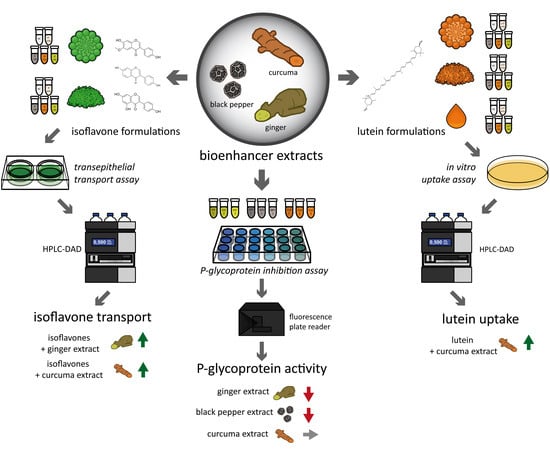
Improved Bioavailability and Bioaccessibility of Lutein and Isoflavones in Cultured Cells In Vitro through Interaction with Ginger, Curcuma and Black Pepper Extracts
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. CaCo-2 Cell Culture
2.3. Cell Viability Assay
2.4. Lutein and Curcumin Uptake Studies
2.5. Isoflavone Transport Studies
2.6. Determination of Incorporation of Lutein and Isoflavones into Mixed Micelles
2.7. Rhodamine Efflux Assay
2.8. HPLC Analysis
2.9. Statistics
3. Results
3.1. Quantitation of Bioactive Lead Compounds in Lutein and Isoflavone Extracts
3.2. Curcuma Extract Increases Lutein Uptake in Intestinal Cells
3.3. CuE and GiE Enhance Isoflavone Transport by CaCo-2 Cells
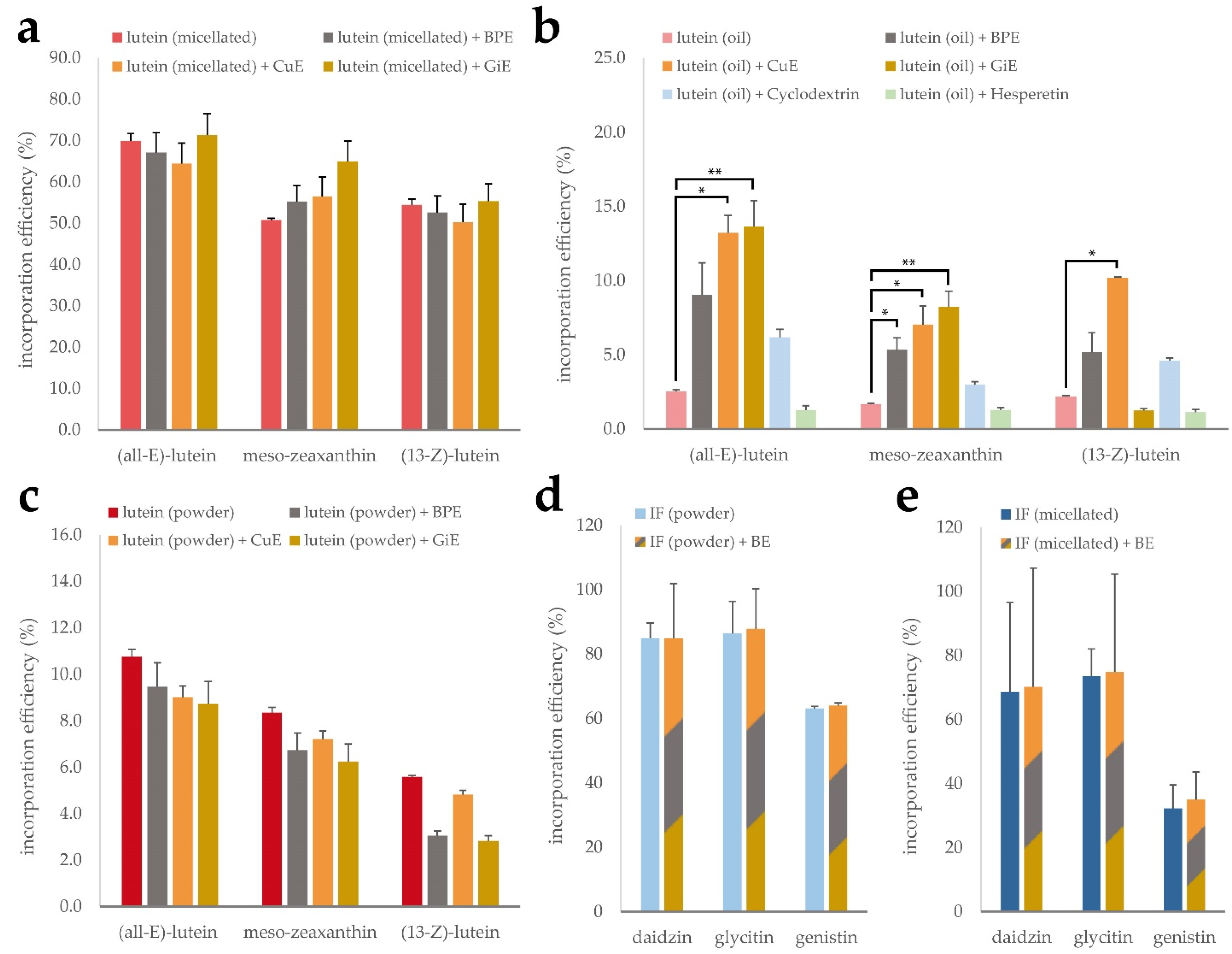
3.4. Impact of Bioenhancer Extracts on Mixed Micelle Incorpartion Efficiency of Lutein and Isoflavones
3.5. Rhodamine Efflux Assay
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Krzyzanowska, J.; Czubacka, A.; Oleszek, W. Dietary phytochemicals and human health. Adv. Exp. Med. Biol. 2010, 698, 74–98. [Google Scholar] [CrossRef] [PubMed]
- Neilson, A.P.; Goodrich, K.M.; Ferruzzi, M.G. Bioavailability and Metabolism of Bioactive Compounds from Foods. Nutrition in the Prevention and Treatment of Disease; Elsevier: Amsterdam, The Netherlands, 2017; pp. 301–319. ISBN 9780128029282. [Google Scholar]
- Phelan, D.; Prado-Cabrero, A.; Nolan, J.M. Analysis of Lutein, Zeaxanthin, and Meso-Zeaxanthin in the Organs of Carotenoid-Supplemented Chickens. Foods 2018, 7, 20. [Google Scholar] [CrossRef] [PubMed]
- Alves-Rodrigues, A.; Shao, A. The science behind lutein. Toxicol. Lett. 2004, 150, 57–83. [Google Scholar] [CrossRef] [PubMed]
- Kamoshita, M.; Toda, E.; Osada, H.; Narimatsu, T.; Kobayashi, S.; Tsubota, K.; Ozawa, Y. Lutein acts via multiple antioxidant pathways in the photo-stressed retina. Sci. Rep. 2016, 6, 30226. [Google Scholar] [CrossRef]
- Yu, J.; Bi, X.; Yu, B.; Chen, D. Isoflavones: Anti-Inflammatory Benefit and Possible Caveats. Nutrients 2016, 8, 361. [Google Scholar] [CrossRef]
- Ruiz-Larrea, M.B.; Mohan, A.R.; Paganga, G.; Miller, N.J.; Bolwell, G.P.; Rice-Evans, C.A. Antioxidant activity of phytoestrogenic isoflavones. Free Radic. Res. 1997, 26, 63–70. [Google Scholar] [CrossRef]
