The Peanut Skin Procyanidins Attenuate DSS-Induced Ulcerative Colitis in C57BL/6 Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Extraction and Determination of PSPc
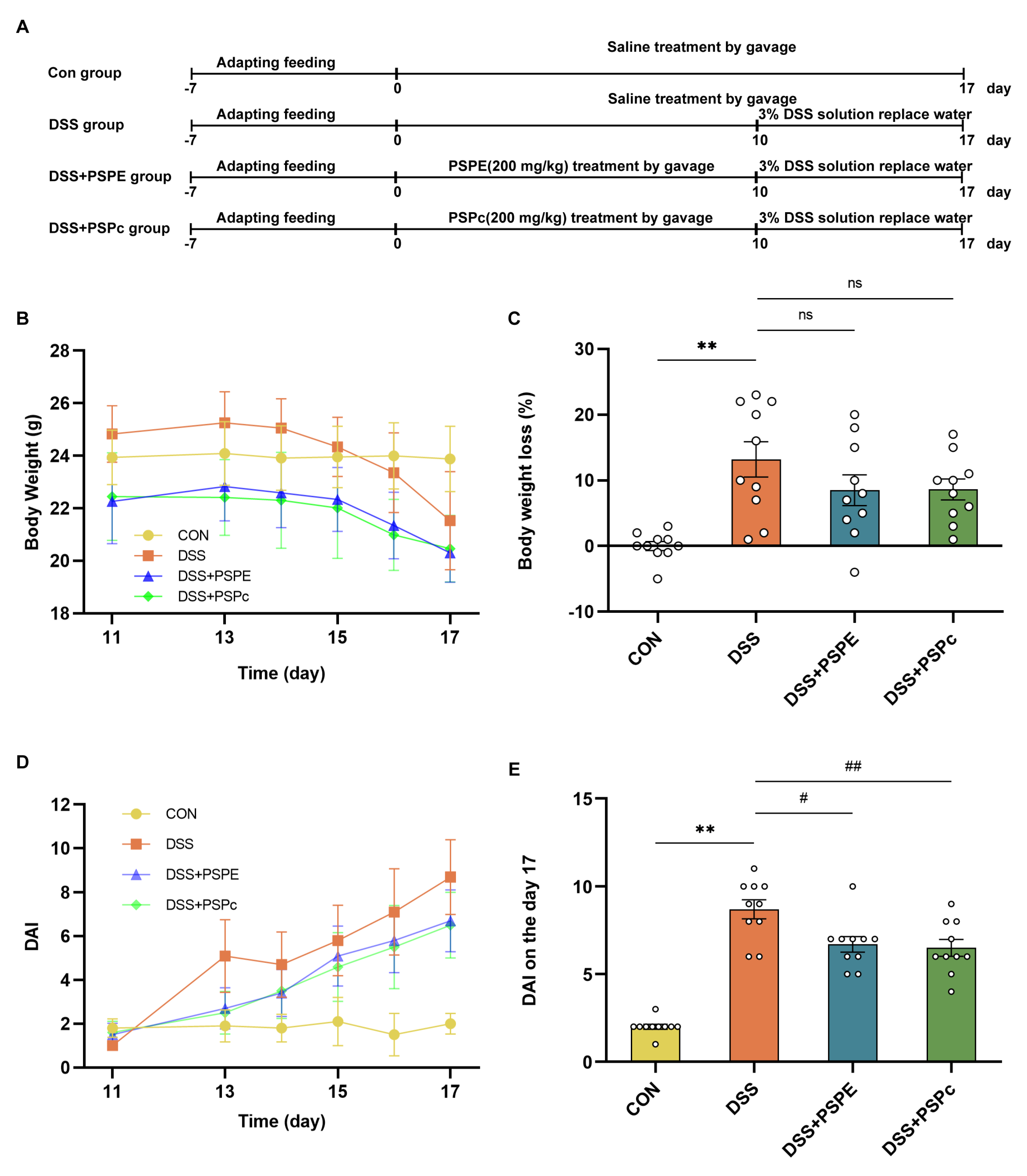
2.2. Animal Experiment
2.3. Disease Activity Index (DAI) Evaluation
2.4. Colon Histopathology Analysis
2.5. Alcian Blue Staining
2.6. Immunohistochemistry Analysis
2.7. ELISA Assay
2.8. qRT-PCR
2.9. Analysis of 16S rRNA Sequencing
2.10. SCFAs Content in Feces
2.11. Statistical Analysis
3. Results
3.1. Composition of PSPc
3.2. Effects of PSPE and PSPc Supplementation on Pathological Changes in DSS-Induced Colitis Mice
3.3. Effect of PSPE and PSPc Supplementation on the Inflammatory Factor Expressions and Biomarker of Oxidative Stress in DSS-Induced Colitis Mice
3.4. Effects of PSPE and PSPc Supplementation on the Histopathological Changes in the Colon of the DSS-Induced Colitis Mice
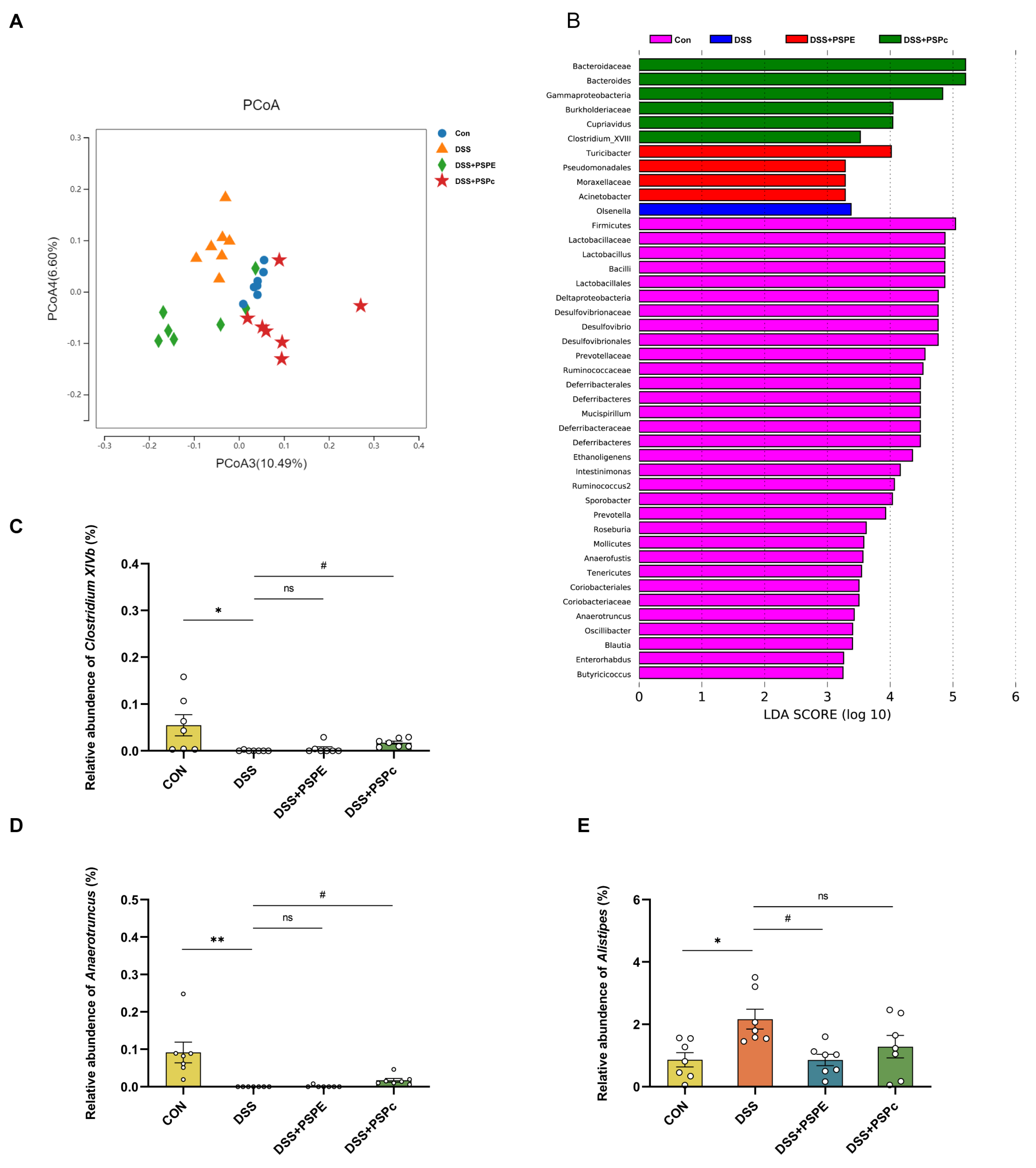
3.5. Effects of PSPE and PSPc Supplementation on the Gut Microbiome Diversity in DSS-Induced Colitis Mice
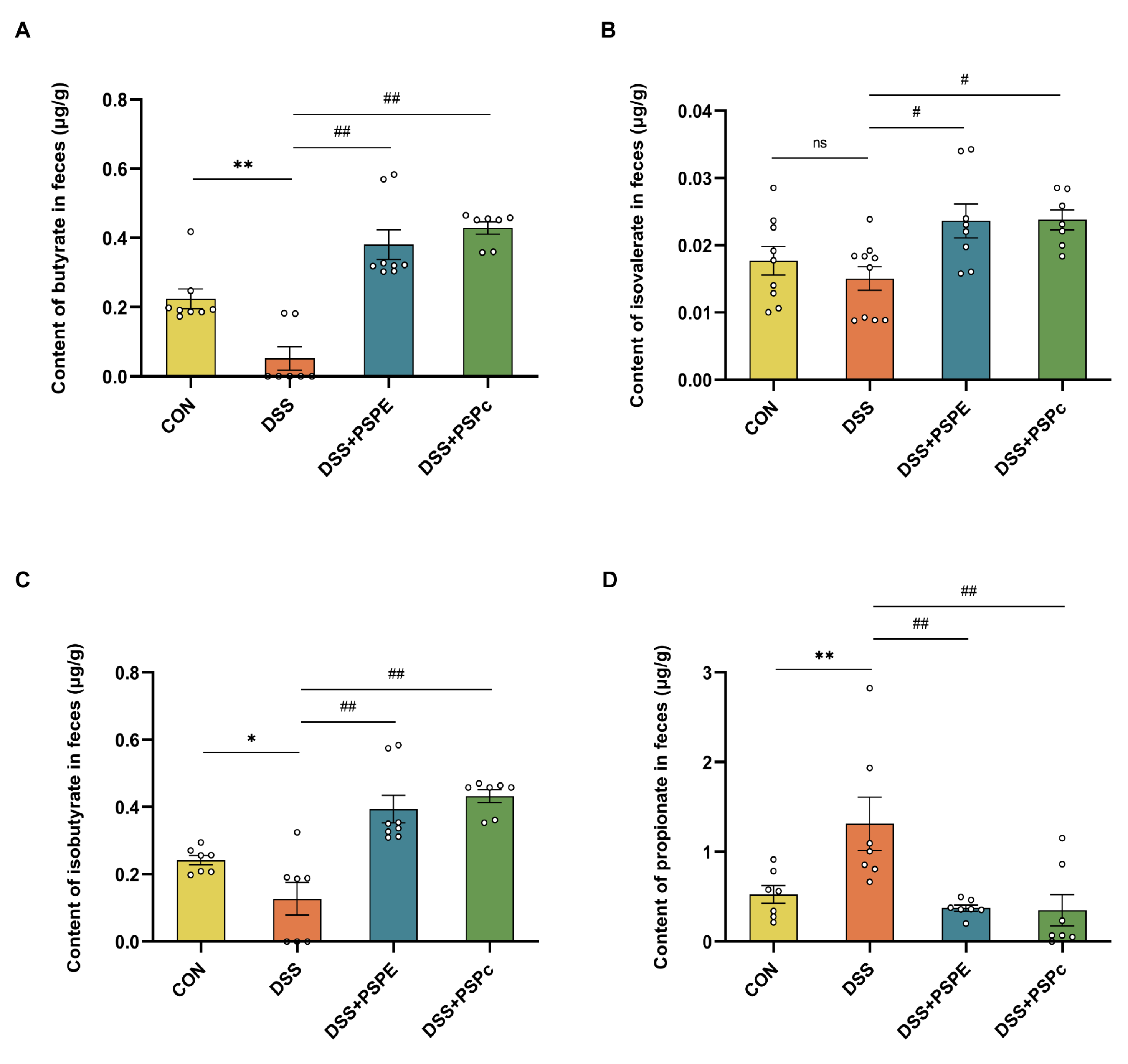
3.6. Effect of PSPE and PSPc Supplementation on the SCFAs Generation in DSS-Induced Colitis Mice
3.7. Correlation Analysis among the DAI, Fecal SCFAs Levels, Colon IL-1β, TNF-α, MDA Level, and Gut Microbiota
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hodson, R. Inflammatory Bowel Disease. Nature 2016, 540, S97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baumgart, D.C.; Sandborn, W.J. Inflammatory Bowel Disease: Clinical Aspects and Established and Evolving Therapies. Lancet 2007, 369, 1641–1657. [Google Scholar] [CrossRef]
- Ananthakrishnan, A.N. Epidemiology and Risk Factors for IBD. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 205–217. [Google Scholar] [CrossRef] [PubMed]
- Feuerstein, J.D.; Moss, A.C.; Farraye, F.A. Ulcerative Colitis. Mayo Clin. Proc. 2019, 94, 1357–1373. [Google Scholar] [CrossRef] [Green Version]
- Zhao, B.; Wu, J.; Li, J.; Bai, Y.; Luo, Y.; Ji, B.; Xia, B.; Liu, Z.; Tan, X.; Lv, J.; et al. Lycopene Alleviates DSS-Induced Colitis and Behavioral Disorders via Mediating Microbes-Gut–Brain Axis Balance. J. Agric. Food Chem. 2020, 68, 3963–3975. [Google Scholar] [CrossRef]
- Chiu, H.-F.; Venkatakrishnan, K.; Golovinskaia, O.; Wang, C.-K. Gastroprotective Effects of Polyphenols against Various Gastro-Intestinal Disorders: A Mini-Review with Special Focus on Clinical Evidence. Molecules 2021, 26, 2090. [Google Scholar] [CrossRef]
- Oz, H.S.; Chen, T.; de Villiers, W.J.S. Green Tea Polyphenols and Sulfasalazine Have Parallel Anti-Inflammatory Properties in Colitis Models. Front. Immunol. 2013, 4, 132. [Google Scholar] [CrossRef] [Green Version]
