Determination of Redox Status in Different Tissues of Lambs and Kids and Their in-between Relationship
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Sample Collection
2.3. Blood Fractionation
2.4. Tissue Homogenization and the Measurement of the Total Protein Concentration
2.5. Redox Biomarkers Determination
2.5.1. Reduced Form of Glutathione (GSH)
2.5.2. Catalase (CAT)
2.5.3. Total Antioxidant Activity (TAC)
2.5.4. Thiobarbituric Acid Reactive Substances (TBARS)
2.5.5. Protein Carbonyls
2.6. Statistical Analyses
3. Results
3.1. Redox Biomarkers Evaluated in 4 Developmental Stages
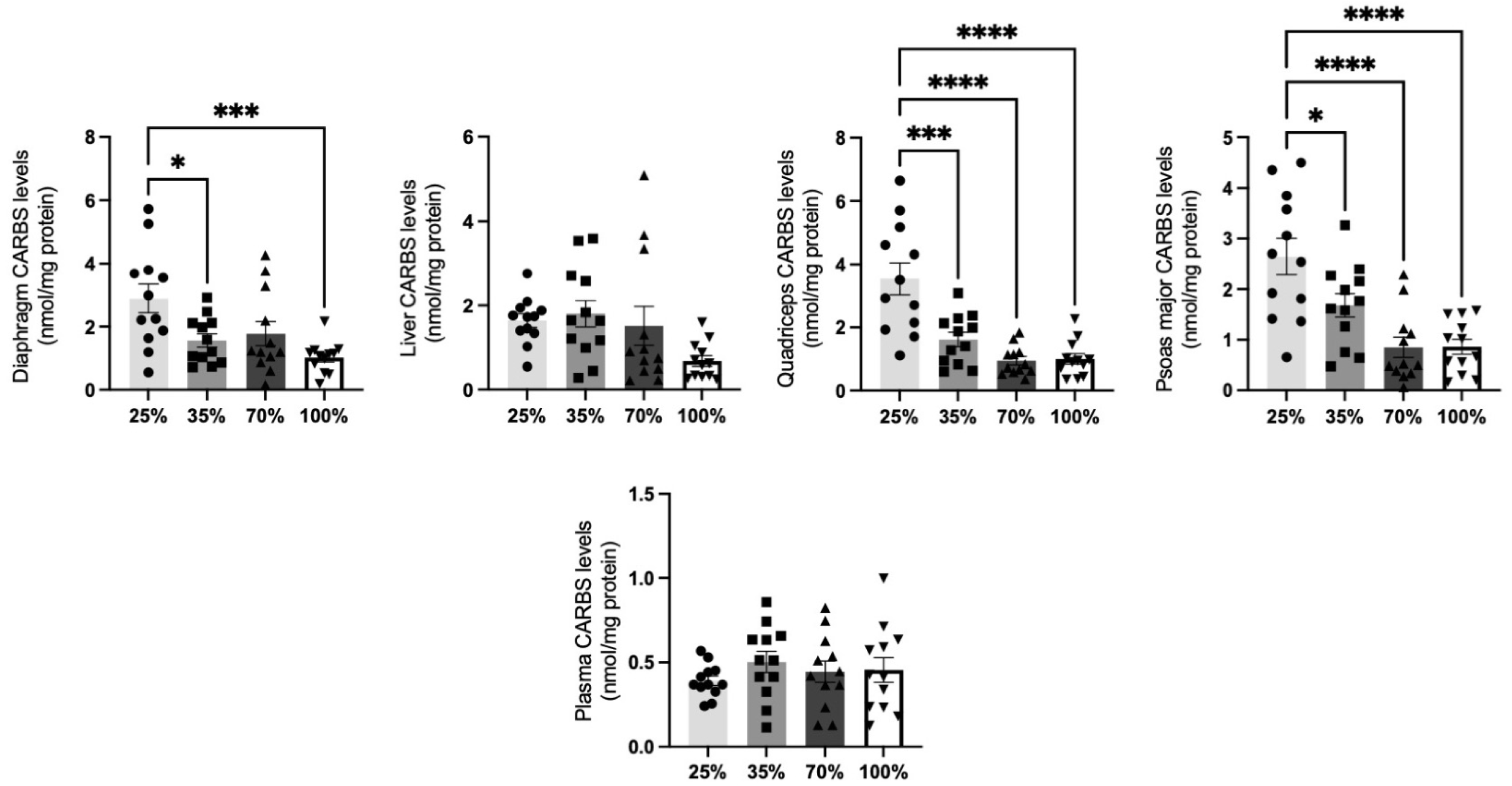
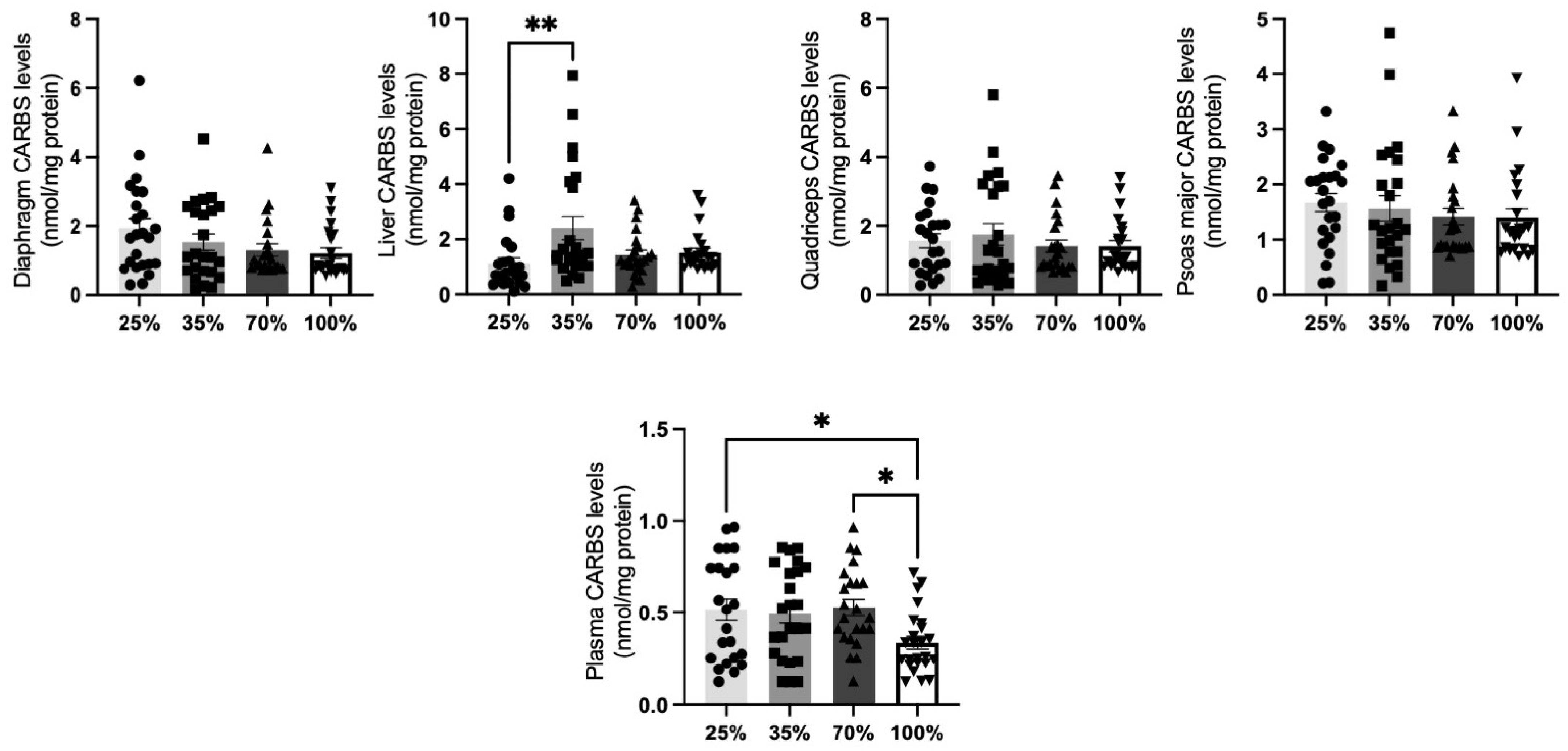
3.1.1. CARBS
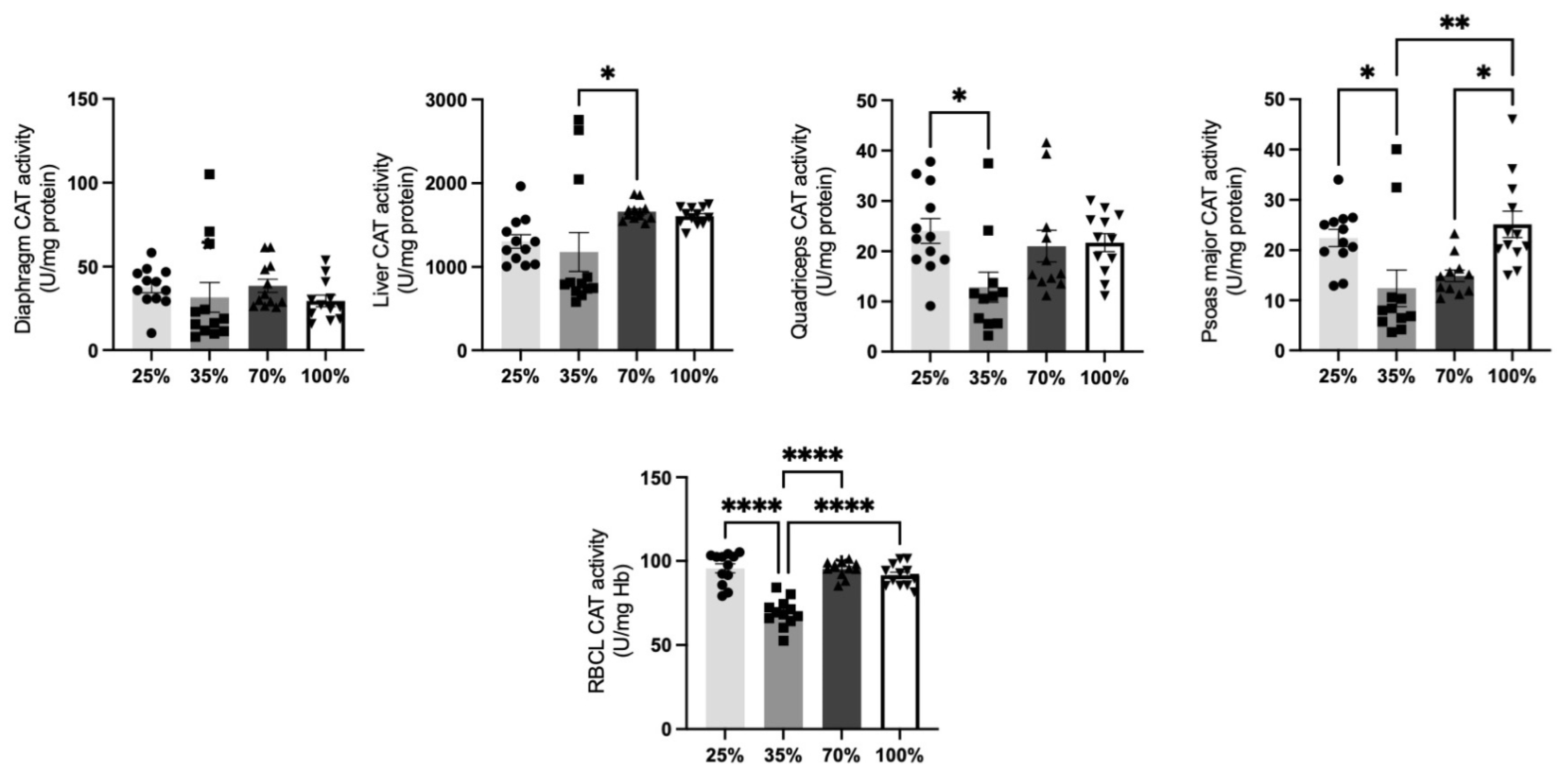
3.1.2. Catalase Activity
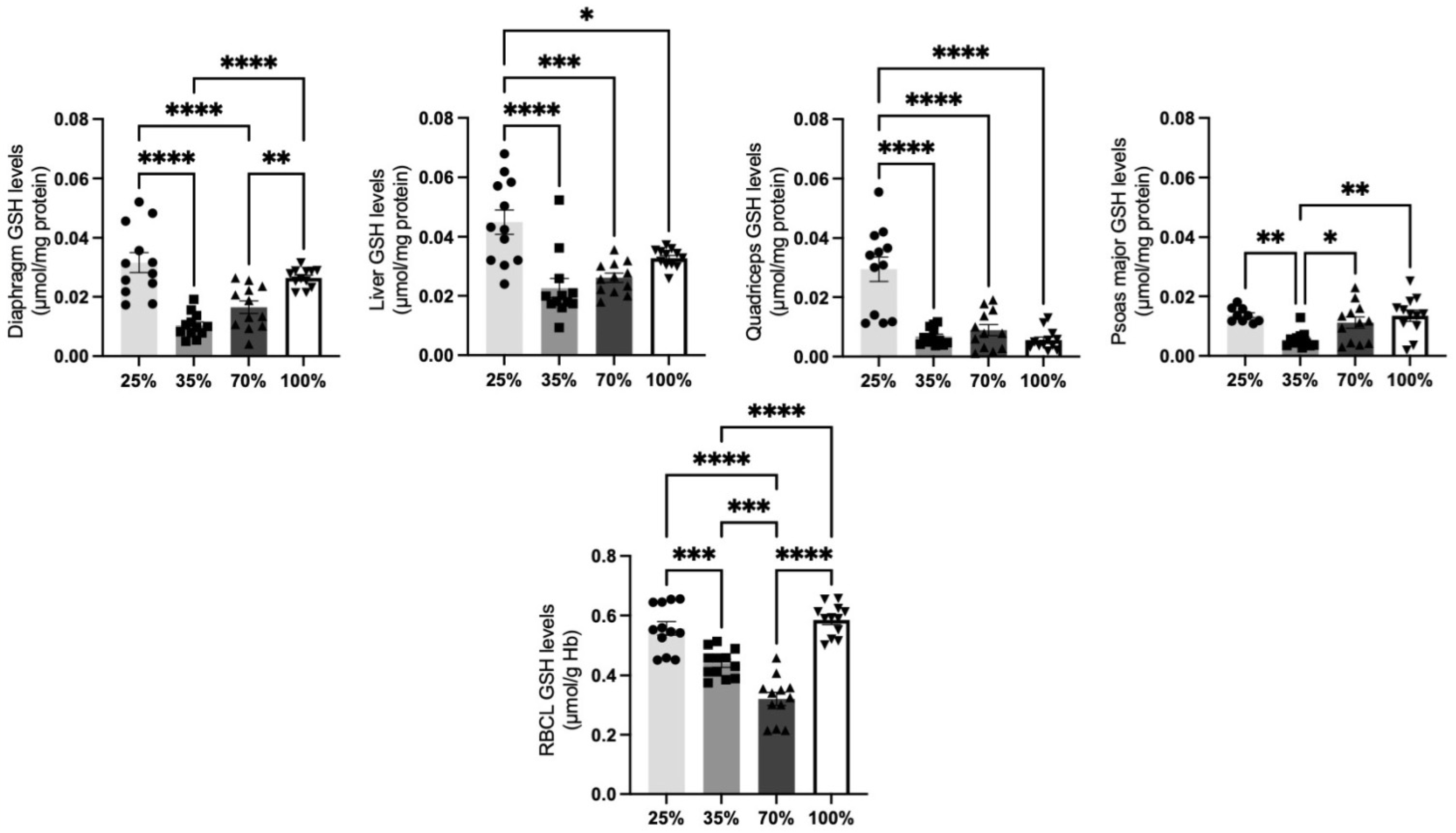
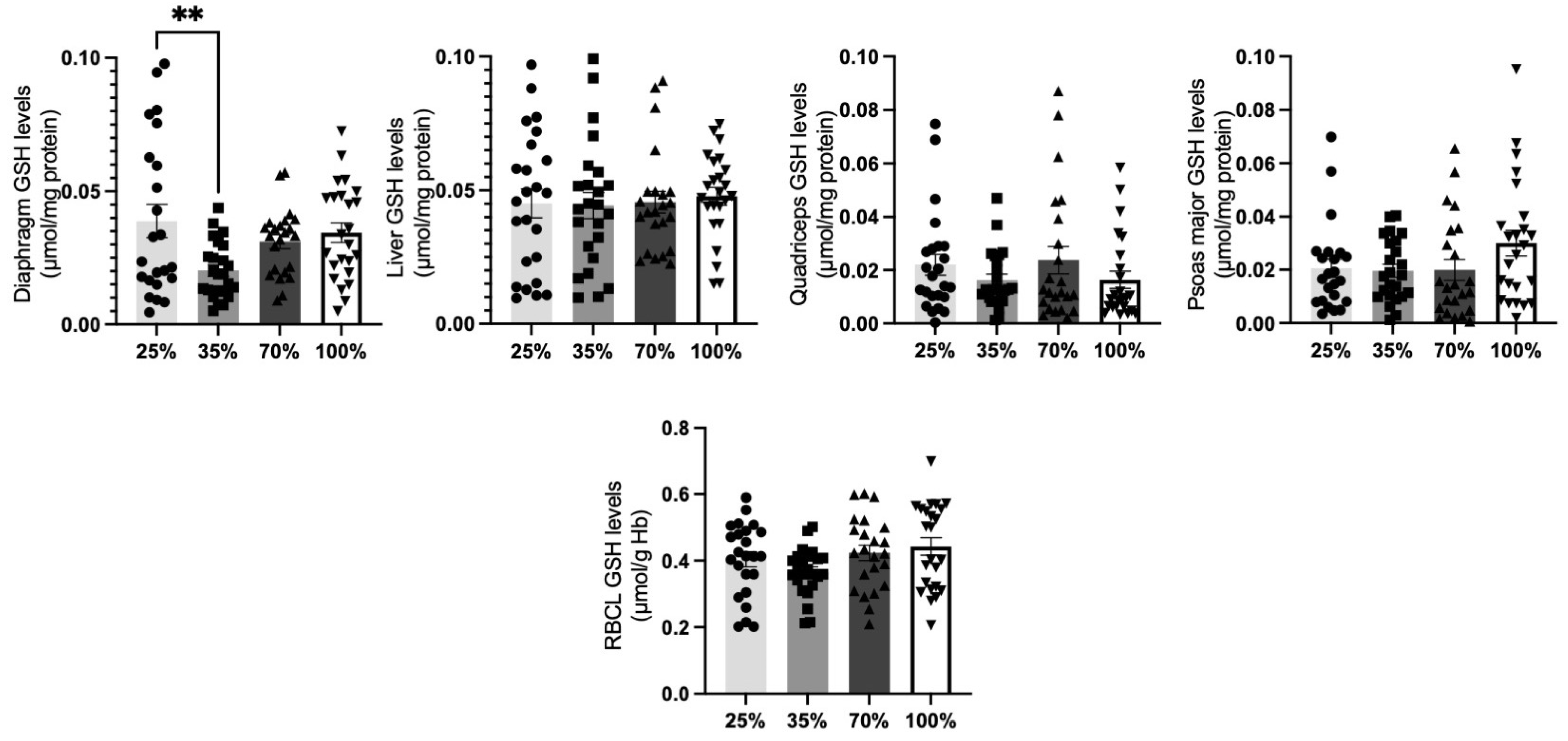
3.1.3. Glutathione Levels
3.1.4. Total Antioxidant Capacity