- Jiang, H.; Fan, J.; Cheng, L.; Hu, P.; Liu, R. The anticancer activity of genistein is increased in estrogen receptor beta 1-positive breast cancer cells. OncoTargets Ther. 2018, 11, 8153–8163. [Google Scholar] [CrossRef]
- Thangavel, P.; Puga-Olguín, A.; Rodríguez-Landa, J.F.; Zepeda, R.C. Genistein as Potential Therapeutic Candidate for Menopausal Symptoms and Other Related Diseases. Molecules 2019, 24, 3892. [Google Scholar] [CrossRef]
- Atal, N.; Bedi, K.L. Bioenhancers: Revolutionary concept to market. J. Ayurveda Integr. Med. 2010, 1, 96–99. [Google Scholar] [CrossRef]
- Bi, X.; Yuan, Z.; Qu, B.; Zhou, H.; Liu, Z.; Xie, Y. Piperine enhances the bioavailability of silybin via inhibition of efflux transporters BCRP and MRP2. Phytomedicine 2019, 54, 98–108. [Google Scholar] [CrossRef]
- Cao, X.; Gibbs, S.T.; Fang, L.; Miller, H.A.; Landowski, C.P.; Shin, H.-C.; Lennernas, H.; Zhong, Y.; Amidon, G.L.; Yu, L.X.; et al. Why is it challenging to predict intestinal drug absorption and oral bioavailability in human using rat model. Pharm. Res. 2006, 23, 1675–1686. [Google Scholar] [CrossRef] [PubMed]
- Chu, X.; Bleasby, K.; Evers, R. Species differences in drug transporters and implications for translating preclinical findings to humans. Expert Opin. Drug Metab. Toxicol. 2013, 9, 237–252. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-R.; Park, S.-W.; Cho, H.-J.; Chae, K.-A.; Sung, J.-M.; Kim, J.-S.; Landowski, C.P.; Sun, D.; Abd El-Aty, A.M.; Amidon, G.L.; et al. Comparative gene expression profiles of intestinal transporters in mice, rats and humans. Pharmacol. Res. 2007, 56, 224–236. [Google Scholar] [CrossRef] [PubMed]
- Hubatsch, I.; Ragnarsson, E.G.E.; Artursson, P. Determination of drug permeability and prediction of drug absorption in CaCO2 monolayers. Nat. Protoc. 2007, 2, 2111–2119. [Google Scholar] [CrossRef] [PubMed]
- Röhrl, C.; Stübl, F.; Maier, M.; Schwarzinger, B.; Schwarzinger, C.; Pitsch, J.; Lanzerstorfer, P.; Iken, M.; Weghuber, J. Increased Cellular Uptake of Polyunsaturated Fatty Acids and Phytosterols from Natural Micellar Oil. Nutrients 2020, 12, 150. [Google Scholar] [CrossRef]
- Marziano, M.; Tonello, S.; Cantù, E.; Abate, G.; Vezzoli, M.; Rungratanawanich, W.; Serpelloni, M.; Lopomo, N.F.; Memo, M.; Sardini, E.; et al. Monitoring CaCO2 to enterocyte-like cells differentiation by means of electric impedance analysis on printed sensors. Biochim. Biophys. Acta Gen. Subj. 2019, 1863, 893–902. [Google Scholar] [CrossRef]
