Markers of Endothelial Dysfunction Are Attenuated by Resveratrol in Preeclampsia
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Cell Culture
2.3. Umbilical Arteries
2.4. Intracellular NO
2.5. eNOS Uncoupling
2.6. Total Antioxidant Capacity
2.7. Total Arginase Activity
2.8. Reverse Transcriptase Reaction and Real-Time qPCR
2.9. Statistical Analyses
3. Results
3.1. Clinical Parameters of HP and PE Pregnant Women Plasma of the Study
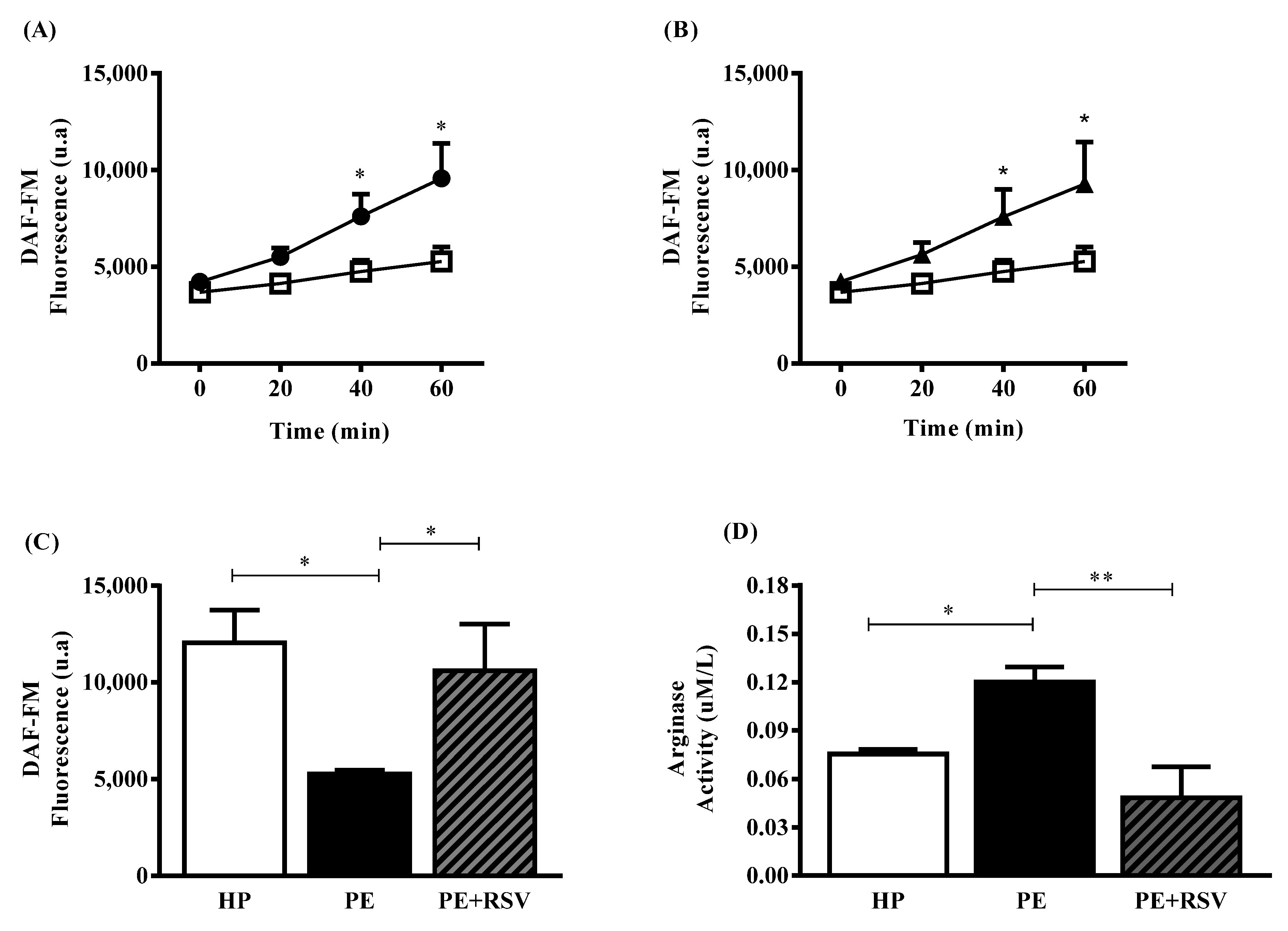
3.2. RSV Increases NO Levels and Decreases Arginase Activity in Endothelial Cells Incubated with Plasma from PE Pregnant Women
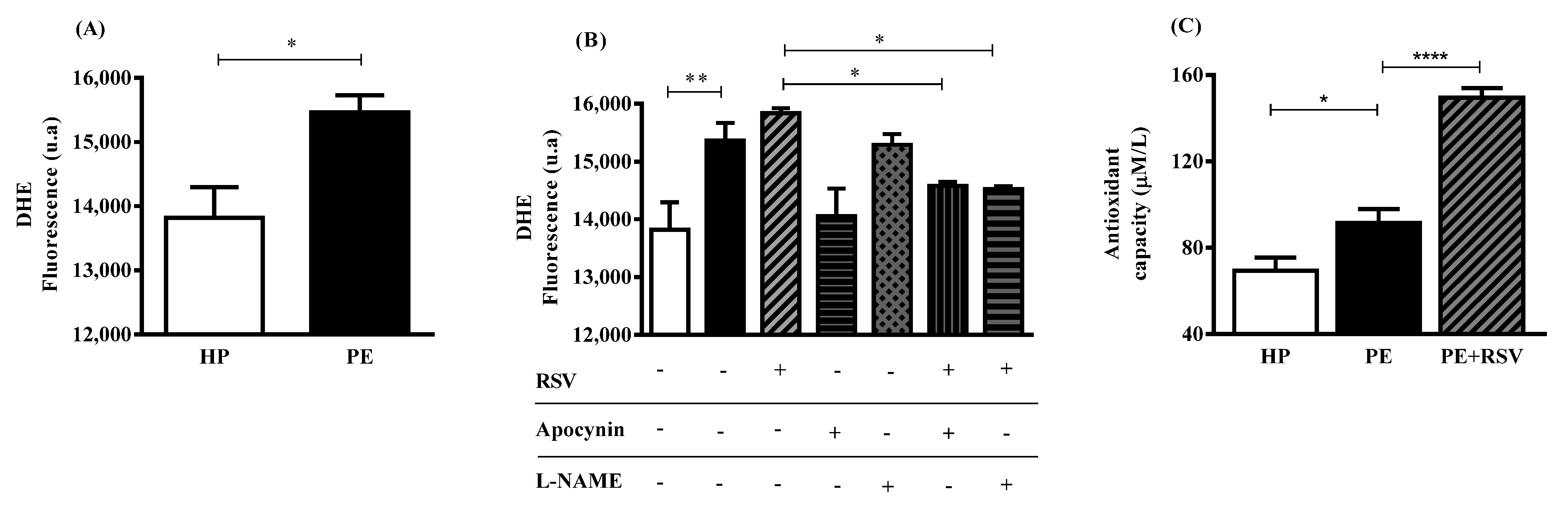
3.3. RSV Combined with eNOS (L-NAME) Inhibitor Decreases DHE Fluorescence, Suggesting Uncoupling eNOS
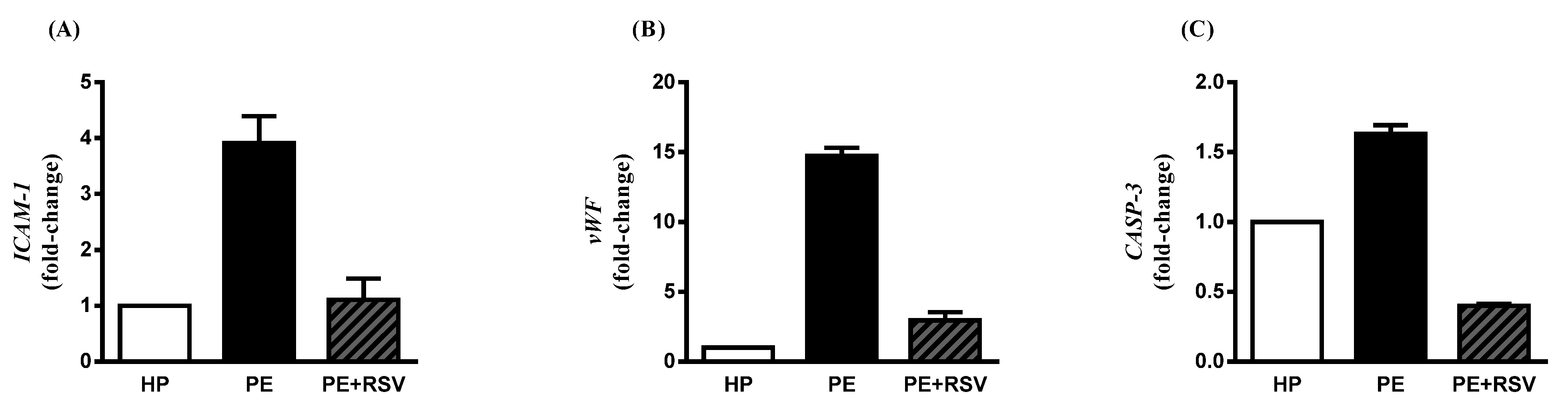
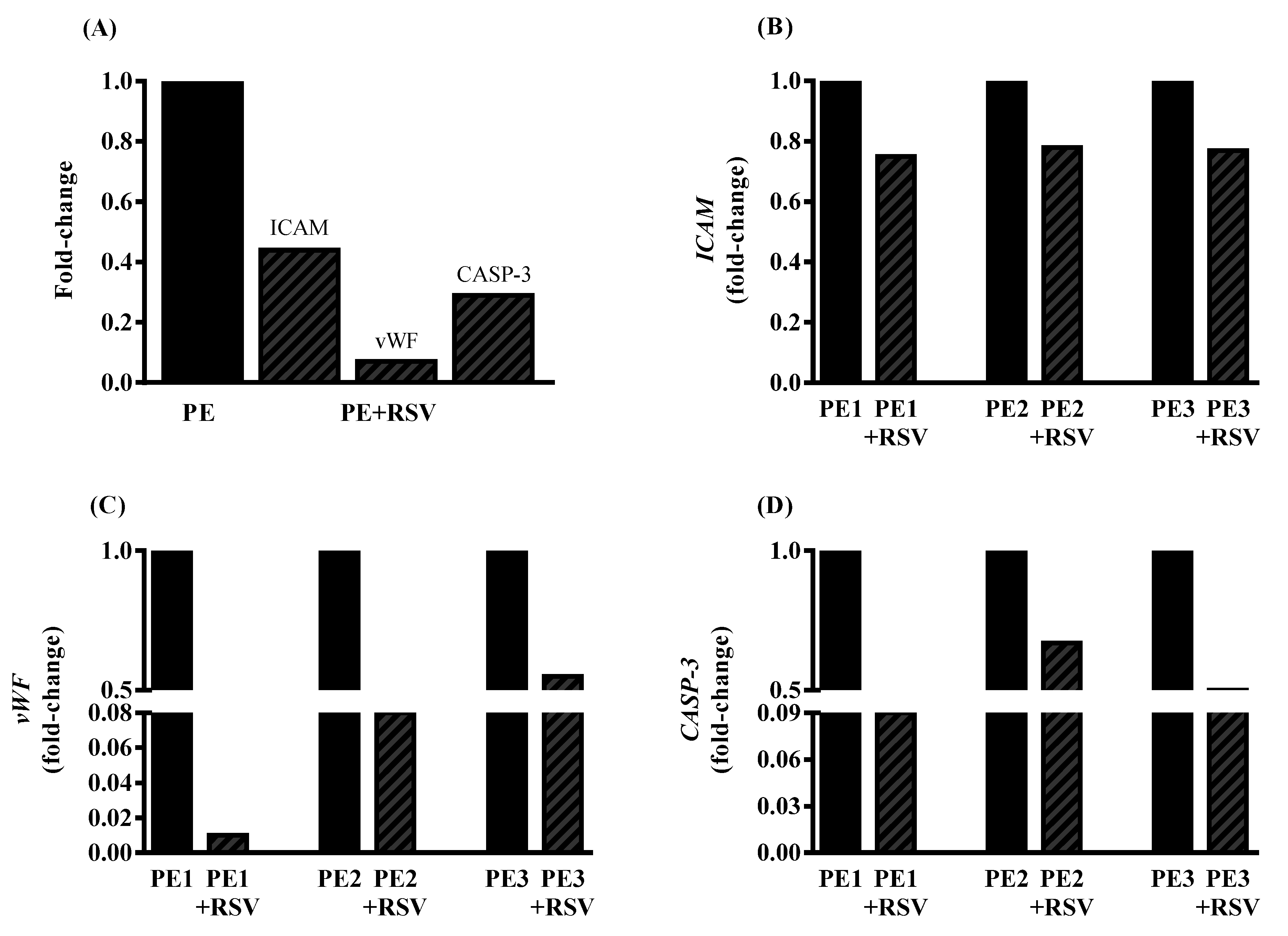
3.4. RSV Downregulates Endothelial Dysfunction Biomarkers in Endothelial Cells Incubated with PE Plasma and Umbilical Arteries from PE Patients
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- ACOG. ACOG Practice Bulletin Clinical Management Guidelines for Obstetrician Gynecologists. Gestational Hypertension and Preeclampsia. Obstet. Gynecol. 2020, 135, e237–e260. [Google Scholar] [CrossRef] [PubMed]
- Bartsch, E.; Medcalf, K.E.; Park, A.L.; Ray, J.G.; Al-Rubaie, Z.T.A.; Askie, L.M.; Berger, H.; Blake, J.; Graves, L.; Kingdom, J.C.; et al. Clinical risk factors for pre-eclampsia determined in early pregnancy: Systematic review and meta-analysis of large cohort studies. BMJ 2016, 353, i1753. [Google Scholar] [CrossRef] [PubMed]
- Brosens, I.A.; Robertson, W.B.; Dixon, H.G. The role of the spiral arteries in the pathogenesis of preeclampsia. Obs. Gynecol. Annu. 1972, 101, 177–191. [Google Scholar] [CrossRef]
