Antioxidant Potential of Resveratrol as the Result of Radiation Exposition
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Irradiation
2.3. Electron Paramagnetic Resonance (EPR) Spectroscopy
2.4. Fourier Transform Infrared Spectroscopy (FTIR)
2.5. Computation
2.6. HPLC and HPLC-MS Analysis
2.7. Antioxidant Assay
3. Results
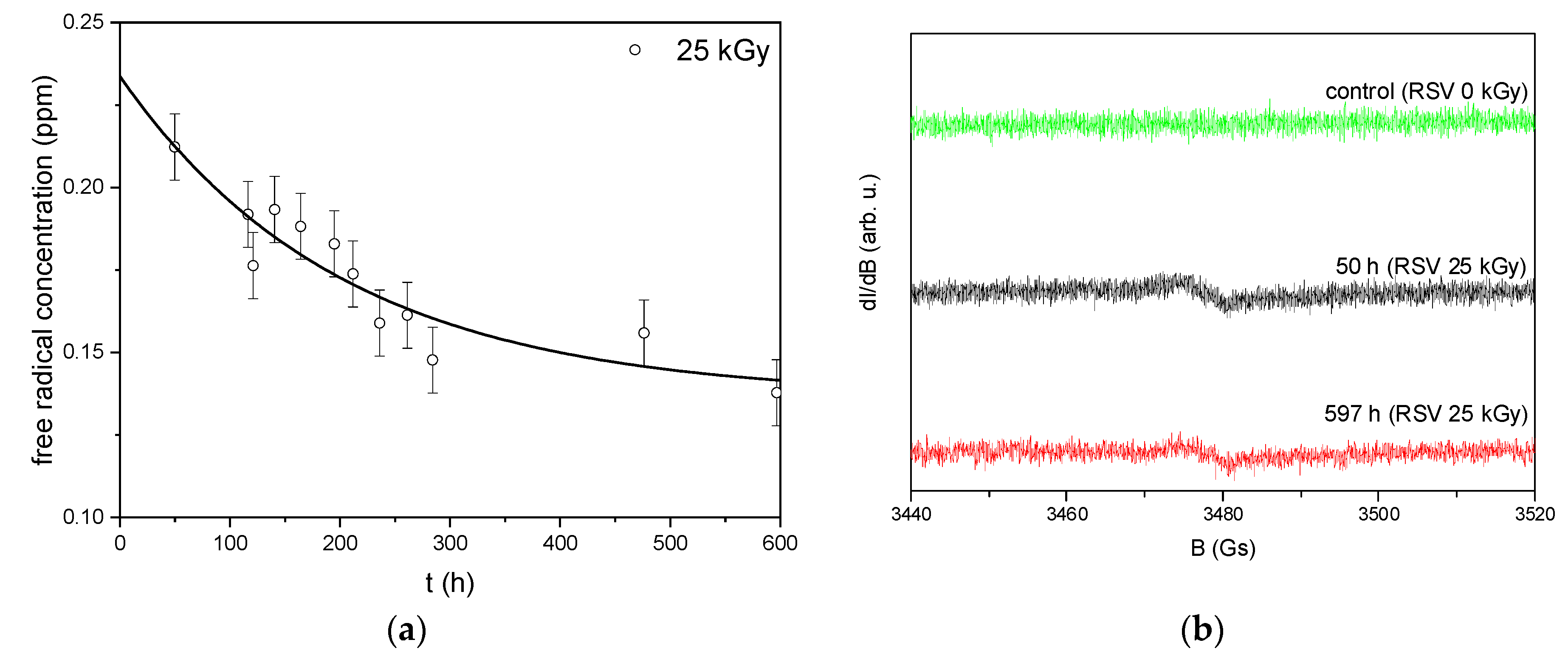
3.1. Electron Paramagnetic Resonance (EPR)
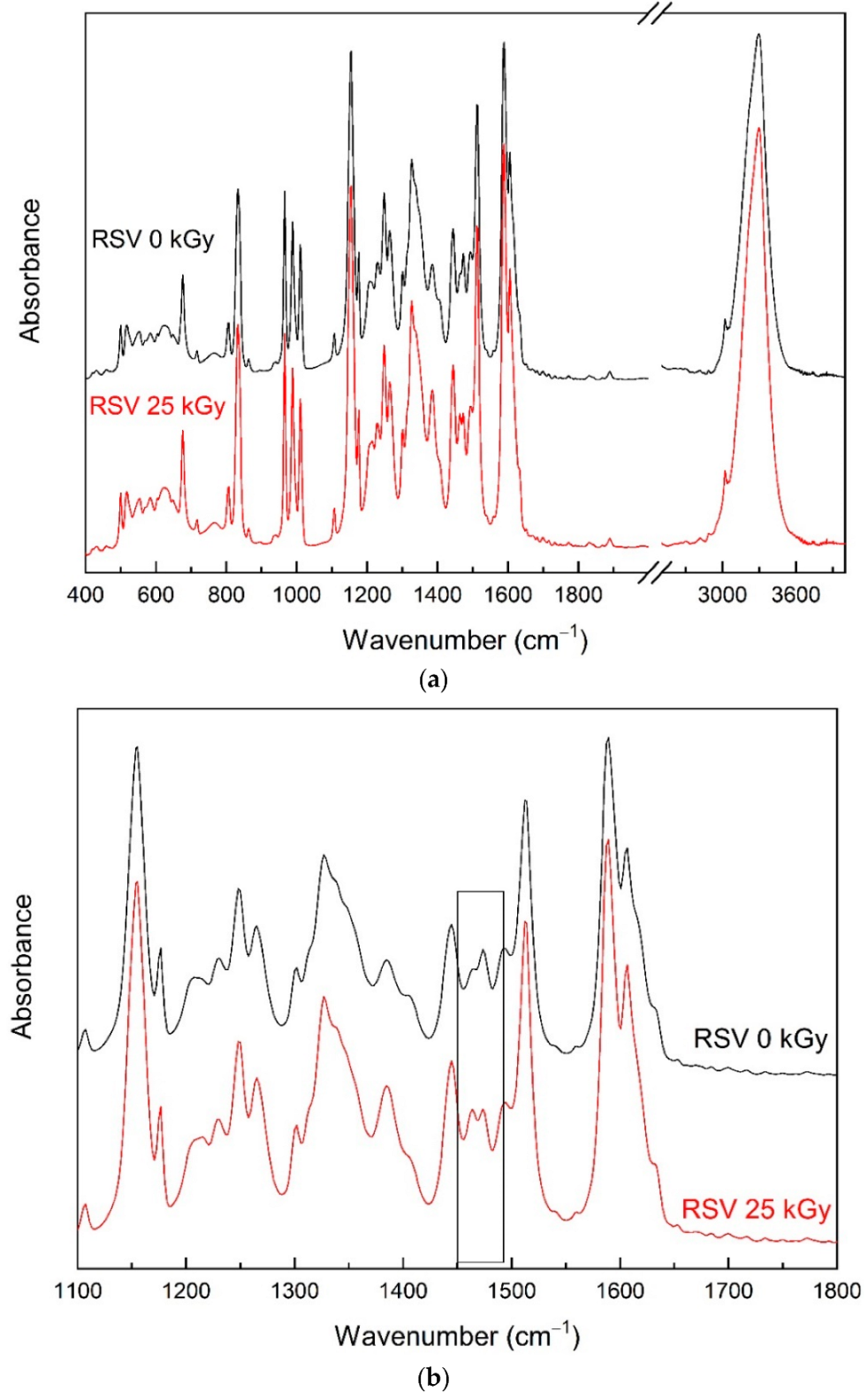
3.2. Fourier Transform Infrared Spectroscopy (FTIR) Analysis
3.3. HPLC and HPLC-MS Analysis
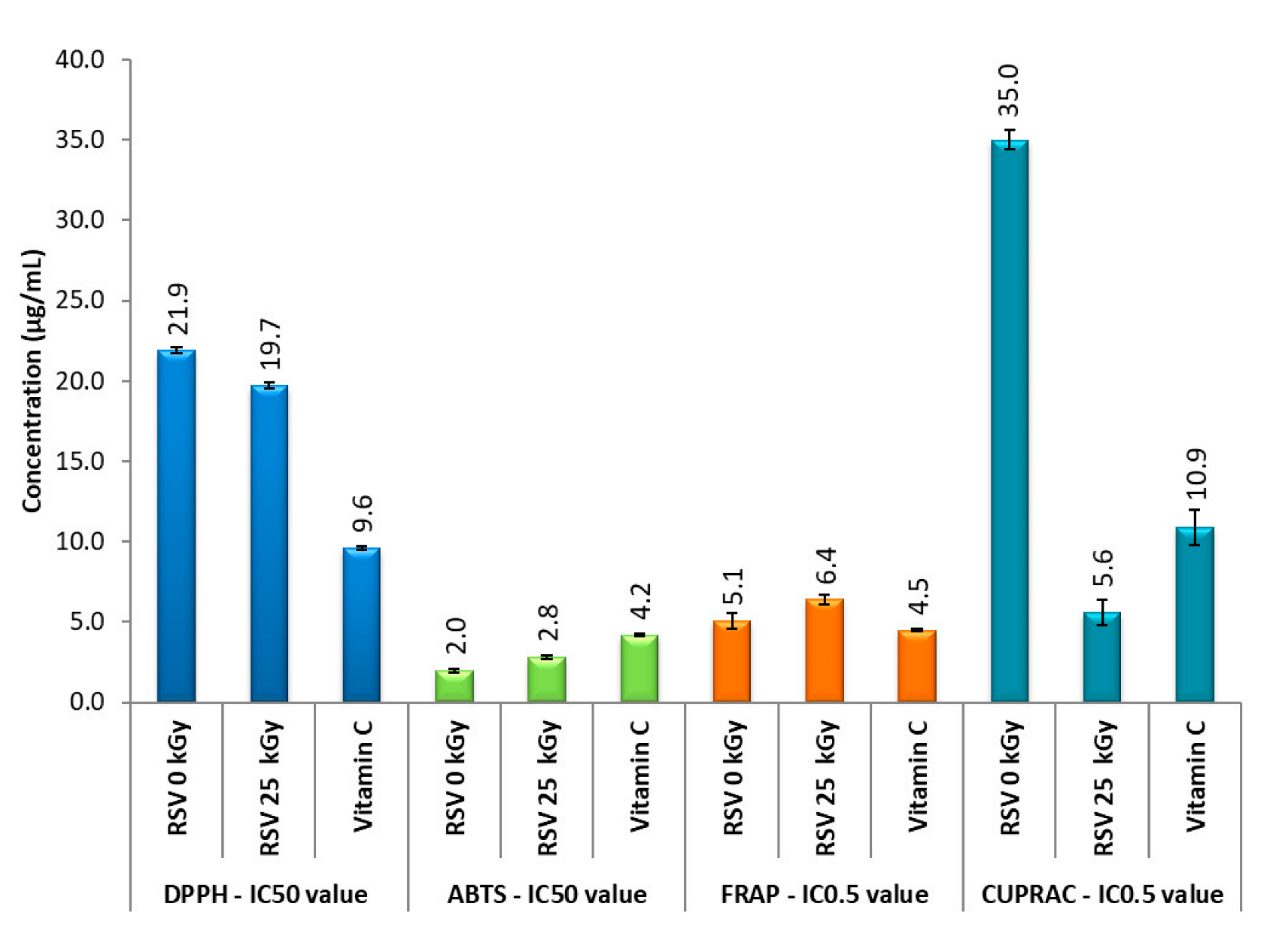
3.4. Antioxidant Properties
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Berman, A.Y.; Motechin, R.A.; Wiesenfeld, M.Y.; Holz, M.K. The therapeutic potential of resveratrol: A review of clinical trials. NPJ Precis. Oncol. 2017, 1, 35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, A.P.; Singh, R.; Verma, S.S.; Rai, V.; Kaschula, C.H.; Maiti, P.; Gupta, S.C. Health benefits of resveratrol: Evidence from clinical studies. Med. Res. Rev. 2019, 39, 1851–1891. [Google Scholar] [CrossRef] [PubMed]
- Rossi, D.; Guerrini, A.; Bruni, R.; Brognara, E.; Borgatti, M.; Gambari, R.; Maietti, S.; Sacchetti, G. Trans-resveratrol in nutraceuticals: Issues in retail quality and effectiveness. Molecules 2012, 17, 12393–12405. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.-X.; Li, C.-X.; Kakar, M.U.; Khan, M.S.; Wu, P.-F.; Amir, R.M.; Dai, D.-F.; Naveed, M.; Li, Q.-Y.; Saeed, M. Resveratrol (RV): A pharmacological review and call for further research. Biomed. Pharmacother. 2021, 143, 112164. [Google Scholar] [PubMed]
