Abstract
The imbalance between pro-oxidants and antioxidants is thought to be responsible for aging and cognitive impairment in many degenerative diseases, including schizophrenia (SZ). As the first antioxidant enzyme to detoxify superoxide radicals in mitochondria, manganese superoxide dismutase (MnSOD) activity and its functional polymorphism of Ala-9Val have been found to be associated with SZ. In this study, we explored the association between MnSOD activity, MnSOD Ala-9Val polymorphism and cognitive dysfunction in unmedicated first-episode (UMFE) SZ patients, which has not been examined. We recruited 234 UMFE SZ patients and 232 healthy controls (HC) and evaluated them with Repeated Battery for the Assessment of Neuropsychological Status (RBANS), plasma MnSOD activity and MnSOD Ala-9Val (rs4880) polymorphism. In addition, we used the Positive and Negative Syndrome Scale (PANSS) to assess the severity of patients’ psychopathological symptoms. Compared with HC, UMFE patients showed extensive cognitive impairment on RBANS, and had higher MnSOD activity. MnSOD Ala-9Val polymorphism was not associated with SZ susceptibility and cognitive impairment, but only affected MnSOD activity in patients. Moreover, only in SZ patients with Val homozygotes, MnSOD activity was significantly correlated with cognitive impairment, especially in RBANS total score, visuospatial/constructional and attention index scores. Our results suggest that cognitive impairment is associated with MnSOD activity in patients with first-episode SZ, which may be regulated by MnSOD Ala-9Val polymorphism.
1. Introduction
Cognitive impairment is a pervasive feature of schizophrenia (SZ). It occurs before the onset of psychosis and is independent of the clinical symptoms of SZ [1]. More than 80% of SZ individuals exhibit deficits in at least one cognitive domain, manifested as overall changes in attention, language ability, memory, executive function and social function [2,3]. Cognitive impairment has an important functional impact on the daily life of patients, and previous studies have shown that cognitive impairment is the best predictor of long-term functional outcomes [3,4]. Current antipsychotics are effective in relieving psychotic symptoms and reducing the risk of relapse, but they cannot treat cognitive impairment of SZ patients [5]. To a certain extent, this is due to a lack of understanding of the underlying pathological mechanisms associated with cognitive impairment in SZ patients.
Oxidative stress (OS) is a general oxidation-related imbalance that is known to result in cell dysfunction or cell death [6]. Neurons in the brain are particularly sensitive to OS because of their high lipid content and high metabolic rate [7]. Several studies have shown that OS is related with the severity of symptoms in SZ patients [8,9]. The antioxidant system can neutralize reactive oxygen species (ROS) by supplying reductive equivalents (electrons) to ROS and reverse oxidative damage in the brain [10]. As the first antioxidant enzyme in the antioxidant defense system, superoxide dismutase (SOD) promotes superoxide anion radicals (O2−) to produce hydrogen peroxide (H2O2), which is subsequently detoxified by catalase to oxygen and water [11]. SOD includes three different metal cofactor isomers in the human brain: copper (Cu) and zinc (Zn) isomers exist in the cytoplasm and extracellular space, while manganese isomer (MnSOD) is located in the mitochondrial substrate. Due to its location, MnSOD is an important enzyme that controls ROS formation and plays an important role in numerous biochemical and molecular processes essential for the survival of aerobic life [12,13,14]. It has been reported that the activity of MnSOD in peripheral blood is disrupted in chronic [15,16] and first-episode SZ patients [17]. Another important study using the postmortem brain tissue of SZ patients found that the level of MnSOD was elevated in the prefrontal cortex [18]. The human MnSOD gene is located on chromosome 6 and has been identified as a linkage candidate in genome-wide linkage studies in SZ patients [19,20]. The Ala-9Vla polymorphism (rs4880) is the most widely investigated functional polymorphism of the MnSOD coding gene. The substitution from T to C (GTT to GCT), that is, from valine to alanine, causes the structure of the mitochondrial targeting domain to change from β-sheet to α-helix, thereby increasing the formation of active MnSOD tetramer in mitochondria [21,22]. Previous studies on the contribution of MnSOD polymorphism to SZ susceptibility are inconsistent [23,24,25,26,27]. The inclusion of subjects of different races in different studies may explain the inconsistent results. A recent meta-analysis study has shown that the Ala-9Val genotype is not associated with SZ susceptibility, or tardive dyskinesia, which is a common motor syndrome in SZ patients [15].