- Klein, S. The use of biorelevant dissolution media to forecast the in vivo performance of a drug. AAPS J. 2010, 12, 397–406. [Google Scholar] [CrossRef]
- Ilardia-Arana, D.; Kristensen, H.G.; Müllertz, A. Biorelevant dissolution media: Aggregation of amphiphiles and solubility of estradiol. J. Pharm. Sci. 2006, 95, 248–255. [Google Scholar] [CrossRef]
- Ollinger, N.; Neuhauser, C.; Schwarzinger, B.; Wallner, M.; Schwarzinger, C.; Blank-Landeshammer, B.; Hager, R.; Sadova, N.; Drotarova, I.; Mathmann, K.; et al. Anti-Hyperglycemic Effects of Oils and Extracts Derived from Sea Buckthorn—A Comprehensive Analysis Utilizing In Vitro and In Vivo Models. Mol. Nutr. Food Res. 2022, 66, e2101133. [Google Scholar] [CrossRef]
- Homan, R.; Hamelehle, K.L. Phospholipase A2 relieves phosphatidylcholine inhibition of micellar cholesterol absorption and transport by human intestinal cell line CaCO2. J. Lipid Res. 1998, 39, 1197–1209. [Google Scholar] [CrossRef]
- Schiller, C.; Fröhlich, C.-P.; Giessmann, T.; Siegmund, W.; Mönnikes, H.; Hosten, N.; Weitschies, W. Intestinal fluid volumes and transit of dosage forms as assessed by magnetic resonance imaging. Aliment. Pharmacol. Ther. 2005, 22, 971–979. [Google Scholar] [CrossRef] [PubMed]
- Khachik, F.; Englert, G.; Daitch, C.E.; Beecher, G.R.; Tonucci, L.H.; Lusby, W.R. Isolation and structural elucidation of the geometrical isomers of lutein and zeaxanthin in extracts from human plasma. J. Chromatogr. B Biomed. Sci. Appl. 1992, 582, 153–166. [Google Scholar] [CrossRef]
- Levy, E.; Mehran, M.; Seidman, E. CaCO2 cells as a model for intestinal lipoprotein synthesis and secretion. FASEB J. 1995, 9, 626–635. [Google Scholar] [CrossRef]
- Müller, U.; Stübl, F.; Schwarzinger, B.; Sandner, G.; Iken, M.; Himmelsbach, M.; Schwarzinger, C.; Ollinger, N.; Stadlbauer, V.; Höglinger, O.; et al. In Vitro and In Vivo Inhibition of Intestinal Glucose Transport by Guava (Psidium guajava) Extracts. Mol. Nutr. Food Res. 2018, 62, e1701012. [Google Scholar] [CrossRef] [PubMed]
- Boni, J.E.; Brickl, R.S.; Dressman, J.; Pfefferle, M.L. Instant FaSSIF and FeSSIF—Biorelevance Meets Practicality. Dissolution Technol. 2009, 16, 41–45. [Google Scholar] [CrossRef]
- Rusznyák, Á.; Malanga, M.; Fenyvesi, É.; Szente, L.; Váradi, J.; Bácskay, I.; Vecsernyés, M.; Vasvári, G.; Haimhoffer, Á.; Fehér, P.; et al. Investigation of the Cellular Effects of β-Cyclodextrin Derivatives on CaCO2 Intestinal Epithelial Cells. Pharmaceutics 2021, 13, 157. [Google Scholar] [CrossRef]