- Morimoto, M.; Watanabe, T.; Yamori, M.; Takebe, M.; Wakatsuki, Y. Isoflavones Regulate Innate Immunity and Inhibit Experimental Colitis. J. Gastroenterol. Hepatol. 2009, 24, 1123–1129. [Google Scholar] [CrossRef]
- Andújar, I.; Recio, M.C.; Giner, R.M.; Cienfuegos-Jovellanos, E.; Laghi, S.; Muguerza, B.; Ríos, J.L. Inhibition of Ulcerative Colitis in Mice after Oral Administration of a Polyphenol-Enriched Cocoa Extract Is Mediated by the Inhibition of STAT1 and STAT3 Phosphorylation in Colon Cells. J. Agric. Food Chem. 2011, 59, 6474–6483. [Google Scholar] [CrossRef]
- Aldini, R.; Budriesi, R.; Roda, G.; Micucci, M.; Ioan, P.; D’Errico-Grigioni, A.; Sartini, A.; Guidetti, E.; Marocchi, M.; Cevenini, M.; et al. Curcuma Longa Extract Exerts a Myorelaxant Effect on the Ileum and Colon in a Mouse Experimental Colitis Model, Independent of the Anti-Inflammatory Effect. PLoS One 2012, 7, e44650. [Google Scholar] [CrossRef]
- Larrosa, M.; González-Sarrías, A.; Yáñez-Gascón, M.J.; Selma, M.V.; Azorín-Ortuño, M.; Toti, S.; Tomás-Barberán, F.; Dolara, P.; Espín, J.C. Anti-Inflammatory Properties of a Pomegranate Extract and Its Metabolite Urolithin-A in a Colitis Rat Model and the Effect of Colon Inflammation on Phenolic Metabolism. J. Nutr. Biochem. 2010, 21, 717–725. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Kerr, W.L.; Swanson, R.B.; Hargrove, J.L.; Pegg, R.B. Peanut Skins-Fortified Peanut Butters: Effect of Processing on the Phenolics Content, Fibre Content and Antioxidant Activity. Food Chem. 2014, 145, 883–891. [Google Scholar] [CrossRef] [PubMed]
- Constanza, K.E.; White, B.L.; Davis, J.P.; Sanders, T.H.; Dean, L.L. Value-Added Processing of Peanut Skins: Antioxidant Capacity, Total Phenolics, and Procyanidin Content of Spray-Dried Extracts. J. Agric. Food Chem. 2012, 60, 10776–10783. [Google Scholar] [CrossRef] [PubMed]
- Sobolev, V.S.; Cole, R.J. Note on Utilisation of Peanut Seed Testa. J. Sci. Food Agric. 2004, 84, 105–111. [Google Scholar] [CrossRef]
- Yang, K.; Hashemi, Z.; Han, W.; Jin, A.; Yang, H.; Ozga, J.; Li, L.; Chan, C.B. Hydrolysis Enhances Bioavailability of Proanthocyanidin-Derived Metabolites and Improves β-Cell Function in Glucose Intolerant Rats. J. Nutr. Biochem. 2015, 26, 850–859. [Google Scholar] [CrossRef]
- Appeldoorn, M.M.; Vincken, J.-P.; Sanders, M.; Hollman, P.C.H.; Gruppen, H. Combined Normal-Phase and Reversed-Phase Liquid Chromatography/ESI-MS as a Tool to Determine the Molecular Diversity of A-Type Procyanidins in Peanut Skins. J. Agric. Food Chem. 2009, 57, 6007–6013. [Google Scholar] [CrossRef]
- Chang, M.; Sun, X.; Guo, X.; Bai, H.; Liu, R.; Jin, Q.; Wang, X. Composition and Antioxidant Study of Procyanidins from Peanut Skins. J. Food Meas. Charact. 2020, 14, 2781–2789. [Google Scholar] [CrossRef]