Excessive production of ROS in mitochondria can lead to DNA mutations and protein/lipid oxidation, which in turn leads to mitochondrial dysfunction [28], being considered to be a crucial potential cause of cognitive decline in brain aging and several diseases (i.e., Alzheimer’s disease (AD) and Parkinson) [29,30,31]. Superoxide anions are a member of ROS and SOD protects cells from the deleterious effects of superoxide anions in the organism [13]. There are many studies regarding the relationship between SOD and cognitive performance. For example, a recent study shows that SOD is an independent factor leading to cognitive deficits in patients with cerebral small vessel disease [32]. In an animal study, it was found that overexpression of SOD prevented AD-related learning and memory deficits, and reduced hippocampus superoxide radicals [33]. In addition, antioxidant treatment can also reverse the working-learning deficits and hippocampal long-term potentiation (LTP) and significantly increase the activities of SOD and another antioxidant enzyme in the hippocampus and cerebral cortex in AD animal model [34]. However, studies on the link between SOD and cognitive impairment in SZ individuals are inconsistent [16,35,36,37,38]. Our previous studies have shown that SOD activity, especially MnSOD activity, is closely related to cognitive performance of patients with chronic SZ [16,23,37], but these results are not repeated in other chronic [35,39] or first-episode [36] SZ patients. The history of antipsychotic drugs, patient status (chronic or acute episode), sample size and genetic polymorphisms may be the main reasons for these inconsistencies [40,41].
In this study, patients with unmedicated first-episode (UMFE) SZ were included to maximize control of confounding factors, including disease duration, the effect of antipsychotic drugs and related psychiatric and medical comorbidities [42]. Therefore, we studied the association between cognitive performance and MnSOD activity of individuals with UMFE SZ, and further examined whether the MnSOD Ala-9Val polymorphism modulated this relationship. We hypothesized that the MnSOD Ala-9Val polymorphism may affect the activity of MnSOD, which may lead to cognitive impairment in SZ patients. The purposes of this study were: (1) to analyze whether MnSOD Ala-9Val variant regulates MnSOD activity in SZ patients and healthy controls (HC); (2) to explore the association between MnSOD activity and cognitive performance in SZ patients and HC; (3) to detect the effect of MnSOD Ala-9Val polymorphism on cognitive performance of these two groups; and (4) to investigate whether the MnSOD Ala-9Val genotype would regulate the association between MnSOD activity and cognitive function.
2. Material and Methods
2.1. Subjects
Our sample included 234 patients who were hospitalized in Beijing Hui-Long-Guan Hospital and followed up for about 3 months to confirm the diagnosis of SZ. All participants were assessed for SZ by two psychiatrists according to the Structured Clinical Interview for DSM-IV. The criteria for selecting patients included: (1) age between 18 and 55 years old; (2) acute onset at admission; (3) course of disease ≤ 2 years; (4) never taking antipsychotic drugs; (5) no physical disease. Patients with a personal history of alcohol or substance dependence (except for smoking) were excluded.
A total of 232 age- and sex-matched HC were also recruited from the communities in Beijing. Through the questionnaire survey, the researcher obtained demographic data, lifestyle (such as smoking history, drug abuse) and physical conditions. Those with a personal history of mental illness, alcohol or substance dependence (except for smoking) were excluded.
The study was approved by the Ethical Board of Beijing Hui-Long-Guan hospital (SCH-A01), and all participants filled out and signed a consent form after understanding the detailed research plan.
2.2. Clinical Symptom and Cognitive Function Measurements
Two experienced psychiatrists used PANSS to assess patients’ psychiatric symptoms [43]. These two psychiatrists attended a training curriculum on the use of PANSS. After the course, the inter-rater reliability was examined by inter-rater correlation coefficient (ICC) of the total PANSS score, which was 0.82. RBANS was applied to assess cognitive function of participants [44]. The Chinese version of RBANS has been translated by our research team, and we have also examined its test reliability in HC and SZ patients [45]. RBANS contains 12 subtests, which can be converted into five subscales (immediate memory, visuospatial/constructional, language, attention, delayed memory), and a total score.
