The Status of Oxidative Stress in Patients with Alcohol Dependence: A Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Literature Screening
2.2. Quality Assessment
2.3. Data Extraction
2.4. Data Analysis
3. Results
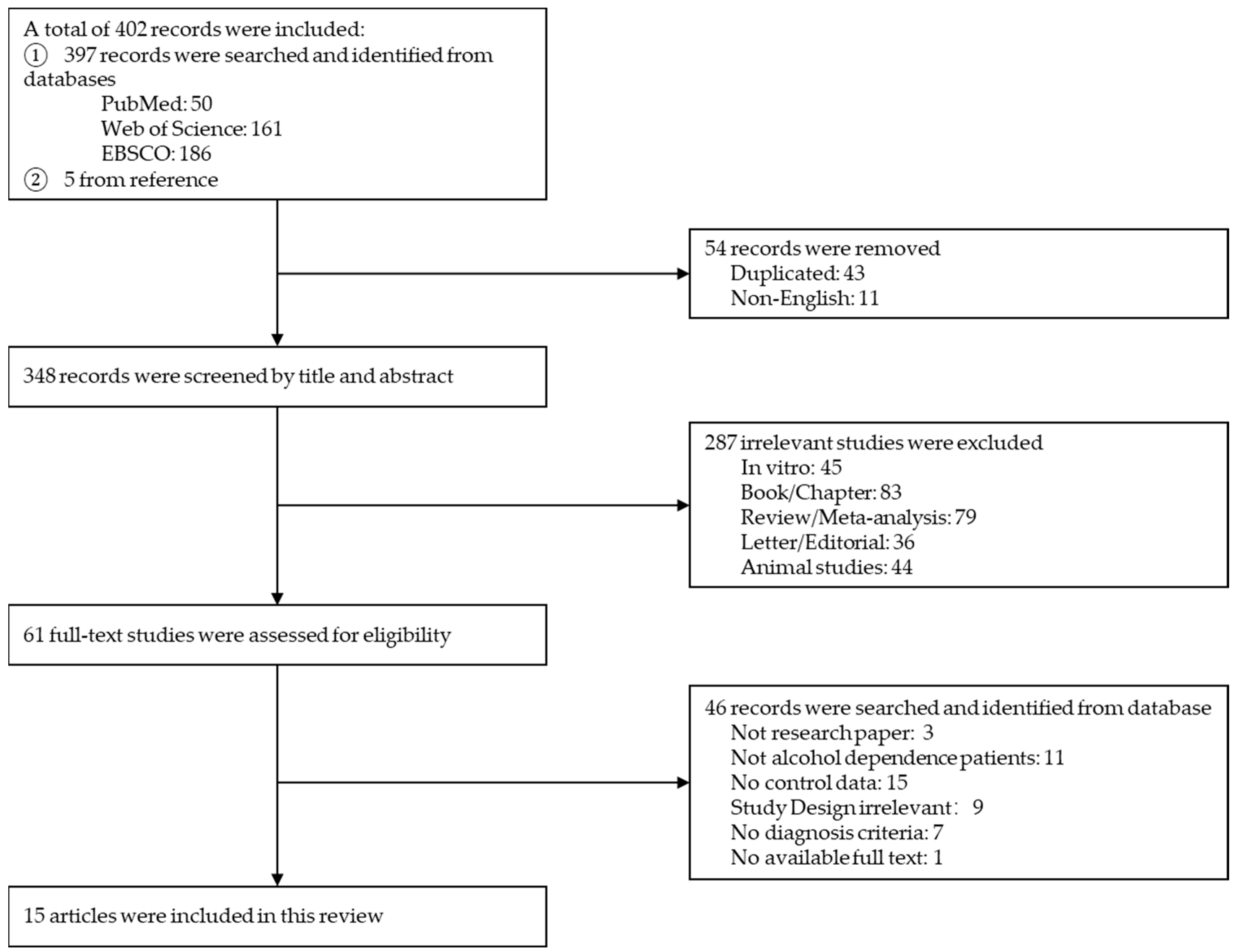
3.1. Literature Screening
3.2. Quality Assessment
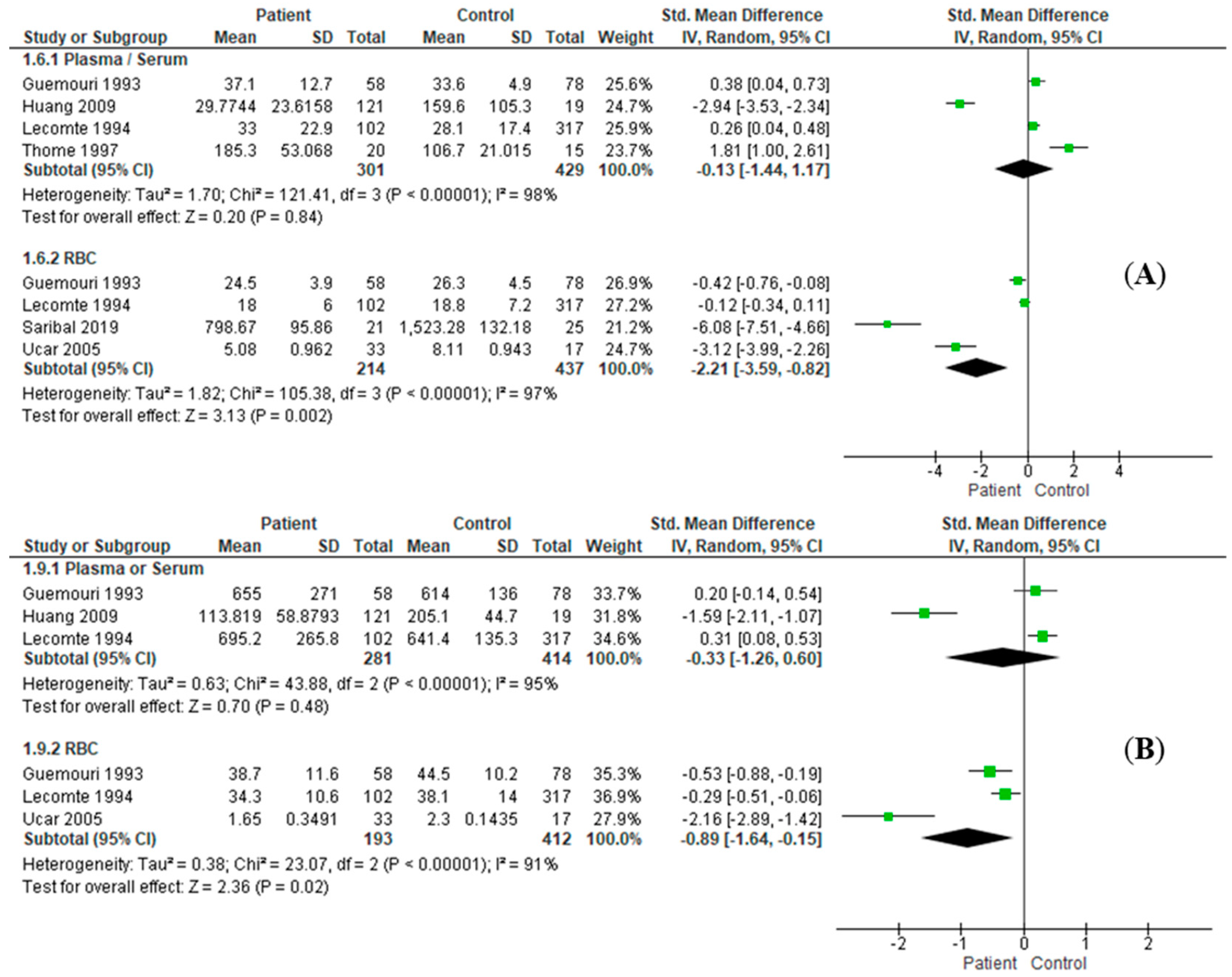
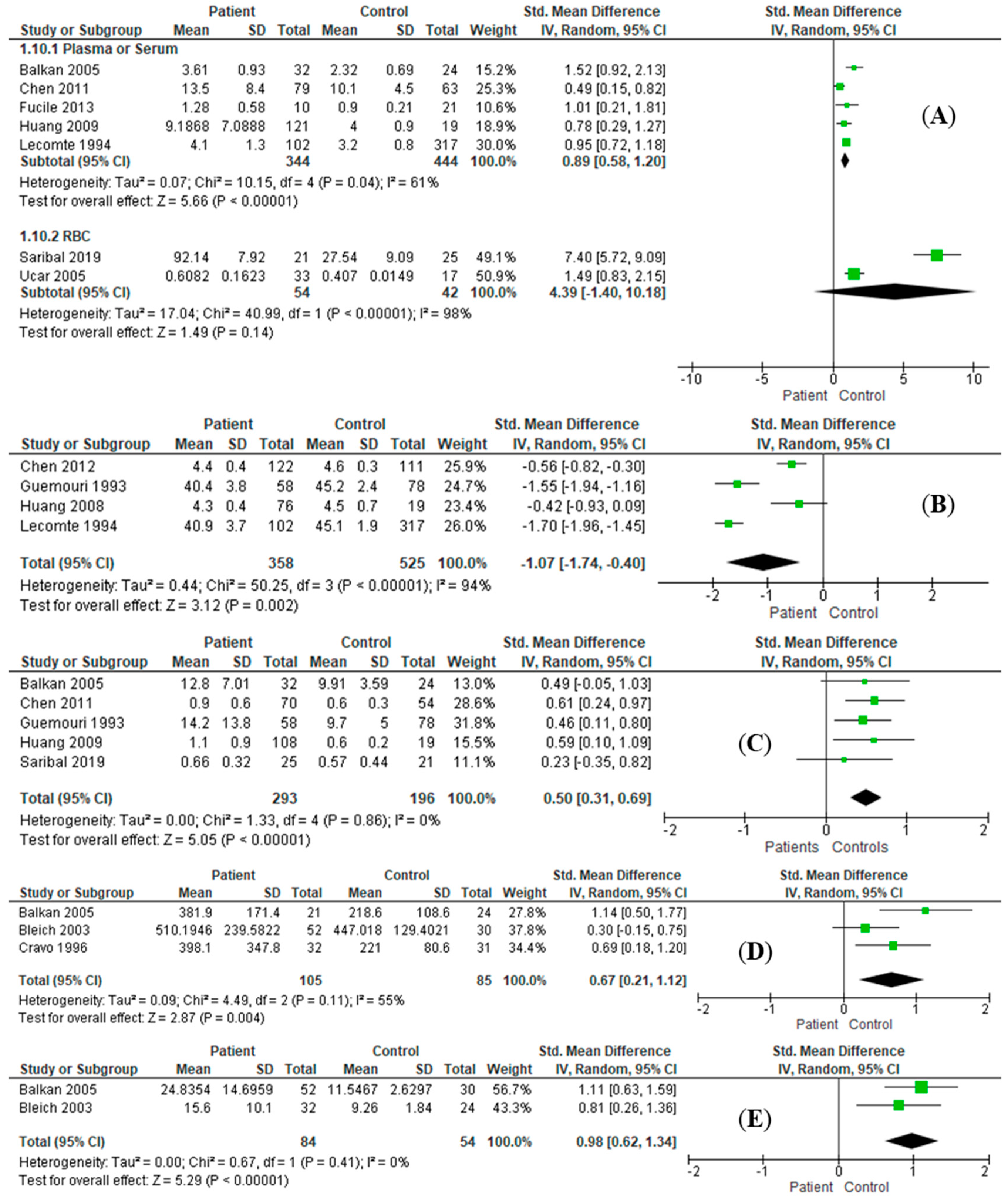
3.3. Effect Size Estimation
3.4. Sensitivity and Publication Bias Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Griswold, M.G.; Fullman, N.; Hawley, C.; Arian, N.; Zimsen, S.R.M.; Tymeson, H.D.; Venkateswaran, V.; Tapp, A.D.; Forouzanfar, M.H.; Salama, J.S.; et al. Alcohol use and burden for 195 countries and territories, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet 2018, 392, 1015–1035. [Google Scholar] [CrossRef]
- World Health Organization. Global Status Report on Alcohol and Health 2018; World Health Organization: Geneva, Switzerland, 2019. [Google Scholar]
- Teesson, M.; Hall, W.; Slade, T.; Mills, K.; Grove, R.; Mewton, L.; Baillie, A.; Haber, P. Prevalence and correlates of DSM-IV alcohol abuse and dependence in Australia: Findings of the 2007 National Survey of Mental Health and Wellbeing. Addiction 2010, 105, 2085–2094. [Google Scholar] [CrossRef]
- Cheng, H.G.; Deng, F.; Xiong, W.; Phillips, M.R. Prevalence of alcohol use disorders in mainland China: A systematic review. Addiction 2015, 110, 761–774. [Google Scholar] [CrossRef] [PubMed]
- Rehm, J.; Guiraud, J.; Poulnais, R.; Shield, K.D. Alcohol dependence and very high risk level of alcohol consumption: A life-threatening and debilitating disease. Addict. Biol. 2018, 23, 961–968. [Google Scholar] [CrossRef]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef] [PubMed]
- Sies, H.; Jones, D.P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Biol. 2020, 21, 363–383. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Sun, L.; Chen, X.; Zhang, D. Oxidative stress, mitochondrial damage and neurodegenerative diseases. Neural Regen. Res. 2013, 8, 2003–2014. [Google Scholar] [CrossRef]
- Kieroń, M.; Żekanowski, C.; Falk, A.; Wężyk, M. Oxidative DNA Damage Signalling in Neural Stem Cells in Alzheimer’s Disease. Oxidative Med. Cell. Longev. 2019, 2019, 2149812. [Google Scholar] [CrossRef]
- Koga, M.; Serritella, A.V.; Sawa, A.; Sedlak, T.W. Implications for reactive oxygen species in schizophrenia pathogenesis. Schizophr. Res. 2016, 176, 52–71. [Google Scholar] [CrossRef]
- Dorszewska, J.; Kowalska, M.; Prendecki, M.; Piekut, T.; Kozłowska, J.; Kozubski, W. Oxidative stress factors in Parkinson’s disease. Neural Regen. Res. 2021, 16, 1383–1391. [Google Scholar] [CrossRef]
- Kim, G.H.; Kim, J.E.; Rhie, S.J.; Yoon, S. The Role of Oxidative Stress in Neurodegenerative Diseases. Exp. Neurobiol. 2015, 24, 325–340. [Google Scholar] [CrossRef] [PubMed]
- Akanji, M.A.; Rotimi, D.E.; Elebiyo, T.C.; Awakan, O.J.; Adeyemi, O.S. Redox Homeostasis and Prospects for Therapeutic Targeting in Neurodegenerative Disorders. Oxidative Med. Cell. Longev. 2021, 2021, 9971885. [Google Scholar] [CrossRef] [PubMed]
- Behl, T.; Makkar, R.; Sehgal, A.; Singh, S.; Sharma, N.; Zengin, G.; Bungau, S.; Andronie-Cioara, F.L.; Munteanu, M.A.; Brisc, M.C.; et al. Current Trends in Neurodegeneration: Cross Talks between Oxidative Stress, Cell Death, and Inflammation. Int. J. Mol. Sci. 2021, 22, 7432. [Google Scholar] [CrossRef] [PubMed]
- Aragon, C.M.; Rogan, F.; Amit, Z. Ethanol metabolism in rat brain homogenates by a catalase-H2O2 system. Biochem. Pharmacol. 1992, 44, 93–98. [Google Scholar] [CrossRef]
- Wilson, D.F.; Matschinsky, F.M. Ethanol metabolism: The good, the bad, and the ugly. Med. Hypotheses 2020, 140, 109638. [Google Scholar] [CrossRef]
- Haorah, J.; Ramirez, S.H.; Floreani, N.; Gorantla, S.; Morsey, B.; Persidsky, Y. Mechanism of alcohol-induced oxidative stress and neuronal injury. Free Radic. Biol. Med. 2008, 45, 1542–1550. [Google Scholar] [CrossRef] [PubMed]
- Zimatkin, S.M.; Buben, A.L. Ethanol oxidation in the living brain. Alcohol Alcohol. 2007, 42, 529–532. [Google Scholar] [CrossRef]
- Birkova, A.; Hubkova, B.; Cizmarova, B.; Bolerazska, B. Current View on the Mechanisms of Alcohol-Mediated Toxicity. Int. J. Mol. Sci. 2021, 22, 9686. [Google Scholar] [CrossRef]
- Heit, C.; Dong, H.; Chen, Y.; Thompson, D.C.; Deitrich, R.A.; Vasiliou, V.K. The role of CYP2E1 in alcohol metabolism and sensitivity in the central nervous system. Subcell. Biochem. 2013, 67, 235–247. [Google Scholar] [CrossRef]
- Zhong, Y.; Dong, G.; Luo, H.; Cao, J.; Wang, C.; Wu, J.; Feng, Y.Q.; Yue, J. Induction of brain CYP2E1 by chronic ethanol treatment and related oxidative stress in hippocampus, cerebellum, and brainstem. Toxicology 2012, 302, 275–284. [Google Scholar] [CrossRef]