- Islam, M.A.; Punt, A.; Spenkelink, B.; Murk, A.J.; van Rolaf Leeuwen, F.X.; Rietjens, I.M.C.M. Conversion of major soy isoflavone glucosides and aglycones in in vitro intestinal models. Mol. Nutr. Food Res. 2014, 58, 503–515. [Google Scholar] [CrossRef]
- Setchell, K.D.; Brown, N.M.; Desai, P.; Zimmer-Nechemias, L.; Wolfe, B.E.; Brashear, W.T.; Kirschner, A.S.; Cassidy, A.; Heubi, J.E. Bioavailability of pure isoflavones in healthy humans and analysis of commercial soy isoflavone supplements. J. Nutr. 2001, 131, 1362S–1375S. [Google Scholar] [CrossRef]
- Borel, P. Factors affecting intestinal absorption of highly lipophilic food microconstituents (fat-soluble vitamins, carotenoids and phytosterols). Clin. Chem. Lab. Med. 2003, 41, 979–994. [Google Scholar] [CrossRef]
- Nie, M.; Zhang, Z.; Liu, C.; Li, D.; Huang, W.; Liu, C.; Jiang, N. Hesperetin and Hesperidin Improved β-Carotene Incorporation Efficiency, Intestinal Cell Uptake, and Retinoid Concentrations in Tissues. J. Agric. Food Chem. 2019, 67, 3363–3371. [Google Scholar] [CrossRef]
- Shoba, G.; Joy, D.; Joseph, T.; Majeed, M.; Rajendran, R.; Srinivas, P.S. Influence of piperine on the pharmacokinetics of curcumin in animals and human volunteers. Planta Med. 1998, 64, 353–356. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.V.; Godbole, M.M.; Misra, K. A plausible explanation for enhanced bioavailability of P-gp substrates in presence of piperine: Simulation for next generation of P-gp inhibitors. J. Mol. Model. 2013, 19, 227–238. [Google Scholar] [CrossRef] [PubMed]
- Jouan, E.; Le Vée, M.; Mayati, A.; Denizot, C.; Parmentier, Y.; Fardel, O. Evaluation of P-Glycoprotein Inhibitory Potential Using a Rhodamine 123 Accumulation Assay. Pharmaceutics 2016, 8, 12. [Google Scholar] [CrossRef] [PubMed]
- Forster, S.; Thumser, A.E.; Hood, S.R.; Plant, N. Characterization of rhodamine-123 as a tracer dye for use in in vitro drug transport assays. PLoS ONE 2012, 7, e33253. [Google Scholar] [CrossRef]
- Phan, M.A.T.; Paterson, J.; Bucknall, M.; Arcot, J. Interactions between phytochemicals from fruits and vegetables: Effects on bioactivities and bioavailability. Crit. Rev. Food Sci. Nutr. 2018, 58, 1310–1329. [Google Scholar] [CrossRef]