- Sircar, M.; Thadhani, R.; Karumanchi, S.A. Pathogenesis of preeclampsia. Curr. Opin. Nephrol. Hypertens. 2015, 24, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Taylor, R.N.; De Groot, C.J.M.; Cho, Y.K.; Lim, K.H. Circulating factors as markers and mediators of endothelial cell dysfunction in preeclampsia. Semin. Reprod. Endocrinol. 1998, 16, 17–31. [Google Scholar] [CrossRef] [PubMed]
- Lowe, D.T. Nitric oxide dysfunction in the pathophysiology of preeclampsia. Nitric. Oxide Biol. Chem. 2000, 4, 441–458. [Google Scholar] [CrossRef]
- Mori, M.; Gotoh, T.; Nagasaki, A.; Takiguchi, M.; Miyanaka, K. Arginine metabolism and nitric oxide production. Pathophysiology 1998, 5, 60. [Google Scholar] [CrossRef]
- White, A.R.; Ryoo, S.; Li, D.; Champion, H.C.; Steppan, J.; Wang, D.; Nyhan, D.; Shoukas, A.A.; Hare, J.M.; Berkowitz, D.E. Knockdown of arginase I restores NO signaling in the vasculature of old rats. Hypertension 2006, 47, 245–251. [Google Scholar] [CrossRef]
- Berkowitz, D.E.; White, R.; Li, D.; Minhas, K.M.; Cernetich, A.; Kim, S.; Burke, S.; Shoukas, A.A.; Nyhan, D.; Champion, H.C.; et al. Arginase Reciprocally Regulates Nitric Oxide Synthase Activity and Contributes to Endothelial Dysfunction in Aging Blood Vessels. Circulation 2003, 108, 2000–2006. [Google Scholar] [CrossRef]
- Bertozzi-Matheus, M.; Bueno-Pereira, T.O.; Viana-Mattioli, S.; Carlström, M.; de Cavalli, R.C.; Sandrim, V.C. Different profiles of circulating arginase 2 in subtypes of preeclampsia pregnant women. Clin. Biochem. 2021, 92, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Vásquez-Vivar, J.; Kalyanaraman, B.; Martásek, P.; Hogg, N.; Masters, B.S.S.; Karoui, H.; Tordo, P.; Pritchard, K.A. Superoxide generation by endothelial nitric oxide synthase: The influence of cofactors. Proc. Natl. Acad. Sci. USA 1998, 95, 9220–9225. [Google Scholar] [CrossRef]