- Pan, X.; Zhu, Y.; Lin, N.; Zhang, J.; Ye, Q.; Huang, H.; Chen, X. Microglial phagocytosis induced by fibrillar β-amyloid is attenuated by oligomeric β-amyloid: Implications for Alzheimer’s disease. Mol. Neurodegener. 2011, 6, 45. [Google Scholar] [CrossRef] [Green Version]
- Sevcsik, E.; Trexler, A.J.; Dunn, J.M.; Rhoades, E. Allostery in a Disordered Protein: Oxidative Modifications to α-Synuclein Act Distally To Regulate Membrane Binding. J. Am. Chem. Soc. 2011, 133, 7152–7158. [Google Scholar] [CrossRef] [Green Version]
- Zhao, W.; Varghese, M.; Yemul, S.; Pan, Y.; Cheng, A.; Marano, P.; Hassan, S.; Vempati, P.; Chen, F.; Qian, X.; et al. Peroxisome proliferator activator receptor gamma coactivator-1alpha (PGC-1α) improves motor performance and survival in a mouse model of amyotrophic lateral sclerosis. Mol. Neurodegener. 2011, 6, 51. [Google Scholar] [CrossRef] [Green Version]
- Schmatz, R.; Perreira, L.B.; Stefanello, N.; Mazzanti, C.; Spanevello, R.; Gutierres, J.; Bagatini, M.; Martins, C.C.; Abdalla, F.H.; Daci da Silva Serres, J.; et al. Effects of resveratrol on biomarkers of oxidative stress and on the activity of delta aminolevulinic acid dehydratase in liver and kidney of streptozotocin-induced diabetic rats. Biochimie 2012, 94, 374–383. [Google Scholar] [CrossRef] [Green Version]
- Moussa, C.; Hebron, M.; Huang, X.; Ahn, J.; Rissman, R.A.; Aisen, P.S.; Turner, R.S. Resveratrol regulates neuro-inflammation and induces adaptive immunity in Alzheimer’s disease. J. Neuroinflamm. 2017, 14, 1. [Google Scholar] [CrossRef] [Green Version]
