Prevention of LPS-Induced Acute Kidney Injury in Mice by Bavachin and Its Potential Mechanisms
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal Model
2.2. Renal Histological Assessment and Immunostaining
2.3. Biochemical Analyses in Blood
2.4. Cell Culture
2.5. Reactive Oxygen Species (ROS) Measurement
2.6. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
2.7. Western Blotting
2.8. Transient Transfection
2.9. Statistical Analysis
3. Results
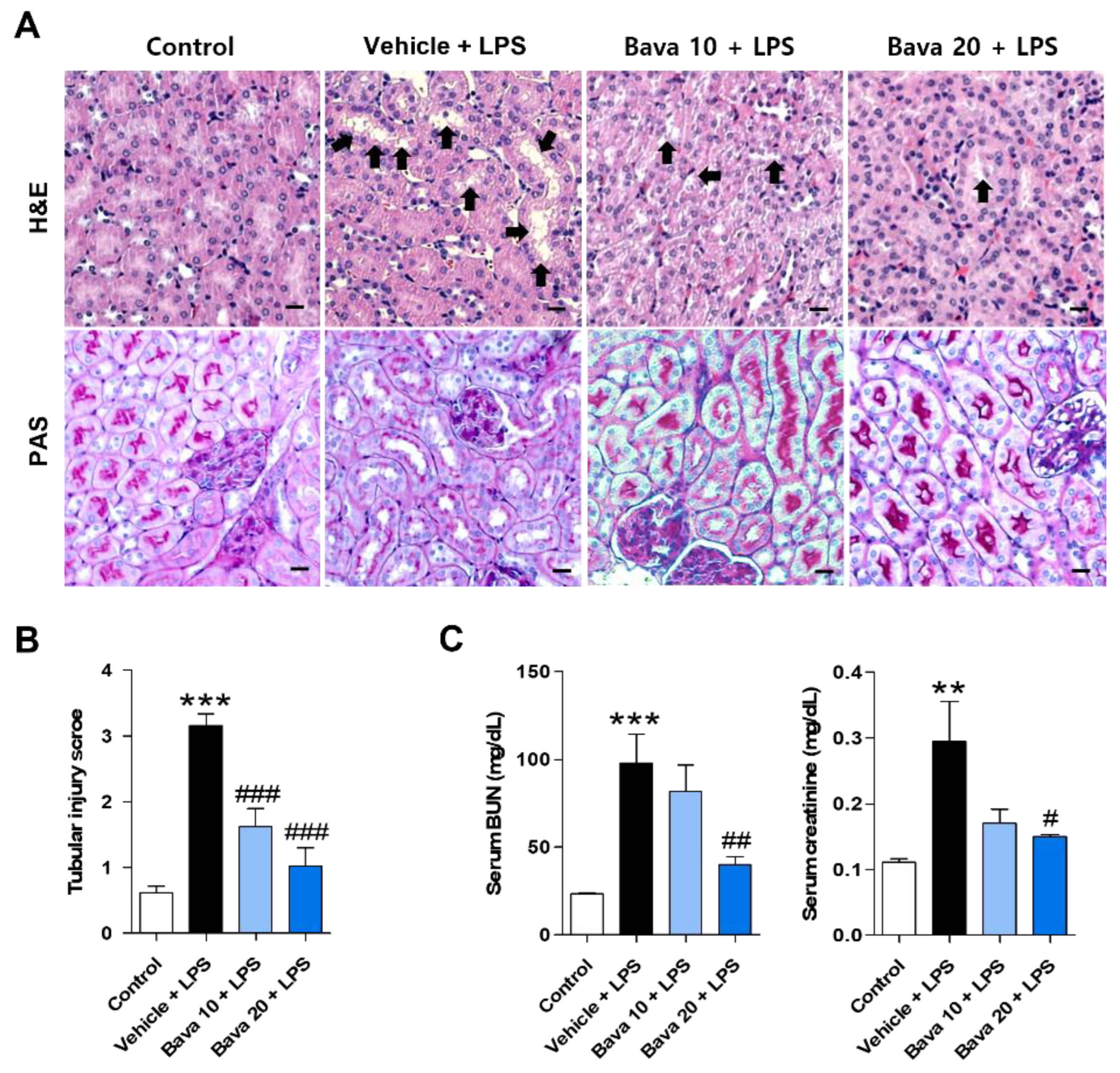
3.1. Bavachin Restores Histological Changes and Kidney Function in LPS-Induced AKI Mice
3.2. Bavachin Decreases the Expression of Tubular Injury Markers in LPS-Induced AKI Mice and LPS-Treated HK-2 Cells
3.3. Bavachin Decreases ROS Production in LPS-Induced AKI Mice and LPS-Treated HK-2 Cells
3.4. Bavachin Decreases PKCβ Activation and NOX4 Expression in LPS-Induced AKI Mice and LPS-Treated HK-2 Cells
3.5. Bavachin Decreases the Expression of Inflammatory Cytokines by Downregulating the MAPK/NF-κB Pathway in LPS-Induced AKI Mice and LPS-Treated HK-2 Cells
3.6. Bavachin Decreases NF-κB Signaling via Down-Regulation of KLF5 in LPS-Induced AKI Mice and LPS-Treated HK-2 Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Napolitano, L.M. Sepsis 2018: Definitions and Guideline Changes. Surg. Infect. 2018, 19, 117–125. [Google Scholar] [CrossRef] [Green Version]
- Zhang, D.; Lu, H.; Hou, W.; Bai, Y.; Wu, X. Effect of miR-132-3p on sepsis-induced acute kidney injury in mice via regulating HAVCR1/KIM-1. Am. J. Transl. Res. 2021, 13, 7794–7803. [Google Scholar]
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef]
- Singbartl, K.; Kellum, J.A. AKI in the ICU: Definition, epidemiology, risk stratification, and outcomes. Kidney Int. 2012, 81, 819–825. [Google Scholar] [CrossRef] [Green Version]
- Wang, F.; Zhang, G.; Lu, Z.; Geurts, A.M.; Usa, K.; Jacob, H.J.; Cowley, A.W.; Wang, N.; Liang, M. Antithrombin III/SerpinC1 insufficiency exacerbates renal ischemia/reperfusion injury. Kidney Int. 2015, 88, 796–803. [Google Scholar] [CrossRef] [Green Version]
- Wang, K.; Xie, S.; Xiao, K.; Yan, P.; He, W.; Xie, L. Biomarkers of Sepsis-Induced Acute Kidney Injury. Biomed. Res. Int. 2018, 2018, 6937947. [Google Scholar] [CrossRef] [Green Version]
- Torregrosa, I.; Montoliu, C.; Urios, A.; Andrés-Costa, M.J.; Giménez-Garzó, C.; Juan, I.; Puchades, M.J.; Blasco, M.L.; Carratalá, A.; Sanjuán, R.; et al. Urinary KIM-1, NGAL and L-FABP for the diagnosis of AKI in patients with acute coronary syndrome or heart failure undergoing coronary angiography. Heart Vessels 2015, 30, 703–711. [Google Scholar] [CrossRef]
