Novel Active Food Packaging Films Based on Gelatin-Sodium Alginate Containing Beetroot Peel Extract
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Beetroot Peels Extracts (BPE)
2.2. Betalains Quantification and Antibacterial Activity
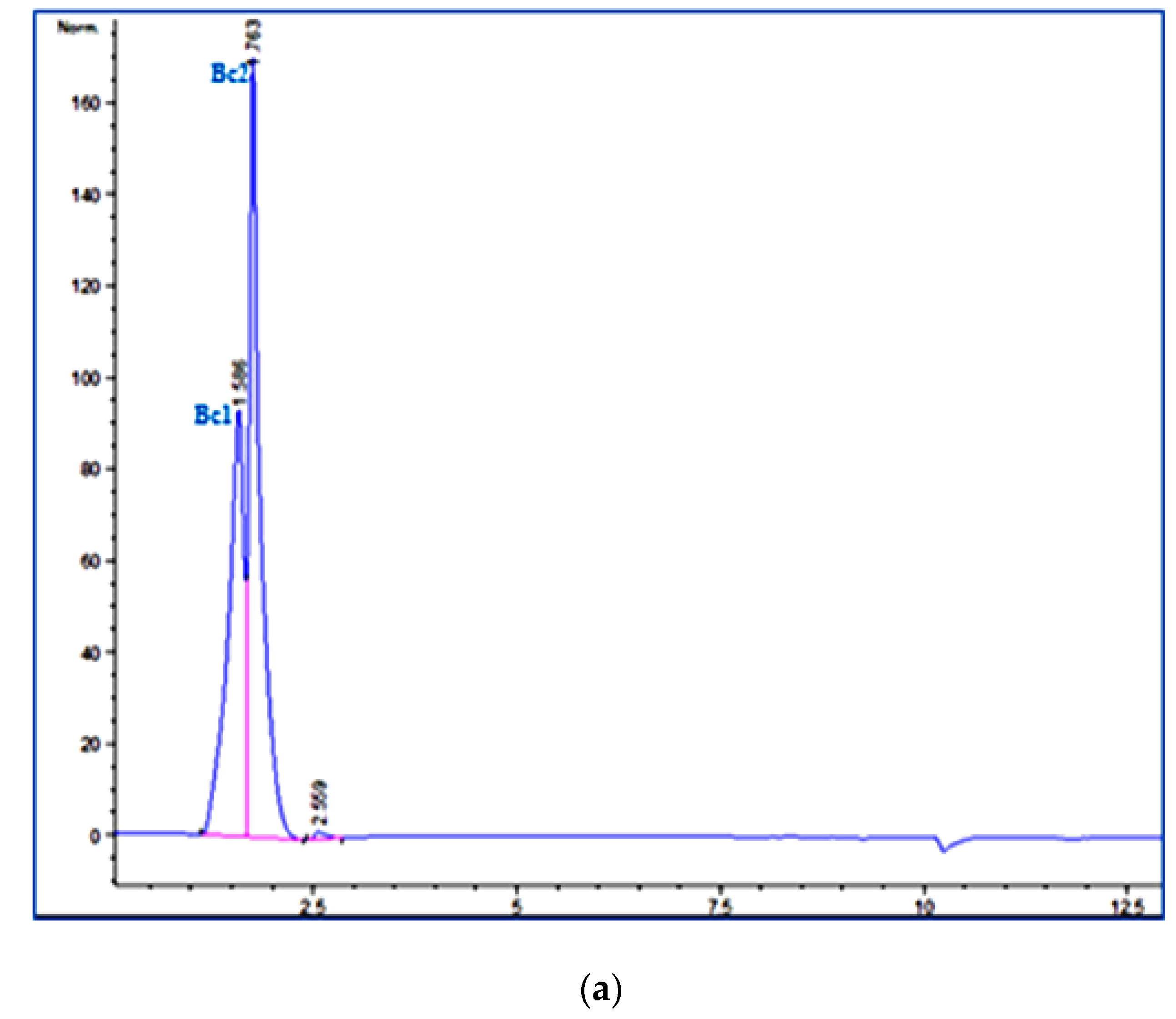
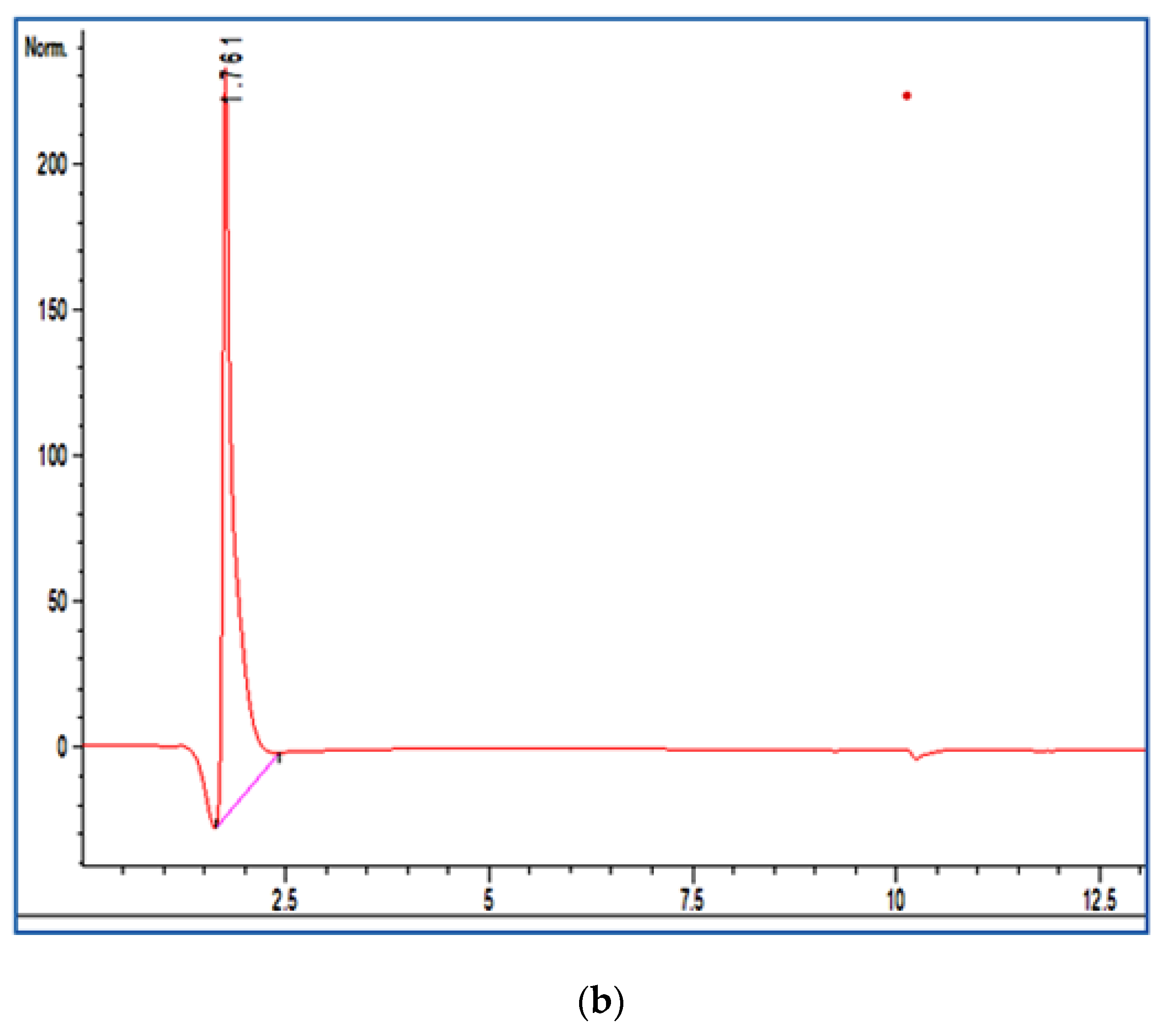
2.3. HPLC Analysis
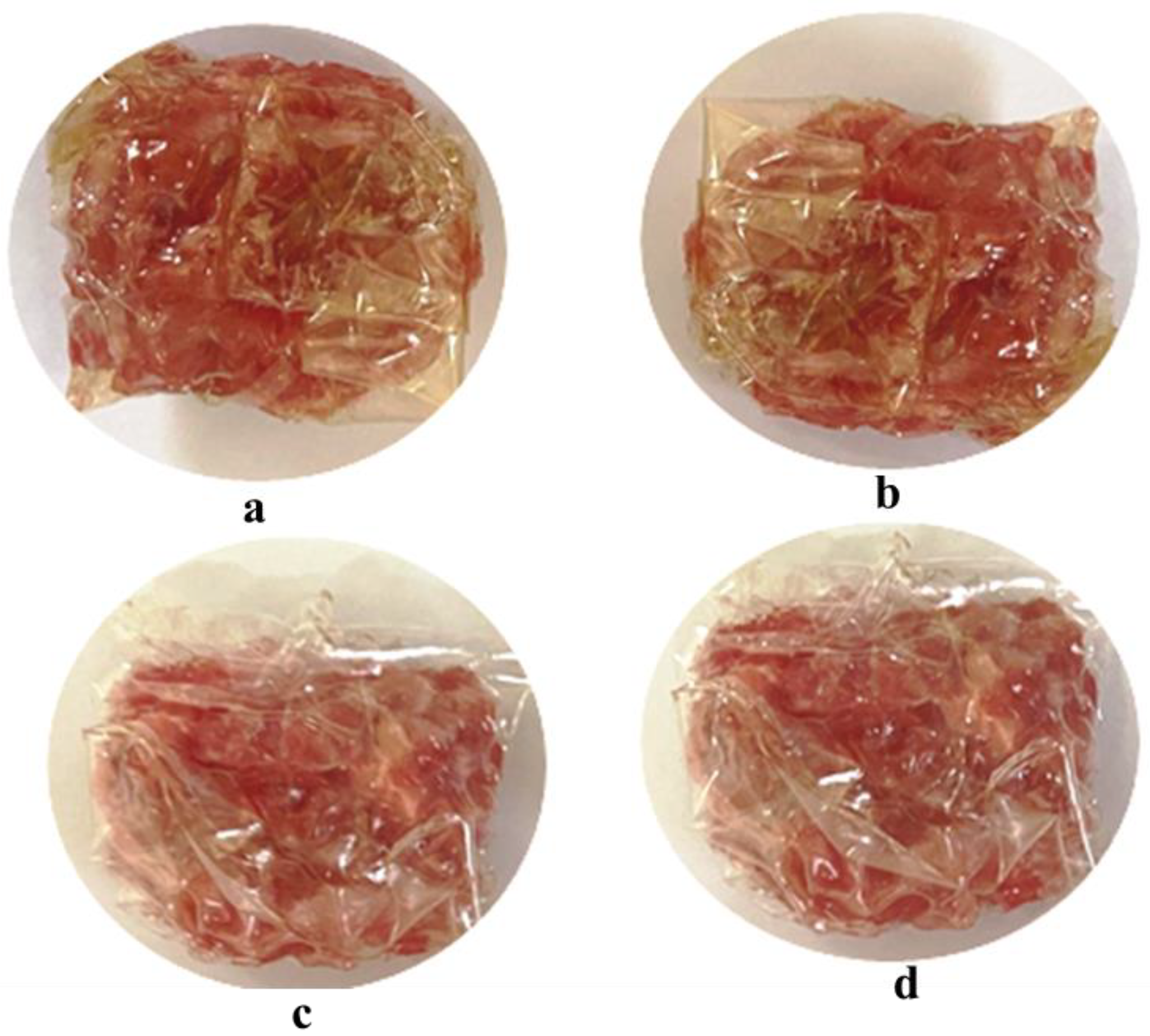
2.4. Preparation of Film
2.5. Film Characterization
2.5.1. Mechanical Properties
2.5.2. Physical Characterization
- -
- Swelling index (SI)
- -
- Water solubility (WS)
- -
- Moisture content (MC)
- -
- Biodegradability
- -
- Film instrumental color evaluation
2.5.3. Biological Characterization
- -
- Antibacterial activity
- -
- Total phenolic content and antioxidant activity
2.6. Analysis of Beef Meat Samples
2.6.1. Microbiological Analysis
2.6.2. Physiochemical Analysis
- -
- pH analysis
- -
- Evaluation of protein and lipid oxidation
2.6.3. Instrumental Color Evaluation and Sensory Evaluation
2.7. Statistical Analysis
3. Results and Discussion
3.1. Betalains Content
3.2. Antibacterial Activity of BPE
3.3. Film Properties
3.3.1. Mechanical Characterization
3.3.2. Physical Characterization
3.3.3. Optical Film’s Characterization
3.3.4. Biological Characterization of Developed Films
- -
- Antibacterial activity
- -
- Total phenolic content (TPC) and antioxidant activity (DPPH)
3.4. Minced Beef Meat Analysis
3.4.1. Microbiological Analysis
3.4.2. Physiochemical Analysis
- -
- pH analysis
- -
- Evaluation of protein oxidation
- -
- Evaluation of lipid oxidation
3.4.3. Instrumental Color
3.4.4. Sensory Evaluation
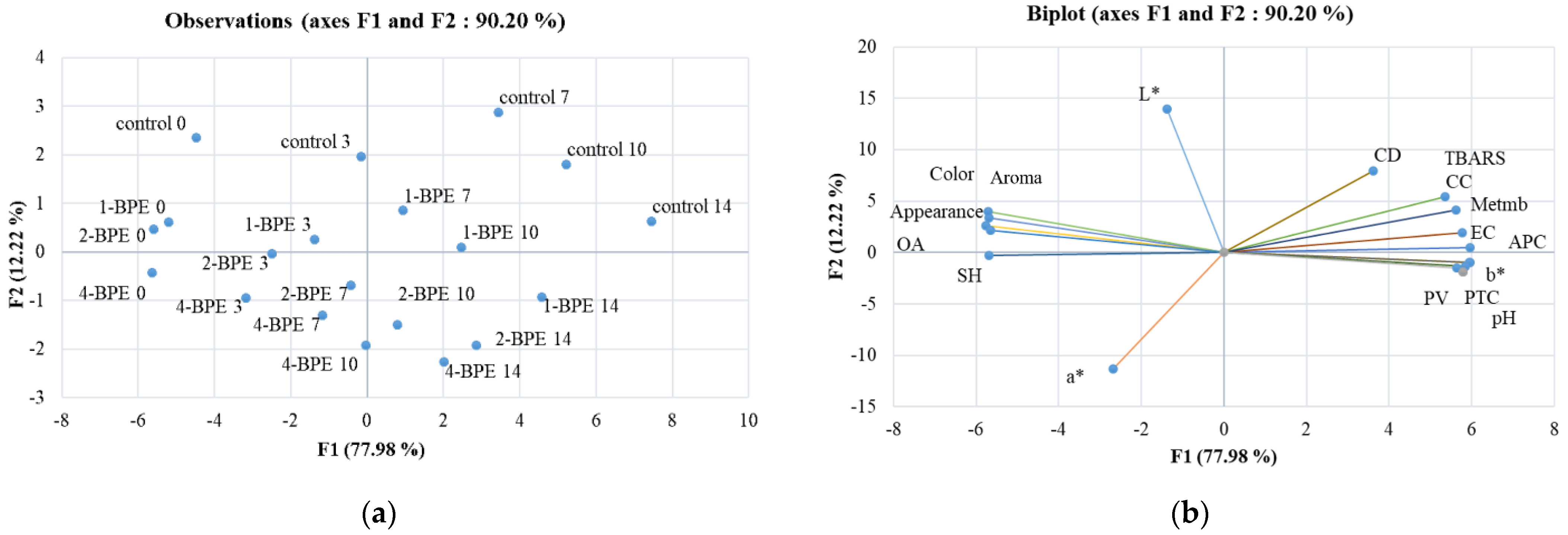
3.5. Chemometric Analysis
- -
- Principal component analysis
- -
- Heat maps
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Smaoui, S.; Ben Hlima, H.; Tavares, L.; Ben Braïek, O.; Ennouri, K.; Abdelkafi, S.; Mellouli, L.; Mousavi Khaneghah, A. Application of Eco-Friendly Active Films and Coatings Based on Natural Antioxidant in Meat Products: A Review. Prog. Org. Coat. 2022, 166, 106780. [Google Scholar] [CrossRef]
- Liu, J.; Yao, X.; Yun, D.; Zhang, M.; Qian, C.; Liu, J. Development of Active Packaging Films Based on Quaternary Ammonium Chitosan, Polyvinyl Alcohol and Litchi (Litchi chinensis Sonn.) Pericarp Extract. Qual. Assur. Saf. Crop. Foods 2021, 13, 9–19. [Google Scholar] [CrossRef]
- La, D.D.; Nguyen-Tri, P.; Le, K.H.; Nguyen, P.T.M.; Nguyen, M.D.-B.; Vo, A.T.K.; Nguyen, M.T.H.; Chang, S.W.; Tran, L.D.; Chung, W.J.; et al. Effects of Antibacterial ZnO Nanoparticles on the Performance of a Chitosan/Gum Arabic Edible Coating for Post-Harvest Banana Preservation. Prog. Org. Coat. 2021, 151, 106057. [Google Scholar] [CrossRef]
- Chamas, A.; Moon, H.; Zheng, J.; Qiu, Y.; Tabassum, T.; Jang, J.H.; Abu-Omar, M.; Scott, S.L.; Suh, S. Degradation Rates of Plastics in the Environment. ACS Sustain. Chem. Eng. 2020, 8, 3494–3511. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Liu, Y.; Chen, W.-Q.; Zhu, B.; Qu, S.; Xu, M. Critical Review of Global Plastics Stock and Flow Data. J. Ind. Ecol. 2021, 25, 1300–1317. [Google Scholar] [CrossRef]
- Taha, T.H.; Abu-Saied, M.A.; Elnouby, M.; Hashem, M.; Alamri, S.; Desouky, E.A.E.; Morsy, K. Profitable Exploitation of Biodegradable Polymer Including Chitosan Blended Potato Peels’ Starch Waste as an Alternative Source of Petroleum Plastics. Biomass Conv. Bioref. 2022. [Google Scholar] [CrossRef]
- Deshmukh, R.K.; Gaikwad, K.K. Natural Antimicrobial and Antioxidant Compounds for Active Food Packaging Applications. Biomass Conv. Bioref. 2022. [Google Scholar] [CrossRef]
- Pietrosanto, A.; Scarfato, P.; Di Maio, L.; Incarnato, L. Development of Eco-Sustainable PBAT-Based Blown Films and Performance Analysis for Food Packaging Applications. Materials 2020, 13, 5395. [Google Scholar] [CrossRef]
- Balart, R.; Montanes, N.; Dominici, F.; Boronat, T.; Torres-Giner, S. Environmentally Friendly Polymers and Polymer Composites. Materials 2020, 13, 4892. [Google Scholar] [CrossRef] [PubMed]