- Turner, R.S.; Thomas, R.G.; Craft, S.; Van Dyck, C.H.; Mintzer, J.; Reynolds, B.A.; Brewer, J.B.; Rissman, R.A.; Raman, R.; Aisen, P.S. A randomized, double-blind, placebo-controlled trial of resveratrol for Alzheimer disease. Neurology 2015, 85, 1383–1391. [Google Scholar] [CrossRef]
- Brasnyó, P.; Molnár, G.A.; Mohás, M.; Markó, L.; Laczy, B.; Cseh, J.; Mikolás, E.; Szijártó, I.A.; Mérei, A.; Halmai, R. Resveratrol improves insulin sensitivity, reduces oxidative stress and activates the Akt pathway in type 2 diabetic patients. Br. J. Nutr. 2011, 106, 383–389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thazhath, S.S.; Wu, T.; Bound, M.J.; Checklin, H.L.; Standfield, S.; Jones, K.L.; Horowitz, M.; Rayner, C.K. Administration of resveratrol for 5 wk has no effect on glucagon-like peptide 1 secretion, gastric emptying, or glycemic control in type 2 diabetes: A randomized controlled trial. Am. J. Clin. Nutr. 2016, 103, 66–70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhatt, J.K.; Thomas, S.; Nanjan, M.J. Resveratrol supplementation improves glycemic control in type 2 diabetes mellitus. Nutr. Res. 2012, 32, 537–541. [Google Scholar] [CrossRef] [PubMed]
- Izzo, C.; Annunziata, M.; Melara, G.; Sciorio, R.; Dallio, M.; Masarone, M.; Federico, A.; Persico, M. The role of resveratrol in liver disease: A comprehensive review from in vitro to clinical trials. Nutrients 2021, 13, 933. [Google Scholar] [CrossRef]
