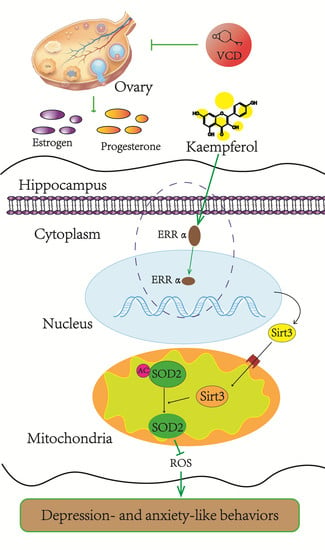
Sirtuin 3 Plays a Critical Role in the Antidepressant- and Anxiolytic-like Effects of Kaempferol
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. VCD Induced Ovary Failure Modelling [24,25]
2.3. Estrous Cycle Examination
2.4. Ovarian Embedding and H&E Staining
2.5. Follicle Counts
2.6. Radioimmunoassay of Estradiol and Progesterone
2.7. Kaempferol Treatment
2.8. Behavioral Tests
2.8.1. Forced Swimming Test (FST)
2.8.2. Open Field Test (OFT)
2.8.3. Elevated Plus Maze Test (EPM)
2.9. Superoxide Dismutase (SOD) Activity
2.10. Mitochondrial Membrane Potential Test
2.11. Total Antioxidant Capacity (TAC)
2.12. Protein Isolation and Western Blotting
2.13. Nuclear Protein Isolation and Western Blotting
2.14. Chronic Unpredictable Mild Stress (CUMS)
2.15. Overexpression of Sirt3
2.16. Statistical Analysis

3. Results
3.1. VCD Exposure Induces Ovarian Damage, Depression- and Anxiety-like Behaviors in Female Mice (Figure 1)
3.2. Kaempferol Exhibits Antidepressant- and Anxiolytic-like Effects in Two Mice Models of Menopausal Depression (Figure 2)

3.3. Kaempferol Alleviates Hippocampal Oxidative Stress and Increases the Deacetylation of SOD2 and the Protein Level of Sirt3 (Figure 3)

3.4. Sirt3 Depletion Increases SOD2 Acetylation and Partially Prevents the Antidepressant- and Anxiolytic-like Effects as Well as Antioxidant Effects of Kaempferol (Figure 4)

3.5. Overexpressing Sirt3 Exhibits Antidepressant- and Anxiolytic-like Effects Similar to Kaempferol (Figure 5)

3.6. Kaempferol Promotes ERRα Nuclear Translocation (Figure 6)

4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Monteleone, P.; Mascagni, G.; Giannini, A.; Genazzani, A.R.; Simoncini, T. Symptoms of menopause—Global prevalence, physiology and implications. Nat. Rev. Endocrinol. 2018, 14, 199–215. [Google Scholar] [CrossRef] [PubMed]
- Georgakis, M.K.; Thomopoulos, T.P.; Diamantaras, A.A.; Kalogirou, E.I.; Skalkidou, A.; Daskalopoulou, S.S.; Petridou, E.T. Association of Age at Menopause and Duration of Reproductive Period With Depression After Menopause: A Systematic Review and Meta-analysis. Jama Psychiatry 2016, 73, 139–149. [Google Scholar] [CrossRef] [PubMed]
- Gordon, J.L.; Girdler, S.S.; Meltzer-Brody, S.E.; Stika, C.S.; Thurston, R.C.; Clark, C.T.; Prairie, B.A.; Moses-Kolko, E.; Joffe, H.; Wisner, K.L. Ovarian hormone fluctuation, neurosteroids, and HPA axis dysregulation in perimenopausal depression: A novel heuristic model. Am. J. Psychiatry 2015, 172, 227–236. [Google Scholar] [CrossRef]
- Hwang, W.J.; Lee, T.Y.; Kim, N.S.; Kwon, J.S. The Role of Estrogen Receptors and Their Signaling across Psychiatric Disorders. Int. J. Mol. Sci. 2020, 22, 373. [Google Scholar] [CrossRef] [PubMed]
- Lobo, R.A. Hormone-replacement therapy: Current thinking. Nat. Rev. Endocrinol. 2017, 13, 220–231. [Google Scholar] [CrossRef]