- Hundshammer, C.; Schön, C.; Kimura, M.; Furune, T.; Terao, K.; Elgeti, D.; Mohr, R. Enhanced metabolic bioavailability of tetrahydrocurcumin after oral supplementation of a γ-cyclodextrin curcumin complex. J. Funct. Foods 2021, 79, 104410. [Google Scholar] [CrossRef]
- Kopec, R.E.; Failla, M.L. Recent advances in the bioaccessibility and bioavailability of carotenoids and effects of other dietary lipophiles. J. Food Compos. Anal. 2018, 68, 16–30. [Google Scholar] [CrossRef]
- Chitchumroonchokchai, C.; Failla, M.L. Hydrolysis of zeaxanthin esters by carboxyl ester lipase during digestion facilitates micellarization and uptake of the xanthophyll by CaCO2 human intestinal cells. J. Nutr. 2006, 136, 588–594. [Google Scholar] [CrossRef]
- Chen, F.-Y.; Zhou, J.; Guo, N.; Ma, W.-G.; Huang, X.; Wang, H.; Yuan, Z.-Y. Curcumin retunes cholesterol transport homeostasis and inflammation response in M1 macrophage to prevent atherosclerosis. Biochem. Biophys. Res. Commun. 2015, 467, 872–878. [Google Scholar] [CrossRef]
- Werder, M.; Han, C.H.; Wehrli, E.; Bimmler, D.; Schulthess, G.; Hauser, H. Role of scavenger receptors SR-BI and CD36 in selective sterol uptake in the small intestine. Biochemistry 2001, 40, 11643–11650. [Google Scholar] [CrossRef]
- Cai, S.F.; Kirby, R.J.; Howles, P.N.; Hui, D.Y. Differentiation-dependent expression and localization of the class B type I scavenger receptor in intestine. J. Lipid Res. 2001, 42, 902–909. [Google Scholar] [CrossRef]
- Jiang, W.; Hu, M. Mutual interactions between flavonoids and enzymatic and transporter elements responsible for flavonoid disposition via phase II metabolic pathways. RSC Adv. 2012, 2, 7948–7963. [Google Scholar] [CrossRef] [PubMed]
- Järvinen, E.; Deng, F.; Kiander, W.; Sinokki, A.; Kidron, H.; Sjöstedt, N. The Role of Uptake and Efflux Transporters in the Disposition of Glucuronide and Sulfate Conjugates. Front. Pharmacol. 2021, 12, 802539. [Google Scholar] [CrossRef]
- Fang, Y.; Cao, W.; Liang, F.; Xia, M.; Pan, S.; Xu, X. Structure affinity relationship and docking studies of flavonoids as substrates of multidrug-resistant associated protein 2 (MRP2) in MDCK/MRP2 cells. Food Chem. 2019, 291, 101–109. [Google Scholar] [CrossRef] [PubMed]




| Curcuma Extract (Regular) | Curcuma Extract (CD Complexed) | |
|---|---|---|
| curcumin (µg/mg) | 404.8 ± 11.1 (78.2%) | 168.7 ± 1.2 (81.4%) |