- Kovačević, Z.; Flinčec Grgac, S.; Bischof, S. Progress in Biodegradable Flame Retardant Nano-Biocomposites. Polymers 2021, 13, 741. [Google Scholar] [CrossRef]
- Ramos, M.; Valdés, A.; Beltrán, A.; Garrigós, M.C. Gelatin-Based Films and Coatings for Food Packaging Applications. Coatings 2016, 6, 41. [Google Scholar] [CrossRef] [Green Version]
- Riahi, Z.; Priyadarshi, R.; Rhim, J.-W.; Bagheri, R. Gelatin-Based Functional Films Integrated with Grapefruit Seed Extract and TiO2 for Active Food Packaging Applications. Food Hydrocoll. 2021, 112, 106314. [Google Scholar] [CrossRef]
- Lu, Y.; Luo, Q.; Chu, Y.; Tao, N.; Deng, S.; Wang, L.; Li, L. Application of Gelatin in Food Packaging: A Review. Polymers 2022, 14, 436. [Google Scholar] [CrossRef]
- Liu, F.; Antoniou, J.; Li, Y.; Ma, J.; Zhong, F. Effect of Sodium Acetate and Drying Temperature on Physicochemical and Thermomechanical Properties of Gelatin Films. Food Hydrocoll. 2015, 45, 140–149. [Google Scholar] [CrossRef]
- Boughriba, S.; Souissi, N.; Jridi, M.; Li, S.; Nasri, M. Thermal, Mechanical and Microstructural Characterization and Antioxidant Potential of Rhinobatos Cemiculus Gelatin Films Supplemented by Titanium Dioxide Doped Silver Nanoparticles. Food Hydrocoll. 2020, 103, 105695. [Google Scholar] [CrossRef]
- Haghighi, H.; De Leo, R.; Bedin, E.; Pfeifer, F.; Siesler, H.W.; Pulvirenti, A. Comparative Analysis of Blend and Bilayer Films Based on Chitosan and Gelatin Enriched with LAE (Lauroyl Arginate Ethyl) with Antimicrobial Activity for Food Packaging Applications. Food Packag. Shelf Life 2019, 19, 31–39. [Google Scholar] [CrossRef]
- Ahmad, T.; Ismail, A.; Ahmad, S.A.; Khalil, K.A.; Kumar, Y.; Adeyemi, K.D.; Sazili, A.Q. Recent Advances on the Role of Process Variables Affecting Gelatin Yield and Characteristics with Special Reference to Enzymatic Extraction: A Review. Food Hydrocoll. 2017, 63, 85–96. [Google Scholar] [CrossRef]
- Dou, L.; Li, B.; Zhang, K.; Chu, X.; Hou, H. Physical Properties and Antioxidant Activity of Gelatin-Sodium Alginate Edible Films with Tea Polyphenols. Int. J. Biol. Macromol. 2018, 118, 1377–1383. [Google Scholar] [CrossRef] [PubMed]
- Gheorghita, R.; Gutt, G.; Amariei, S. The Use of Edible Films Based on Sodium Alginate in Meat Product Packaging: An Eco-Friendly Alternative to Conventional Plastic Materials. Coatings 2020, 10, 166. [Google Scholar] [CrossRef] [Green Version]
- Purcell-Meyerink, D.; Packer, M.A.; Wheeler, T.T.; Hayes, M. Aquaculture Production of the Brown Seaweeds Laminaria Digitata and Macrocystis Pyrifera: Applications in Food and Pharmaceuticals. Molecules 2021, 26, 1306. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Mascaraque, L.G.; Martínez-Sanz, M.; Martínez-López, R.; Martínez-Abad, A.; Panikuttira, B.; López-Rubio, A.; Tuohy, M.G.; Hogan, S.A.; Brodkorb, A. Characterization and Gelling Properties of a Bioactive Extract from Ascophyllum Nodosum Obtained Using a Chemical-Free Approach. Curr. Res. Food Sci. 2021, 4, 354–364. [Google Scholar] [CrossRef] [PubMed]
- Senturk Parreidt, T.; Müller, K.; Schmid, M. Alginate-Based Edible Films and Coatings for Food Packaging Applications. Foods 2018, 7, 170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdel Aziz, M.S.; Salama, H.E. Developing Multifunctional Edible Coatings Based on Alginate for Active Food Packaging. Int. J. Biol. Macromol. 2021, 190, 837–844. [Google Scholar] [CrossRef] [PubMed]
- Alves, Z.; Ferreira, N.M.; Mendo, S.; Ferreira, P.; Nunes, C. Design of Alginate-Based Bionanocomposites with Electrical Conductivity for Active Food Packaging. Int. J. Mol. Sci. 2021, 22, 9943. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Yuan, L.; Han, L.; Li, S.; Song, L. Characterization of Antioxidant and Antibacterial Gelatin Films Incorporated with Ginkgo Biloba Extract. RSC Adv. 2019, 9, 27449–27454. [Google Scholar] [CrossRef] [Green Version]