- Agarwal, B.; Campen, M.J.; Channell, M.M.; Wherry, S.J.; Varamini, B.; Davis, J.G.; Baur, J.A.; Smoliga, J.M. Resveratrol for primary prevention of atherosclerosis: Clinical trial evidence for improved gene expression in vascular endothelium. Int. J. Cardiol. 2013, 166, 246–248. [Google Scholar] [CrossRef] [Green Version]
- Magyar, K.; Halmosi, R.; Palfi, A.; Feher, G.; Czopf, L.; Fulop, A.; Battyany, I.; Sumegi, B.; Toth, K.; Szabados, E. Cardioprotection by resveratrol: A human clinical trial in patients with stable coronary artery disease. Clin. Hemorheol. Microcirc. 2012, 50, 179–187. [Google Scholar] [CrossRef]
- Ogrodowczyk, M.; Dettlaff, K.; Bednarski, W.; Ćwiertnia, B.; Stawny, M.; Spólnik, G.; Adamski, J.; Danikiewicz, W. Radiodegradation of nadolol in the solid state and identification of its radiolysis products by UHPLC–MS method. Chem. Pap. 2018, 72, 349–357. [Google Scholar] [CrossRef]
- Zalewski, P.; Rosiak, N.; Kilińska, K.; Skibiński, R.; Szymanowska, D.; Tykarska, E.; Piekara-Sady, L.; Lewandowska, K.; Miklaszewski, A.; Piontek, J. The radiolytic studies of panipenem in the solid state. Acta Pol. Pharm. Drug Res. 2020, 77, 241–250. [Google Scholar] [CrossRef]
- Ogrodowczyk, M.; Dettlaff, K.; Kachlicki, P.; Marciniec, B. Identification of Radiodegradation Products of Acebutolol and Alprenolol by HPLC/MS/MS. J. AOAC Int. 2015, 98, 46–50. [Google Scholar] [CrossRef]
- Zhuan, R.; Wang, J. Degradation of sulfamethoxazole by ionizing radiation: Kinetics and implications of additives. Sci. Total Environ. 2019, 668, 67–73. [Google Scholar] [CrossRef]