- Félétou, M.; Vanhoutte, P.M. Endothelial dysfunction: A multifaceted disorder. Am. J. Physiol. Heart Circ. Physiol. 2006, 291, H985–H1002. [Google Scholar] [CrossRef] [PubMed]
- Hg, W.; Kathy, K.G.; Ushio-Fukai, M. NADH/NADPH Oxidase and Vascular Function. Trends Cardiovasc. Med. 1997, 7, 1456–1462. [Google Scholar]
- Sankaralingam, S.; Xu, H.; Davidge, S.T. Arginase contributes to endothelial cell oxidative stress in response to plasma from women with preeclampsia. Cardiovasc. Res. 2010, 85, 194–203. [Google Scholar] [CrossRef] [PubMed]
- Roggensack, A.M.; Zhang, Y.; Davidge, S.T. Evidence for Peroxynitrite Formation in the Vasculature of Women with Preeclampsia. Hypertension 1999, 33, 83–89. [Google Scholar] [CrossRef]
- Aggarwal, B.B.; Bhardwaj, A.; Aggarwal, R.S.; Seeram, N.P.; Shishodia, S.; Takada, Y. Role of resveratrol in prevention and therapy of cancer: Preclinical and clinical studies. Anticancer Res. 2004, 24, 2783–2840. [Google Scholar]
- Chow, S.E.; Hshu, Y.C.; Wang, J.S.; Chen, J.K. Resveratrol attenuates oxLDL-stimulated NADPH oxidase activity and protects endothelial cells from oxidative functional damages. J. Appl. Physiol. 2007, 102, 1520–1527. [Google Scholar] [CrossRef] [PubMed]
- Wallerath, T.; Deckert, G.; Ternes, T.; Anderson, H.; Li, H.; Witte, K.; Förstermann, U. Resveratrol, a polyphenolic phytoalexin present in red wine, enhances expression and activity of endothelial nitric oxide synthase. Circulation 2002, 106, 1652–1658. [Google Scholar] [CrossRef] [PubMed]