- Kanatt, S.R. Development of Active/Intelligent Food Packaging Film Containing Amaranthus Leaf Extract for Shelf Life Extension of Chicken/Fish during Chilled Storage. Food Packag. Shelf Life 2020, 24, 100506. [Google Scholar] [CrossRef]
- Ho, T.C.; Kim, M.H.; Cho, Y.-J.; Park, J.-S.; Nam, S.Y.; Chun, B.-S. Gelatin-Sodium Alginate Based Films with Pseuderanthemum Palatiferum (Nees) Radlk. Freeze-Dried Powder Obtained by Subcritical Water Extraction. Food Packag. Shelf Life 2020, 24, 100469. [Google Scholar] [CrossRef]
- Kazemi, S.M.; Rezaei, M. Antimicrobial Effectiveness of Gelatin–Alginate Film Containing Oregano Essential Oil for Fish Preservation. J. Food Saf. 2015, 35, 482–490. [Google Scholar] [CrossRef]
- Syarifuddin, A.; Hasmiyani; Dirpan, A.; Mahendradatta, M. Physical, Mechanical, and Barrier Properties of Sodium Alginate/Gelatin Emulsion Based-Films Incorporated with Canola Oil. IOP Conf. Ser. Earth Environ. Sci. 2017, 101, 012019. [Google Scholar] [CrossRef]
- Elhadef, K.; Smaoui, S.; Ben Hlima, H.; Ennouri, K.; Fourati, M.; Chakchouk Mtibaa, A.; Ennouri, M.; Mellouli, L. Effects of Ephedra Alata Extract on the Quality of Minced Beef Meat during Refrigerated Storage: A Chemometric Approach. Meat Sci. 2020, 170, 108246. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.-R.; Zhang, Y.-Q.; Wang, S.; Wang, W.-D.; Yu, N.-N.; Gong, H.; Ni, Z.-Z. Optimization of Functional Compounds Extraction from Ginkgo Biloba Seeds Using Response Surface Methodology. Qual. Assur. Saf. Crop. Foods 2022, 14, 102–112. [Google Scholar] [CrossRef]
- Tayebeh, B.; Soraya, K.; Khaneghah, A.M. Antioxidant and Antibacterial Activity of Ethanolic Extract of Safflower with Contrasting Seed Coat Colors. Qual. Assur. Saf. Crop. Foods 2021, 13, 94–100. [Google Scholar] [CrossRef]
- Torres Vargas, O.L.; Galeano Loaiza, Y.V.; González, M.L. Effect of Incorporating Extracts from Natural Pigments in Alginate/Starch Films. J. Mater. Res. Technol. 2021, 13, 2239–2250. [Google Scholar] [CrossRef]
- Xie, Q.; Liu, G.; Zhang, Y.; Yu, J.; Wang, Y.; Ma, X. Active Edible Films with Plant Extracts: A Updated Review of Their Types, Preparations, Reinforcing Properties, and Applications in Muscle Foods Packaging and Preservation. Crit. Rev. Food Sci. Nutr. 2022, 1–23. [Google Scholar] [CrossRef]
- Tan, Y.M.; Lim, S.H.; Tay, B.Y.; Lee, M.W.; Thian, E.S. Functional Chitosan-Based Grapefruit Seed Extract Composite Films for Applications in Food Packaging Technology. Mater. Res. Bull. 2015, 69, 142–146. [Google Scholar] [CrossRef]
- Kadam, D.; Shah, N.; Palamthodi, S.; Lele, S.S. An Investigation on the Effect of Polyphenolic Extracts of Nigella Sativa Seedcake on Physicochemical Properties of Chitosan-Based Films. Carbohydr. Polym. 2018, 192, 347–355. [Google Scholar] [CrossRef]
- de Jesus Cenobio-Galindo, A.; Pimentel-González, D.J.; Del Razo-Rodríguez, O.E.; Medina-Pérez, G.; Carrillo-Inungaray, M.L.; Reyes-Munguía, A.; Campos-Montiel, R.G. Antioxidant and Antibacterial Activities of a Starch Film with Bioextracts Microencapsulated from Cactus Fruits (Opuntia oligacantha). Food Sci. Biotechnol. 2019, 28, 1553–1561. [Google Scholar] [CrossRef] [PubMed]
- Nirmal, N.P.; Mereddy, R.; Maqsood, S. Recent Developments in Emerging Technologies for Beetroot Pigment Extraction and Its Food Applications. Food Chem. 2021, 356, 129611. [Google Scholar] [CrossRef] [PubMed]
- Aykın-Dinçer, E.; Güngör, K.K.; Çağlar, E.; Erbaş, M. The Use of Beetroot Extract and Extract Powder in Sausages as Natural Food Colorant. Int. J. Food Eng. 2021, 17, 75–82. [Google Scholar] [CrossRef]
- Chaari, M.; Akermi, S.; Elhadef, K.; Ennouri, K.; Hlima, H.B.; Fourati, M.; Chakchouk-Mtibaa, A.; Sarka, T.; Shariati, M.A.; Mellouli, L.; et al. From Modeling and Optimizing Extraction of Peels Beetroot (Beta vulgaris L.) Betalains to in Silico Probing of Their Antibacterial Multitarget Mechanisms. Biomass Conv. Bioref. 2022. [Google Scholar] [CrossRef]