- Rozenberg, S.; Vandromme, J.; Antoine, C. Postmenopausal hormone therapy: Risks and benefits. Nat. Rev. Endocrinol. 2013, 9, 216–227. [Google Scholar] [CrossRef] [PubMed]
- Schiller, C.E.; Meltzer-Brody, S.; Rubinow, D.R. The role of reproductive hormones in postpartum depression. CNS Spectr. 2015, 20, 48–59. [Google Scholar] [CrossRef]
- Vavakova, M.; Durackova, Z.; Trebaticka, J. Markers of Oxidative Stress and Neuroprogression in Depression Disorder. Oxid. Med. Cell. Longev. 2015, 2015, 898393. [Google Scholar] [CrossRef]
- Luo, J.; Mills, K.; le Cessie, S.; Noordam, R.; van Heemst, D. Ageing, age-related diseases and oxidative stress: What to do next? Ageing Res. Rev. 2020, 57, 100982. [Google Scholar] [CrossRef]
- Thaung Zaw, J.J.; Howe, P.R.C.; Wong, R.H.X. Postmenopausal health interventions: Time to move on from the Women’s Health Initiative? Ageing Res. Rev. 2018, 48, 79–86. [Google Scholar] [CrossRef]
- Conti, V.; Izzo, V.; Corbi, G.; Russomanno, G.; Manzo, V.; De Lise, F.; Di Donato, A.; Filippelli, A. Antioxidant Supplementation in the Treatment of Aging-Associated Diseases. Front. Pharm. 2016, 7, 24. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.H. Health-promoting components of fruits and vegetables in the diet. Adv. Nutr. 2013, 4, 384S–392S. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.S.; Shen, C.Y.; Jiang, J.G. Antidepressant active ingredients from herbs and nutraceuticals used in TCM: Pharmacological mechanisms and prospects for drug discovery. Pharm. Res. 2019, 150, 104520. [Google Scholar] [CrossRef]
- Lin, H.Y.; Tsai, J.C.; Wu, L.Y.; Peng, W.H. Reveals of New Candidate Active Components in Hemerocallis Radix and Its Anti-Depression Action of Mechanism Based on Network Pharmacology Approach. Int. J. Mol. Sci. 2020, 21, 1868. [Google Scholar] [CrossRef]
- Yuan, N.; Gong, L.; Tang, K.; He, L.; Hao, W.; Li, X.; Ma, Q.; Chen, J. An Integrated Pharmacology-Based Analysis for Antidepressant Mechanism of Chinese Herbal Formula Xiao-Yao-San. Front. Pharm. 2020, 11, 284. [Google Scholar] [CrossRef] [PubMed]
- Silva Dos Santos, J.; Goncalves Cirino, J.P.; de Oliveira Carvalho, P.; Ortega, M.M. The Pharmacological Action of Kaempferol in Central Nervous System Diseases: A Review. Front. Pharm. 2020, 11, 565700. [Google Scholar] [CrossRef]
- Rajendran, P.; Rengarajan, T.; Nandakumar, N.; Palaniswami, R.; Nishigaki, Y.; Nishigaki, I. Kaempferol, a potential cytostatic and cure for inflammatory disorders. Eur. J. Med. Chem. 2014, 86, 103–112. [Google Scholar] [CrossRef]
- Devi, K.P.; Malar, D.S.; Nabavi, S.F.; Sureda, A.; Xiao, J.; Nabavi, S.M.; Daglia, M. Kaempferol and inflammation: From chemistry to medicine. Pharm. Res. 2015, 99, 1–10. [Google Scholar] [CrossRef]
- Wang, J.; Li, H.Y.; Shen, S.Y.; Zhang, J.R.; Liang, L.F.; Huang, H.J.; Li, B.; Wu, G.C.; Zhang, Y.Q.; Yu, J. The antidepressant and anxiolytic effect of GPER on translocator protein (TSPO) via protein kinase a (PKA) signaling in menopausal female rats. J. Steroid Biochem. Mol. Biol. 2021, 207, 105807. [Google Scholar] [CrossRef]