- Cos, P.; De Bruyne, T.; Hermans, N.; Apers, S.; Berghe, D.V.; Vlietinck, A.J. Proanthocyanidins in Health Care: Current and New Trends. Curr. Med. Chem. 2004, 11, 1345–1359. [Google Scholar] [CrossRef]
- Pan, C.; Wang, C.; Zhang, L.; Song, L.; Chen, Y.; Liu, B.; Liu, W.-T.; Hu, L.; Pan, Y. Procyanidins Attenuate Neuropathic Pain by Suppressing Matrix Metalloproteinase-9/2. J. Neuroinflammation 2018, 15, 187. [Google Scholar] [CrossRef]
- Jia, Z.; Song, Z.; Zhao, Y.; Wang, X.; Liu, P. Grape Seed Proanthocyanidin Extract Protects Human Lens Epithelial Cells from Oxidative Stress via Reducing NF-kB and MAPK Protein Expression. Mol. Vis. 2011, 17, 210–217. [Google Scholar]
- Kramer, K.; Yeboah-Awudzi, M.; Magazine, N.; King, J.M.; Xu, Z.; Losso, J.N. Procyanidin B2 Rich Cocoa Extracts Inhibit Inflammation in Caco-2 Cell Model of in Vitro Celiac Disease by down-Regulating Interferon-Gamma-or Gliadin Peptide 31-43-Induced Transglutaminase-2 and Interleukin-15. J. Funct. Foods 2019, 57, 112–120. [Google Scholar] [CrossRef]
- Bansode, R.R.; Randolph, P.; Ahmedna, M.; Hurley, S.; Hanner, T.; Baxter, S.A.S.; Johnston, T.A.; Su, M.; Holmes, B.M.; Yu, J.; et al. Bioavailability of Polyphenols from Peanut Skin Extract Associated with Plasma Lipid Lowering Function. Food Chem. 2014, 148, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Xu, L.; Li, B.; Zhang, W.; Zhang, C.; Feng, H.; Cui, X.; Gao, H. Protective Effects of Grape Seed Proanthocyanidin Extracts on Cerebral Cortex of Streptozotocin-Induced Diabetic Rats through Modulating AGEs/RAGE/NF-KappaB Pathway. J. Nutr. Sci. Vitaminol. 2010, 56, 87–97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, X.; Liu, J.; Lin, Q.; Mao, K.; Tian, F.; Jing, C.; Wang, C.; Ding, L.; Pang, C. Proanthocyanidin Prevents Lipopolysaccharide-Induced Depressive-like Behavior in Mice via Neuroinflammatory Pathway. Brain Res. Bull. 2017, 135, 40–46. [Google Scholar] [CrossRef]
- Ungaro, R.; Mehandru, S.; Allen, P.B.; Peyrin-Biroulet, L.; Colombel, J.-F. Ulcerative Colitis. Lancet 2017, 389, 1756–1770. [Google Scholar] [CrossRef]
- Larrosa, M.; Yañéz-Gascón, M.J.; Selma, M.V.; González-Sarrías, A.; Toti, S.; Cerón, J.J.; Tomás-Barberán, F.; Dolara, P.; Espín, J.C. Effect of a Low Dose of Dietary Resveratrol on Colon Microbiota, Inflammation and Tissue Damage in a DSS-Induced Colitis Rat Model. J. Agric. Food Chem. 2009, 57, 2211–2220. [Google Scholar] [CrossRef]
- Liu, M.; Huang, B.; Wang, L.; Lu, Q.; Liu, R. Peanut Skin Procyanidins Ameliorate Insulin Resistance via Modulation of Gut Microbiota and Gut Barrier in Type 2 Diabetic Mice. J. Sci. Food Agric. 2022. [Google Scholar] [CrossRef]
- Lewis, W.E.; Harris, G.K.; Sanders, T.H.; White, B.L.; Dean, L.L. Antioxidant and Anti-Inflammatory Effects of Peanut Skin Extracts. Sci. Res. 2013, 4, 35240. [Google Scholar] [CrossRef] [Green Version]