2.3. Evaluation of MnSOD Activity
The researchers collected blood from the forearm veins of all participants from 7 a.m. to 9 a.m. after fasting the night before, and then coded each sample. An assistant, who was blind for clinical condition of all participants measured MnSOD activity according to established procedures [46,47]. Briefly, the activity of MnSOD in plasma was recorded in units per milliliter of plasma, and was measured by the cyanide inhibition method, which was derived from the nitrite method [48]. The coefficients of variation of MnSOD were 8% (inter-assay) and 6% (intra-assay).
2.4. Genotyping
DNA was extracted according to the routine procedures. The MnSOD Ala-9Val polymorphism genotypes were determined as previously reported [23,49]. The following primers were used: 5′-ACGTTGGATGCTGTGCTTTCTCGTCTTCAG-3′ and 5′-ACGTTGGATGTTCTGCCTGGAGCCCAGATA-3′. An assistant performed genotyping tests twice (20%) to ensure accuracy. All concordance rates were at least 91.3%, and call rates were higher than 93.3%.
2.5. Statistical Analysis
Deviations of Hardy-Weinberg equilibrium (HWE) were evaluated by χ2 test of goodness of fit. MnSOD Ala-9Val allele and genotype frequencies were calculated for SZ patients and HC using chi-square test. Chi-square test and Analysis of Variance (ANOVA) were used to compare categorical variables and continuous variables, respectively. Pearson’s product-moment correlation was used to examine the correlation between variables. Since there was no homozygous variant Ala/Ala genotype in patients, and it only occurred in 0.9% of HC, the Ala/Ala genotype and Val/Ala genotype were combined into one group in the analysis. In addition, we used MnSOD genotype (Val/Val vs. Ala/Ala + Val/Ala) and diagnosis (patients vs. HC) as between-group factors, and used RBANS total and subscale scores or MnSOD activity as dependent variables, with sex, education, age, BMI and smoking status as covariates. The main effects of genotype, diagnosis and genotype × diagnosis interaction was examined. In addition, we modified multiple tests by using Bonferroni corrections. Finally, we applied stepwise regression analysis to assess whether MnSOD genotype could regulate the relation between MnSOD activity and cognitive performance. The RBANS total and its subscale scores were used as the dependent variables, and MnSOD activity was used as the independent variable in each MnSOD genotype group. Age, sex, education, smoking status, BMI, and age at first onset were used as covariates in the above-mentioned stepwise forward entry models. We adopted SPSS 22 version for data analysis. The significance degree of all p values was set to less than 0.05 (with two tails).
3. Results
The general and clinical characteristics of the patients and HC by MnSOD Ala-9Val genotype subgroup are shown in Table 1. No differences were found in age, sex and smoking status between genotype groups in patients, HC, or in the entire sample. Compared with the HC, SZ patients had a lower BMI (p < 0.001). Further, we found that there were significant differences in education level (years) between genotypes in the whole sample or patients (both p < 0.05), indicating that the education level of Ala carriers was significantly higher than that of Val homozygotes. In addition, in SZ patients no differences were found in the PANSS total score and its subscale scores or the age of onset between different Ala-9Val polymorphism.

Table 1.
Demographics and clinical characteristics in first-episode patients and healthy controls grouped by MnSOD Val-9Ala genotype.
The MnSOD allele and genotype frequencies are shown in Table 2. The genotype distributions of MnSOD gene in patients and HC were consistent with HWE (both p > 0.05). No significant difference was found in the MnSOD genotype and allele distribution between patients and healthy individuals. In addition, there were no significant gender differences in MnSOD allele distribution and genotype among all participants or when healthy participants and patients were analyzed separately (all p > 0.05).

Table 2.
MnSOD allele and genotype distributions in patients with schizophrenia and healthy controls.
3.1. Genotype Effects on Performance of Cognition between Patients and HC
Table 3 summarizes the RBANS total and RBANS subscale scores grouped by MnSOD Ala-9Val polymorphism. As expected, we found that patients had lower total RBANS scores and lower scores on all subscales than HC, except for the visuospatial/constructional index (all p < 0.001). After adding sex, age, education, BMI and smoking as covariates, these differences were still significant (all p < 0.01).

Table 3.