- Wang, J.; Yu, R.; Han, Q.Q.; Huang, H.J.; Wang, Y.L.; Li, H.Y.; Wang, H.M.; Chen, X.R.; Ma, S.L.; Yu, J. G-1 exhibit antidepressant effect, increase of hippocampal ERs expression and improve hippocampal redox status in aged female rats. Behav. Brain Res. 2019, 359, 845–852. [Google Scholar] [CrossRef]
- Dikalova, A.E.; Itani, H.A.; Nazarewicz, R.R.; McMaster, W.G.; Flynn, C.R.; Uzhachenko, R.; Fessel, J.P.; Gamboa, J.L.; Harrison, D.G.; Dikalov, S.I. Sirt3 Impairment and SOD2 Hyperacetylation in Vascular Oxidative Stress and Hypertension. Circ. Res. 2017, 121, 564–574. [Google Scholar] [CrossRef] [PubMed]
- Zefzoufi, M.; Fdil, R.; Bouamama, H.; Gadhi, C.; Katakura, Y.; Mouzdahir, A.; Sraidi, K. Effect of extracts and isolated compounds derived from Retama monosperma (L.) Boiss. on anti-aging gene expression in human keratinocytes and antioxidant activity. J. Ethnopharmacol. 2021, 280, 114451. [Google Scholar] [CrossRef] [PubMed]
- Someya, S.; Yu, W.; Hallows, W.C.; Xu, J.; Vann, J.M.; Leeuwenburgh, C.; Tanokura, M.; Denu, J.M.; Prolla, T.A. Sirt3 mediates reduction of oxidative damage and prevention of age-related hearing loss under caloric restriction. Cell 2010, 143, 802–812. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, R.D.; Hall, A.; Ferguson, M.; Lorenzen-Schmidt, I.; Balasubramaniam, V.; Pyle, W.G. Cardiac changes during the peri-menopausal period in a VCD-induced murine model of ovarian failure. Acta Physiol. 2019, 227, e13290. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Y.; Xiong, J.; Di, T.; Yuan, Z.; Wu, J.; Chen, L. Reduced serotonin impairs long-term depression in basolateral amygdala complex and causes anxiety-like behaviors in a mouse model of perimenopause. Exp. Neurol. 2019, 321, 113030. [Google Scholar] [CrossRef]
- McLean, A.C.; Valenzuela, N.; Fai, S.; Bennett, S.A. Performing vaginal lavage, crystal violet staining, and vaginal cytological evaluation for mouse estrous cycle staging identification. J. Vis. Exp. 2012, 67, e4389. [Google Scholar] [CrossRef]
- Byers, S.L.; Wiles, M.V.; Dunn, S.L.; Taft, R.A. Mouse estrous cycle identification tool and images. PLoS ONE 2012, 7, e35538. [Google Scholar] [CrossRef]
- Cima, G. AVMA Guidelines for the Euthanasia of Animal: 2013 Edition. Javma-J. Am. Vet. Med. A. 2013, 242, 715–716. [Google Scholar]
- Fitzmaurice, C.; Abate, D.; Abbasi, N.; Abbastabar, H.; Abd-Allah, F.; Abdel-Rahman, O.; Abdelalim, A.; Abdoli, A.; Abdollahpour, I.; Abdulle, A.S.M.; et al. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived with Disability, and Disability-Adjusted Life-Years for 29 Cancer Groups, 1990 to 2017: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol. 2019, 4, 1553–1568. [Google Scholar] [CrossRef]