- Endre, Z.H.; Kellum, J.A.; Di Somma, S.; Doi, K.; Goldstein, S.L.; Koyner, J.L.; Macedo, E.; Mehta, R.L.; Murray, P.T. Differential diagnosis of AKI in clinical practice by functional and damage biomarkers: Workgroup statements from the tenth Acute Dialysis Quality Initiative Consensus Conference. Contrib. Nephrol. 2013, 182, 30–44. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Luan, L.; Wang, C.; Song, M.; Zhao, Y.; Yao, Y.; Yang, H.; Ma, B.; Fan, H. Dexmedetomidine protects against lipopolysaccharide-induced early acute kidney injury by inhibiting the iNOS/NO signaling pathway in rats. Nitric Oxide 2019, 85, 1–9. [Google Scholar] [CrossRef]
- Chen, Y.; Jin, S.; Teng, X.; Hu, Z.; Zhang, Z.; Qiu, X.; Tian, D.; Wu, Y. Hydrogen Sulfide Attenuates LPS-Induced Acute Kidney Injury by Inhibiting Inflammation and Oxidative Stress. Oxid. Med. Cell. Longev. 2018, 2018, 6717212. [Google Scholar] [CrossRef] [Green Version]
- Nair, A.R.; Masson, G.S.; Ebenezer, P.J.; Del Piero, F.; Francis, J. Role of TLR4 in lipopolysaccharide-induced acute kidney injury: Protection by blueberry. Free Radic. Biol. Med. 2014, 71, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.G.; Lee, H.K.; Cho, K.B.; Park, S.I. A Review of Natural Products for Prevention of Acute Kidney Injury. Medicina 2021, 57, 1266. [Google Scholar] [CrossRef] [PubMed]
- Alam, F.; Khan, G.N.; Asad, M. Psoralea corylifolia L: Ethnobotanical, biological, and chemical aspects: A review. Phytother. Res. 2018, 32, 597–615. [Google Scholar] [CrossRef] [PubMed]
- Hung, Y.L.; Wang, S.C.; Suzuki, K.; Fang, S.H.; Chen, C.S.; Cheng, W.C.; Su, C.C.; Yeh, H.C.; Tu, H.P.; Liu, P.L.; et al. Bavachin attenuates LPS-induced inflammatory response and inhibits the activation of NLRP3 inflammasome in macrophages. Phytomedicine 2019, 59, 152785. [Google Scholar] [CrossRef]
- Zhu, W.Y.; Yue, X.; Lv, X.; Yi, C.; Liu, J. Bavachin antioxidant effects on the cardiac myocytes of mice in exhaustive exercise. Bulg. Chem. Commun. 2017, 49, 136–142. [Google Scholar]
- Chang, Y.K.; Choi, H.; Jeong, J.Y.; Na, K.R.; Lee, K.W.; Lim, B.J.; Choi, D.E. Dapagliflozin, SGLT2 Inhibitor, Attenuates Renal Ischemia-Reperfusion Injury. PLoS ONE 2016, 11, e0158810. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.Y.; Leem, J.; Hong, H.L. Protective Effects of SPA0355, a Thiourea Analogue, Against Lipopolysaccharide-Induced Acute Kidney Injury in Mice. Antioxidants 2020, 9, 585. [Google Scholar] [CrossRef]
- Raghavan, V.; Weisz, O.A. Flow stimulated endocytosis in the proximal tubule. Curr. Opin. Nephrol. Hypertens. 2015, 24, 359–365. [Google Scholar] [CrossRef] [Green Version]
- Welling, L.W.; Welling, D.J. Surface areas of brush border and lateral cell walls in the rabbit proximal nephron. Kidney Int. 1975, 8, 343–348. [Google Scholar] [CrossRef] [Green Version]