- Rodriguez, F.D.; Covenas, R. Biochemical Mechanisms Associating Alcohol Use Disorders with Cancers. Cancers 2021, 13, 3548. [Google Scholar] [CrossRef]
- Das, S.K.; Vasudevan, D.M. Alcohol-induced oxidative stress. Life Sci. 2007, 81, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Bitanihirwe, B.K.; Woo, T.U. Oxidative stress in schizophrenia: An integrated approach. Neurosci. Biobehav. Rev. 2011, 35, 878–893. [Google Scholar] [CrossRef] [PubMed]
- Thome, J.; Foley, P.; Gsell, W.; Davids, E.; Wodarz, N.; Wiesbeck, G.A.; Boning, J.; Riederer, P. Increased concentrations of manganese superoxide dismutase in serum of alcohol-dependent patients. Alcohol Alcohol. 1997, 32, 65–69. [Google Scholar] [CrossRef] [PubMed]
- Guemouri, L.; Lecomte, E.; Herbeth, B.; Pirollet, P.; Paille, F.; Siest, G.; Artur, Y. Blood activities of antioxidant enzymes in alcoholics before and after withdrawal. J. Stud. Alcohol 1993, 54, 626–629. [Google Scholar] [CrossRef] [PubMed]
- Demirci-Cekic, S.; Ozkan, G.; Avan, A.N.; Uzunboy, S.; Capanoglu, E.; Apak, R. Biomarkers of Oxidative Stress and Antioxidant Defense. J. Pharm. Biomed. Anal. 2022, 209, 114477. [Google Scholar] [CrossRef]
- Birben, E.; Sahiner, U.M.; Sackesen, C.; Erzurum, S.; Kalayci, O. Oxidative stress and antioxidant defense. World Allergy Organ. J. 2012, 5, 9–19. [Google Scholar] [CrossRef]
- Balkan, J.; Vural, P.; Oztezcan, S.; Mirsal, H.; Beyazyurek, M.; Aykac-Toker, G.; Uysal, M. Increased LDL+VLDL oxidizability and plasma homocysteine levels in chronic alcoholic patients. J. Nutr. Sci. Vitaminol. 2005, 51, 99–103. [Google Scholar] [CrossRef][Green Version]
- Saribal, D.; Hocaoglu-Emre, F.S.; Karaman, F.; Mirsal, H.; Akyolcu, M.C. Trace Element Levels and Oxidant/Antioxidant Status in Patients with Alcohol Abuse. Biol. Trace Elem. Res. 2020, 193, 7–13. [Google Scholar] [CrossRef]
- Cravo, M.L.; Gloria, L.M.; Selhub, J.; Nadeau, M.R.; Camilo, M.E.; Resende, M.P.; Cardoso, J.N.; Leitao, C.N.; Mira, F.C. Hyperhomocysteinemia in chronic alcoholism: Correlation with folate, vitamin B-12, and vitamin B-6 status. Am. J. Clin. Nutr. 1996, 63, 220–224. [Google Scholar] [CrossRef]
- Huang, M.C.; Chen, C.H.; Peng, F.C.; Tang, S.H.; Chen, C.C. Alterations in oxidative stress status during early alcohol withdrawal in alcoholic patients. J. Formos. Med. Assoc. 2009, 108, 560–569. [Google Scholar] [CrossRef]
- Lecomte, E.; Herbeth, B.; Pirollet, P.; Chancerelle, Y.; Arnaud, J.; Musse, N.; Paille, F.; Siest, G.; Artur, Y. Effect of alcohol consumption on blood antioxidant nutrients and oxidative stress indicators. Am. J. Clin. Nutr. 1994, 60, 255–261. [Google Scholar] [CrossRef]
- Heymann, H.M.; Gardner, A.M.; Gross, E.R. Aldehyde-Induced DNA and Protein Adducts as Biomarker Tools for Alcohol Use Disorder. Trends Mol. Med. 2018, 24, 144–155. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.H.; Pan, C.H.; Chen, C.C.; Huang, M.C. Increased oxidative DNA damage in patients with alcohol dependence and its correlation with alcohol withdrawal severity. Alcohol. Clin. Exp. Res. 2011, 35, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Fucile, C.; Marini, V.; Zuccoli, M.L.; Leone, S.; Robbiano, L.; Martelli, A.; Mattioli, F. HPLC determination of malondialdehyde as biomarker for oxidative stress: Application in patients with alcohol dependence. Clin. Lab. 2013, 59, 837–841. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.C.; Chen, C.C.; Peng, F.C.; Tang, S.H.; Chen, C.H. The correlation between early alcohol withdrawal severity and oxidative stress in patients with alcohol dependence. Prog. Neuropsychopharmacol. Biol. Psychiatry 2009, 33, 66–69. [Google Scholar] [CrossRef]
- Kapaki, E.; Liappas, I.; Lyras, L.; Paraskevas, G.P.; Mamali, I.; Theotoka, I.; Bourboulis, N.; Liosis, I.; Petropoulou, O.; Soldatos, K. Oxidative damage to plasma proteins in patients with chronic alcohol dependence: The effect of smoking. In Vivo 2007, 21, 523–528. [Google Scholar]
- Jacob, A.; Wang, P. Alcohol Intoxication and Cognition: Implications on Mechanisms and Therapeutic Strategies. Front. Neurosci. 2020, 14, 102. [Google Scholar] [CrossRef]
- Beck, A.; Wustenberg, T.; Genauck, A.; Wrase, J.; Schlagenhauf, F.; Smolka, M.N.; Mann, K.; Heinz, A. Effect of brain structure, brain function, and brain connectivity on relapse in alcohol-dependent patients. Arch. Gen. Psychiatry 2012, 69, 842–852. [Google Scholar] [CrossRef]
- Mattson, S.N.; Schoenfeld, A.M.; Riley, E.P. Teratogenic effects of alcohol on brain and behavior. Alcohol Res. Health 2001, 25, 185–191. [Google Scholar]