- Elefantova, K.; Lakatos, B.; Kubickova, J.; Sulova, Z.; Breier, A. Detection of the Mitochondrial Membrane Potential by the Cationic Dye JC-1 in L1210 Cells with Massive Overexpression of the Plasma Membrane ABCB1 Drug Transporter. Int. J. Mol. Sci. 2018, 19, 1985. [Google Scholar] [CrossRef]
- Mitchell, S.J.; Madrigal-Matute, J.; Scheibye-Knudsen, M.; Fang, E.; Aon, M.; Gonzalez-Reyes, J.A.; Cortassa, S.; Kaushik, S.; Gonzalez-Freire, M.; Patel, B.; et al. Effects of Sex, Strain, and Energy Intake on Hallmarks of Aging in Mice. Cell Metab. 2016, 23, 1093–1112. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.R.; Lee, Y.J.; Kim, T.W.; Lim, R.N.; Hwang, D.Y.; Moffat, J.J.; Kim, S.; Seo, J.W.; Ka, M. Asparagus cochinchinensis extract ameliorates menopausal depression in ovariectomized rats under chronic unpredictable mild stress. BMC Complement. Med. Ther. 2020, 20, 325. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhang, L.; Cao, L.L.; Qi, J.; Li, P.; Wang, X.P.; Sun, X.L. MicroRNA-99a is a Potential Target for Regulating Hypothalamic Synaptic Plasticity in the Peri/Postmenopausal Depression Model. Cellsl 2019, 8, 1081. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.E.; Borkowski, K.; Newman, J.W.; Park, Y. N-3 PUFA improved post-menopausal depression induced by maternal separation and chronic mild stress through serotonergic pathway in rats-effect associated with lipid mediators. J. Nutr. Biochem. 2021, 91, 108599. [Google Scholar] [CrossRef] [PubMed]
- Khayum, M.A.; Moraga-Amaro, R.; Buwalda, B.; Koole, M.; den Boer, J.A.; Dierckx, R.; Doorduin, J.; de Vries, E.F.J. Ovariectomy-induced depressive-like behavior and brain glucose metabolism changes in female rats are not affected by chronic mild stress. Psychoneuroendocrinology 2020, 115, 104610. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.J.; Chen, X.R.; Han, Q.Q.; Wang, J.; Pilot, A.; Yu, R.; Liu, Q.; Li, B.; Wu, G.C.; Wang, Y.Q.; et al. The protective effects of Ghrelin/GHSR on hippocampal neurogenesis in CUMS mice. Neuropharmacology 2019, 155, 31–43. [Google Scholar] [CrossRef]
- Huang, H.J.; Zhu, X.C.; Han, Q.Q.; Wang, Y.L.; Yue, N.; Wang, J.; Yu, R.; Li, B.; Wu, G.C.; Liu, Q.; et al. Ghrelin alleviates anxiety- and depression-like behaviors induced by chronic unpredictable mild stress in rodents. Behav. Brain Res. 2017, 326, 33–43. [Google Scholar] [CrossRef]
- Kappeler, C.J.; Hoyer, P.B. 4-vinylcyclohexene diepoxide: A model chemical for ovotoxicity. Syst. Biol. Reprod. Med. 2012, 58, 57–62. [Google Scholar] [CrossRef]
- Brooks, H.L.; Pollow, D.P.; Hoyer, P.B. The VCD Mouse Model of Menopause and Perimenopause for the Study of Sex Differences in Cardiovascular Disease and the Metabolic Syndrome. Physiology 2016, 31, 250–257. [Google Scholar] [CrossRef]
- Doshi, S.B.; Agarwal, A. The role of oxidative stress in menopause. J. Midlife Health 2013, 4, 140–146. [Google Scholar] [CrossRef]