Cognitive performance and plasma MnSOD activity in first-episode schizophrenia patients and normal controls grouped by MnSOD Ala-9Val genotype.
According to Table 3, there was no genotype × diagnosis interaction on the RBANS total score or any index scores. We also did not observe significant differences in RBANS total or any index scores between the genotype subgroups of the combined group or when HC and SZ patients and were examined separately (all p > 0.05).
3.2. Effect of Ala-9Val Polymorphism on MnSOD Activity between Patients and Healthy Participants
The activity of MnSOD was analyzed in 215 UMFE SZ patients and 205 HC. Showing in the Table 3, the activity of plasma MnSOD in SZ patients was higher than that of HC (24.3 ± 15.3 U/mL vs. 22.0 ± 13.5 U/mL, F (1,416) = 8.51, p < 0.01). After controlling for age, sex, education level, smoking status and BMI, the difference remained significant (F (6,375) = 4.63, p < 0.05). In addition, there was a genotype × diagnosis interaction on MnSOD activity (F (1,416) = 9.15, p = 0.003), which was still significant after Bonferroni correction (Table 3).
Patients with Ala alleles had higher MnSOD activity than patients with Val homozygote (F (1,213) = 8.46, p = 0.004; Bonferroni corrected p < 0.05). After controlling for age, sex, education level, BMI and smoking status, there was still a significant difference in MnSOD activity between patients with Ala alleles and those with Val homozygote (F (5,180) = 11.35, p < 0.01, Bonferroni corrected, p < 0.05). However, there was no difference in MnSOD activity between MnSOD genotypes in the HC (F (1,203) = 1.80, p = 0.18).
3.3. MnSOD Activity and Performance of Cognition: Associations with MnSOD Ala-9Val Polymorphism
Pearson correlation analysis exhibited that only in patients, MnSOD activity was inversely correlated with immediate memory (r = −0.15, df = 209, p = 0.027), visuospatial/constructional (r = −0.17, df = 209, p = 0.012), attention (r = −0.19, df = 209, p < 0.01), and RBANS total scores (r = −0.19, df = 209, p < 0.01). Further, using gender and age as covariates, partial correlation analysis showed that these correlations remained significant (all p < 0.05). However, only the p value between MnSOD activity and attention score or RBANS total score passed Bonferroni correction (both corrected p < 0.05).
As shown in Table 4 and Figure 1, in patients with Val homozygote, MnSOD activity was remarkably correlated with RBANS total score (r = −0.16, df = 151, p = 0.046), attention (r = −0.18, df = 151, p = 0.024), or visuospatial/constructional index (r = −0.17, df = 151, p = 0.038). Further, partial correlation analysis showed that these correlations remain significant when gender and age were used as covariates (all p < 0.05). However, there was no significant association between MnSOD activity and RBANSS scores in Ala allele carriers. Further regression analysis showed that MnSOD activity was significantly correlated with RBANS total score (beta = −0.17, t = −0.38, p = 0.04), attention index (beta = −0.23, t = −2.01, p = 0.045) and visuospatial/constructional index (beta = −0.22, t = −2.39, p = 0.018).

Table 4.
Relationships between MnSOD activity and cognitive domain scores across MnSOD genotype in schizophrenia patients.
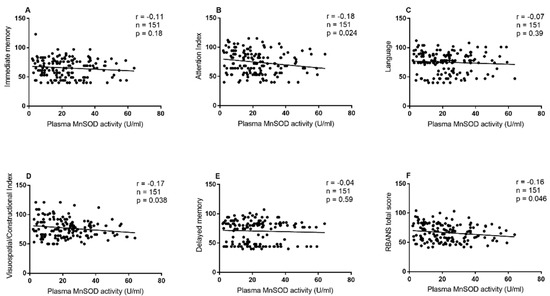
Figure 1.
Association between MnSOD activity and cognitive performance in schizophrenia patients with the Val homozygote. Association between MnSOD activity and (A) Immediate memory, (B) Attention index, (C) Language, (D) Visuospatial/Constructional Index, (E) Delayed memory and (F) RBANS total score in schizophrenia patients with the Val homozygote.
In the HC, there was no significant correlation between MnSOD activity and RBANS scores in the whole group or in the MnSOD genotype subgroups (all p > 0.05).