- Caldeira-Dias, M.; Viana-Mattioli, S.; de Souza Rangel Machado, J.; Carlström, M.; de Carvalho Cavalli, R.; Sandrim, V.C. Resveratrol and grape juice: Effects on redox status and nitric oxide production of endothelial cells in in vitro preeclampsia model. Pregnancy Hypertens. 2021, 23, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Sankaralingam, S.; Xu, H.; Jiang, Y.; Sawamura, T.; Davidge, S.T. Evidence for increased methylglyoxal in the vasculature of women with preeclampsia: Role in upregulation of LOX-1 and arginase. Hypertension 2009, 54, 897–904. [Google Scholar] [CrossRef]
- Taylor, N.E.; Maier, K.G.; Roman, R.J.; Cowley, A.W. NO synthase uncoupling in the kidney of Dahl S rats: Role of dihydrobiopterin. Hypertension 2006, 48, 1066–1071. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Sandrim, V.C.; Palei, A.C.T.; Metzger, I.F.; Gomes, V.A.; Cavalli, R.C.; Tanus-Santos, J.E. Nitric oxide formation is inversely related to serum levels of antiangiogenic factors soluble fms-like tyrosine kinase-1 and soluble endogline in preeclampsia. Hypertension 2008, 52, 402–407. [Google Scholar] [CrossRef] [PubMed]
- Cho, W.K.; Lee, C.M.; Kang, M.J.; Huang, Y.; Giordano, F.J.; Lee, P.J.; Trow, T.K.; Homer, R.J.; Sessa, W.C.; Elias, J.A.; et al. IL-13 receptor α2-arginase 2 pathway mediates IL-13-induced pulmonary hypertension. Am. J. Physiol. Lung Cell. Mol. Physiol. 2013, 304, 112–124. [Google Scholar] [CrossRef] [PubMed]
- Quirié, A.; Demougeot, C.; Bertrand, N.; Mossiat, C.; Garnier, P.; Marie, C.; Prigent-Tessier, A. Effect of stroke on arginase expression and localization in the rat brain. Eur. J. Neurosci. 2013, 37, 1193–1202. [Google Scholar] [CrossRef]
- Ryoo, S.; Bhunia, A.; Chang, F.; Shoukas, A.; Berkowitz, D.E.; Romer, L.H. OxLDL-dependent activation of arginase II is dependent on the LOX-1 receptor and downstream RhoA signaling. Atherosclerosis 2011, 214, 279–287. [Google Scholar] [CrossRef]