- Zin, M.M.; Bánvölgyi, S. Emerging Technology Approach for Extractability and Stability of Betalains from the Peel of Beetroot (Beta vulgaris L.). Biomass Conv. Bioref. 2021. [Google Scholar] [CrossRef]
- Song, D.-H.; Hoa, V.B.; Kim, H.W.; Khang, S.M.; Cho, S.-H.; Ham, J.-S.; Seol, K.-H. Edible Films on Meat and Meat Products. Coatings 2021, 11, 1344. [Google Scholar] [CrossRef]
- Guo, Z.; Ge, X.; Li, W.; Yang, L.; Han, L.; Yu, Q. Active-Intelligent Film Based on Pectin from Watermelon Peel Containing Beetroot Extract to Monitor the Freshness of Packaged Chilled Beef. Food Hydrocoll. 2021, 119, 106751. [Google Scholar] [CrossRef]
- Mojaddar Langroodi, A.; Nematollahi, A.; Sayadi, M. Chitosan Coating Incorporated with Grape Seed Extract and Origanum Vulgare Essential Oil: An Active Packaging for Turkey Meat Preservation. Food Meas. 2021, 15, 2790–2804. [Google Scholar] [CrossRef]
- Contini, C.; Álvarez, R.; O’Sullivan, M.; Dowling, D.P.; Gargan, S.Ó.; Monahan, F.J. Effect of an Active Packaging with Citrus Extract on Lipid Oxidation and Sensory Quality of Cooked Turkey Meat. Meat Sci. 2014, 96, 1171–1176. [Google Scholar] [CrossRef] [PubMed]
- Marrone, R.; Smaldone, G.; Ambrosio, R.L.; Festa, R.; Ceruso, M.; Chianese, A.; Anastasio, A. Effect of Beetroot (Beta vulgaris) Extract on Black Angus Burgers Shelf Life. Ital. J. Food Saf. 2021, 10, 9031. [Google Scholar] [CrossRef] [PubMed]
- Righi Pessoa da Silva, H.; da Silva, C.; Bolanho, B.C. Ultrasonic-Assisted Extraction of Betalains from Red Beet (Beta vulgaris L.). J. Food Process Eng. 2018, 41, e12833. [Google Scholar] [CrossRef]
- Fourati, M.; Smaoui, S.; Ennouri, K.; Ben Hlima, H.; Elhadef, K.; Chakchouk-Mtibaa, A.; Sellem, I.; Mellouli, L. Multiresponse Optimization of Pomegranate Peel Extraction by Statistical versus Artificial Intelligence: Predictive Approach for Foodborne Bacterial Pathogen Inactivation. Evid.-Based Complement. Altern. Med. 2019, 2019, 1542615. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daei, S.; Mohtarami, F.; Pirsa, S. A Biodegradable Film Based on Carrageenan Gum/Plantago Psyllium Mucilage/Red Beet Extract: Physicochemical Properties, Biodegradability and Water Absorption Kinetic. Polym. Bull. 2022. [Google Scholar] [CrossRef]
- Nouraddini, M.; Esmaiili, M.; Mohtarami, F. Development and Characterization of Edible Films Based on Eggplant Flour and Corn Starch. Int. J. Biol. Macromol. 2018, 120, 1639–1645. [Google Scholar] [CrossRef] [PubMed]
- Zahedi, Y.; Fathi-Achachlouei, B.; Yousefi, A.R. Physical and Mechanical Properties of Hybrid Montmorillonite/Zinc Oxide Reinforced Carboxymethyl Cellulose Nanocomposites. Int. J. Biol. Macromol. 2018, 108, 863–873. [Google Scholar] [CrossRef] [PubMed]
- Piñeros-Hernandez, D.; Medina-Jaramillo, C.; López-Córdoba, A.; Goyanes, S. Edible Cassava Starch Films Carrying Rosemary Antioxidant Extracts for Potential Use as Active Food Packaging. Food Hydrocoll. 2017, 63, 488–495. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, Y.; Yong, H.; Qin, Y.; Liu, J.; Liu, J. Development of Multifunctional Food Packaging Films Based on Chitosan, TiO2 Nanoparticles and Anthocyanin-Rich Black Plum Peel Extract. Food Hydrocoll. 2019, 94, 80–92. [Google Scholar] [CrossRef]
- Liang, S.; Wang, L. A Natural Antibacterial-Antioxidant Film from Soy Protein Isolate Incorporated with Cortex Phellodendron Extract. Polymers 2018, 10, 71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parveen, S.; Chaudhury, P.; Dasmahapatra, U.; Dasgupta, S. Biodegradable Protein Films from Gallic Acid and the Cataractous Eye Protein Isolate. Int. J. Biol. Macromol. 2019, 139, 12–20. [Google Scholar] [CrossRef] [PubMed]
- ISO 4833-1:2013; Microbiology of the Food Chain—Horizontal Method for the Enumeration of Microorganisms. Part 1: Colony Count at 30 °C by the Pour Plate Technique. International Organization for Standardization: Geneva, Switzerland, 2013.