- Arnold, N.; Girke, T.; Sureshchandra, S.; Messaoudi, I. Acute Simian Varicella Virus Infection Causes Robust and Sustained Changes in Gene Expression in the Sensory Ganglia. J. Virol. 2016, 90, 10823–10843. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Lu, J.; Liao, Y.; Liu, S.; Chen, Y.; He, R.; Men, L.; Lu, C.; Chen, Z.; Li, S.; et al. Dihydroartemisinin attenuates lipopolysaccharide-induced acute kidney injury by inhibiting inflammation and oxidative stress. Biomed. Pharmacother. 2019, 117, 109070. [Google Scholar] [CrossRef] [PubMed]
- Breitzig, M.; Bhimineni, C.; Lockey, R.; Kolliputi, N. 4-Hydroxy-2-nonenal: A critical target in oxidative stress? Am. J. Physiol. Cell Physiol. 2016, 311, C537–C543. [Google Scholar] [CrossRef] [PubMed]
- Gaweł, S.; Wardas, M.; Niedworok, E.; Wardas, P. Malondialdehyde (MDA) as a lipid peroxidation marker. Wiad. Lek. 2004, 57, 453–455. [Google Scholar] [PubMed]
- Yoo, J.Y.; Cha, D.R.; Kim, B.; An, E.J.; Lee, S.R.; Cha, J.J.; Kang, Y.S.; Ghee, J.Y.; Han, J.Y.; Bae, Y.S. LPS-Induced Acute Kidney Injury Is Mediated by Nox4-SH3YL1. Cell Rep. 2020, 33, 108245. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Zhang, W.; Xu, S.; Peng, L.; Wang, Z.; Liu, H.; Fang, Q.; Deng, T.; Men, X.; Lou, J. TRB3 mediates advanced glycation end product-induced apoptosis of pancreatic β-cells through the protein kinase C β pathway. Int. J. Mol. Med. 2017, 40, 130–136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gorin, Y.; Block, K. Nox4 and diabetic nephropathy: With a friend like this, who needs enemies? Free Radic. Biol. Med. 2013, 61, 130–142. [Google Scholar] [CrossRef] [Green Version]
- Torres, J.; Enriquez-de-Salamanca, A.; Fernandez, I.; Rodriguez-Ares, M.T.; Quadrado, M.J.; Murta, J.; Benitez del Castillo, J.M.; Stern, M.E.; Calonge, M. Activation of MAPK signaling pathway and NF-kappaB activation in pterygium and ipsilateral pterygium-free conjunctival specimens. Investig. Ophthalmol. Vis. Sci. 2011, 52, 5842–5852. [Google Scholar] [CrossRef]
- Li, J.; Liu, L.; Zhou, W.Q.; Cai, L.; Xu, Z.G.; Rane, M.J. Roles of Kruppel-like factor 5 in kidney disease. J. Cell. Mol. Med. 2021, 25, 2342–2355. [Google Scholar] [CrossRef]
- Gómez, H.; Kellum, J.A. Sepsis-induced acute kidney injury. Curr. Opin. Crit. Care 2016, 22, 546–553. [Google Scholar] [CrossRef] [Green Version]
- Peerapornratana, S.; Manrique-Caballero, C.L.; Gomez, H.; Kellum, J.A. Acute kidney injury from sepsis: Current concepts, epidemiology, pathophysiology, prevention and treatment. Kidney Int. 2019, 96, 1083–1099. [Google Scholar] [CrossRef]
- Godin, M.; Murray, P.; Mehta, R.L. Clinical approach to the patient with AKI and sepsis. Semin. Nephrol. 2015, 35, 12–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ronco, C.; Bellomo, R.; Kellum, J.A. Acute kidney injury. Lancet 2019, 394, 1949–1964. [Google Scholar] [CrossRef]