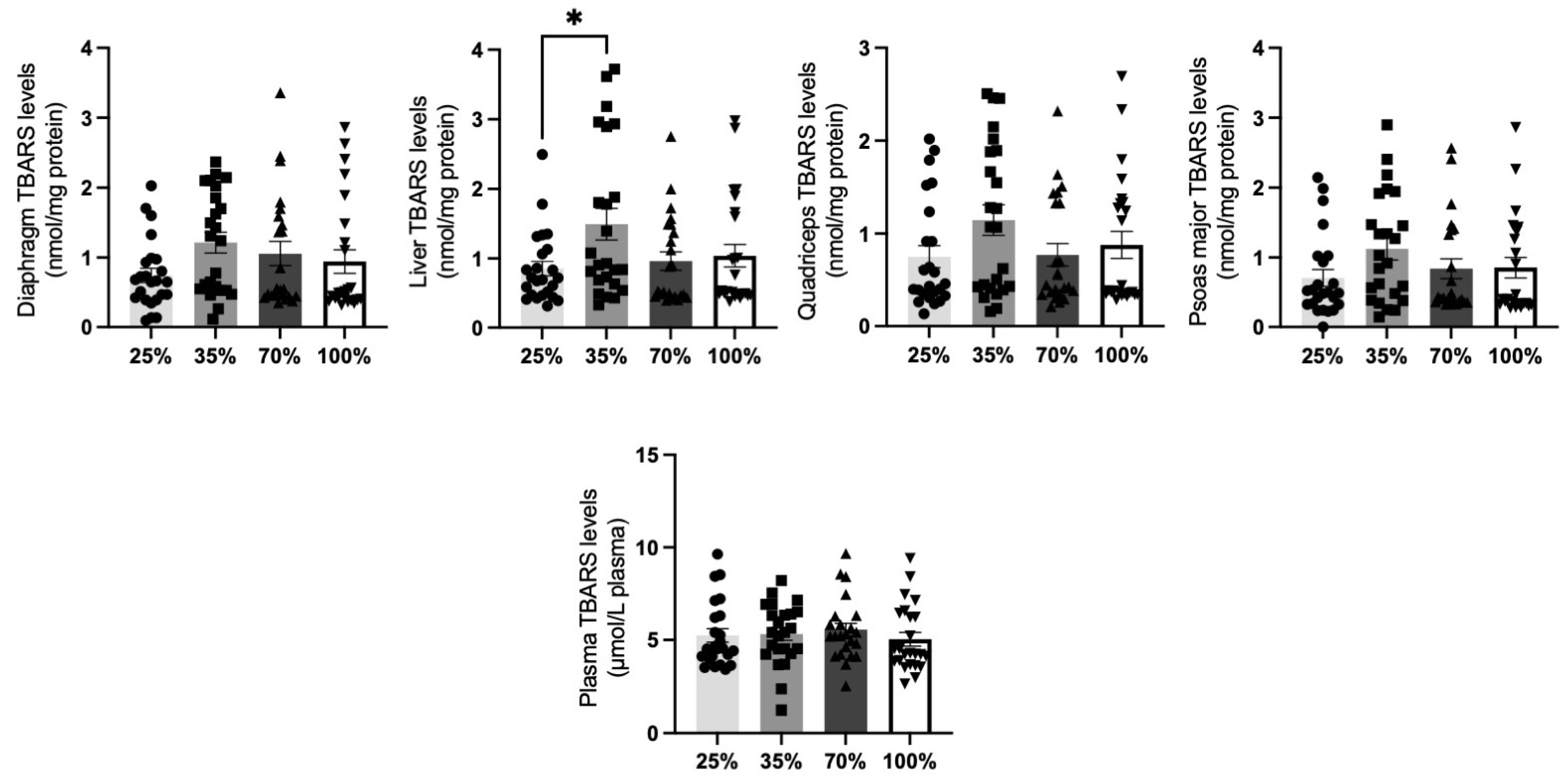
3.1.5. Thiobarbituric Acid Reactive Substances (TBARS)
3.2. Correlation Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| GSH | reduced glutathione |
| CAT | catalase |
| TAC | total antioxidant capacity |
| TBARS | thiobarbituric acid reactive substances |
| CARBs | protein carbonyls |
| d.s. | developmental stage |
| LWC | live weight category |
References
- Ritchie, H.; Rosado, R.; Roser, M. Our World in Data. Meat and Dairy Production. 2017. Available online: https://ourworldindata.org/meat-production#global-meat-production (accessed on 1 September 2022).