- Sechi, G.; Serra, A. Wernicke’s encephalopathy: New clinical settings and recent advances in diagnosis and management. Lancet Neurol. 2007, 6, 442–455. [Google Scholar] [CrossRef]
- Maillard, A.; Laniepce, A.; Cabe, N.; Boudehent, C.; Chetelat, G.; Urso, L.; Eustache, F.; Vabret, F.; Segobin, S.; Pitel, A.L. Temporal Cognitive and Brain Changes in Korsakoff Syndrome. Neurology 2021, 96, e1987–e1998. [Google Scholar] [CrossRef]
- El Haj, M.; Moustafa, A.A.; Nandrino, J.L. Future Thinking in Korsakoff Syndrome. Alcohol Alcohol. 2019, 54, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative stress, aging, and diseases. Clin. Interv. Aging 2018, 13, 757–772. [Google Scholar] [CrossRef] [PubMed]
- Praud, D.; Rota, M.; Rehm, J.; Shield, K.; Zatonski, W.; Hashibe, M.; La Vecchia, C.; Boffetta, P. Cancer incidence and mortality attributable to alcohol consumption. Int. J. Cancer 2016, 138, 1380–1387. [Google Scholar] [CrossRef]
- Shield, K.; Manthey, J.; Rylett, M.; Probst, C.; Wettlaufer, A.; Parry, C.D.H.; Rehm, J. National, regional, and global burdens of disease from 2000 to 2016 attributable to alcohol use: A comparative risk assessment study. Lancet Public Health 2020, 5, e51–e61. [Google Scholar] [CrossRef]
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A.; Group, P.-P. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 2015, 4, 1. [Google Scholar] [CrossRef]
- Luo, D.; Wan, X.; Liu, J.; Tong, T. Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile range. Stat. Methods Med. Res. 2018, 27, 1785–1805. [Google Scholar] [CrossRef]
- Wan, X.; Wang, W.; Liu, J.; Tong, T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med. Res. Methodol. 2014, 14, 135. [Google Scholar] [CrossRef]
- Cai, S.; Zhou, J.; Pan, J. Estimating the sample mean and standard deviation from order statistics and sample size in meta-analysis. Stat. Methods Med. Res. 2021, 30, 2701–2719. [Google Scholar] [CrossRef]
- Bleich, S.; Bandelow, B.; Javaheripour, K.; Müller, A.; Degner, D.; Wilhelm, J.; Havemann-Reinecke, U.; Sperling, W.; Rüther, E.; Kornhuber, J. Hyperhomocysteinemia as a new risk factor for brain shrinkage in patients with alcoholism. Neurosci. Lett. 2003, 335, 179–182. [Google Scholar] [CrossRef]
- Peng, F.C.; Tang, S.H.; Huang, M.C.; Chen, C.C.; Kuo, T.L.; Yin, S.J. Oxidative status in patients with alcohol dependence: A clinical study in Taiwan. J. Toxicol. Environ. Health A 2005, 68, 1497–1509. [Google Scholar] [CrossRef] [PubMed]
- Ucar, G.; Demir, B.; Ulug, B. Lipid peroxidation and antioxidant enzyme activities in erythrocytes of type I and II alcoholics. Cell Biochem. Funct. 2005, 23, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.H.; Walker, J.; Momenan, R.; Rawlings, R.; Heilig, M.; Hommer, D.W. Relationship between liver function and brain shrinkage in patients with alcohol dependence. Alcohol. Clin. Exp. Res. 2012, 36, 625–632. [Google Scholar] [CrossRef]
- Ahern, J.; Galea, S.; Hubbard, A.; Midanik, L.; Syme, S.L. “Culture of Drinking” and Individual Problems with Alcohol Use. Am. J. Epidemiol. 2008, 167, 1041–1049. [Google Scholar] [CrossRef]
- Savic, M.; Room, R.; Mugavin, J.; Pennay, A.; Livingston, M. Defining “drinking culture”: A critical review of its meaning and connotation in social research on alcohol problems. Drugs Educ. Prev. Policy 2016, 23, 270–282. [Google Scholar] [CrossRef]
- Burton, R.; Sheron, N. No level of alcohol consumption improves health. Lancet 2018, 392, 987–988. [Google Scholar] [CrossRef]
- Devries, K.M.; Child, J.C.; Bacchus, L.J.; Mak, J.; Falder, G.; Graham, K.; Watts, C.; Heise, L. Intimate partner violence victimization and alcohol consumption in women: A systematic review and meta-analysis. Addiction 2014, 109, 379–391. [Google Scholar] [CrossRef]
- Collaborators, G.B.D.C.O.D. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980-2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1736–1788. [Google Scholar] [CrossRef]
- Darvishi, N.; Farhadi, M.; Haghtalab, T.; Poorolajal, J. Alcohol-related risk of suicidal ideation, suicide attempt, and completed suicide: A meta-analysis. PLoS ONE 2015, 10, e0126870. [Google Scholar] [CrossRef]
- Romano, E.; Torres-Saavedra, P.A.; Calderon Cartagena, H.I.; Voas, R.B.; Ramirez, A. Alcohol-Related Risk of Driver Fatalities in Motor Vehicle Crashes: Comparing Data From 2007 and 2013–2014. J. Stud. Alcohol Drugs 2018, 79, 547–552. [Google Scholar] [CrossRef] [PubMed]
- Diseases, G.B.D.; Injuries, C. Global burden of 369 diseases and injuries in 204 countries and territories, 1990-2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1204–1222. [Google Scholar] [CrossRef]