- Xu, M.; Lv, C.; Wang, H.; Lu, Q.; Ye, M.; Zhu, X.; Liu, R. Peanut Skin Extract Ameliorates High-Fat Diet-Induced Atherosclerosis by Regulating Lipid Metabolism, Inflammation Reaction and Gut Microbiota in ApoE−/− Mice. Food Res. Int. 2022, 154, 111014. [Google Scholar] [CrossRef]
- Shen, Z.-H.; Zhu, C.-X.; Quan, Y.-S.; Yang, Z.-Y.; Wu, S.; Luo, W.-W.; Tan, B.; Wang, X.-Y. Relationship between Intestinal Microbiota and Ulcerative Colitis: Mechanisms and Clinical Application of Probiotics and Fecal Microbiota Transplantation. World J. Gastroenterol. 2018, 24, 5–14. [Google Scholar] [CrossRef]
- Liu, Z.; Dai, X.; Zhang, H.; Shi, R.; Hui, Y.; Jin, X.; Zhang, W.; Wang, L.; Wang, Q.; Wang, D.; et al. Gut Microbiota Mediates Intermittent-Fasting Alleviation of Diabetes-Induced Cognitive Impairment. Nat. Commun. 2020, 11, 855. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.M.; Howitt, M.R.; Panikov, N.; Michaud, M.; Gallini, C.A.; Bohlooly-Y, M.; Glickman, J.N.; Garrett, W.S. The Microbial Metabolites, Short-Chain Fatty Acids, Regulate Colonic Treg Cell Homeostasis. Science 2013, 341, 569–573. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, Y.; Yan, Y.; Wan, P.; Chen, D.; Ding, Y.; Ran, L.; Mi, J.; Lu, L.; Zhang, Z.; Li, X.; et al. Gut Microbiota Modulation and Anti-Inflammatory Properties of Anthocyanins from the Fruits of Lycium Ruthenicum Murray in Dextran Sodium Sulfate-Induced Colitis in Mice. Free Radic. Biol. Med. 2019, 136, 96–108. [Google Scholar] [CrossRef] [PubMed]
- Mo, J.; Ni, J.; Zhang, M.; Xu, Y.; Li, Y.; Karim, N.; Chen, W. Mulberry Anthocyanins Ameliorate DSS-Induced Ulcerative Colitis by Improving Intestinal Barrier Function and Modulating Gut Microbiota. Antioxidants 2022, 11, 1674. [Google Scholar] [CrossRef] [PubMed]
- Rangan, P.; Choi, I.; Wei, M.; Navarrete, G.; Guen, E.; Brandhorst, S.; Enyati, N.; Pasia, G.; Maesincee, D.; Ocon, V.; et al. Fasting-Mimicking Diet Modulates Microbiota and Promotes Intestinal Regeneration to Reduce Inflammatory Bowel Disease Pathology. Cell Rep. 2019, 26, 2704–2719.e6. [Google Scholar] [CrossRef] [Green Version]
- Alipour, M.; Zaidi, D.; Valcheva, R.; Jovel, J.; Martínez, I.; Sergi, C.; Walter, J.; Mason, A.L.; Wong, G.K.-S.; Dieleman, L.A.; et al. Mucosal Barrier Depletion and Loss of Bacterial Diversity Are Primary Abnormalities in Paediatric Ulcerative Colitis. J. Crohn’s Colitis 2015, 10, 462–471. [Google Scholar] [CrossRef]
- Zou, Q.; Zhang, X.; Liu, X.; Li, Y.; Tan, Q.; Dan, Q.; Yuan, T.; Liu, X.; Liu, R.H.; Liu, Z. Ficus Carica Polysaccharide Attenuates DSS-Induced Ulcerative Colitis in C57BL/6 Mice. Food Funct. 2020, 11, 6666–6679. [Google Scholar] [CrossRef]