- Zalewski, P.; Skibiński, R.; Szymanowska-Powałowska, D.; Piotrowska, H.; Kozak, M.; Pietralik, Z.; Bednarski, W.; Cielecka-Piontek, J. The radiolytic studies of cefpirome sulfate in the solid state. J. Pharm. Biomed. Anal. 2016, 118, 410–416. [Google Scholar] [CrossRef] [PubMed]
- Janiaczyk, M.; Jelińska, A.; Woźniak-Braszak, A.; Bilski, P.; Popielarz-Brzezińska, M.; Wachowiak, M.; Baranowski, M.; Tomczak, S.; Ogrodowczyk, M. Electron Beam Radiation as a Safe Method for the Sterilization of Aceclofenac and Diclofenac—The Usefulness of EPR and 1H-NMR Methods in Determination of Molecular Structure and Dynamics. Pharmaceutics 2022, 14, 1331. [Google Scholar] [CrossRef] [PubMed]
- Kilińska, K.; Cielecka-Piontek, J.; Skibiński, R.; Szymanowska, D.; Miklaszewski, A.; Lewandowska, K.; Bednarski, W.; Mizera, M.; Tykarska, E.; Zalewski, P. The Radiation Sterilization of Ertapenem Sodium in the Solid State. Molecules 2019, 24, 2944. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kilińska, K.; Cielecka-Piontek, J.; Skibiński, R.; Szymanowska, D.; Miklaszewski, A.; Bednarski, W.; Tykarska, E.; Stasiłowicz, A.; Zalewski, P. The Radiostability of Meropenem Trihydrate in Solid State. Molecules 2018, 23, 2738. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- ISO 11137-2:2013; Sterilization of Health Care Products—Radiation—Part 2: Establishing the Sterilization Dose. International Organization for Standardization: Geneva, Switzerland, 2013.