- Driscoll, T.R.; Harrison, J.A.; Steenkamp, M. Review of the role of alcohol in drowning associated with recreational aquatic activity. Inj. Prev. 2004, 10, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Chikritzhs, T.; Livingston, M. Alcohol and the Risk of Injury. Nutrients 2021, 13, 2777. [Google Scholar] [CrossRef]
- McNeilly, B.; Ibrahim, J.E.; Bugeja, L.; Ozanne-Smith, J. The prevalence of work-related deaths associated with alcohol and drugs in Victoria, Australia, 2001–2006. Inj. Prev. 2010, 16, 423–428. [Google Scholar] [CrossRef]
- de la Monte, S.M.; Kril, J.J. Human alcohol-related neuropathology. Acta Neuropathol. 2014, 127, 71–90. [Google Scholar] [CrossRef]
- Mukamal, K.; Lazo, M. Alcohol and cardiovascular disease. BMJ 2017, 356, j1340. [Google Scholar] [CrossRef]
- Dey, A.; Cederbaum, A.I. Alcohol and oxidative liver injury. Hepatology 2006, 43, S63–S74. [Google Scholar] [CrossRef]
- Pohl, K.; Moodley, P.; Dhanda, A.D. Alcohol’s Impact on the Gut and Liver. Nutrients 2021, 13, 3170. [Google Scholar] [CrossRef]
- Ogden, G.R. Alcohol and mouth cancer. Br. Dent. J. 2018, 225, 880–883. [Google Scholar] [CrossRef]
- Morojele, N.K.; Shenoi, S.V.; Shuper, P.A.; Braithwaite, R.S.; Rehm, J. Alcohol Use and the Risk of Communicable Diseases. Nutrients 2021, 13, 3317. [Google Scholar] [CrossRef] [PubMed]
- Rosa, A.C.; Corsi, D.; Cavi, N.; Bruni, N.; Dosio, F. Superoxide Dismutase Administration: A Review of Proposed Human Uses. Molecules 2021, 26, 1844. [Google Scholar] [CrossRef]
- Zhao, H.; Zhang, R.; Yan, X.; Fan, K. Superoxide dismutase nanozymes: An emerging star for anti-oxidation. J. Mater. Chem. B 2021, 9, 6939–6957. [Google Scholar] [CrossRef] [PubMed]
- Eleutherio, E.C.A.; Magalhães, R.S.S.; de Araujo Brasil, A.; Neto, J.R.M.; de Holanda Paranhos, L. SOD1, more than just an antioxidant. Arch. Biochem. Biophys. 2021, 697, 108701. [Google Scholar] [CrossRef] [PubMed]
- Islinger, M.; Li, K.W.; Seitz, J.; Völkl, A.; Lüers, G.H. Hitchhiking of Cu/Zn Superoxide Dismutase to Peroxisomes - Evidence for a Natural Piggyback Import Mechanism in Mammals. Traffic 2009, 10, 1711–1721. [Google Scholar] [CrossRef] [PubMed]
- Sturtz, L.A.; Diekert, K.; Jensen, L.T.; Lill, R.; Culotta, V.C. A fraction of yeast Cu,Zn-superoxide dismutase and its metallochaperone, CCS, localize to the intermembrane space of mitochondria. A physiological role for SOD1 in guarding against mitochondrial oxidative damage. J. Biol. Chem. 2001, 276, 38084–38089. [Google Scholar] [CrossRef] [PubMed]
- Jabri, M.A.; Sani, M.; Rtibi, K.; Marzouki, L.; El-Benna, J.; Sakly, M.; Sebai, H. Chamomile decoction extract inhibits human neutrophils ROS production and attenuates alcohol-induced haematological parameters changes and erythrocytes oxidative stress in rat. Lipids Health Dis. 2016, 15, 65. [Google Scholar] [CrossRef]
- Reddy, K.R.; Reddy, V.D.; Padmavathi, P.; Kavitha, G.; Saradamma, B.; Varadacharyulu, N.C. Gender differences in alcohol-induced oxidative stress and altered membrane properties in erythrocytes of rats. Indian J. Biochem. Biophys. 2013, 50, 32–39. [Google Scholar]
- Zweier, J.L.; Hemann, C.; Kundu, T.; Ewees, M.G.; Khaleel, S.A.; Samouilov, A.; Ilangovan, G.; El-Mahdy, M.A. Cytoglobin has potent superoxide dismutase function. Proc. Natl. Acad. Sci. USA 2021, 118, e2105053118. [Google Scholar] [CrossRef]
- Hillesund, E.R.; Overby, N.C.; Valen, E.L.; Engeset, D. Alcohol consumption among students and its relationship with nutritional intake: A cross-sectional study. Public Health Nutr. 2021, 24, 2877–2888. [Google Scholar] [CrossRef]
- Tardelli, V.S.; Lago, M.; Silveira, D.X.D.; Fidalgo, T.M. Vitamin D and alcohol: A review of the current literature. Psychiatry Res. 2017, 248, 83–86. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.Q.; Bo, Q.L.; Chu, L.L.; Hu, Y.D.; Fu, L.; Wang, G.X.; Lu, Y.; Liu, X.J.; Wang, H.; Xu, D.X. Vitamin D Deficiency Aggravates Hepatic Oxidative Stress and Inflammation during Chronic Alcohol-Induced Liver Injury in Mice. Oxid. Med. Cell. Longev. 2020, 2020, 5715893. [Google Scholar] [CrossRef] [PubMed]
- Santiard, D.; Ribiere, C.; Nordmann, R.; Houee-Levin, C. Inactivation of Cu,Zn-superoxide dismutase by free radicals derived from ethanol metabolism: A gamma radiolysis study. Free Radic. Biol. Med. 1995, 19, 121–127. [Google Scholar] [CrossRef]
- Reddy, V.D.; Padmavathi, P.; Bulle, S.; Hebbani, A.V.; Marthadu, S.B.; Venugopalacharyulu, N.C.; Maturu, P.; Varadacharyulu, N.C. Association between alcohol-induced oxidative stress and membrane properties in synaptosomes: A protective role of vitamin E. Neurotoxicol. Teratol. 2017, 63, 60–65. [Google Scholar] [CrossRef]