| desmethoxycurcumin (µg/mg) | 92.0 ± 3.3 (17.8%) | 31.9 ± 0.69 (15.4%) |
| bisdemethoxycurcumin (µg/mg) | 21.0 ± 0.43 (4.0%) | 6.6 ± 0.05 (3.2%) |
| total (µg/mg) | 517.7 ± 14.4 | 207.2 ± 1.9 |
| Lutein (Oil) | Lutein (Micellated) | Lutein (Powder) | |
|---|---|---|---|
| (all-E)-lutein (µg/mg) | 266.7 ± 22.8 | 7.2 ± 0.5 | 65.1 ± 6.3 |
| meso-zeaxanthin (µg/mg) | 23.2 ± 0.4 | 0.3 ± 0.04 | 5.5 ± 0.2 |
| (13-Z)-lutein (µg/mg) | 44.3 ± 1.8 | 1.5 ± 0.2 | 3.7 ± 0.1 |
| total (µg/mg) | 334.3 ± 24.3 | 9.1 ± 0.7 | 74.3 ± 6.2 |
| Isoflavones (Micellated) | Isoflavones (Powder) | |
|---|---|---|
| daidzin (µg/mg) | 9.6 ± 0.3 | 265.7 ± 6.7 |
| glycitin (µg/mg) | 3.4 ± 0.2 | 135.5 ± 4.4 |
| genisitin (µg/mg) | 1.7 ± 0.05 | 35.5 ± 1.2 |
| daidzein (µg/mg) | 0.34 ± 0.04 | 12.8 ± 1.0 |
| glycitein (µg/mg) | 0.14 ± 0.03 | 4.9 ± 0.5 |
| genistein (µg/mg) | 0.09 ± 0.02 | 3.7 ± 0.6 |
| total (µg/mg) | 15.3 ± 0.7 | 458.2 ± 21 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blank-Landeshammer, B.; Klanert, G.; Mitter, L.; Turisser, S.; Nusser, N.; König, A.; Iken, M.; Weghuber, J. Improved Bioavailability and Bioaccessibility of Lutein and Isoflavones in Cultured Cells In Vitro through Interaction with Ginger, Curcuma and Black Pepper Extracts. Antioxidants 2022, 11, 1917. https://doi.org/10.3390/antiox11101917
Blank-Landeshammer B, Klanert G, Mitter L, Turisser S, Nusser N, König A, Iken M, Weghuber J. Improved Bioavailability and Bioaccessibility of Lutein and Isoflavones in Cultured Cells In Vitro through Interaction with Ginger, Curcuma and Black Pepper Extracts. Antioxidants. 2022; 11(10):1917. https://doi.org/10.3390/antiox11101917
Chicago/Turabian StyleBlank-Landeshammer, Bernhard, Gerald Klanert, Lisa Mitter, Sophia Turisser, Nicolas Nusser, Alice König, Marcus Iken, and Julian Weghuber. 2022. "Improved Bioavailability and Bioaccessibility of Lutein and Isoflavones in Cultured Cells In Vitro through Interaction with Ginger, Curcuma and Black Pepper Extracts" Antioxidants 11, no. 10: 1917. https://doi.org/10.3390/antiox11101917
APA StyleBlank-Landeshammer, B., Klanert, G., Mitter, L., Turisser, S., Nusser, N., König, A., Iken, M., & Weghuber, J. (2022). Improved Bioavailability and Bioaccessibility of Lutein and Isoflavones in Cultured Cells In Vitro through Interaction with Ginger, Curcuma and Black Pepper Extracts. Antioxidants, 11(10), 1917. https://doi.org/10.3390/antiox11101917