- Zhang, C.; Hein, T.W.; Wang, W.; Chang, C.; Kuo, L. Constitutive expression of arginase in microvascular endothelial cells counteracts nitric oxide-mediated vasodilatory function. FASEB J. 2001, 15, 1264–1266. [Google Scholar] [CrossRef]
- Jae, H.K.; Bugaj, L.J.; Young, J.O.; Bivalacqua, T.J.; Ryoo, S.; Soucy, K.G.; Santhanam, L.; Webb, A.; Camara, A.; Sikka, G.; et al. Arginase inhibition restores NOS coupling and reverses endothelial dysfunction and vascular stiffness in old rats. J. Appl. Physiol. 2009, 107, 1249–1257. [Google Scholar] [CrossRef]
- Yang, Z.; Ming, X.F. Arginase: The emerging therapeutic target for vascular oxidative stress and inflammation. Front. Immunol. 2013, 4, 149. [Google Scholar] [CrossRef]
- Konior, A.; Schramm, A.; Czesnikiewicz-Guzik, M.; Guzik, T.J. NADPH oxidases in vascular pathology. Antioxid. Redox Signal. 2014, 20, 2794–2814. [Google Scholar] [CrossRef]
- Kim, S.Y.; Ryu, H.M.; Jae, H.Y.; Kim, M.Y.; Ahn, H.K.; Lim, H.J.; Shin, J.S.; Woo, H.J.; Park, S.Y.; Kim, Y.M.; et al. Maternal serum levels of VCAM-1, ICAM-1 and E-selectin in preeclampsia. J. Korean Med. Sci. 2004, 19, 688–692. [Google Scholar] [CrossRef]
- Sharp, A.N.; Heazell, A.E.P.; Baczyk, D.; Dunk, C.E.; Lacey, H.A.; Jones, C.J.P.; Perkins, J.E.; Kingdom, J.C.P.; Baker, P.N.; Crocker, I.P. Preeclampsia is associated with alterations in the p53-pathway in villous trophoblast. PLoS ONE 2014, 9, e87621. [Google Scholar] [CrossRef]
- Calvin, S.; Corrigan, J.; Weinstein, L.; Jeter, M. Factor VIII: Von Willebrand Factor Patterns in the Plasma of Patients with Pre-Eclampsia. Am. J. Perinatol. 1988, 5, 29–32. [Google Scholar] [CrossRef] [PubMed]