4. Discussion
Our study had several main results. (1) MnSOD Ala-9Val polymorphism may not directly contribute to SZ susceptibility. (2) Cognitive impairment was not dependent on MnSOD polymorphism in SZ patients. (3) Ala variant was associated with higher MnSOD activity in SZ patients, but not in HC. (4) Only in SZ patients with Val homozygote, MnSOD activity was remarkably associated with cognitive performance.
4.1. Effects of Val-9Ala Polymorphism on the Susceptibility of SZ
Previous studies on MnSOD Ala-9Val polymorphism and SZ susceptibility are inconsistent [23,24,25,26,27]. For example, some studies have found that MnSOD Ala-9Val genotype may be concerned with the physiological pathogenesis of SZ [24,25]. However, several other studies have not revealed the association between Ala-9Val polymorphism and SZ [15,23,26,27]. Different ethnic backgrounds may be the main reason for the inconsistency of these results. It is noteworthy that compared with Caucasians and African Americans, most Asians have higher frequencies of Val/Val genotypes and Val alleles. It is reported that the frequency of Ala alleles in Asians ranges from 9.4% to 16.3% [27,50,51], while the frequency in Caucasians is around 50% [26,52]. In this study, the allele frequency in the healthy group was 12.9%, which was similar to the results of other studies among Asian populations. In addition to ethnic differences in the MnSOD Ala-9Val polymorphism, the general heterogeneity of SZ diagnosis, small gene effect and population stratification may be important factors that lead to inconsistent results.
4.2. MnSOD Ala-9Val Polymorphism on Cognitive Function in Participants with SZ
Patients with UMFE SZ showed significant cognitive decline in the RBANS total score and four subscales including immediate memory, attention, language and delayed memory, which replicate our previous results in first-episode [53,54,55] and chronic SZ patients [56,57]. These consistent results indicate that cognitive impairment appears in the early stages s of the disease and seems to be an essential feature of SZ patients.
In this study, there were no significant differences in all RBANS scores in the three genotypes of the patient and healthy groups, suggesting that the MnSOD genotype may have no significant regulatory effect on the cognitive dimensions we measured. Our study is the first to systematically focus on the association between MnSOD variant and cognitive function in patients with UMFE SZ. This result is inconsistent with our previous study, which showed that the MnSOD Val-9Ala genotype was associated with the attention domain of patients with chronic SZ [23]. This inconsistent result may be due to the fact that the average age of first-episode patients is younger than that of chronic SZ patients (approximately 27 years vs. 48 years). Based on the resource regulation hypothesis, with the increase of age, the gradual shortage of brain resources leads to enhanced regulation of genetic variation on cognition, brain structure and function, so genetic variation plays a more important role in the aging process [58,59].
There is growing evidence showing that compared with the elderly, the genetic effects of young people are moderate or non-existent [60]. Previous studies have shown that the functional MnSOD variants are not an independent risk factor for amnestic mild cognitive impairment, which is similar to our results [61]. Further studies are needed to recruit relatively older samples to determine whether there is an age-dependent relationship between MnSOD variants and cognitive performance.
4.3. Increased MnSOD Level and Dysfunction of Cognition: Relationship to MnSOD Val-9Ala Genotype
We found that compared with HC, UMFE SZ patients had higher MnSOD activity, which was in line with our previous research results [17] and other research results [18,51]. However, some studies have found that there is no change or decrease in MnSOD activity in patients with first episode [62] or chronic SZ [15,16,23]. A variety of factors may cause these inconsistent results, including but not limited to disease state (chronic or acute onset), history of antipsychotic drugs, test sources (serum, plasma, platelet or brain tissue), obesity and MnSOD polymorphism [63,64]. In addition, it has been found that 8-hydroxy-2 deoxyguanosine, a common biomarker in DNA oxidative damage, has increased in patients with first-episode SZ [65]. Therefore, we speculate that the increased MnSOD activity is a compensatory response to excessive detoxification of ROS.