- Pari, L.; Suresh, A. Effect of grape (Vitis vinifera L.) leaf extract on alcohol induced oxidative stress in rats. Food Chem. Toxicol. 2008, 46, 1627–1634. [Google Scholar] [CrossRef] [PubMed]
- Margis, R.; Dunand, C.; Teixeira, F.K.; Margis-Pinheiro, M. Glutathione peroxidase family—An evolutionary overview. FEBS J. 2008, 275, 3959–3970. [Google Scholar] [CrossRef]
- Brigelius-Flohe, R.; Flohe, L. Regulatory Phenomena in the Glutathione Peroxidase Superfamily. Antioxid. Redox Signal. 2020, 33, 498–516. [Google Scholar] [CrossRef]
- Brigelius-Flohe, R.; Maiorino, M. Glutathione peroxidases. Biochim. Biophys. Acta 2013, 1830, 3289–3303. [Google Scholar] [CrossRef]
- Pastori, D.; Pignatelli, P.; Farcomeni, A.; Menichelli, D.; Nocella, C.; Carnevale, R.; Violi, F. Aging-Related Decline of Glutathione Peroxidase 3 and Risk of Cardiovascular Events in Patients With Atrial Fibrillation. J. Am. Heart Assoc. 2016, 5, e003682. [Google Scholar] [CrossRef]
- Abarikwu, S.O.; Duru, Q.C.; Chinonso, O.V.; Njoku, R.C. Antioxidant enzymes activity, lipid peroxidation, oxidative damage in the testis and epididymis, and steroidogenesis in rats after co-exposure to atrazine and ethanol. Andrologia 2016, 48, 548–557. [Google Scholar] [CrossRef]
- Soylu, A.R.; Altaner, S.; Aydodu, N.; Basaran, U.N.; Tarcin, O.; Gedik, N.; Umit, H.; Tezel, A.; Ture, M.; Kutlu, K.; et al. Effects of vitamins E and C supplementation on hepatic glutathione peroxidase activity and tissue injury associated with ethanol ingestion in malnourished rats. Curr. Ther. Res. Clin. Exp. 2006, 67, 118–137. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pivetta, L.A.; Pereira, R.P.; Farinon, M.; de Bem, A.F.; Perottoni, J.; Soares, J.C.; Duarte, M.M.; Zeni, G.; Rocha, J.B.; Farina, M. Ethanol inhibits delta-aminolevulinate dehydratase and glutathione peroxidase activities in mice liver: Protective effects of ebselen and N-acetylcysteine. Environ. Toxicol. Pharmacol. 2006, 21, 338–343. [Google Scholar] [CrossRef]
- Guan, T.; Song, J.; Wang, Y.; Guo, L.; Yuan, L.; Zhao, Y.; Gao, Y.; Lin, L.; Wang, Y.; Wei, J. Expression and characterization of recombinant bifunctional enzymes with glutathione peroxidase and superoxide dismutase activities. Free Radic. Biol. Med. 2017, 110, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Brasiliano, S.; Carezzato, F.; Hochgraf, P.B. Gender, Alcohol Dependence, and Public Policies. In Drugs and Human Behavior: Biopsychosocial Aspects of Psychotropic Substances Use; De Micheli, D., Andrade, A.L.M., Reichert, R.A., Silva, E.A.D., Pinheiro, B.D.O., Lopes, F.M., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 333–343. [Google Scholar]
- Ribiere, C.; Hininger, I.; Rouach, H.; Nordmann, R. Effects of chronic ethanol administration on free radical defence in rat myocardium. Biochem. Pharmacol. 1992, 44, 1495–1500. [Google Scholar] [CrossRef]
- Rhoads, D.E.; Contreras, C.; Fathalla, S. Brain Levels of Catalase Remain Constant through Strain, Developmental, and Chronic Alcohol Challenges. Enzyme Res. 2012, 2012, 572939. [Google Scholar] [CrossRef]
- Del Rio, D.; Stewart, A.J.; Pellegrini, N. A review of recent studies on malondialdehyde as toxic molecule and biological marker of oxidative stress. Nutr. Metab. Cardiovasc. Dis. 2005, 15, 316–328. [Google Scholar] [CrossRef] [PubMed]
- Draper, H.H.; Hadley, M. A review of recent studies on the metabolism of exogenous and endogenous malondialdehyde. Xenobiotica 1990, 20, 901–907. [Google Scholar] [CrossRef]
- Ayala, A.; Munoz, M.F.; Arguelles, S. Lipid peroxidation: Production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid. Med. Cell. Longev. 2014, 2014, 360438. [Google Scholar] [CrossRef]
- Hebbani, A.V.; Vaddi, D.R.; Dd, P.P.; Varadacharyulu, N.C. Protective effect of Terminalia arjuna against alcohol induced oxidative damage of rat erythrocyte membranes. J. Ayurveda Integr. Med. 2021, 12, 330–339. [Google Scholar] [CrossRef]
- Rabai, M.; Detterich, J.A.; Wenby, R.B.; Toth, K.; Meiselman, H.J. Effects of ethanol on red blood cell rheological behavior. Clin. Hemorheol. Microcirc. 2014, 56, 87–99. [Google Scholar] [CrossRef]
- Bulle, S.; Reddy, V.D.; Padmavathi, P.; Maturu, P.; Puvvada, P.K.; Nallanchakravarthula, V. Association between alcohol-induced erythrocyte membrane alterations and hemolysis in chronic alcoholics. J. Clin. Biochem. Nutr. 2017, 60, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Janero, D.R.; Burghardt, B. Thiobarbituric acid-reactive malondialdehyde formation during superoxide-dependent, iron-catalyzed lipid peroxidation: Influence of peroxidation conditions. Lipids 1989, 24, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Yang, Y.J.; Yang, Y.K.; Oh, S.Y.; Hong, Y.C.; Lee, E.K.; Kwon, O. Diet quality scores and oxidative stress in Korean adults. Eur. J. Clin. Nutr. 2011, 65, 1271–1278. [Google Scholar] [CrossRef] [PubMed]