- Tomé-Carneiro, J.; Gonzálvez, M.; Larrosa, M.; Yáñez-Gascón, M.J.; García-Almagro, F.J.; Ruiz-Ros, J.A.; García-Conesa, M.T.; Tomás-Barberán, F.A.; Espín, J.C. One-year consumption of a grape nutraceutical containing resveratrol improves the inflammatory and fibrinolytic status of patients in primary prevention of cardiovascular disease. Am. J. Cardiol. 2012, 110, 356–363. [Google Scholar] [CrossRef]
- Mai, V.C.; Bednarski, W.; Borowiak-Sobkowiak, B.; Wilkaniec, B.; Samardakiewicz, S.; Morkunas, I. Oxidative stress in pea seedling leaves in response to Acyrthosiphon pisum infestation. Phytochemistry 2013, 93, 49–62. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 09, Revision C. 01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Dennington, R.; Keith, T.; Millam, J. GaussView, version 5; Semichem Inc.: Shawnee Mission, KS, USA, 2009. [Google Scholar]
- Kikowska, M.A.; Chmielewska, M.; Włodarczyk, A.; Studzińska-Sroka, E.; Żuchowski, J.; Stochmal, A.; Kotwicka, M.; Thiem, B. Effect of pentacyclic triterpenoids-rich callus extract of Chaenomeles japonica (Thunb.) Lindl. ex spach on viability, morphology, and proliferation of normal human skin fibroblasts. Molecules 2018, 23, 3009. [Google Scholar] [CrossRef] [Green Version]
- Chanaj-Kaczmarek, J.; Wysocki, M.; Karachitos, A.; Wojcińska, M.; Bartosz, G.; Matławska, I.; Kmita, H. Effects of plant extract antioxidative phenolic compounds on energetic status and viability of Saccharomyces cerevisiae cells undergoing oxidative stress. J. Funct. Foods 2015, 16, 364–377. [Google Scholar] [CrossRef]
- Özyürek, M.; Güçlü, K.; Apak, R. The main and modified CUPRAC methods of antioxidant measurement. TrAC Trends Anal. Chem. 2011, 30, 652–664. [Google Scholar] [CrossRef]
- Benzie, I.F.; Devaki, M. The ferric reducing/antioxidant power (FRAP) assay for non-enzymatic antioxidant capacity: Concepts, procedures, limitations and applications. In Measurement of Antioxidant Activity & Capacity: Recent Trends and Applications; Wiley: New York, NY, USA, 2018; Chapter 5; pp. 77–106. [Google Scholar]
- Leopoldini, M.; Marino, T.; Russo, N.; Toscano, M. Antioxidant properties of phenolic compounds: H-atom versus electron transfer mechanism. J. Phys. Chem. A 2004, 108, 4916–4922. [Google Scholar] [CrossRef]
- Hussein, M.A. A convenient mechanism for the free radical scavenging activity of resveratrol. Int. J. Phytomed. 2011, 3, 459. [Google Scholar]
- Kitazuru, E.R.; Moreira, A.V.B.; Mancini-Filho, J.; Delincée, H.; Villavicencio, A.L.C.H. Effects of irradiation on natural antioxidants of cinnamon (Cinnamomum zeylanicum N.). Radiat. Phys. Chem. 2004, 71, 39–41. [Google Scholar] [CrossRef]
- Byun, M.W.; Son, J.H.; Yook, H.S.; Jo, C.; Kim, D.H. Effect of gamma irradiation on the physiological activity of Korean soybean fermented foods, Chungkookjang and Doenjang. Radiat. Phys. Chem. 2002, 64, 245–248. [Google Scholar] [CrossRef]