Furthermore, our study found that the MnSOD activity in plasma was regulated by MnSOD genotype, indicating that the MnSOD activity of Ala carriers was higher than that of Val homozygous patients, but there was no such effect in the HC. As far as we know, this study is one of the first to reveal that plasma MnSOD activity is regulated by MnSOD genotype in patients with UMFE SZ. This result is different from our previous finding that MnSOD genotype has no effect on MnSOD activity in chronic SZ patients [23]. Several factors, such as antipsychotic treatment, course of disease, and disease status (acute or chronic) may lead to this inconsistent result. Several authors have found similar results in other diseases, such as diabetes mellitus and prostate cancer, suggesting that the -9Ala allele may be associated with higher MnSOD activity, which is similar to previous results [64,66]. Ala-9Val as a functional polymorphism of the MnSOD gene, its variant from C to T represents the amino acid change from Val to Ala, resulting in the chromosome structure alteration in the targeted domain from the beta-sheet to the alpha-helix. Compared with the beta-sheet structure, the alpha-helical structure containing the Ala precursor enhances the transport efficiency of the enzyme to the mitochondria matrix and increases the antioxidant defense enzyme against ROS [22]. In addition, our study did not detect the effect of Ala-9Val polymorphism on SZ susceptibility. Considering the influence of Ala alleles on MnSOD activity, it is speculated that Ala-9Val mutants may affect the OS process involved in MnSOD, thereby participating in the development of SZ.
We also found that increased MnSOD activity was inversely related to general cognitive performance and several cognitive domains, especially with the poor attention index in UMFE SZ patients, but not in HC. This result is similar to our previous studies in the first-episode [62] and chronic SZ patients [16,37]. Another study found that total antioxidant capacity (TAC) was particularly associated with attention index in non-affective SZ patients [67]. Furthermore, a previous study also found that in patients with recurrent depressive disorder, the MnSOD expression was significantly negatively correlated with some cognitive performance tests including Trial Making test and Stroop test [41]. These studies indicate that OS may play an essential role in cognitive impairment of patients with mental illness. However, the negative correlation between MnSOD activity and cognition exceeded our expectation. It should be pointed out that OS is caused by oxidative damage due to the imbalance between pro-oxidants and antioxidants, so high levels of antioxidant enzymes may not indicate low OS damage [68]. A previous study demonstrated that the activity of antioxidant enzymes was found enhanced in the elderly, while indicators representing OS damage, including 8-hydroxy-2′-deoxyguanosine and protein carbonyls, were also increased [69]. Based on these findings, we suppose that the increased MnSOD is a compensatory response to OS to protect the human body from free radical damage. The cognitive impairment of SZ patients may be partly attributed to the damage of OS. As an antioxidant enzyme that scavenges ROS in mitochondria, MnSOD participates in neurocognitive processes mainly through the interaction of antioxidant enzymes, increased OS and cellular destruction [70,71].
It has been found that in neurodegenerative diseases such as AD, the expression of MnSOD in hippocampal neurons is downregulated, while overexpression of MnSOD reduces hippocampal superoxide and Aβ plaques, and prevents learning and memory deficits associated with AD [33,72]. At present, it is not clear how MnSOD activity affects cognitive function, but when the balance of ROS and antioxidant enzymes is disrupted, it will hinder the formation of LTP, the molecular basis of learning [73,74]. In addition, ROS is also an important signaling molecule, which is necessary for synaptic plasticity and construction of memory [75].
Another impressive finding of this study was that MnSOD polymorphism regulated the relationship between cognitive performance and MnSOD activity in UMFE SZ patients, that is, a remarkable inverse relationship between MnSOD activity and attention, visuospatial/constructure index or RBANS total scores was observed only in patients with Val homozygote, but there was no such correlation in Ala carriers. The difference in plasma MnSOD-cognitive function between different MnSOD genotypes in UMFE SZ patients gives a hint about the unexpected discovery of higher MnSOD activity with more damage of cognitive performance. It was known that the MnSOD gene-9Ala variant, but not the -9Val allele, has an alpha-helical structure. This alpha-helical structure helps to transport the MnSOD precursor protein into the mitochondria more effectively, that is, individuals with MnSOD -9Ala genotypes can transfer more MnSOD precursor to mitochondria than individuals with-9Val genotypes, thus eliminating more excessive detoxification of ROS [22]. As a result, only patients with Ala carrier produced additional MnSOD, while patients with Val/Val did not produce more MnSOD to protect against the cognitive impairment caused by ROS-induced neuronal damage. Interestingly, we did find that MnSOD activity in Val homozygous patients was lower than that in Ala carriers, suggesting that patients with Val homozygotes may suffer more severe ROS damage. Therefore, in UMFE SZ patients with Val homozygote, when ROS production increases, leading to cognitive impairment, it induces more MnSOD production as a compensatory increase, thus showing a negative interrelationship between MnSOD activity and cognitive performance. Certainly, these are our speculations, and further longitudinal studies are needed in SZ patients to clarify the relationship between peripheral blood MnSOD activity and cognitive impairment, and to further investigate how MnSOD genotype affects the relationship between cognitive deficits and MnSOD activity.