- ISO 17410:2001; Microbiology of Food and Animal Feeding Stuffs-Horizontal Method for the Enumeration of Psychrotrophic Microorganisms. International Organization for Standardization: Geneva, Switzerland, 2001.
- ISO 21528-2:2004; Microbiology of Food and Animal Feeding Stuffs—Horizontal Methods for the Detection and Enumeration of Enterobacteriaceae. International Organization for Standardization: Geneva, Switzerland, 2004.
- Smaoui, S.; Hsouna, A.B.; Lahmar, A.; Ennouri, K.; Mtibaa-Chakchouk, A.; Sellem, I.; Najah, S.; Bouaziz, M.; Mellouli, L. Bio-Preservative Effect of the Essential Oil of the Endemic Mentha Piperita Used Alone and in Combination with BacTN635 in Stored Minced Beef Meat. Meat Sci. 2016, 117, 196–204. [Google Scholar] [CrossRef]
- Mtibaa, A.C.; Smaoui, S.; Ben Hlima, H.; Sellem, I.; Ennouri, K.; Mellouli, L. Enterocin BacFL31 from a Safety Enterococcus faecium FL31: Natural Preservative Agent Used Alone and in Combination with Aqueous Peel Onion (Allium cepa) Extract in Ground Beef Meat Storage. BioMed Res. Int. 2019, 2019, 4094890. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.; He, Z.; Gan, X.; Li, H. Interrelationship among Ferrous Myoglobin, Lipid and Protein Oxidations in Rabbit Meat during Refrigerated and Superchilled Storage. Meat Sci. 2018, 146, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Eymard, S.; Carcouët, E.; Rochet, M.-J.; Dumay, J.; Chopin, C.; Genot, C. Development of Lipid Oxidation during Manufacturing of Horse Mackerel Surimi. J. Sci. Food Agric. 2005, 85, 1750–1756. [Google Scholar] [CrossRef] [Green Version]
- Sucu, C.; Turp, G.Y. The Investigation of the Use of Beetroot Powder in Turkish Fermented Beef Sausage (sucuk) as Nitrite Alternative. Meat Sci. 2018, 140, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Vieira Teixeira da Silva, D.; dos Santos Baião, D.; de Oliveira Silva, F.; Alves, G.; Perrone, D.; Mere Del Aguila, E.; Paschoalin, V.M.F. Betanin, a Natural Food Additive: Stability, Bioavailability, Antioxidant and Preservative Ability Assessments. Molecules 2019, 24, 458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skalicky, M.; Kubes, J.; Shokoofeh, H.; Tahjib-Ul-Arif, M.; Vachova, P.; Hejnak, V. Betacyanins and Betaxanthins in Cultivated Varieties of Beta vulgaris L. Compared to Weed Beets. Molecules 2020, 25, 5395. [Google Scholar] [CrossRef] [PubMed]
- Maqbool, H.; Visnuvinayagam, S.; Zynudheen, A.A.; Safeena, M.P.; Kumar, S. Antibacterial Activity of Beetroot Peel and Whole Radish Extract by Modified Well Diffusion Assay. Int. J. Curr. Microbiol. App. Sci. 2020, 9, 1222–1231. [Google Scholar] [CrossRef]
- Pranoto, Y.; Salokhe, V.M.; Rakshit, S.K. Physical and Antibacte Rial Properties of Alginate-Based Edible Film Incorporated with Garlic Oil. Food Res. Int. 2005, 38, 267–272. [Google Scholar] [CrossRef]
- Aloui, H.; Deshmukh, A.R.; Khomlaem, C.; Kim, B.S. Novel Composite Films Based on Sodium Alginate and Gallnut Extract with Enhanced Antioxidant, Antimicrobial, Barrier and Mechanical Properties. Food Hydrocoll. 2021, 113, 106508. [Google Scholar] [CrossRef]
- Bitencourt, C.M.; Fávaro-Trindade, C.S.; Sobral, P.J.A.; Carvalho, R.A. Gelatin-Based Films Additivated with Curcuma Ethanol Extract: Antioxidant Activity and Physical Properties of Films. Food Hydrocoll. 2014, 40, 145–152. [Google Scholar] [CrossRef]
- Thakur, R.; Pristijono, P.; Scarlett, C.J.; Bowyer, M.; Singh, S.P.; Vuong, Q.V. Starch-Based Films: Major Factors Affecting Their Properties. Int. J. Biol. Macromol. 2019, 132, 1079–1089. [Google Scholar] [CrossRef] [PubMed]
- Koosha, M.; Hamedi, S. Intelligent Chitosan/PVA Nanocomposite Films Containing Black Carrot Anthocyanin and Bentonite Nanoclays with Improved Mechanical, Thermal and Antibacterial Properties. Prog. Org. Coat. 2019, 127, 338–347. [Google Scholar] [CrossRef]
- Chen, W.; Ma, S.; Wang, Q.; McClements, D.J.; Liu, X.; Ngai, T.; Liu, F. Fortification of Edible Films with Bioactive Agents: A Review of Their Formation, Properties, and Application in Food Preservation. Crit. Rev. Food Sci. Nutr. 2022, 62, 5029–5055. [Google Scholar] [CrossRef]
- Aydin, G. Characterisation and Antibacterial Properties of Novel Biodegradable Films Based on Alginate and Roselle (Hibiscus sabdariffa L.) Extract. Waste Biomass Valorization 2022, 13, 2991–3002. [Google Scholar] [CrossRef]
- Luo, Y.; Liu, H.; Yang, S.; Zeng, J.; Wu, Z. Sodium Alginate-Based Green Packaging Films Functionalized by Guava Leaf Extracts and Their Bioactivities. Materials 2019, 12, 2923. [Google Scholar] [CrossRef] [Green Version]
- de Alvarenga Pinto Cotrim, M.; Mottin, A.C.; Ayres, E. Preparation and Characterization of Okra Mucilage (Abelmoschus esculentus) Edible Films. Macromol. Symp. 2016, 367, 90–100. [Google Scholar] [CrossRef]
- Chen, Y.; Lu, W.; Guo, Y.; Zhu, Y.; Lu, H.; Wu, Y. Superhydrophobic Coatings on Gelatin-Based Films: Fabrication, Characterization and Cytotoxicity Studies. RSC Adv. 2018, 8, 23712–23719. [Google Scholar] [CrossRef] [Green Version]
- Coy-Barrera, E. Analysis of Betalains (Betacyanins and Betaxanthins). In Recent Advances in Natural Products Analysis; Elsevier: Amsterdam, The Netherlands, 2020; pp. 593–619. ISBN 978-0-12-816455-6. [Google Scholar]