- Byun, M.W.; Yook, H.S.; Kim, K.S.; Chung, C.K. Effects of gamma irradiation on physiological effectiveness of Korean medicinal herbs. Radiat. Phys. Chem. 1999, 54, 291–300. [Google Scholar] [CrossRef]
- Al-Kuraieef, A.N.; Alshawi, A.H. The effect of gamma irradiation on the essential oils and antioxidants in dried thyme. Int. J. Food Stud. 2020, 9, 203–212. [Google Scholar] [CrossRef] [Green Version]
- Lampart-Szczapa, E.; Korczak, J.; Nogala-Kalucka, M.; Zawirska-Wojtasiak, R. Antioxidant properties of lupin seed products. Food Chem. 2003, 83, 279–285. [Google Scholar] [CrossRef]
- Ahn, H.J.; Kim, J.H.; Kim, J.K.; Kim, D.H.; Yook, H.S.; Byun, M.W. Combined effects of irradiation and modified atmosphere packaging on minimally processed Chinese cabbage (Brassica rapa L.). Food Chem. 2005, 89, 589–597. [Google Scholar] [CrossRef]
- Rajurkar, N.S.; Gaikwad, K.N. Effect of gamma irradiation on antioxidant activity of Amoora rohitaka. J. Radioanal. Nucl. Chem. 2012, 293, 409–413. [Google Scholar] [CrossRef]
- Hussein, S.Z.; Yusoff, K.M.; Makpol, S.; Yusof, Y.A.M. Antioxidant Capacities and Total Phenolic Contents Increase with Gamma Irradiation in Two Types of Malaysian Honey. Molecules 2011, 16, 6378–6395. [Google Scholar] [CrossRef]
- Khalil, M.I.; Sulaiman, S.A.; Alam, N.; Moniruzzaman, M.; Bai’e, S.; Man, C.N.; Jamalullail, S.M.S.; Gan, S.H. Gamma irradiation increases the antioxidant properties of tualang honey stored under different conditions. Molecules 2012, 17, 674–687. [Google Scholar] [CrossRef] [Green Version]
- Jung, H.J.; Park, H.R.; Jung, U.; Jo, S.K. Radiolysis study of genistein in methanolic solution. Radiat. Phys. Chem. 2009, 78, 386–393. [Google Scholar] [CrossRef]
- Choi, J.I.; Kim, J.K.; Srinivasan, P.; Kim, J.H.; Park, H.J.; Byun, M.W.; Lee, J.W. Comparison of gamma ray and electron beam irradiation on extraction yield, morphological and antioxidant properties of polysaccharides from tamarind seed. Radiat. Phys. Chem. 2009, 78, 605–609. [Google Scholar] [CrossRef]
- Lee, H.J.; Yoon, M.; Sung, N.Y.; Choi, J. il Spirogyra varians mutant generated by high dose gamma-irradiation shows increased antioxidant properties. Radiat. Phys. Chem. 2012, 81, 1017–1019. [Google Scholar] [CrossRef]
- Byun, E.-B.; Sung, N.-Y.; Park, J.-N.; Yang, M.-S.; Park, S.-H.; Byun, E.-H. Gamma-irradiated resveratrol negatively regulates LPS-induced MAPK and NF-κB signaling through TLR4 in macrophages. Int. Immunopharmacol. 2015, 25, 249–259. [Google Scholar] [CrossRef]
- López-Nicolás, J.M.; García-Carmona, F. Aggregation state and p K a values of (E)-resveratrol as determined by fluorescence spectroscopy and UV-visible absorption. J. Agric. Food Chem. 2008, 56, 7600–7605. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.; Masoodi, F.A.; Gani, A.; Ashwar, B.A. Effect of γ-irradiation on antioxidant and antiproliferative properties of oat β-glucan. Radiat. Phys. Chem. 2015, 117, 120–127. [Google Scholar] [CrossRef]