- Coata, G.; Pennacchi, L.; Bini, V.; Liotta, L.; Di Renzo, G.C. Soluble adhesion molecules: Marker of pre-eclampsia and intrauterine growth restriction. J. Matern. Neonatal Med. 2002, 12, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Holthe, M.R.; Staff, A.C.; Berge, L.N.; Lyberg, T. Different levels of platelet activation in preeclamptic, normotensive pregnant, and nonpregnant women. Am. J. Obstet. Gynecol. 2004, 190, 1128–1134. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Park, H.S.; Lee, H.Y.; Ha, E.H.; Suh, S.H.; Oh, S.K.; Yoo, H.S. Reduced L-arginine level and decreased placental eNOS activity in preeclampsia. Placenta 2006, 27, 438–444. [Google Scholar] [CrossRef] [PubMed]
- Smith-Jackson, K.; Hentschke, M.R.; Poli-De-Figueiredo, C.E.; Pinheiro Da Costa, B.E.; Kurlak, L.O.; Broughton Pipkin, F.; Czajka, A.; Mistry, H.D. Placental expression of eNOS, iNOS and the major protein components of caveolae in women with pre-eclampsia. Placenta 2015, 36, 607–610. [Google Scholar] [CrossRef] [PubMed]
- Orange, S.J.; Painter, D.; Horvath, J.; Yu, B.; Trent, R.; Hennessy, A. Placental endothelial nitric oxide synthase localization and expression in normal human pregnancy and pre-eclampsia. Clin. Exp. Pharmacol. Physiol. 2003, 30, 376–381. [Google Scholar] [CrossRef]
- Tenório, M.B.; Ferreira, R.C.; Moura, F.A.; Bueno, N.B.; Goulart, M.O.F.; Oliveira, A.C.M. Oral antioxidant therapy for prevention and treatment of preeclampsia: Meta-analysis of randomized controlled trials. Nutr. Metab. Cardiovasc. Dis. 2018, 28, 865–876. [Google Scholar] [CrossRef]
- Bordage, S.; Pham, T.N.; Zedet, A.; Gugglielmetti, A.S.; Nappey, M.; Demougeot, C.; Girard-Thernier, C. Investigation of Mammal Arginase Inhibitory Properties of Natural Ubiquitous Polyphenols by Using an Optimized Colorimetric Microplate Assay. Planta Med. 2017, 83, 647–653. [Google Scholar] [CrossRef] [PubMed]