- Zebrowska, E.; Maciejczyk, M.; Zendzian-Piotrowska, M.; Zalewska, A.; Chabowski, A. High Protein Diet Induces Oxidative Stress in Rat Cerebral Cortex and Hypothalamus. Int. J. Mol. Sci. 2019, 20, 1547. [Google Scholar] [CrossRef]
- Barrera, G.; Pizzimenti, S.; Daga, M.; Dianzani, C.; Arcaro, A.; Cetrangolo, G.P.; Giordano, G.; Cucci, M.A.; Graf, M.; Gentile, F. Lipid Peroxidation-Derived Aldehydes, 4-Hydroxynonenal and Malondialdehyde in Aging-Related Disorders. Antioxidants 2018, 7, 102. [Google Scholar] [CrossRef] [PubMed]
- Toto, A.; Wild, P.; Graille, M.; Turcu, V.; Creze, C.; Hemmendinger, M.; Sauvain, J.J.; Bergamaschi, E.; Guseva Canu, I.; Hopf, N.B. Urinary Malondialdehyde (MDA) Concentrations in the General Population—A Systematic Literature Review and Meta-Analysis. Toxics 2022, 10, 160. [Google Scholar] [CrossRef]
- Mancuso, C.; Pani, G.; Calabrese, V. Bilirubin: An endogenous scavenger of nitric oxide and reactive nitrogen species. Redox Rep. 2006, 11, 207–213. [Google Scholar] [CrossRef]
- Vitek, L.; Ostrow, J.D. Bilirubin chemistry and metabolism; harmful and protective aspects. Curr. Pharm. Des. 2009, 15, 2869–2883. [Google Scholar] [CrossRef]
- Keshavan, P.; Deem, T.L.; Schwemberger, S.J.; Babcock, G.F.; Cook-Mills, J.M.; Zucker, S.D. Unconjugated bilirubin inhibits VCAM-1-mediated transendothelial leukocyte migration. J. Immunol. 2005, 174, 3709–3718. [Google Scholar] [CrossRef]
- Salma, N.U.; Peddha, M.S.; Setty, J.L.A. Ameliorative effect of flaxseed (Linum usitatissimum) and its protein on ethanol-induced hepatotoxicity in Wistar rats. J. Food Biochem. 2019, 43, e13047. [Google Scholar] [CrossRef]
- Reddy, V.D.; Padmavathi, P.; Paramahamsa, M.; Varadacharyulu, N.C. Amelioration of alcohol-induced oxidative stress by Emblica officinalis (amla) in rats. Indian J. Biochem. Biophys. 2010, 47, 20–25. [Google Scholar] [PubMed]
- Wang, G.; Fu, Y.; Li, J.; Li, Y.; Zhao, Q.; Hu, A.; Xu, C.; Shao, D.; Chen, W. Aqueous extract of Polygonatum sibiricum ameliorates ethanol-induced mice liver injury via regulation of the Nrf2/ARE pathway. J. Food Biochem. 2021, 45, e13537. [Google Scholar] [CrossRef] [PubMed]
- Foti, R.S.; Fisher, M.B. Assessment of UDP-glucuronosyltransferase catalyzed formation of ethyl glucuronide in human liver microsomes and recombinant UGTs. Forensic Sci. Int. 2005, 153, 109–116. [Google Scholar] [CrossRef]
- Creeden, J.F.; Gordon, D.M.; Stec, D.E.; Hinds, T.D., Jr. Bilirubin as a metabolic hormone: The physiological relevance of low levels. Am. J. Physiol. Endocrinol. Metab. 2021, 320, E191–E207. [Google Scholar] [CrossRef] [PubMed]
- Adin, C.A. Bilirubin as a Therapeutic Molecule: Challenges and Opportunities. Antioxidants 2021, 10, 1536. [Google Scholar] [CrossRef]
- Silva, W.R.D.; Dos Santos, A.A.; Xerez, M.C.; de Morais, E.F.; de Oliveira, P.T.; Silveira, E. Recognition and management of vitamin B12 deficiency: Report of four cases with oral manifestations. Spec. Care Dentist. 2021, 42, 410–415. [Google Scholar] [CrossRef] [PubMed]
- Langan, R.C.; Goodbred, A.J. Vitamin B12 Deficiency: Recognition and Management. Am. Fam. Physician 2017, 96, 384–389. [Google Scholar] [PubMed]
- Ermens, A.A.; Vlasveld, L.T.; Lindemans, J. Significance of elevated cobalamin (vitamin B12) levels in blood. Clin. Biochem. 2003, 36, 585–590. [Google Scholar] [CrossRef]
- Andres, E.; Serraj, K.; Zhu, J.; Vermorken, A.J. The pathophysiology of elevated vitamin B12 in clinical practice. QJM 2013, 106, 505–515. [Google Scholar] [CrossRef]
- Baker, H.; Leevy, C.B.; DeAngelis, B.; Frank, O.; Baker, E.R. Cobalamin (vitamin B12) and holotranscobalamin changes in plasma and liver tissue in alcoholics with liver disease. J. Am. Coll. Nutr. 1998, 17, 235–238. [Google Scholar] [CrossRef]
- Cylwik, B.; Czygier, M.; Daniluk, M.; Chrostek, L.; Szmitkowski, M. Vitamin B12 concentration in the blood of alcoholics. Pol. Merkur Lek. 2010, 28, 122–125. [Google Scholar]
- Selhub, J. Homocysteine metabolism. Annu. Rev. Nutr. 1999, 19, 217–246. [Google Scholar] [CrossRef] [PubMed]
- Shinohara, M.; Ji, C.; Kaplowitz, N. Differences in betaine-homocysteine methyltransferase expression, endoplasmic reticulum stress response, and liver injury between alcohol-fed mice and rats. Hepatology 2010, 51, 796–805. [Google Scholar] [CrossRef] [PubMed]
- Tsuchiya, M.; Ji, C.; Kosyk, O.; Shymonyak, S.; Melnyk, S.; Kono, H.; Tryndyak, V.; Muskhelishvili, L.; Pogribny, I.P.; Kaplowitz, N.; et al. Interstrain differences in liver injury and one-carbon metabolism in alcohol-fed mice. Hepatology 2012, 56, 130–139. [Google Scholar] [CrossRef] [PubMed]