- Khan, A.A.; Gani, A.; Shah, A.; Masoodi, F.A.; Hussain, P.R.; Wani, I.A.; Khanday, F.A. Effect of γ-irradiation on structural, functional and antioxidant properties of β-glucan extracted from button mushroom (Agaricus bisporus). Innov. Food Sci. Emerg. Technol. 2015, 31, 123–130. [Google Scholar] [CrossRef]
- Variyar, P.S.; Limaye, A.; Sharma, A. Radiation-induced enhancement of antioxidant contents of soybean (Glycine max Merrill). J. Agric. Food Chem. 2004, 52, 3385–3388. [Google Scholar] [CrossRef]
- Jo, C.; Son, J.H.; Lee, H.J.; Byun, M.W. Irradiation application for color removal and purification of green tea leaves extract. Radiat. Phys. Chem. 2003, 66, 179–184. [Google Scholar] [CrossRef]
- Huang, S.J.; Mau, J.L. Antioxidant properties of methanolic extracts from Antrodia camphorata with various doses of γ-irradiation. Food Chem. 2007, 105, 1702–1710. [Google Scholar] [CrossRef]
- Ahn, H.J.; Kim, J.H.; Jo, C.; Kim, M.J.; Byun, M.W. Comparison of irradiated phytic acid and other antioxidants for antioxidant activity. Food Chem. 2004, 88, 173–178. [Google Scholar] [CrossRef]
| Method | Solution of Resveratrol | Solution of Ascorbic Acid |
|---|---|---|
| DPPH assay | 0.4–0.025 mg·mL−1 | 100−10 μg·mL−1 |
| ABTS assay | 0.2–0.005 mg·mL−1 | 100−10 μg·mL−1 |
| CUPRAC assay | 0.4–0.025 mg·mL−1 | 125−8 μg·mL−1 |
| FRAP assay | 0.2–0.025 mg·mL−1 | 300−100 μg·mL−1 |
| Method | Sample Solution + Working Solution | Incubation | Measured |
|---|---|---|---|
| DPPH | 25 µL + 175 µL | 30 min reaction, 5 min: 600 rpm, 25 °C | 517 nm |
| ABTS | 10 µL + 200 µL | 10 min reaction, 10 min: 600 rpm, 25 °C | 734 nm |
| CUPRAC | 50 µL + 150 µL | 30 min reaction, 5 min: 600 rpm, 25 °C | 450 nm |
| FRAP | 25 µL + 175 µL | 30 min reaction, 30 min: 100 rpm, 37 °C | 593 nm |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rosiak, N.; Cielecka-Piontek, J.; Skibiński, R.; Lewandowska, K.; Bednarski, W.; Zalewski, P. Antioxidant Potential of Resveratrol as the Result of Radiation Exposition. Antioxidants 2022, 11, 2097. https://doi.org/10.3390/antiox11112097
Rosiak N, Cielecka-Piontek J, Skibiński R, Lewandowska K, Bednarski W, Zalewski P. Antioxidant Potential of Resveratrol as the Result of Radiation Exposition. Antioxidants. 2022; 11(11):2097. https://doi.org/10.3390/antiox11112097
Chicago/Turabian StyleRosiak, Natalia, Judyta Cielecka-Piontek, Robert Skibiński, Kornelia Lewandowska, Waldemar Bednarski, and Przemysław Zalewski. 2022. "Antioxidant Potential of Resveratrol as the Result of Radiation Exposition" Antioxidants 11, no. 11: 2097. https://doi.org/10.3390/antiox11112097
APA StyleRosiak, N., Cielecka-Piontek, J., Skibiński, R., Lewandowska, K., Bednarski, W., & Zalewski, P. (2022). Antioxidant Potential of Resveratrol as the Result of Radiation Exposition. Antioxidants, 11(11), 2097. https://doi.org/10.3390/antiox11112097