- Xia, N.; Daiber, A.; Förstermann, U.; Li, H. Antioxidant effects of resveratrol in the cardiovascular system. Br. J. Pharmacol. 2017, 174, 1633–1646. [Google Scholar] [CrossRef] [PubMed]
- Lou, Z.; Li, X.; Zhao, X.; Du, K.; Li, X.; Wang, B. Resveratrol attenuates hydrogen peroxide-induced apoptosis, reactive oxygen species generation, and PSGL-1 and VWF activation in human umbilical vein endothelial cells, potentially via MAPK signalling pathways. Mol. Med. Rep. 2018, 17, 2479–2487. [Google Scholar] [CrossRef] [PubMed]
- Ou, H.C.; Chou, F.P.; Sheen, H.M.; Lin, T.M.; Yang, C.H.; Huey-Herng Sheu, W. Resveratrol, a polyphenolic compound in red wine, protects against oxidized LDL-induced cytotoxicity in endothelial cells. Clin. Chim. Acta 2006, 364, 196–204. [Google Scholar] [CrossRef] [PubMed]
- Harikumar, K.B.; Aggarwal, B.B. Resveratrol: A multitargeted agent for age-associated chronic diseases. Cell Cycle 2008, 7, 1020–1035. [Google Scholar] [CrossRef] [PubMed]
| Parameters | Healthy Pregnant | Preeclampsia | p |
|---|---|---|---|
| n | 10 | 10 | |
| Age (years) | 24.0 ± 1 | 26.0 ± 1 | 0.2011 |
| Caucasian (%) | 100 | 90 | 0.3161 |
| Primiparae (%) | 70 | 60 | 0.9581 |
| Smoker (%) | 10 | 0 | 0.3161 |
| GAS (weeks) | 36.6 ± 0.3 | 34.6 ± 1.3 | 0.9893 |
| SBP (mmHg) | 110.9 ± 3 | 136.9 ± 6.9 | 0.0040 |
| DPB (mmHg) | 71.2 ± 3.1 | 85.8 ± 3.7 | 0.0076 |
| BMI (kg/m2) | 24.8 ± 0.5 | 30.3 ± 1.2 | 0.0015 |
| Uric acid (mg/dL) | ND | 9.1 ± 3.2 | - |
| Hemoglobin (g/dL) | 12.8 ± 1.2 | 11.3 ± 2.3 | 0.0951 |
| Hematocrit (%) | 39.0 ± 4.0 | 34.5 ± 7.0 | 0.1227 |
| Platelets (×103/mm3) | ND | 192.9 ± 41.7 | - |
| Creatinine (mg/dL) | ND | 0.7 ± 0.1 | - |
| Newborn weight (g) | 3175.0 ± 497.9 | 2053.0 ± 1074 | 0.0157 |
| Placental weight (g) | 572.2 ± 147.4 | 472.5 ± 147.1 | 0.3054 |