- Martucci, J.F.; Ruseckaite, R.A. Biodegradation Behavior of Three-Layer Sheets Based on Gelatin and Poly (Lactic Acid) Buried under Indoor Soil Conditions. Polym. Degrad. Stab. 2015, 116, 36–44. [Google Scholar] [CrossRef]
- Cejudo-Bastante, M.J.; Cejudo-Bastante, C.; Cran, M.J.; Heredia, F.J.; Bigger, S.W. Optical, Structural, Mechanical and Thermal Characterization of Antioxidant Ethylene Vinyl Alcohol Copolymer Films Containing Betalain-Rich Beetroot. Food Packag. Shelf Life 2020, 24, 100502. [Google Scholar] [CrossRef]
- Otálora González, C.M.; De’Nobili, M.D.; Rojas, A.M.; Basanta, M.F.; Gerschenson, L.N. Development of Functional Pectin Edible Films with Fillers Obtained from Red Cabbage and Beetroot. Int. J. Food Sci. Technol. 2021, 56, 3662–3669. [Google Scholar] [CrossRef]
- Jridi, M.; Mora, L.; Souissi, N.; Aristoy, M.-C.; Nasri, M.; Toldrá, F. Effects of Active Gelatin Coated with Henna (L. inermis) Extract on Beef Meat Quality during Chilled Storage. Food Control 2018, 84, 238–245. [Google Scholar] [CrossRef]
- Afnor, N. Hygiene and Safety Foods-Validation of the Microbiological Shelf Life-Perishable and Cooled Foods; AFNOR: La Plaine-saint-denis, France, 2004. [Google Scholar]
- Lozano-Navarro, J.I.; Díaz-Zavala, N.P.; Velasco-Santos, C.; Martínez-Hernández, A.L.; Tijerina-Ramos, B.I.; García-Hernández, M.; Rivera-Armenta, J.L.; Páramo-García, U.; Reyes-de la Torre, A.I. Antimicrobial, Optical and Mechanical Properties of Chitosan–Starch Films with Natural Extracts. Int. J. Mol. Sci. 2017, 18, 997. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, V.; Kumar, A. Betalain. In Nutraceuticals and Health Care; Elsevier: Amsterdam, The Netherlands, 2022; pp. 87–104. ISBN 978-0-323-89779-2. [Google Scholar]
- Čanadanović-Brunet, J.M.; Savatović, S.S.; Ćetković, G.S.; Vulić, J.J.; Djilas, S.M.; Markov, S.L.; Cvetković, D.D. Antioxidant and Antimicrobial Activities of Beet Root Pomace Extracts. Czech J. Food Sci. 2011, 29, 575–585. [Google Scholar] [CrossRef] [Green Version]
- Rawdkuen, S. Edible Films Incorporated with Active Compounds: Their Properties and Application. In Active Antimicrobial Food Packaging; Isıl, V., Uzunlu, S., Eds.; IntechOpen: London, UK, 2019; pp. 71–85. [Google Scholar]
- Berizi, E.; Hosseinzadeh, S.; Shekarforoush, S.S.; Barbieri, G. Microbial, Chemical, Textural and Sensory Properties of Coated Rainbow Trout by Chitosan Combined with Pomegranate Peel Extract during Frozen Storage. Int. J. Biol. Macromol. 2018, 106, 1004–1013. [Google Scholar] [CrossRef]
- Xiong, Y.; Chen, M.; Warner, R.D.; Fang, Z. Incorporating Nisin and Grape Seed Extract in Chitosan-Gelatine Edible Coating and Its Effect on Cold Storage of Fresh Pork. Food Control 2020, 110, 107018. [Google Scholar] [CrossRef]
- Guan, W.; Ren, X.; Li, Y.; Mao, L. The Beneficial Effects of Grape Seed, Sage and Oregano Extracts on the Quality and Volatile Flavor Component of Hairtail Fish Balls during Cold Storage at 4 °C. LWT 2019, 101, 25–31. [Google Scholar] [CrossRef]
- Rashidaie Abandansarie, S.S.; Ariaii, P.; Charmchian Langerodi, M. Effects of Encapsulated Rosemary Extract on Oxidative and Microbiological Stability of Beef Meat during Refrigerated Storage. Food Sci. Nutr. 2019, 7, 3969–3978. [Google Scholar] [CrossRef]
- Guo, Z.; Han, L.; Yu, Q.; Lin, L. Effect of a Sea Buckthorn Pomace Extract-Esterified Potato Starch Film on the Quality and Spoilage Bacteria of Beef Jerky Sold in Supermarket. Food Chem. 2020, 326, 127001. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, R.H.; Ida, E.I.; Madruga, M.S.; Martínez, S.L.; Shimokomaki, M.; Estévez, M. Underlying Connections between the Redox System Imbalance, Protein Oxidation and Impaired Quality Traits in Pale, Soft and Exudative (PSE) Poultry Meat. Food Chem. 2017, 215, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Mohan, C.C.; Harini, K.; Sudharsan, K.; Krishnan, K.R.; Sukumar, M. Quorum Quenching Effect and Kinetics of Active Compound from S. Aromaticum and C. Cassia Fused Packaging Films in Shelf Life of Chicken Meat. LWT 2019, 105, 87–102. [Google Scholar] [CrossRef]
- Chauhan, P.; Pradhan, S.R.; Das, A.; Nanda, P.K.; Bandyopadhyay, N.; Das, A.K. Inhibition of Lipid and Protein Oxidation in Raw Ground Pork by Terminalia Arjuna Fruit Extract during Refrigerated Storage. Asian-Australas. J. Anim. Sci. 2019, 32, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Gandemer, G. Lipids in Muscles and Adipose Tissues, Changes during Processing and Sensory Properties of Meat Products. Meat Sci. 2002, 62, 309–321. [Google Scholar] [CrossRef]
- Domínguez, R.; Pateiro, M.; Gagaoua, M.; Barba, F.J.; Zhang, W.; Lorenzo, J.M. A Comprehensive Review on Lipid Oxidation in Meat and Meat Products. Antioxidants 2019, 8, 429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sallam, K.I.; Ishioroshi, M.; Samejima, K. Antioxidant and Antimicrobial Effects of Garlic in Chicken Sausage. LWT-Food Sci. Technol. 2004, 37, 849–855. [Google Scholar] [CrossRef] [PubMed]
- Maqsood, S.; Abushelaibi, A.; Manheem, K.; Al Rashedi, A.; Kadim, I.T. Lipid Oxidation, Protein Degradation, Microbial and Sensorial Quality of Camel Meat as Influenced by Phenolic Compounds. LWT-Food Sci. Technol. 2015, 63, 953–959. [Google Scholar] [CrossRef]