- He, H.Q.; Law, B.Y.K.; Zhang, N.; Qiu, C.L.; Qu, Y.Q.; Wu, A.G.; Han, Y.; Song, Q.; Zheng, W.L.; Liu, Y.; et al. Bavachin Protects Human Aortic Smooth Muscle Cells Against β-Glycerophosphate-Mediated Vascular Calcification and Apoptosis via Activation of mTOR-Dependent Autophagy and Suppression of β-Catenin Signaling. Front. Pharmacol. 2019, 10, 1427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flower, D.R. The lipocalin protein family: A role in cell regulation. FEBS Lett. 1994, 354, 7–11. [Google Scholar] [CrossRef] [Green Version]
- Mirzoyan, K.; Denis, C.; Casemayou, A.; Gilet, M.; Marsal, D.; Goudouneche, D.; Faguer, S.; Bascands, J.L.; Schanstra, J.P.; Saulnier-Blache, J.S. Lysophosphatidic Acid Protects Against Endotoxin-Induced Acute Kidney Injury. Inflammation 2017, 40, 1707–1716. [Google Scholar] [CrossRef]
- Han, W.K.; Bonventre, J.V. Biologic markers for the early detection of acute kidney injury. Curr. Opin. Crit. Care 2004, 10, 476–482. [Google Scholar] [CrossRef]
- Ichimura, T.; Hung, C.C.; Yang, S.A.; Stevens, J.L.; Bonventre, J.V. Kidney injury molecule-1: A tissue and urinary biomarker for nephrotoxicant-induced renal injury. Am. J. Physiol. Ren. Physiol. 2004, 286, F552–F563. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.G.; Sohn, E.J.; Moon, M.K.; Lee, Y.M.; Lee, H.S. Rehmannia glutinose ameliorates renal function in the ischemia/reperfusion-induced acute renal failure rats. Biol. Pharm. Bull. 2005, 28, 1662–1667. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schrier, R.W.; Wang, W. Acute renal failure and sepsis. N. Engl. J. Med. 2004, 351, 159–169. [Google Scholar] [CrossRef]
- Gorin, Y.; Cavaglieri, R.C.; Khazim, K.; Lee, D.Y.; Bruno, F.; Thakur, S.; Fanti, P.; Szyndralewiez, C.; Barnes, J.L.; Block, K.; et al. Targeting NADPH oxidase with a novel dual Nox1/Nox4 inhibitor attenuates renal pathology in type 1 diabetes. Am. J. Physiol. Ren. Physiol. 2015, 308, F1276–F1287. [Google Scholar] [CrossRef] [Green Version]
- Eid, A.A.; Ford, B.M.; Block, K.; Kasinath, B.S.; Gorin, Y.; Ghosh-Choudhury, G.; Barnes, J.L.; Abboud, H.E. AMP-activated protein kinase (AMPK) negatively regulates Nox4-dependent activation of p53 and epithelial cell apoptosis in diabetes. J. Biol. Chem. 2010, 285, 37503–37512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, K.; Lin, L.; Ai, Q.; Wan, J.; Dai, J.; Liu, G.; Tang, L.; Yang, Y.; Ge, P.; Jiang, R.; et al. Lipopolysaccharide-Induced Dephosphorylation of AMPK-Activated Protein Kinase Potentiates Inflammatory Injury via Repression of ULK1-Dependent Autophagy. Front. Immunol. 2018, 9, 1464. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, H.; Miyamoto, Y.; Honda, D.; Tanaka, H.; Wu, Q.; Endo, M.; Noguchi, T.; Kadowaki, D.; Ishima, Y.; Kotani, S.; et al. p-Cresyl sulfate causes renal tubular cell damage by inducing oxidative stress by activation of NADPH oxidase. Kidney Int. 2013, 83, 582–592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xia, L.; Wang, H.; Goldberg, H.J.; Munk, S.; Fantus, I.G.; Whiteside, C.I. Mesangial cell NADPH oxidase upregulation in high glucose is protein kinase C dependent and required for collagen IV expression. Am. J. Physiol. Ren. Physiol. 2006, 290, F345–F356. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Cheon, J.; Yoon, H.; Jun, H.S. Cudrania tricuspidata Root Extract Prevents Methylglyoxal-Induced Inflammation and Oxidative Stress via Regulation of the PKC-NOX4 Pathway in Human Kidney Cells. Oxid. Med. Cell. Longev. 2021, 2021, 5511881. [Google Scholar] [CrossRef]
- Lu, Q.B.; Du, Q.; Wang, H.P.; Tang, Z.H.; Wang, Y.B.; Sun, H.J. Salusin-beta mediates tubular cell apoptosis in acute kidney injury: Involvement of the PKC/ROS signaling pathway. Redox Biol. 2020, 30, 101411. [Google Scholar] [CrossRef]
- Yang, C.C.; Yao, C.A.; Yang, J.C.; Chien, C.T. Sialic acid rescues repurified lipopolysaccharide-induced acute renal failure via inhibiting TLR4/PKC/gp91-mediated endoplasmic reticulum stress, apoptosis, autophagy, and pyroptosis signaling. Toxicol. Sci. 2014, 141, 155–165. [Google Scholar] [CrossRef] [Green Version]
- Simmons, E.M.; Himmelfarb, J.; Sezer, M.T.; Chertow, G.M.; Mehta, R.L.; Paganini, E.P.; Soroko, S.; Freedman, S.; Becker, K.; Spratt, D.; et al. Plasma cytokine levels predict mortality in patients with acute renal failure. Kidney Int. 2004, 65, 1357–1365. [Google Scholar] [CrossRef] [Green Version]
- Zheng, C.; Zhou, Y.; Huang, Y.; Chen, B.; Wu, M.; Xie, Y.; Chen, X.; Sun, M.; Liu, Y.; Chen, C.; et al. Effect of ATM on inflammatory response and autophagy in renal tubular epithelial cells in LPS-induced septic AKI. Exp. Ther. Med. 2019, 18, 4707–4717. [Google Scholar] [CrossRef] [Green Version]
- Song, N.; Thaiss, F.; Guo, L. NFkappaB and Kidney Injury. Front. Immunol. 2019, 10, 815. [Google Scholar] [CrossRef] [Green Version]
- Marko, L.; Vigolo, E.; Hinze, C.; Park, J.K.; Roel, G.; Balogh, A.; Choi, M.; Wubken, A.; Cording, J.; Blasig, I.E.; et al. Tubular Epithelial NF-kappaB Activity Regulates Ischemic AKI. J. Am. Soc. Nephrol. 2016, 27, 2658–2669. [Google Scholar] [CrossRef] [Green Version]