| APGAR Score 1 (1 min) <7 | 0.0 | 10.0 | 0.0015 |
| APGAR Score 2 (5 min) <7 | 0.0 | 0.0 | >0.9999 |
| Parameters | #PE1 | #PE2 | #PE3 |
|---|---|---|---|
| Age (years) | 29 | 30 | 26 |
| GAS (weeks) | 39 | 37 | 37 |
| SBP (mmHg) | 140 | 140 | 140 |
| DPB (mmHg) | 90 | 90 | 90 |
| Uric acid (mg/dL) | 6.4 | 3.5 | 4.2 |
| Platelets (×103/mm3) | 248 | 239 | 288 |
| Creatinin (mg/dL) | 0.9 | 0.6 | 0.5 |
| Newborn weight (g) | 3690 | 3545 | 3050 |
| APGAR Score 1 (1 min) <7 | 9 | 8 | 8 |
| APGAR Score 2 (5 min) <7 | 10 | 9 | 8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bueno-Pereira, T.O.; Bertozzi-Matheus, M.; Zampieri, G.M.; Abbade, J.F.; Cavalli, R.C.; Nunes, P.R.; Sandrim, V.C. Markers of Endothelial Dysfunction Are Attenuated by Resveratrol in Preeclampsia. Antioxidants 2022, 11, 2111. https://doi.org/10.3390/antiox11112111
Bueno-Pereira TO, Bertozzi-Matheus M, Zampieri GM, Abbade JF, Cavalli RC, Nunes PR, Sandrim VC. Markers of Endothelial Dysfunction Are Attenuated by Resveratrol in Preeclampsia. Antioxidants. 2022; 11(11):2111. https://doi.org/10.3390/antiox11112111
Chicago/Turabian StyleBueno-Pereira, Thaina Omia, Mariana Bertozzi-Matheus, Gabriela Morelli Zampieri, Joelcio Francisco Abbade, Ricardo C. Cavalli, Priscila Rezeck Nunes, and Valeria Cristina Sandrim. 2022. "Markers of Endothelial Dysfunction Are Attenuated by Resveratrol in Preeclampsia" Antioxidants 11, no. 11: 2111. https://doi.org/10.3390/antiox11112111
APA StyleBueno-Pereira, T. O., Bertozzi-Matheus, M., Zampieri, G. M., Abbade, J. F., Cavalli, R. C., Nunes, P. R., & Sandrim, V. C. (2022). Markers of Endothelial Dysfunction Are Attenuated by Resveratrol in Preeclampsia. Antioxidants, 11(11), 2111. https://doi.org/10.3390/antiox11112111