- da Nóbrega Santos, E.; Cesar de Albuquerque Sousa, T.; Cassiano de Santana Neto, D.; Brandão Grisi, C.V.; Cardoso da Silva Ferreira, V.; Pereira da Silva, F.A. Edible Active Film Based on Gelatin and Malpighia Emarginata Waste Extract to Inhibit Lipid and Protein Oxidation in Beef Patties. LWT 2022, 154, 112837. [Google Scholar] [CrossRef]
- Sun, X.; Guo, X.; Ji, M.; Wu, J.; Zhu, W.; Wang, J.; Cheng, C.; Chen, L.; Zhang, Q. Preservative Effects of Fish Gelatin Coating Enriched with CUR/ΒCD Emulsion on Grass Carp (Ctenopharyngodon idellus) Fillets during Storage at 4 °C. Food Chem. 2019, 272, 643–652. [Google Scholar] [CrossRef]
- Campo, M.M.; Nute, G.R.; Hughes, S.I.; Enser, M.; Wood, J.D.; Richardson, R.I. Flavour Perception of Oxidation in Beef. Meat Sci. 2006, 72, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Liu, Y.; Wang, H.; Liu, W.; Cheong, K.; Teng, B. Effect of Sodium Alginate-Agar Coating Containing Ginger Essential Oil on the Shelf Life and Quality of Beef. Food Control 2021, 130, 108216. [Google Scholar] [CrossRef]
- Wang, B.; Li, F.; Pan, N.; Kong, B.; Xia, X. Effect of Ice Structuring Protein on the Quality of Quick-Frozen Patties Subjected to Multiple Freeze-Thaw Cycles. Meat Sci. 2021, 172, 108335. [Google Scholar] [CrossRef]
- Estévez, M. Critical Overview of the Use of Plant Antioxidants in the Meat Industry: Opportunities, Innovative Applications and Future Perspectives. Meat Sci. 2021, 181, 108610. [Google Scholar] [CrossRef] [PubMed]
- Smaoui, S.; Ennouri, K.; Chakchouk-Mtibaa, A.; Karray-Rebai, I.; Hmidi, M.; Bouchaala, K.; Mellouli, L. Relationships Between Textural Modifications, Lipid and Protein Oxidation and Sensory Attributes of Refrigerated Turkey Meat Sausage Treated with Bacteriocin BacTN635. Food Bioprocess Technol. 2017, 10, 1655–1667. [Google Scholar] [CrossRef]
- Fourati, M.; Smaoui, S.; Ben Hlima, H.; Ennouri, K.; Chakchouk Mtibaa, A.; Sellem, I.; Elhadef, K.; Mellouli, L. Synchronised Interrelationship between Lipid/Protein Oxidation Analysis and Sensory Attributes in Refrigerated Minced Beef Meat Formulated with Punica Granatum Peel Extract. Int. J. Food Sci. Technol. 2020, 55, 1080–1087. [Google Scholar] [CrossRef]
- Zhao, B.; Zhou, H.; Zhang, S.; Pan, X.; Li, S.; Zhu, N.; Wu, Q.; Wang, S.; Qiao, X.; Chen, W. Changes of Protein Oxidation, Lipid Oxidation and Lipolysis in Chinese Dry Sausage with Different Sodium Chloride Curing Salt Content. Food Sci. Hum. Wellness 2020, 9, 328–337. [Google Scholar] [CrossRef]
- Nishad, J.; Koley, T.K.; Varghese, E.; Kaur, C. Synergistic Effects of Nutmeg and Citrus Peel Extracts in Imparting Oxidative Stability in Meat Balls. Food Res. Int. 2018, 106, 1026–1036. [Google Scholar] [CrossRef] [PubMed]
- Turcu, R.P.; Panaite, T.D.; Untea, A.E.; Vlaicu, P.A.; Badea, I.A.; Mironeasa, S. Effects of Grape Seed Oil Supplementation to Broilers Diets on Growth Performance, Meat Fatty Acids, Health Lipid Indices and Lipid Oxidation Parameters. Agriculture 2021, 11, 404. [Google Scholar] [CrossRef]





| Sample | EB (%) | TS (MPa) |
|---|---|---|
| Control | 61.21 ± 2.75 a | 14.11 ± 0.69 a |
| 1-BPE | 66.10 ± 3.31 a | 23.20 ± 1.14 b |
| 2-BPE | 70.10 ± 3.51 a | 25.03 ± 1.23 b |
| 4-BPE | 91.10 ± 4.56 c | 36.43 ± 1.79 c |
| Samples | MC (%) | WS (%) | SI (%) |
|---|---|---|---|
| Control | 19 ± 0.99 c | 100.00 ± 4.80 d | 88.11 ± 4.67 d |
| 1-BPE | 18 ± 0.94 bc | 55.70 ± 2.73 c | 67.53 ± 3.58 c |
| 2-BPE | 16 ± 0.83 b | 40.80 ± 2.00 b | 60.86 ± 3.23 b |
| 4-BPE | 13 ± 0.68 a | 32.62 ± 1.60 a | 56.06 ± 2.97 a |
| Samples | L* | a* | b* |
|---|---|---|---|
| Control | 47.920 ± 2.16 c | 40.780 ± 0.03 a | −0.80 ± 0.04 a |
| 1-BPE | 44.327 ± 1.99 b | 43.537 ± 1.87 b | 1.47 ± 0.07 b |
| 2-BPE | 46.757 ± 2.10 bc | 44.157 ± 1.90 bc | 2.23 ± 0.10 c |
| 4-BPE | 42.350 ± 1.91 a | 45.052 ± 1.94 c | 3.60 ± 0.17 d |
| Antibacterial Activity (mm) | ||||
|---|---|---|---|---|
| Sample | Anti-S. aureus | Anti-L. monocytogenes | Anti-S. enterica | Anti-E. coli |
| Control | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a |
| 1-BPE | 15 ± 0.675 b | 18 ± 0.756 b | 19 ± 0.817 b | 16 ± 0.80 b |
| 2-BPE | 16 ± 0.720 bc | 19 ± 0.798 bc | 21 ± 0.903 c | 21 ± 1.05 c |
| 4-BPE | 18 ± 0.81 c | 20 ± 0.840 c | 25 ± 1.07 d | 23 ± 1.15 d |
| Samples | TPC (mg GAE/mL) | DPPH (%) |
|---|---|---|
| 1-BPE | 21.85 ± 0.98 a | 30.32 ± 1.42 c |
| 2-BPE | 22.81 ± 1.03 a | 46.18 ± 2.17 b |
| 4-BPE | 23.00 ± 1.04 a | 70.68 ± 3.32 a |
| Days of Storage | ||||||
|---|---|---|---|---|---|---|
| Sample | 0 | 3 | 7 | 10 | 14 | |
| APC | Control | 2.08 ± 0.094 aA | 4.99 ± 0.224 bB | 6.789 ± 0.312 cC | 7.810 ± 0.366 dD | 8.990 ± 0.431 eE |
| 1-BPE | 2.05 ± 0.092 aA | 4.68 ± 0.210 bB | 5.432 ± 0.249 cC | 6.018 ± 0.282 dD | 7.242 ± 0.362 eE | |
| 2-BPE | 2.02 ± 0.091 aA | 4.38 ± 0.197 bB | 5.221 ± 0.240 cC | 5.520 ± 0.270 dD | 6.530 ± 0.326 dD | |
| 4-BPE | 2.01 ± 0.09 aA | 4.00 ± 0.180 bB | 4.980 ± 0.229 cC | 5.102 ± 0.239 cC | 6.172 ± 0.296 dD | |
| PTC | Control | 2.36 ± 0.104 aA | 4.32 ± 0.194 cB | 5.96 ± 0.280 cC | 6.483 ± 0.311 cD | 7.50 ± 0.367 cE |
| 1-BPE | 2.35 ± 0.106 aA | 3.74 ± 0.168 bB | 4.23 ± 0.198 bC | 5.66 ± 0.272 bD | 6.98 ± 0.342 bE | |
| 2-BPE | 2.35 ± 0.108 aA | 3.07 ± 0.138 aB | 3.57 ± 0.167 aB | 5.32 ± 0.255 abC | 6.71 ± 0.328 bD | |
| 4-BPE | 2.34 ± 0.105 aA | 2.89 ± 0.130 aAB | 3.19 ± 0.149 aB | 5.06 ± 0.243 aC | 6.18 ± 0.302 aD | |
| EC | Control | <1 | 2.168 ± 0.101dA | 3.147 ± 0.151 cB | 3.675 ± 0.180 cB | 4.161 ± 0.208 dC |