- Jung, Y.S.; Park, J.H.; Kim, H.; Kim, S.Y.; Hwang, J.Y.; Hong, K.W.; Bae, S.S.; Choi, B.T.; Lee, S.W.; Shin, H.K. Probucol inhibits LPS-induced microglia activation and ameliorates brain ischemic injury in normal and hyperlipidemic mice. Acta Pharmacol. Sin. 2016, 37, 1031–1044. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Qu, R.; Zhu, S.; Zhang, R.; Ma, S. Rhynchophylline attenuates LPS-induced pro-inflammatory responses through down-regulation of MAPK/NF-kappaB signaling pathways in primary microglia. Phytother. Res. 2012, 26, 1528–1533. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.; Lang, W.; Wang, S.; Li, G.; Bai, X.; Yan, X.; Zhang, H. Echinacea polysaccharide attenuates lipopolysaccharideinduced acute kidney injury via inhibiting inflammation, oxidative stress and the MAPK signaling pathway. Int. J. Mol. Med. 2021, 47, 243–255. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Zeng, M.; Wang, Y.; Li, M.; Wu, Y.; Xu, R.; Zhang, Q.; Jia, J.; Huang, Y.; Zheng, X.; et al. Oleic acid alleviates LPS-induced acute kidney injury by restraining inflammation and oxidative stress via the Ras/MAPKs/PPAR-gamma signaling pathway. Phytomedicine 2022, 94, 153818. [Google Scholar] [CrossRef]
- Lim, H.S.; Kim, Y.J.; Kim, B.Y.; Park, G.; Jeong, S.J. The Anti-neuroinflammatory Activity of Tectorigenin Pretreatment via Downregulated NF-κB and ERK/JNK Pathways in BV-2 Microglial and Microglia Inactivation in Mice With Lipopolysaccharide. Front. Pharmacol. 2018, 9, 462. [Google Scholar] [CrossRef] [PubMed]
- Pollak, N.M.; Hoffman, M.; Goldberg, I.J.; Drosatos, K. Kruppel-like factors: Crippling and un-crippling metabolic pathways. JACC Basic Transl. Sci. 2018, 3, 132–156. [Google Scholar] [CrossRef] [PubMed]
- Conkright, M.D.; Wani, M.A.; Anderson, K.P.; Lingrel, J.B. A gene encoding an intestinal-enriched member of the Kruppel-like factor family expressed in intestinal epithelial cells. Nucleic Acids Res. 1999, 27, 1263–1270. [Google Scholar] [CrossRef] [Green Version]
- Sogawa, K.; Imataka, H.; Yamasaki, Y.; Kusume, H.; Abe, H.; Fujii-Kuriyama, Y. cDNA cloning and transcriptional properties of a novel GC box-binding protein, BTEB2. Nucleic Acids Res. 1993, 21, 1527–1532. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.L.; Chong, I.W.; Lee, Y.C.; Tsai, J.R.; Yuan, S.S.; Wang, H.M.; Liu, W.L.; Liu, P.L. Kruppel-like factor 5 mediates proinflammatory cytokine expression in lipopolysaccharide-induced acute lung injury through upregulation of nuclear factor-kappaB phosphorylation in vitro and in vivo. Mediat. Inflamm. 2014, 2014, 281984. [Google Scholar] [CrossRef] [Green Version]
- Yan, W.; Wu, J.; Song, B.; Luo, Q.; Xu, Y. Treatment with a brain-selective prodrug of 17beta-estradiol improves cognitive function in Alzheimer’s disease mice by regulating klf5-NF-kappaB pathway. Naunyn Schmiedebergs Arch. Pharmacol. 2019, 392, 879–886. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chanchevalap, S.; Nandan, M.O.; McConnell, B.B.; Charrier, L.; Merlin, D.; Katz, J.P.; Yang, V.W. Kruppel-like factor 5 is an important mediator for lipopolysaccharide-induced proinflammatory response in intestinal epithelial cells. Nucleic Acids Res. 2006, 34, 1216–1223. [Google Scholar] [CrossRef] [PubMed]






