels and reduce the risk of coronary heart disease [
56] and the antioxidants ergothioneine [
11] and selenium [
57] have been detected in relatively high amounts in
P. eryngii. Added to the above, the increase in vitamin D2 content via UV radiation has been shown to ameliorate inflammation in humans [
58,
59]. However, when examining the effects of such complex matrices on metabolic health, it should be emphasized that the overall activity may be due to the synergistic effects of all compounds rather than the individual activities of specific constituents. DPPH and FRAP experiments in the extracts from the snack have shown a noticeable antioxidant capacity (399.92 ±10.14 μmol Trolox equivalents/100 g and 16.31 ± 0.07 μmol ascorbic acid equivalents/100 g, respectively). This may be due to the remaining antioxidant content in mushrooms, but also due to the antioxidants that arise from baking (i.e., the Maillard reaction products) and contribute to the overall antioxidant capacity.
With regard to the quality of life, PCS-12 was significantly higher in the intervention group at follow-up with a significant difference in the degree of mean change between the two groups. PCS-12 is a very good marker for explaining variations in the quality of life across BMI and seems to be lower in obese patients compared to normal-weight controls [
60]. In our study, the 4.1-point increase in PCS-12 in the intervention group may be explained by the regulation of several metabolic parameters, such as glucose, weight, BMI, fat, and of course by the overall improvement in the inflammatory and oxidative stress status. Additionally, the quality of life may have been improved due to an improvement in the quality of sleep and, more specifically, in snoring (snoring was stopped successfully in 23% of the participants at follow-up in the intervention group, whereas no participant stopped snoring in the control group).
Overall, the results of our randomized controlled clinical trial should be viewed in light of the fact that the trial could not be blinded; thus, a degree of bias is inevitable. However, we believe that the above is counterbalanced by several strengths, such as the adequate power of the study, and the satisfying degree of compliance, as verified by consistent phone calls with the participants, as well as with follow-up increased levels of vitamin 25(OH)D2 only in the intervention group. Finally, another strength was the very careful selection of the participants according to tight inclusion and exclusion criteria, as well as the successful randomization process mitigating possible bias in the study.