- Zhang, X.; Zou, Q.; Zhao, B.; Zhang, J.; Zhao, W.; Li, Y.; Liu, R.; Liu, X.; Liu, Z. Effects of Alternate-Day Fasting, Time-Restricted Fasting and Intermittent Energy Restriction DSS-Induced on Colitis and Behavioral Disorders. Redox Biol. 2020, 32, 101535. [Google Scholar] [CrossRef]
- Yan, S.; Shi, R.; Li, L.; Ma, S.; Zhang, H.; Ye, J.; Wang, J.; Pan, J.; Wang, Q.; Jin, X.; et al. Mannan Oligosaccharide Suppresses Lipid Accumulation and Appetite in Western-Diet-Induced Obese Mice Via Reshaping Gut Microbiome and Enhancing Short-Chain Fatty Acids Production. Mol. Nutr. Food Res. 2019, 63, e1900521. [Google Scholar] [CrossRef]
- Yao, D.; Dong, M.; Dai, C.; Wu, S. Inflammation and Inflammatory Cytokine Contribute to the Initiation and Development of Ulcerative Colitis and Its Associated Cancer. Inflamm. Bowel Dis. 2019, 25, 1595–1602. [Google Scholar] [CrossRef]
- Samarghandian, S.; Azimi-Nezhad, M.; Farkhondeh, T.; Samini, F. Anti-Oxidative Effects of Curcumin on Immobilization-Induced Oxidative Stress in Rat Brain, Liver and Kidney. Biomed. Pharmacother. 2017, 87, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Han, J.; Li, L.; Wang, Y.; Li, Y.; Zhang, S. Claudin Family Participates in the Pathogenesis of Inflammatory Bowel Diseases and Colitis-Associated Colorectal Cancer. Front. Immunol. 2019, 10, 1441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Segain, J.P.; Raingeard de la Blétière, D.; Bourreille, A.; Leray, V.; Gervois, N.; Rosales, C.; Ferrier, L.; Bonnet, C.; Blottière, H.M.; Galmiche, J.P. Butyrate Inhibits Inflammatory Responses through NFkappaB Inhibition: Implications for Crohn’s Disease. Gut 2000, 47, 397–403. [Google Scholar] [CrossRef] [Green Version]
- Zhao, L.; Zhu, X.; Yu, Y.; He, L.; Li, Y.; Zhang, L.; Liu, R. Comprehensive Analysis of the Anti-Glycation Effect of Peanut Skin Extract. Food Chem. 2021, 362, 130169. [Google Scholar] [CrossRef]
- Fernandes, A.C.F.; Vieira, N.C.; de Santana, Á.L.; de Pádua Gandra, R.L.; Rubia, C.; Castro-Gamboa, I.; Macedo, J.A.; Macedo, G.A. Peanut Skin Polyphenols Inhibit Toxicity Induced by Advanced Glycation End-Products in RAW264.7 Macrophages. Food Chem. Toxicol. 2020, 145, 111619. [Google Scholar] [CrossRef] [PubMed]
- Xiang, L.; Wu, Q.; Osada, H.; Yoshida, M.; Pan, W.; Qi, J. Peanut Skin Extract Ameliorates the Symptoms of Type 2 Diabetes Mellitus in Mice by Alleviating Inflammation and Maintaining Gut Microbiota Homeostasis. Aging 2020, 12, 13991–14018. [Google Scholar] [CrossRef] [PubMed]
- Gam, D.H.; Hong, J.W.; Kim, J.H.; Kim, J.W. Skin-Whitening and Anti-Wrinkle Effects of Bioactive Compounds Isolated from Peanut Shell Using Ultrasound-Assisted Extraction. Molecules 2021, 26, 1231. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Zhang, M.; Chen, H.; Peng, J.; Jiang, F.; Shi, X.; Bai, Y.; Jian, M.; Jia, Y. Anthocyanins from Black Peanut Skin Protect against UV-B Induced Keratinocyte Cell and Skin Oxidative Damage through Activating Nrf 2 Signaling. Food Funct. 2019, 10, 6815–6828. [Google Scholar] [CrossRef]