- FAO. World Livestock 2011—Livestock in Food Security; FAO: Rome, Italy, 2011. [Google Scholar]
- Mulvihill, B. Ruminant meat as a source of conjugated linoleic acid (CLA). Nutr. Bull. 2001, 26, 295–299. [Google Scholar] [CrossRef]
- Benjamin, S.; Prakasan, P.; Sreedharan, S.; Wright, A.-D.G.; Spener, F. Pros and cons of CLA consumption: An insight from clinical evidences. Nutr. Metab. 2015, 12, 4. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Ahn, D.U. Lipid oxidation and its implications to meat quality and human health. Food Sci. Biotechnol. 2019, 28, 1275–1285. [Google Scholar] [CrossRef] [PubMed]
- Celi, P.; Gabai, G. Oxidant/antioxidant balance in animal nutrition and health: The role of protein oxidation. Front. Vet. Sci. 2015, 2, 48. [Google Scholar] [CrossRef] [PubMed]
- McGrath, J.; Duval, S.M.; Tamassia, L.F.M.; Kindermann, M.; Stemmler, R.T.; de Gouvea, V.N.; Acedo, T.S.; Immig, I.; Williams, S.N.; Celi, P. Nutritional strategies in ruminants: A lifetime approach. Res. Vet. Sci. 2018, 116, 28–39. [Google Scholar] [CrossRef]
- Chauhan, S.S.; Celi, P.; Ponnampalam, E.N.; Leury, B.J.; Liu, F.; Dunshea, F.R. Antioxidant dynamics in the live animal and implications for ruminant health and product (meat/milk) quality: Role of vitamin E and selenium. Anim. Prod. Sci. 2014, 54, 1525–1536. [Google Scholar] [CrossRef]
- Aurousseau, B.; Gruffat, D.; Durand, D. Gestation linked radical oxygen species fluxes and vitamins and trace mineral deficiencies in the ruminant. Reprod. Nutr. Dev. 2006, 46, 601–620. [Google Scholar] [CrossRef]
- Bešlo, D.; Došlić, G.; Agić, D.; Rastija, V.; Šperanda, M.; Gantner, V.; Lučić, B. Polyphenols in Ruminant Nutrition and Their Effects on Reproduction. Antioxidants 2022, 11, 970. [Google Scholar] [CrossRef]
- Hashem, N.M.; Gonzalez-Bulnes, A.; Simal-Gandara, J. Polyphenols in Farm Animals: Source of Reproductive Gain or Waste? Antioxidants 2020, 9, 1023. [Google Scholar] [CrossRef]
- Ionescu, J.G.; Novotny, J.; Stejskal, V.; Lätsch, A.; Blaurock-Busch, E.; Eisenmann-Klein, M. Increased levels of transition metals in breast cancer tissue. Neuro Endocrinol. Lett. 2006, 27 (Suppl. 1), 36–39. [Google Scholar]
- González, M.J.; Miranda-Massari, J.R.; Mora, E.M.; Guzmán, A.; Riordan, N.H.; Riordan, H.D.; Casciari, J.J.; Jackson, J.A.; Román-Franco, A. Orthomolecular oncology review: Ascorbic acid and cancer 25 years later. Integr. Cancer Ther. 2005, 4, 32–44. [Google Scholar] [CrossRef] [PubMed]
- Degroote, J.; Vergauwen, H.; Wang, W.; Van Ginneken, C.; De Smet, S.; Michiels, J. Changes of the glutathione redox system during the weaning transition in piglets, in relation to small intestinal morphology and barrier function. J. Anim. Sci. Biotechnol. 2020, 11, 45. [Google Scholar] [CrossRef] [PubMed]
- Colson, V.; Martin, E.; Orgeur, P.; Prunier, A. Influence of housing and social changes on growth, behaviour and cortisol in piglets at weaning. Physiol. Behav. 2012, 107, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.C.G.; Regazzi, F.M.; Lúcio, C.F.; Veiga, G.A.L.; Angrimani, D.S.R.; Fernandes, C.B.; Vannucchi, C.I. Redox, acid-base and clinical analysis of preterm and term neonatal lambs. Anim. Reprod. 2018, 15, 51–55. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chun, O.K.; Song, W.O. Plasma and Dietary Antioxidant Status as Cardiovascular Disease Risk Factors: A Review of Human Studies. Nutrients 2013, 5, 2969–3004. [Google Scholar] [CrossRef]
- Shahidi, F.; Zhong, Y. Lipid oxidation and improving the oxidative stability. Chem. Soc. Rev. 2010, 39, 4067–4079. [Google Scholar] [CrossRef] [PubMed]
- Papuc, C.; Goran, G.V.; Predescu, C.N.; Nicorescu, V. Mechanisms of Oxidative Processes in Meat and Toxicity Induced by Postprandial Degradation Products: A Review. Compr. Rev. Food Sci. Food Saf. 2017, 16, 96–123. [Google Scholar] [CrossRef]
- Nair, M.B.; Latha, M.S. Glutathione antioxidant status of frozen meat samples. J. Environ. Biol. 2005, 26, 681–685. [Google Scholar]
- Echegaray, N.; Pateiro, M.; Munekata, P.E.S.; Lorenzo, J.M.; Chabani, Z.; Farag, M.A.; Domínguez, R. Measurement of Antioxidant Capacity of Meat and Meat Products: Methods and Applications. Molecules 2021, 26, 3880. [Google Scholar] [CrossRef]
- Halliwell, B.; Gutteridge, J.M.C. Antioxidant defences synthesized in vivo. In Free Radicals in Biology and Medicine; Oxford University Press: Oxford, UK, 2015; ISBN 9780198717478. [Google Scholar]