- Kamat, P.K.; Mallonee, C.J.; George, A.K.; Tyagi, S.C.; Tyagi, N. Homocysteine, Alcoholism, and Its Potential Epigenetic Mechanism. Alcohol. Clin. Exp. Res. 2016, 40, 2474–2481. [Google Scholar] [CrossRef]
- Bleich, S.; Degner, D.; Javaheripour, K.; Kurth, C.; Kornhuber, J. Homocysteine and alcoholism. J. Neural Transm. Suppl. 2000, 187–196. [Google Scholar] [CrossRef]
- Arques, S. Human serum albumin in cardiovascular diseases. Eur. J. Intern. Med. 2018, 52, 8–12. [Google Scholar] [CrossRef]
- Roche, M.; Rondeau, P.; Singh, N.R.; Tarnus, E.; Bourdon, E. The antioxidant properties of serum albumin. FEBS Lett. 2008, 582, 1783–1787. [Google Scholar] [CrossRef]
- Aprioku, J.S.; Gospel, P. Concurrent administration of acetaminophen and ethanol: Impact on mouse liver and testis. J. Basic Clin. Physiol. Pharmacol. 2020, 32, 1065–1074. [Google Scholar] [CrossRef]
- Farashbandi, A.L.; Shariati, M.; Mokhtari, M. Comparing the Protective Effects of Curcumin and Ursodeoxycholic Acid after Ethanol-Induced Hepatotoxicity in Rat Liver. Ethiop. J. Health Sci. 2021, 31, 673–682. [Google Scholar] [CrossRef]
- Jagdish, R.K.; Maras, J.S.; Sarin, S.K. Albumin in Advanced Liver Diseases: The Good and Bad of a Drug! Hepatology 2021, 74, 2848–2862. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, R.; Wang, Y.; Li, H.; Han, Q.; Wu, Y.; Wang, T.; Liu, F. The Level of Serum Albumin Is Associated with Renal Prognosis in Patients with Diabetic Nephropathy. J. Diabetes Res. 2019, 2019, 7825804. [Google Scholar] [CrossRef] [PubMed]
- Kjaergard, L.L.; Villumsen, J.; Gluud, C. Reported methodologic quality and discrepancies between large and small randomized trials in meta-analyses. Ann. Intern. Med. 2001, 135, 982–989. [Google Scholar] [CrossRef] [PubMed]
- Poole, C.; Greenland, S. Random-effects meta-analyses are not always conservative. Am. J. Epidemiol. 1999, 150, 469–475. [Google Scholar] [CrossRef] [PubMed]

| Study | Year | Patient | Control | Diagnosis Criteria | Drink History (Years) | Alcohol Consumption | Oxidative Index | ||
|---|---|---|---|---|---|---|---|---|---|
| Size (M/F) | Age (Mn ± SD) | Size (M/F) | Age (Mn ± SD) | ||||||
| Balkan | 2005 | 32 (26/6) | 48.6 ± 9.86 (40–60) | 24 (18/6) | 52.3 ± 10.2 (42–62) | DSM-IV | 18.8 ± 9.04 (5–30) | 225.9 ± 88.2 (g/day) | Plasma: bilirubin, MDA, diene conjugate, homocysteine, folic acid, vitamin B12 |
| Bleich | 2003 | 52 (34/18) | M: 45.59 ± 8.41 F: 48.44 ± 9.98 | 30 (16/14) | M: 48.34 ± 8.13 F: 48.00 ± 11.36 | DSM-IV | M: 13.35 ± 5.74 F:12.61 ± 3.71 | Lifetime drinking (kg) M: 1652.56 ± 1572.94 F: 685.44 ± 320.52 | Plasma: total homocysteine, folic acid, B12, B6 |
| Chen | 2011 | 79 (67/12) | 41 ± 7.0 | 63 (58/5) | 40.7 ± 8.3 | DSM-IV-IR | 11.0 ± 7.5 (n = 75) | 196.5 ± 105.0 (g/day) | Serum: MDA, total bilirubin, 8-OHdG |
| Chen | 2012 | 124 (79/45) | 40.0 ± 8.5 | 111 (73/38) | 34.0 ± 9.8 | DSM-IV-IR | 11.6 ± 7.1 (n = 103) | Lifetime drinking (kg) 566.0 ± 484.0 (n = 103) | Serum: albumin |
| Cravo | 1996 | 32 (24/8) | 43(29–60) | 31 (19/12) | 36 (25–63) | DSM-III-R | ≥5 years | P: 2.78 ± 1.32 (g/kg/day) C: ≤30 g/day | Serum: folate, pyridoxal phosphate, vitamin B12 RBC: Folate |
| Fucile | 2013 | 10 (8/2) | 45.7 ± 11.0 | 20 (16/4) | 42.8 ± 8.4 | DSM-IV-TR | NA | NA | Plasma: MDA |
| Guemouri | 1993 | 58 (58/0) | 42.1 ± 8.2 (26–61) | 78 (78/0) | 35.8 ± 5.7 (24–54) | DSM-III-R | 13.7 ± 8.5 | P: 197.1 ± 132.6 (g/day) C: 19.9 ± 20.6 (g/day, ≤88 g/d) | Plasma: bilirubin, albumin, SOD, GPx RBC: SOD, GPx, CAT |
| Huang | 2008 | 76 | 41.2 ± 8.5 | 19 | 30.4 ± 10.4 | DSM-IV-TR | 12.4 ± 7.7 | 208.9 ± 100.7 (g/day) | Serum: SOD, MDA, total bilirubin, total albumin |
| Huang | 2009 | 121 (113/8) | 42.2 ± 9.0 | 19 (11/8) | 30.4 ± 10.4 | DSM-IV-TR | 13.2 ± 8.5 (n = 113) | 216.6 ± 107.1 (n = 114, g/day) | Serum: SOD, CAT, GSH, MDA, total bilirubin |
| Kapaki | 2007 | 71 (58/13) | 45 ± 11 | 61 (27/34) | 44.8 ± 17.9 | DSM-IV | 16.7 ± 7.6 | 360 ± 258 (g/day) | Serum: protein carbonyl |
| Lecomte | 1994 | 102 (102/0) | 40.5 ± 8.8 | 317 (317/0) | 36.2 ± 6.7 | DSM-III-R | 13.2 ± 8.3 | P: 194.3 ± 140.7 (g/day) C: 10.6 ± 9.2 (g/day, ≤33 g/day) | Plasma: albumin, α-tocopherol, ascorbic acid, selenium, GPx, SOD, MDA RBC: GPx, SOD |
| Peng | 2005 | 29 (28/1) | 43.81 ± 10.41 (25–66) | 19 (11/8) | 30.33 ± 10.93 (21–57) | DSM-III-R | 22.2 ± 10.5 (3–46) | 271 ± 123.6 (120–660) (g/day) | Serum: total-bilirubin, total protein, albumin, uric acid, MDA, SOD, CAT, GR, GPX |
| Saribal | 2019 | 21 (21/0) | 28–52 | 25 (25/0) | 28–52 | DSM-IV-IR | NA | >80 (g/day) | RBC: SOD, CAT, GPx, MDA, Cu, Fe, Zn Serum: total bilirubin |