| 1-BPE | <1 | 1.819 ± 0.085 cA | 2.149 ± 0.103 bB | 2.626 ± 0.128 cbB | 3.544 ± 0.177 cC | |
| 2-BPE | <1 | 1.439 ± 0.067 bA | 1.801 ± 0.086 aAB | 2.066 ± 0.101 aB | 2.884 ± 0.144 bC | |
| 4-BPE | <1 | 1.023 ± 0.048 aA | 1.715 ± 0.082 aB | 1.920 ± 0.094 aBC | 2.530 ± 0.102 aC | |
| Days of Storage | |||||
|---|---|---|---|---|---|
| Samples | 0 | 3 | 7 | 10 | 14 |
| Control | 5.21 ± 0.234 aA | 5.46 ± 0.251 cB | 5.74 ± 0.270 dC | 5.88 ± 0.282 cC | 6.12 ± 0.300 cD |
| 1-BPE | 5.20 ± 0.229 aA | 5.34 ± 0.246 bB | 5.52 ± 0.259 cC | 5.71 ± 0.274 bD | 5.94 ± 0.291 bE |
| 2-BPE | 5.19 ± 0.236 aA | 5.27 ± 0.242 aB | 5.42 ± 0.255 bC | 5.68 ± 0.273 bD | 5.81 ± 0.285 aE |
| 4-BPE | 5.19 ± 0.237 aA | 5.25 ± 0.247 aAB | 5.30 ± 0.249 aB | 5.61 ± 0.269 aC | 5.77 ± 0.283 aD |
| Days of Storage | ||||||
|---|---|---|---|---|---|---|
| Samples | 0 | 3 | 7 | 10 | 14 | |
| L* | Control | 46.213 ± 2.03 aC | 46.123 ± 2.12 bC | 45.750 ± 2.15 bBC | 45.200 ± 2.17 bB | 44.213 ± 2.17 bA |
| 1-BPE | 45.680 ± 2.01 aC | 45.390 ± 2.09 aC | 45.270 ± 2.13 bBC | 45.104 ± 2.16 bB | 44.100 ± 2.16 bA | |
| 2-BPE | 45.406 ± 2.00 aB | 45.130 ± 2.08 aB | 44.106± 2.07 abAB | 43.400 ± 2.08 aA | 43.416 ± 2.18 abA | |
| 4-BPE | 44.100 ± 1.94 aC | 43.780 ± 2.01 aB | 43.465 ± 2.04 aB | 43.115 ± 2.07 aAB | 42.955 ± 2.10 aA | |
| a* | Control | 10.273 ± 0.45 aC | 9.155 ± 0.42 aB | 8.555 ± 0.41 aA | 8.820 ± 0.43 aA | 9.560 ± 0.47 aB |
| 1-BPE | 44.580 ± 1.96 bC | 43.420 ± 1.99 bBC | 42.550 ± 2.04 bB | 41.210 ± 2.01 bAB | 40.490 ± 2.02 bA | |
| 2-BPE | 46.060 ± 2.02 bC | 44.780 ± 2.06 bB | 43.480 ± 2.08 bB | 41.796 ± 2.05 bA | 41.086 ± 2.05 bA | |
| 4-BPE | 46.613 ± 2.05 bD | 45.940 ± 2.11 bC | 44.630 ± 2.14 bB | 42.050 ± 2.06 bA | 42.273 ± 2.11 bA | |
| b* | Control | 11.470 ± 0.50 bA | 11.790 ± 0.54 aAB | 12.515 ± 0.59 bB | 14.115± 0.68 dC | 15.105 ± 0.74 cD |
| 1-BPE | 10.890 ± 0.48 abA | 11.560 ± 0.53 aB | 11.960 ± 0.56 abB | 13.000 ± 0.62 cC | 14.550 ± 0.71 bD | |
| 2-BPE | 10.453 ± 0.46 aA | 11.276 ± 0.52 aB | 11.723 ± 0.55 aBC | 12.343 ± 0.59 bC | 14.253 ± 0.70 bD | |
| 4-BPE | 10.136 ± 0.45 aA | 11.106 ± 0.51 aB | 11.576 ± 0.54 aBC | 11.700 ± 0.56 aC | 13.656 ± 0.67 aC | |
| Days of Storage | ||||||
|---|---|---|---|---|---|---|
| Samples | 0 | 3 | 7 | 10 | 14 | |
| Appearance | Control | 8.2 ± 0.361 aE | 6 ± 0.270 aD | 5.10 ± 0.235 aC | 4.50 ± 0.212 aB | 3.62 ± 0.174 aA |
| 1-BPE | 8.5 ± 0.374 aD | 6.3 ± 0.284 bC | 5.35 ± 0.246 bB | 5.10 ± 0.240 bB | 4.40 ± 0.211 bA | |
| 2-BPE | 8.25 ± 0.396 aD | 6.7 ± 0.302 cC | 5.70 ± 0.262 cB | 5.25 ± 0.247 bcB | 4.50 ± 0.216 bA | |
| 4-BPE | 8.5 ± 0.396 aD | 6.87 ± 0.309 cC | 5.80 ± 0.267 cB | 5.35 ± 0.251 cA | 5.20 ± 0.250 cA | |
| Color | Control | 8.45 ± 0.372 aE | 6.22 ± 0.280 aD | 5.30 ± 0.244 aC | 4.45 ± 0.209 aB | 3.50 ± 0.168 aA |
| 1-BPE | 8.5 ± 0.405 aD | 6.5 ± 0.293 bC | 5.33 ± 0.245 aB | 5 ± 0.235 bB | 4.40 ± 0.211 bA | |
| 2-BPE | 8.33 ± 0.402 aD | 6.88 ± 0.310 cC | 6.35 ± 0.246 bB | 5.12 ± 0.241 bA | 5.01 ± 0.214 cA | |
| 4-BPE | 8.5 ± 0.401 aD | 7.75 ± 0.349 dC | 6.45 ± 0.251 bC | 5.72 ± 0.245 cB | 5.10 ± 0.230 cA | |
| Aroma | Control | 8.5 ± 0.374 aE | 6.01 ± 0.270 aD | 5.25 ± 0.242 aC | 4.31 ± 0.203 aB | 3.30 ± 0.158 aA |
| 1-BPE | 8.5 ± 0.383 aD | 6.20 ± 0.279 abC | 5.35 ± 0.246 aB | 5.25 ± 0.247 bB | 4.27 ± 0.205 bA | |
| 2-BPE | 8.5 ± 0.378 aD | 6.34 ± 0.285 bC | 5.71 ± 0.149 bB | 5.30 ± 0.249 bAB | 5.00 ± 0.207 cA | |
| 4-BPE | 8.5 ± 0.380 aD | 6.57 ± 0.296 cC | 6.01 ± 0.255 cB | 5.73 ± 0.251 cA | 5.40 ± 0.211 dA | |
| OA | Control | 8 ± 0.356 aD | 5 ± 0.225 aC | 5 ± 0.230 aC | 4.71 ± 0.221 aB | 3.60 ± 0.173 aA |
| 1-BPE | 8 ± 0.358 aD | 6 ± 0.270 bC | 5.11 ± 0.235 abB | 5 ± 0.235 abB | 4.57 ± 0.219 aA | |
| 2-BPE | 8.5 ± 0.382 aD | 6.75 ± 0.304 cC | 5.21 ± 0.240 bB | 5.11 ± 0.240 bAB | 5.00 ± 0.226 bA | |
| 4-BPE | 8.5 ± 0.374 aE | 7.25 ± 0.315 dD | 5.65 ± 0.260 cB | 5.50 ± 0.248 cAB | 5.25 ± 0.230 cA | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chaari, M.; Elhadef, K.; Akermi, S.; Ben Akacha, B.; Fourati, M.; Chakchouk Mtibaa, A.; Ennouri, M.; Sarkar, T.; Shariati, M.A.; Rebezov, M.; et al. Novel Active Food Packaging Films Based on Gelatin-Sodium Alginate Containing Beetroot Peel Extract. Antioxidants 2022, 11, 2095. https://doi.org/10.3390/antiox11112095
Chaari M, Elhadef K, Akermi S, Ben Akacha B, Fourati M, Chakchouk Mtibaa A, Ennouri M, Sarkar T, Shariati MA, Rebezov M, et al. Novel Active Food Packaging Films Based on Gelatin-Sodium Alginate Containing Beetroot Peel Extract. Antioxidants. 2022; 11(11):2095. https://doi.org/10.3390/antiox11112095
Chicago/Turabian StyleChaari, Moufida, Khaoula Elhadef, Sarra Akermi, Boutheina Ben Akacha, Mariam Fourati, Ahlem Chakchouk Mtibaa, Monia Ennouri, Tanmay Sarkar, Mohammad Ali Shariati, Maksim Rebezov, and et al. 2022. "Novel Active Food Packaging Films Based on Gelatin-Sodium Alginate Containing Beetroot Peel Extract" Antioxidants 11, no. 11: 2095. https://doi.org/10.3390/antiox11112095
APA StyleChaari, M., Elhadef, K., Akermi, S., Ben Akacha, B., Fourati, M., Chakchouk Mtibaa, A., Ennouri, M., Sarkar, T., Shariati, M. A., Rebezov, M., Abdelkafi, S., Mellouli, L., & Smaoui, S. (2022). Novel Active Food Packaging Films Based on Gelatin-Sodium Alginate Containing Beetroot Peel Extract. Antioxidants, 11(11), 2095. https://doi.org/10.3390/antiox11112095