Some limitations of this research are as follows. First, we examined the activity of only one antioxidant enzyme, which is involved in only one part of the action of the antioxidant defense system. Both antioxidant enzymes and non-enzymes should be included in the measurement to have a more detailed knowledge about the antioxidant defense system in future studies. Second, in this study, we did not measure the protein expression of Mn-SOD. Therefore, we cannot directly demonstrate that the regulation of the activity is due to the presence of the Mn-SOD gene polymorphism. Furthermore, as no other parameters of oxidative stress were measured, it is possible that the change in Mn-SOD activity is due to another imbalance in the redox system. Third, specifically, the higher activity of MnSOD in plasma would be expected to produce higher levels of hydrogen peroxide, H2O2, which could be directly measured with appropriate dyes. Moreover, the excess H2O2 would be expected to lead to elevated markers of oxidative damage to proteins and lipids, for example protein carbonyls and protein-bound 4-hydroxynonenal (HNE) or isoprostanes, but definitely not thiobarbituric acid reactive substance (TBARS). In this study, unfortunately, we did not measure H2O2 levels, which should be remedied in the future studies to explore whether our results in this study are consistent with the expectations of elevated H2O2. Fourth, this study only took Han Chinese as the research subjects. Since there are significant differences in Ala allele frequencies between Asians and whites, our findings need to be verified in different races in future studies. Fifth, we only detected one polymorphism of MnSOD gene in this study. Previous studies have shown that other polymorphisms (such as Ile58Thr) also regulate the expression of MnSOD gene, which should be included in future studies to fully understand the effects of MnSOD gene mutations [76]. Sixth, due to the cross-sectional design of this study, it is largely observational in nature rather than, and we could not draw a causal relationship between MnSOD and cognitive performance in SZ patients, which may be clarified in a future study with a longitudinal design.
In summary, this study provides evidence for the correlation between MnSOD polymorphism, MnSOD activity and cognitive deficits in patients with UMFE SZ. Our study showed that patients with UMFE SZ had extensive cognitive deficits and higher MnSOD activity. Moreover, our results showed that MnSOD activity was negatively associated with general cognitive function, especially attention in SZ patients, but not in HC. More importantly, the relationship between cognitive performance and MnSOD activity was regulated by MnSOD Ala-9Vla polymorphism in these SZ patients. These results indicate that in the early stages of the disease, the peripheral MnSOD activity and Ala-9Vla gene polymorphism may be involved in the pathological mechanism of cognitive dysfunction in SZ patients. Among our findings, the important result was that MnSOD activity and cognitive performance were significantly correlated in SZ patients with Val homozygotes, suggesting that oxidative stress may be implicated in the psychopathological mechanisms of cognitive impairment of schizophrenia patients, and the use of antioxidants is a possible option for the treatment of cognitive decline in patients with first-episode SZ in the clinical practice. In view of the difference in Ala allele frequency between Asians and whites, our finding that the MnSOD genotypes regulated the association between MnSOD activity and cognitive impairment may only be specific for Chinese samples. Future studies need to include people from different ethnic backgrounds to verify the findings of this study.
Author Contributions
Conceptualization, D.M.W. and X.Y.Z.; data curation, R.R.Z. and Y.T.; formal analysis, H.X.Z.; investigation, R.R.Z., Y.T. and K.U.; methodology, K.U., L.W. and X.Y.Z.; project administration, J.J.C.; software, H.X.Z.; supervision, X.Y.Z.; writing—original draft, D.M.W.; writing—review and editing, L.W. and T.R.K. All authors have contributed to and have approved the final manuscript.