- Skaperda, Z.; Argyriadou, A.; Nechalioti, P.M.; Alvanou, M.; Makri, S.; Bouroutzika, E.; Kyriazis, I.D.; Tekos, F.; Veskoukis, A.S.; Kallitsis, T.; et al. Redox Biomarker Baseline Levels in Cattle Tissues and Their Relationships with Meat Quality. Antioxidants 2021, 10, 958. [Google Scholar] [CrossRef]
- Yn, R.; Murthy, S.; Dr, K.; Prabhakar, M.C. Role of free radicals and antioxidants in tuberculosis patients. Indian J. Tuberc. 2004, 51, 213–218. [Google Scholar]
- Veskoukis, A.S.; Kyparos, A.; Paschalis, V.; Nikolaidis, M.G. Spectrophotometric assays for measuring redox biomarkers in blood. Biomarkers 2016, 21, 208–217. [Google Scholar] [CrossRef] [PubMed]
- Aebi, H.B.T.-M.E. [13] Catalase in vitro. In Oxygen Radicals in Biological Systems; Academic Press: Cambridge, MA, USA, 1984; Volume 105, pp. 121–126. ISBN 0076-6879. [Google Scholar]
- Janaszewska, A.; Bartosz, G. Assay of total antioxidant capacity: Comparison of four methods as applied to human blood plasma. Scand. J. Clin. Lab. Investig. 2002, 62, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Keles, M.S.; Taysi, S.; Sen, N.; Aksoy, H.; Akçay, F. Effect of corticosteroid therapy on serum and CSF malondialdehyde and antioxidant proteins in multiple sclerosis. Can. J. Neurol. Sci. 2001, 28, 141–143. [Google Scholar] [CrossRef] [PubMed]
- Spanidis, Y.; Goutzourelas, N.; Stagos, D.; Mpesios, A.; Priftis, A.; Bar-Or, D.; Spandidos, D.A.; Tsatsakis, A.M.; Leon, G.; Kouretas, D. Variations in oxidative stress markers in elite basketball players at the beginning and end of a season. Exp. Ther. Med. 2016, 11, 147–153. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Patsoukis, N.; Zervoudakis, G.; Panagopoulos, N.T.; Georgiou, C.D.; Angelatou, F.; Matsokis, N.A. Thiol redox state (TRS) and oxidative stress in the mouse hippocampus after pentylenetetrazol-induced epileptic seizure. Neurosci. Lett. 2004, 357, 83–86. [Google Scholar] [CrossRef]
- Estévez, M. Protein carbonyls in meat systems: A review. Meat Sci. 2011, 89, 259–279. [Google Scholar] [CrossRef]
- Berlett, B.S.; Stadtman, E.R. Protein oxidation in aging, disease, and oxidative stress. J. Biol. Chem. 1997, 272, 20313–20316. [Google Scholar] [CrossRef]
- Grune, T.; Merker, K.; Sandig, G.; Davies, K.J.A. Selective degradation of oxidatively modified protein substrates by the proteasome. Biochem. Biophys. Res. Commun. 2003, 305, 709–718. [Google Scholar] [CrossRef]
- Pérez-Estrada, J.R.; Hernández-García, D.; Leyva-Castro, F.; Ramos-León, J.; Cuevas-Benítez, O.; Díaz-Muñoz, M.; Castro-Obregón, S.; Ramírez-Solís, R.; García, C.; Covarrubias, L. Reduced lifespan of mice lacking catalase correlates with altered lipid metabolism without oxidative damage or premature aging. Free Radic. Biol. Med. 2019, 135, 102–115. [Google Scholar] [CrossRef]
- Townsend, D.M.; Tew, K.D.; Tapiero, H. The importance of glutathione in human disease. Biomed. Pharmacother. 2003, 57, 145–155. [Google Scholar] [CrossRef]
- Kusano, C.; Ferrari, B. Total antioxidant capacity: A biomarker in biomedical and nutritional studies. J. Cell Mol. Biol. 2008, 7, 1–15. [Google Scholar]
- Chen, K.; Liu, Y.; Cheng, Y.; Yan, Q.; Zhou, C.; He, Z.; Zeng, J.; He, J.; Tan, Z. Supplementation of Lactobacillus plantarum or Macleaya cordata Extract Alleviates Oxidative Damage Induced by Weaning in the Lower Gut of Young Goats. Animals 2020, 10, 548. [Google Scholar] [CrossRef]
- Eckert, E.; Brown, H.E.; Leslie, K.E.; DeVries, T.J.; Steele, M.A. Weaning age affects growth, feed intake, gastrointestinal development, and behavior in Holstein calves fed an elevated plane of nutrition during the preweaning stage. J. Dairy Sci. 2015, 98, 6315–6326. [Google Scholar] [CrossRef]
- Lallès, J.-P.; Bosi, P.; Smidt, H.; Stokes, C.R. Weaning—A challenge to gut physiologists. Livest. Sci. 2007, 108, 82–93. [Google Scholar] [CrossRef]
- Zhu, L.H.; Zhao, K.L.; Chen, X.L.; Xu, J.X. Impact of weaning and an antioxidant blend on intestinal barrier function and antioxidant status in pigs. J. Anim. Sci. 2012, 90, 2581–2589. [Google Scholar] [CrossRef] [PubMed]
- Supplementing Lambs with Minerals Post Weaning. Available online: https://www.teagasc.ie/publications/2020/supplementing-lambs-with-minerals-post-weaning.php (accessed on 7 April 2022).