- Chen, L.; Yan, F.; Chen, W.; Zhao, L.; Zhang, J.; Lu, Q.; Liu, R. Procyanidin from Peanut Skin Induces Antiproliferative Effect in Human Prostate Carcinoma Cells DU145. Chem. Biol. Interact. 2018, 288, 12–23. [Google Scholar] [CrossRef]
- Xiang, L.; Wu, Q.; Cheng, L.; Sun, K.; Li, J.; Yoshida, M.; Qi, J. Leptin and Adiponectin Signaling Pathways Are Involved in the Antiobesity Effects of Peanut Skin Extract. Oxid. Med. Cell. Longev. 2019, 2019, 2935315. [Google Scholar] [CrossRef] [Green Version]
- Bansode, R.R.; Plundrich, N.J.; Randolph, P.D.; Lila, M.A.; Williams, L.L. Peanut Flour Aggregation with Polyphenolic Extracts Derived from Peanut Skin Inhibits IgE Binding Capacity and Attenuates RBL-2H3 Cells Degranulation via MAPK Signaling Pathway. Food Chem. 2018, 263, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Chen, T.; Wang, Q.; Liu, J.; Lu, Y.; Shi, Y. Structure Analysis and Study of Biological Activities of Condensed Tannins from Bruguiera Gymnorhiza (L.) Lam and Their Effect on Fresh-Cut Lotus Roots. Molecules 2021, 26, 1369. [Google Scholar] [CrossRef] [PubMed]
- Hellström, J.K.; Mattila, P.H. HPLC Determination of Extractable and Unextractable Proanthocyanidins in Plant Materials. J. Agric. Food Chem. 2008, 56, 7617–7624. [Google Scholar] [CrossRef]
- Longo, E.; Rossetti, F.; Merkyte, V.; Boselli, E. Disambiguation of Isomeric Procyanidins with Cyclic B-Type and Non-Cyclic A-Type Structures from Wine and Peanut Skin with HPLC-HDX-HRMS/MS. J. Am. Soc. Mass Spectrom. 2018, 29, 2268–2277. [Google Scholar] [CrossRef]
- Donovan, J.L.; Lee, A.; Manach, C.; Rios, L.; Morand, C.; Scalbert, A.; Rémésy, C. Procyanidins Are Not Bioavailable in Rats Fed a Single Meal Containing a Grapeseed Extract or the Procyanidin Dimer B3. Br. J. Nutr. 2002, 87, 299–306. [Google Scholar] [CrossRef]
- Yang, H.; Tuo, X.; Wang, L.; Tundis, R.; Portillo, M.P.; Simal-Gandara, J.; Yu, Y.; Zou, L.; Xiao, J.; Deng, J. Bioactive Procyanidins from Dietary Sources: The Relationship between Bioactivity and Polymerization Degree. Trends Food Sci. Technol. 2021, 111, 114–127. [Google Scholar] [CrossRef]
- Huang, B.; Wang, L.; Liu, M.; Wu, X.; Lu, Q.; Liu, R. The Underlying Mechanism of A-Type Procyanidins from Peanut Skin on DSS-Induced Ulcerative Colitis Mice by Regulating Gut Microbiota and Metabolism. J. Food Biochem. 2022. [Google Scholar] [CrossRef]
- Fan, F.Y.; Sang, L.X.; Jiang, M.; McPhee, D.J. Catechins and Their Therapeutic Benefits to Inflammatory Bowel Disease. Molecules 2017, 22, 484. [Google Scholar] [CrossRef] [Green Version]