- Grimm, A.; Mensah-Nyagan, A.G.; Eckert, A. Alzheimer, mitochondria and gender. Neurosci. Biobehav. Rev. 2016, 67, 89–101. [Google Scholar] [CrossRef] [PubMed]
- Mathieu, L.; Costa, A.L.; Le Bachelier, C.; Slama, A.; Lebre, A.S.; Taylor, R.W.; Bastin, J.; Djouadi, F. Resveratrol attenuates oxidative stress in mitochondrial Complex I deficiency: Involvement of SIRT3. Free Radic. Biol. Med. 2016, 96, 190–198. [Google Scholar] [CrossRef] [PubMed]
- Brinton, R.D. Minireview: Translational animal models of human menopause: Challenges and emerging opportunities. Endocrinology 2012, 153, 3571–3578. [Google Scholar] [CrossRef] [PubMed]
- Van Kempen, T.A.; Milner, T.A.; Waters, E.M. Accelerated ovarian failure: A novel, chemically induced animal model of menopause. Brain Res. 2011, 1379, 176–187. [Google Scholar] [CrossRef] [PubMed]
- Soucek, P.; Kondrova, E.; Hermanek, J.; Stopka, P.; Boumendjel, A.; Ueng, Y.F.; Gut, I. New model system for testing effects of flavonoids on doxorubicin-related formation of hydroxyl radicals. Anti-Cancer Drugs 2011, 22, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Shakya, G.; Manjini, S.; Hoda, M.; Rajagopalan, R. Hepatoprotective role of kaempferol during alcohol- and DeltaPUFA-induced oxidative stress. J. Basic Clin. Physiol. Pharm. 2014, 25, 73–79. [Google Scholar] [CrossRef]
- Crespo, I.; Garcia-Mediavilla, M.V.; Almar, M.; Gonzalez, P.; Tunon, M.J.; Sanchez-Campos, S.; Gonzalez-Gallego, J. Differential effects of dietary flavonoids on reactive oxygen and nitrogen species generation and changes in antioxidant enzyme expression induced by proinflammatory cytokines in Chang Liver cells. Food Chem. Toxicol. 2008, 46, 1555–1569. [Google Scholar] [CrossRef]
- Chen, X.; Yang, X.; Liu, T.; Guan, M.; Feng, X.; Dong, W.; Chu, X.; Liu, J.; Tian, X.; Ci, X.; et al. Kaempferol regulates MAPKs and NF-kappaB signaling pathways to attenuate LPS-induced acute lung injury in mice. Int. Immunopharmacol. 2012, 14, 209–216. [Google Scholar] [CrossRef]
- Wall, C.; Lim, R.; Poljak, M.; Lappas, M. Dietary flavonoids as therapeutics for preterm birth: Luteolin and kaempferol suppress inflammation in human gestational tissues in vitro. Oxid. Med. Cell. Longev. 2013, 2013, 485201. [Google Scholar] [CrossRef]
- Yoon, H.Y.; Lee, E.G.; Lee, H.; Cho, I.J.; Choi, Y.J.; Sung, M.S.; Yoo, H.G.; Yoo, W.H. Kaempferol inhibits IL-1beta-induced proliferation of rheumatoid arthritis synovial fibroblasts and the production of COX-2, PGE2 and MMPs. Int. J. Mol. Med. 2013, 32, 971–977. [Google Scholar] [CrossRef]
- Gao, W.; Wang, W.; Peng, Y.; Deng, Z. Antidepressive effects of kaempferol mediated by reduction of oxidative stress, proinflammatory cytokines and up-regulation of AKT/beta-catenin cascade. Metab. Brain Dis. 2019, 34, 485–494. [Google Scholar] [CrossRef] [PubMed]