| Thome | 1997 | 20 (18/2) | 40 ± 8 (30–59) | 15 (13/2) | 33 ± 10 (23–57) | ICD-10 & DSM-III-R | 14.3 ± 7.6 (4–35) | 249 ± 99 (120–450) (g/day) | Serum: lactoferrin, SOD |
| Ucar | 2005 | 33 (33/0) | I: 45.1 ± 6.3 (34–56) II: 41.6 ± 9.6 (27–62) | 17 (17/0) | 40.3 ± 8.4 (29–56) | ICD-10 | I: 14.1 ± 6.9 II: 22.3 ± 9.7 | NA | RBC (lysates and membranes): lipid peroxidation, GSH, GSSG, protein-bound GSH, GR, CAT, SOD, GPx |
| Study | Selection | Comparability Control for Important Factor # | Exposure | Score * | |||||
|---|---|---|---|---|---|---|---|---|---|
| Case Definition Is Adequate | Representativeness of the Cases | Controls Selection | Controls Definition | Ascertainment of Exposure | Same Method of Ascertain for Cases and Controls | Non-Response Rate | |||
| Balkan 2005 | ★ | ★ | ★ | ★ | ★★ | ★ | ★ | ★ | 9 |
| Bleich 2003 | ★ | ★ | ★ | ★ | ★★ | ★ | 7 | ||
| Chen 2011 | ★ | ★ | ★ | ★ | ★★ | ★ | ★ | ★ | 9 |
| Chen 2012 | ★ | ★ | ★ | ★ | ★★ | ★ | ★ | ★ | 9 |
| Cravo 1996 | ★ | ★ | ★ | ★ | ★ | ★ | ★ | ★ | 8 |
| Fucile 2013 | ★ | ★ | ★ | ★ | ★★ | ★ | ★ | ★ | 9 |
| Guemouri 1993 | ★ | ★ | ★ | ★ | ★★ | ★ | ★ | ★ | 9 |
| Huang 2008 | ★ | ★ | ★ | ★ | ★ | ★ | ★ | 7 | |
| Huang 2009 | ★ | ★ | ★ | ★ | ★ | ★ | ★ | 7 | |
| Kapaki 2007 | ★ | ★ | ★ | ★ | ★ | ★ | ★ | ★ | 8 |
| Lecomte 1994 | ★ | ★ | ★ | ★ | ★★ | ★ | ★ | ★ | 9 |
| Peng 2005 | ★ | ★ | ★ | ★ | ★ | ★ | ★ | ★ | 8 |
| Saribal 2019 | ★ | ★ | ★ | ★ | ★ | ★ | ★ | ★ | 8 |
| Thome 1997 | ★ | ★ | ★ | ★ | ★★ | ★ | ★ | ★ | 9 |
| Ucar 2005 | ★ | ★ | ★ | ★ | ★ | ★ | ★ | ★ | 8 |
| Oxidative Stress Biomarkers | Alcohol-Dependent Patients vs. Healthy Controls | Patients vs. Controls in Male | |
|---|---|---|---|
| SOD | P/S | RE: SMD = 0.13, I2 = 98%, 95%CI = [−1.44, 1.17], Z = 0.2 (p = 0.84) | RE: SMD = 0.30, I2 = 0%, 95%CI = [0.11, 0.48], Z = 3.10 (p = 0.002) |
| RBC | RE: SMD = −2.21, I2 = 97%, 95%CI = [−3.59, −0.82], Z = 3.13 (p = 0.002) | RE: SMD = −2.21, I2 = 97%, 95%CI = [−3.59, −0.82], Z = 3.13 (p = 0.002) | |
| CAT | RBC | RE: SMD = −2.68, I2 = 98%, 95%CI = [−6.21, 0.84], Z = 1.49 (p = 0.14) | RE: SMD = −2.58, I2 = 98%, 95%CI = [−6.00, 0.84], Z = 1.48 (p = 0.14) |
| GPx | P/S | RE: SMD = −0.33, I2 = 95%, 95%CI = [−1.26, 0.60], Z = 0.70 (p = 0.48) | RE: SMD = 0.27, I2 = 0%, 95%CI = [0.09, 0.46], Z = 2.86 (p = 0.004) |
| RBC | RE: SMD = −0.89, I2 = 91%, 95%CI = [−1.64, −0.15], Z = 2.36 (p = 0.02) | RE: SMD = −0.89, I2 = 91%, 95%CI = [−1.64, −0.15], Z = 2.36 (p = 0.02) | |
| GSH | RBC | RE: SMD = 0.15, I2 = 98%, 95%CI = [−3.05, 3.35], Z = 0.09 (p = 0.93) | |
| MDA | P/S | RE: SMD = 0.89, I2 = 61%, 95%CI = [0.58, 1.20], Z = 5.66 (p < 0.001) | RE: SMD = 1.16, I2 = 67%, 95%CI = [0.62, 1.71], Z = 4.21 (p < 0.001) |
| RBC | RE: SMD = 4.39, I2 = 98%, 95%CI = [−1.40, 10.18], Z = 1.49 (p = 0.14) | RE: SMD = 4.39, I2 = 98%, 95%CI = [−1.40, 10.18], Z = 1.49 (p = 0.14) | |
| Albumin | P/S | RE: SMD = −1.07, I2 = 94%, 95%CI = [−1.74, −0.40], Z = 3.12 (p = 0.002) | RE: SMD = −3.04, I2 = 99%, 95%CI = [−6.39, 0.31], Z = 1.78 (p = 0.08) |
| Bilirubin | P/S | RE: SMD = 0.50, I2 = 0%, 95%CI = [0.31, 0.69], Z = 5.05 (p < 0.001) | RE: SMD = 0.40, I2 = 0%, 95%CI = [0.10, 0.70], Z = 2.64 (p = 0.008) |
| B6 | P/S | RE: SMD = −0.86, I2 = 91%, 95%CI = [−2.07, 0.35], Z = 1.39 (p = 0.16) | |
| B12 | P/S | RE: SMD = 0.67, I2 = 55%, 95%CI = [0.21, 1.12], Z = 2.87 (p = 0.004) | |
| Folic Acid | P/S | RE: SMD = −0.45, I2 = 94%, 95%CI = [−1.63, 0.74], Z = 0.74 (p = 0.46) | |
| Homocysteine | P/S | RE: SMD = 0.98, I2 = 0%, 95%CI = [0.62, 1.34], Z = 2.81 (p < 0.001) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, M.; Zhou, X.; Tan, X.; Huang, X.; Yuan, L.; Zhang, Z.; Yang, Y.; Xu, M.; Wan, Y.; Li, Z. The Status of Oxidative Stress in Patients with Alcohol Dependence: A Meta-Analysis. Antioxidants 2022, 11, 1919. https://doi.org/10.3390/antiox11101919
Yang M, Zhou X, Tan X, Huang X, Yuan L, Zhang Z, Yang Y, Xu M, Wan Y, Li Z. The Status of Oxidative Stress in Patients with Alcohol Dependence: A Meta-Analysis. Antioxidants. 2022; 11(10):1919. https://doi.org/10.3390/antiox11101919
Chicago/Turabian StyleYang, Mi, Xiaofei Zhou, Xi Tan, Xincheng Huang, Lu Yuan, Zipeng Zhang, Yan Yang, Min Xu, Ying Wan, and Zezhi Li. 2022. "The Status of Oxidative Stress in Patients with Alcohol Dependence: A Meta-Analysis" Antioxidants 11, no. 10: 1919. https://doi.org/10.3390/antiox11101919
APA StyleYang, M., Zhou, X., Tan, X., Huang, X., Yuan, L., Zhang, Z., Yang, Y., Xu, M., Wan, Y., & Li, Z. (2022). The Status of Oxidative Stress in Patients with Alcohol Dependence: A Meta-Analysis. Antioxidants, 11(10), 1919. https://doi.org/10.3390/antiox11101919