- Fraga, C.G.; Oteiza, P.I. Dietary Flavonoids: Role of (-)-Epicatechin and Related Procyanidins in Cell Signaling. Free Radic. Biol. Med. 2011, 51, 813–823. [Google Scholar] [CrossRef]
- Dryden, G.W.; Lam, A.; Beatty, K.; Qazzaz, H.H.; McClain, C.J. A Pilot Study to Evaluate the Safety and Efficacy of an Oral Dose of (-)-Epigallocatechin-3-Gallate-Rich Polyphenon E in Patients with Mild to Moderate Ulcerative Colitis. Inflamm. Bowel Dis. 2013, 19, 1904–1912. [Google Scholar] [CrossRef]
- Tilg, H.; Zmora, N.; Adolph, T.E.; Elinav, E. The Intestinal Microbiota Fuelling Metabolic Inflammation. Nat. Rev. Immunol. 2020, 20, 40–54. [Google Scholar] [CrossRef] [PubMed]
- Gryaznova, M.V.; Solodskikh, S.A.; Panevina, A.V.; Syromyatnikov, M.Y.; Dvoretskaya, Y.D.; Sviridova, T.N.; Popov, E.S.; Popov, V.N. Study of Microbiome Changes in Patients with Ulcerative Colitis in the Central European Part of Russia. Heliyon 2021, 7, e06432. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Gao, M.; Liu, Z.; Zhang, Y.; Tu, H.; Lei, L.; Wu, P.; Zhang, A.; Yang, C.; Li, G.; et al. Gut Microbiome Composition Linked to Inflammatory Factors and Cognitive Functions in First-Episode, Drug-Naive Major Depressive Disorder Patients. Front. Neurosci. 2021, 15, 800764. [Google Scholar] [CrossRef] [PubMed]
- Guo, N.; Zhou, L.-X.; Meng, N.; Shi, Y.-P. Associations of Oral and Intestinal Florae and Serum Inflammatory Factors with Pathogenesis of Oral Cancer. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 11090–11095. [Google Scholar] [CrossRef]
- Serrano-Villar, S.; Sanchez-Carrillo, S.; Talavera-Rodríguez, A.; Lelouvier, B.; Gutiérrez, C.; Vallejo, A.; Servant, F.; Bernadino, J.I.; Estrada, V.; Madrid, N.; et al. Blood Bacterial Profiles Associated With Human Immunodeficiency Virus Infection and Immune Recovery. J. Infect. Dis. 2021, 223, 471–481. [Google Scholar] [CrossRef]
- Mowat, A.M.; Agace, W.W. Regional Specialization within the Intestinal Immune System. Nat. Rev. Immunol. 2014, 14, 667–685. [Google Scholar] [CrossRef]
- Zhao, S.; Peng, X.; Zhou, Q.; Huang, Y.; Rao, X.; Tu, J.; Xiao, H.; Liu, D. Bacillus Coagulans 13002 and Fructo-Oligosaccharides Improve the Immunity of Mice with Immunosuppression Induced by Cyclophosphamide through Modulating Intestinal-Derived and Fecal Microbiota. Food Res. Int. 2021, 140, 109793. [Google Scholar] [CrossRef]
- Nankova, B.B.; Agarwal, R.; MacFabe, D.F.; La Gamma, E.F. Enteric Bacterial Metabolites Propionic and Butyric Acid Modulate Gene Expression, Including CREB-Dependent Catecholaminergic Neurotransmission, in PC12 Cells--Possible Relevance to Autism Spectrum Disorders. PLoS One 2014, 9, e103740. [Google Scholar] [CrossRef]