| Gene | Accession Number | Primer Sequence |
|---|---|---|
| hCyclophilin | NM_000942 | Forward: TGCCATCGCCAAGGAGTAG |
| Reverse: TGCACAGACGGTCACTCAAA | ||
| h18s RNA | M10098 | Forward: GCCGCTAGAGGTGAAATTCTTG |
| Reverse: CATTCTTGGCAAATGCTTTCG | ||
| hKLF5 | NM_001730 | Forward: CTTCCACAACAGGCCACTTACTT |
| Reverse: TCTGGAGCATCTCTGCTTGTC | ||
| hHavcr1(KIM-1) | NM_012206.3 | Forward: CTTCACCTCAGCCAGCAGAAAC |
| Reverse: GCCATCTGAAGACTCTGTCACG | ||
| hLcn2(NGAL) | NM_005564.5 | Forward: TTTTGTTCCAGGTTGCCAGC |
| Reverse: CTCACCACTCGGACGAGGTA | ||
| hNOX4 | NM_001143836.3 | Forward: TGTGCCGAACACTCTTGGC |
| Reverse: ACATGCACGCCTGAGAAAATA | ||
| hIL-1β | NM_000576.2 | Forward: GCTGCTCTGGGATTCTCTTCA |
| Reverse: TGGCGAGCTCAGGTACTTCTG | ||
| hIL-6 | NM_000600.4 | Forward: GTACATCCTCGACGGCATCTC |
| Reverse: GTGCCTCTTTGCTGCTTTCAC | ||
| hTNF-α | NM_000594.3 | Forward: GAGATCAATCGGCCCGACTA |
| Reverse: ACAGGGCAATGATCCCAAAG | ||
| mCyclophilin | NM_011149 | Forward: TGGAGAGCACCAAGACAGACA |
| Reverse: TGCCGGAGTCGACAATGAT | ||
| mKLF5 | NM_009769 | Forward: CACCCCACCTCCGTCCTAT |
| Reverse: GGGTTGTGAATCGCCAGTTT | ||
| mLcn2(NGAL) | NM_008491.1 | Forward: GGCAGCTTTACGATGTACAGCA |
| Reverse: TCTGATCCAGTAGCGACAGCC | ||
| mHavcr1(KIM-1) | NM_134248.2 | Forward: GCATCTCTAAGCGTGGTTGC |
| Reverse: TCAGCTCGGGAATGCACAA | ||
| mIl-1β | NM_008361 | Forward: CTACAGGCTCCGAGATGAACAAC |
| Reverse: TCCATTGAGGTGGAGAGCTTTC | ||
| mIl-6 | NM_031168.2 | Forward: TCCATCCAGTTGCCTTCT |
| Reverse: GGAGTGGTATCCTCTGTGAA | ||
| mTNF-α | NM_013693.3 | Forward: CCAACGGCATGGATCTCAAAGACA |
| Reverse: AGATAGCAAATCGGCTGACGGTGT |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ban, K.-Y.; Nam, G.-Y.; Kim, D.; Oh, Y.S.; Jun, H.-S. Prevention of LPS-Induced Acute Kidney Injury in Mice by Bavachin and Its Potential Mechanisms. Antioxidants 2022, 11, 2096. https://doi.org/10.3390/antiox11112096
Ban K-Y, Nam G-Y, Kim D, Oh YS, Jun H-S. Prevention of LPS-Induced Acute Kidney Injury in Mice by Bavachin and Its Potential Mechanisms. Antioxidants. 2022; 11(11):2096. https://doi.org/10.3390/antiox11112096
Chicago/Turabian StyleBan, Ka-Yun, Ga-Young Nam, Donghee Kim, Yoon Sin Oh, and Hee-Sook Jun. 2022. "Prevention of LPS-Induced Acute Kidney Injury in Mice by Bavachin and Its Potential Mechanisms" Antioxidants 11, no. 11: 2096. https://doi.org/10.3390/antiox11112096
APA StyleBan, K.-Y., Nam, G.-Y., Kim, D., Oh, Y. S., & Jun, H.-S. (2022). Prevention of LPS-Induced Acute Kidney Injury in Mice by Bavachin and Its Potential Mechanisms. Antioxidants, 11(11), 2096. https://doi.org/10.3390/antiox11112096