- Luo, Z.; Zhu, W.; Guo, Q.; Luo, W.; Zhang, J.; Xu, W.; Xu, J. Weaning Induced Hepatic Oxidative Stress, Apoptosis, and Aminotransferases through MAPK Signaling Pathways in Piglets. Oxid. Med. Cell. Longev. 2016, 2016, 4768541. [Google Scholar] [CrossRef]
- Yin, J.; Wu, M.M.; Xiao, H.; Ren, W.K.; Duan, J.L.; Yang, G.; Li, T.J.; Yin, Y.L. Development of an antioxidant system after early weaning in piglets. J. Anim. Sci. 2014, 92, 612–619. [Google Scholar] [CrossRef]
- Griffith, O.W.; Meister, A. Glutathione: Interorgan translocation, turnover, and metabolism. Proc. Natl. Acad. Sci. USA 1979, 76, 5606–5610. [Google Scholar] [CrossRef]
- Poulianiti, K.P.; Karioti, A.; Kaltsatou, A.; Mitrou, G.I.; Koutedakis, Y.; Tepetes, K.; Christodoulidis, G.; Giakas, G.; Maridaki, M.D.; Stefanidis, I.; et al. Evidence of Blood and Muscle Redox Status Imbalance in Experimentally Induced Renal Insufficiency in a Rabbit Model. Oxid. Med. Cell. Longev. 2019, 2019, 8219283. [Google Scholar] [CrossRef]
- Veskoukis, A.S.; Nikolaidis, M.G.; Kyparos, A.; Kouretas, D. Blood reflects tissue oxidative stress depending on biomarker and tissue studied. Free Radic. Biol. Med. 2009, 47, 1371–1374. [Google Scholar] [CrossRef] [PubMed]




| D.s. | Biomarker | Tissues | r | p-Value |
|---|---|---|---|---|
| 70% | CARBs | Blood-psoas major | 0.6547 | 0.0209 |
| 25% | CAT | Blood-diaphragm | 0.6582 | 0.0200 |
| 100% | GSH | Blood-diaphragm | 0.6364 | 0.0261 |
| 25% | TAC | Blood-diaphragm | −0.5919 | 0.0426 |
| 25% | TBARS | Blood-diaphragm | 0.6583 | 0.0139 |
| 25% | TBARS | Blood-liver | 0.6600 | 0.0195 |
| 25% | TBARS | Blood-quadriceps | 0.6955 | 0.0120 |
| 25% | TBARS | Blood-psoas major | 0.7867 | 0.0024 |
| 100% | TBARS | Blood-liver | 0.8226 | 0.0010 |
| 100% | TBARS | Blood-quadriceps | 0.6406 | 0.0248 |
| 100% | TBARS | Blood-psoas major | 0.5677 | 0.0442 |
| D.s. | Biomarker | Tissues | r | p-Value |
|---|---|---|---|---|
| 25% | CARBS | Blood-liver | 0.4412 | 0.0398 |
| 35% | CAT | Blood-diaphragm | −0.4118 | 0.0456 |
| 100% | CAT | Blood-liver | 0.4276 | 0.0371 |
| 35% | GSH | Blood-quadriceps | −0.4703 | 0.0204 |
| 70% | GSH | Blood-diaphragm | 0.4398 | 0.0357 |
| 100% | GSH | Blood-psoas major | −0.5036 | 0.0121 |
| 35% | TBARS | Blood-psoas major | 0.4809 | 0.0174 |
| 100% | TBARS | Blood-diaphragm | 0.4837 | 0.0166 |
| 100% | TBARS | Blood-liver | 0.4034 | 0.0406 |
| 100% | TBARS | Blood-quadriceps | 0.6030 | 0.0018 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skaperda, Z.; Kyriazis, I.D.; Tekos, F.; Alvanou, M.V.; Nechalioti, P.-M.; Makri, S.; Argyriadou, A.; Vouraki, S.; Kallitsis, T.; Kourti, M.; et al. Determination of Redox Status in Different Tissues of Lambs and Kids and Their in-between Relationship. Antioxidants 2022, 11, 2065. https://doi.org/10.3390/antiox11102065
Skaperda Z, Kyriazis ID, Tekos F, Alvanou MV, Nechalioti P-M, Makri S, Argyriadou A, Vouraki S, Kallitsis T, Kourti M, et al. Determination of Redox Status in Different Tissues of Lambs and Kids and Their in-between Relationship. Antioxidants. 2022; 11(10):2065. https://doi.org/10.3390/antiox11102065
Chicago/Turabian StyleSkaperda, Zoi, Ioannis D. Kyriazis, Fotios Tekos, Maria V. Alvanou, Paraskevi-Maria Nechalioti, Sotiria Makri, Angeliki Argyriadou, Sotiria Vouraki, Theodoros Kallitsis, Maria Kourti, and et al. 2022. "Determination of Redox Status in Different Tissues of Lambs and Kids and Their in-between Relationship" Antioxidants 11, no. 10: 2065. https://doi.org/10.3390/antiox11102065
APA StyleSkaperda, Z., Kyriazis, I. D., Tekos, F., Alvanou, M. V., Nechalioti, P.-M., Makri, S., Argyriadou, A., Vouraki, S., Kallitsis, T., Kourti, M., Irene, V., Arsenos, G., & Kouretas, D. (2022). Determination of Redox Status in Different Tissues of Lambs and Kids and Their in-between Relationship. Antioxidants, 11(10), 2065. https://doi.org/10.3390/antiox11102065