- Parker, B.J.; Wearsch, P.A.; Veloo, A.C.M.; Rodriguez-Palacios, A. The Genus Alistipes: Gut Bacteria With Emerging Implications to Inflammation, Cancer, and Mental Health. Front. Immunol. 2020, 11, 906. [Google Scholar] [CrossRef]
- Polansky, O.; Sekelova, Z.; Faldynova, M.; Sebkova, A.; Sisak, F.; Rychlik, I. Important Metabolic Pathways and Biological Processes Expressed by Chicken Cecal Microbiota. Appl. Environ. Microbiol. 2015, 82, 1569–1576. [Google Scholar] [CrossRef]




| Forward Primers (5′-3′) | Reverse Primer (5′-3′) | |
|---|---|---|
| Occludin | ACGGACCCTGACCACTATGA | TCAGCAGCAGCCATGTACTC |
| IL-6 | CTCTGGCGGAGCTATTGAGA | AAGTCTCCTGCGTGGAGAAA |
| iNOS | GGGCTGACCTGTTTCCTACT | GGAGGTTGAGACCCAATGGA |
| COX-2 | CCCATTAGCAGCCAGTTGTC | CAGGATGCAGTGCTGAGTTC |
| Claudin-1 | AGCTGCCTGTTCCATGTACT | CTCCCATTTGTCTGCTGCTC |
| MUC2 | AGGGCTCGGAACTCCAGAAA | CCAGGGAATCGGTAGACATCG |
| Gapdh | TGGAGAAACCTGCCAAGTATGA | TGGAAGAATGGGAGTTGCTGT |
| Compound | Matter | Rt (min) | MS[M-H] (m/z) | MS/MS (m/z) |
|---|---|---|---|---|
| 1 | Protocatechuic acid | 7.388 | 153 | 109.03 |
| 2 | B-type procyanidins | 8.286 | 577 | 125.02, 287.05, 289.07, 407.07, 425.08, 451.10 |
| 3 | Protocatechualdehyde | 9.052 | 137 | 119.01, 108.02 |
| 4 | A-type procyanidins dimer | 10.665 | 575 | 285.04, 289.07, 407.07, 449.08 |
| 5 | Catechins | 11.741 | 289 | 125.02, 137.02, 165.02, 179.03, 205.05, 245.08 |
| 6 | A-type procyanidins dimer | 12.279 | 575 | 285.04, 289.07, 407.07, 449.08 |
| 7 | A-type procyanidins trimer | 13.648 | 863 | 287.05, 289.07, 411.07, 451.07, 575.12, 711.13 |
| 8 | A-type procyanidins dimer | 14.110 | 575 | 285.04, 289.07, 407.07, 423.07, 49.08 |
| 9 | A-type procyanidins tetramer | 16.126 | 1149 | 573.10, 575.12, 997.17 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, N.; Chen, W.; Cui, C.; Zheng, Y.; Yu, Q.; Ren, H.; Liu, Z.; Xu, C.; Zhang, G. The Peanut Skin Procyanidins Attenuate DSS-Induced Ulcerative Colitis in C57BL/6 Mice. Antioxidants 2022, 11, 2098. https://doi.org/10.3390/antiox11112098
Wang N, Chen W, Cui C, Zheng Y, Yu Q, Ren H, Liu Z, Xu C, Zhang G. The Peanut Skin Procyanidins Attenuate DSS-Induced Ulcerative Colitis in C57BL/6 Mice. Antioxidants. 2022; 11(11):2098. https://doi.org/10.3390/antiox11112098
Chicago/Turabian StyleWang, Na, Weixuan Chen, Chenxu Cui, Yuru Zheng, Qiuying Yu, Hongtao Ren, Zhigang Liu, Chao Xu, and Gaiping Zhang. 2022. "The Peanut Skin Procyanidins Attenuate DSS-Induced Ulcerative Colitis in C57BL/6 Mice" Antioxidants 11, no. 11: 2098. https://doi.org/10.3390/antiox11112098
APA StyleWang, N., Chen, W., Cui, C., Zheng, Y., Yu, Q., Ren, H., Liu, Z., Xu, C., & Zhang, G. (2022). The Peanut Skin Procyanidins Attenuate DSS-Induced Ulcerative Colitis in C57BL/6 Mice. Antioxidants, 11(11), 2098. https://doi.org/10.3390/antiox11112098