- de la Garza, A.L.; Garza-Cuellar, M.A.; Silva-Hernandez, I.A.; Cardenas-Perez, R.E.; Reyes-Castro, L.A.; Zambrano, E.; Gonzalez-Hernandez, B.; Garza-Ocanas, L.; Fuentes-Mera, L.; Camacho, A. Maternal Flavonoids Intake Reverts Depression-Like Behaviour in Rat Female Offspring. Nutrients 2019, 11, 572. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Del-Rio, L.; Nag, A.; Casado, E.G.; Ariza, J.; Awad, A.M.; Joseph, A.I.; Kwon, O.; Verdin, E.; de Cabo, R.; Schneider, C.; et al. Kaempferol increases levels of coenzyme Q in kidney cells and serves as a biosynthetic ring precursor. Free Radic. Biol. Med. 2017, 110, 176–187. [Google Scholar] [CrossRef] [PubMed]
- Cimen, H.; Han, M.J.; Yang, Y.; Tong, Q.; Koc, H.; Koc, E.C. Regulation of succinate dehydrogenase activity by SIRT3 in mammalian mitochondria. Biochemistry 2010, 49, 304–311. [Google Scholar] [CrossRef] [PubMed]
- Giralt, A.; Hondares, E.; Villena, J.A.; Ribas, F.; Diaz-Delfin, J.; Giralt, M.; Iglesias, R.; Villarroya, F. Peroxisome proliferator-activated receptor-gamma coactivator-1alpha controls transcription of the Sirt3 gene, an essential component of the thermogenic brown adipocyte phenotype. J. Biol. Chem. 2011, 286, 16958–16966. [Google Scholar] [CrossRef]
- Zhang, X.; Ren, X.; Zhang, Q.; Li, Z.; Ma, S.; Bao, J.; Li, Z.; Bai, X.; Zheng, L.; Zhang, Z.; et al. PGC-1alpha/ERRalpha-Sirt3 Pathway Regulates DAergic Neuronal Death by Directly Deacetylating SOD2 and ATP Synthase beta. Antioxid. Redox Signal. 2016, 24, 312–328. [Google Scholar] [CrossRef]
- Wang, J.; Fang, F.; Huang, Z.; Wang, Y.; Wong, C. Kaempferol is an estrogen-related receptor alpha and gamma inverse agonist. FEBS Lett. 2009, 583, 643–647. [Google Scholar] [CrossRef]
- Zeng, R.; Wang, X.; Zhou, Q.; Fu, X.; Wu, Q.; Lu, Y.; Shi, J.; Klaunig, J.E.; Zhou, S. Icariin protects rotenone-induced neurotoxicity through induction of SIRT3. Toxicol. Appl. Pharm. 2019, 379, 114639. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.-Y.; Wang, J.; Liang, L.-F.; Shen, S.-Y.; Li, W.; Chen, X.-R.; Li, B.; Zhang, Y.-Q.; Yu, J. Sirtuin 3 Plays a Critical Role in the Antidepressant- and Anxiolytic-like Effects of Kaempferol. Antioxidants 2022, 11, 1886. https://doi.org/10.3390/antiox11101886
Li H-Y, Wang J, Liang L-F, Shen S-Y, Li W, Chen X-R, Li B, Zhang Y-Q, Yu J. Sirtuin 3 Plays a Critical Role in the Antidepressant- and Anxiolytic-like Effects of Kaempferol. Antioxidants. 2022; 11(10):1886. https://doi.org/10.3390/antiox11101886
Chicago/Turabian StyleLi, Hao-Yuan, Jing Wang, Ling-Feng Liang, Shi-Yu Shen, Wei Li, Xiao-Rong Chen, Bing Li, Yu-Qiu Zhang, and Jin Yu. 2022. "Sirtuin 3 Plays a Critical Role in the Antidepressant- and Anxiolytic-like Effects of Kaempferol" Antioxidants 11, no. 10: 1886. https://doi.org/10.3390/antiox11101886
APA StyleLi, H.-Y., Wang, J., Liang, L.-F., Shen, S.-Y., Li, W., Chen, X.-R., Li, B., Zhang, Y.-Q., & Yu, J. (2022). Sirtuin 3 Plays a Critical Role in the Antidepressant- and Anxiolytic-like Effects of Kaempferol. Antioxidants, 11(10), 1886. https://doi.org/10.3390/antiox11101886