Funding
Funding for this study was provided by the National Natural Science Foundation of China (31300848 by D.M.W.), CAS International Cooperation Research Program (153111KYSB20190004 by X.Y.Z), CAS Pioneer Hundred Talents Program and the CAS Key Lab of Mental Health. The authors declare no conflict of interest.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee of Beijing Hui-Long-Guan hospital (SCH-A01).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
The authors thank the subjects whose participation made this study possible.
Conflicts of Interest
The authors declare that there was no conflict of interest.
References
- Reichenberg, A.; Velthorst, E.; Davidson, M. Cognitive impairment and psychosis in schizophrenia: Independent or linked conditions? World Psychiatry 2019, 18, 162–163. [Google Scholar] [CrossRef] [PubMed]
- Gold, J.M.; Robinson, B.; Leonard, C.J.; Hahn, B.; Chen, S.; McMahon, R.P.; Luck, S.J. Selective Attention, Working Memory, and Executive Function as Potential Independent Sources of Cognitive Dysfunction in Schizophrenia. Schizophr. Bull. 2018, 44, 1227–1234. [Google Scholar] [CrossRef] [PubMed]
- Fett, A.K.; Viechtbauer, W.; Dominguez, M.D.; Penn, D.L.; van Os, J.; Krabbendam, L. The relationship between neurocognition and social cognition with functional outcomes in schizophrenia: A meta-analysis. Neurosci. Biobehav. Rev. 2011, 35, 573–588. [Google Scholar] [CrossRef]
- Pelletier-Baldelli, A.; Holt, D.J. Are Negative Symptoms Merely the "Real World" Consequences of Deficits in Social Cognition? Schizophr. Bull. 2020, 46, 236–241. [Google Scholar] [CrossRef]
- Haddad, P.M.; Correll, C.U. The acute efficacy of antipsychotics in schizophrenia: A review of recent meta-analyses. Ther. Adv. Psychopharmacol. 2018, 8, 303–318. [Google Scholar] [CrossRef]
- Rahal, A.; Kumar, A.; Singh, V.; Yadav, B.; Tiwari, R.; Chakraborty, S.; Dhama, K. Oxidative stress, prooxidants, and antioxidants: The interplay. BioMed Res. Int. 2014, 2014, 761264. [Google Scholar] [CrossRef] [PubMed]
- Salim, S. Oxidative stress and psychological disorders. Curr. Neuropharmacol. 2014, 12, 140–147. [Google Scholar] [CrossRef]
- García, S.; Alberich, S.; Martínez-Cengotitabengoa, M.; Arango, C.; Castro-Fornieles, J.; Parellada, M.; Baeza, I.; Moreno, C.; Mico, J.A.; Berrocoso, E.; et al. The complex association between the antioxidant defense system and clinical status in early psychosis. PLoS ONE 2018, 13, e0194685. [Google Scholar] [CrossRef]
- Lin, C.H.; Lane, H.Y. Early Identification and Intervention of Schizophrenia: Insight From Hypotheses of Glutamate Dysfunction and Oxidative Stress. Front. Psychiatry 2019, 10, 93. [Google Scholar] [CrossRef]
- Hardingham, G.E.; Lipton, S.A. Regulation of neuronal oxidative and nitrosative stress by endogenous protective pathways and disease processes. Antioxid. Redox Signal. 2011, 14, 1421–1424. [Google Scholar] [CrossRef]
- Sakamoto, T.; Imai, H. Hydrogen peroxide produced by superoxide dismutase SOD-2 activates sperm in Caenorhabditis elegans. J. Biol. Chem. 2017, 292, 14804–14813. [Google Scholar] [CrossRef] [PubMed]
- Crawford, A.; Fassett, R.G.; Geraghty, D.P.; Kunde, D.A.; Ball, M.J.; Robertson, I.K.; Coomes, J.S. Relationships between single nucleotide polymorphisms of antioxidant enzymes and disease. Gene 2012, 501, 89–103. [Google Scholar] [CrossRef] [PubMed]
- Dhar, S.K.; St Clair, D.K. Manganese superoxide dismutase regulation and cancer. Free Radic. Biol. Med. 2012, 52, 2209–2222. [Google Scholar] [CrossRef]
- Dhar, S.K.; Scott, T.; Wang, C.; Fan, T.W.M.; St Clair, D.K. Mitochondrial superoxide targets energy metabolism to modulate epigenetic regulation of NRF2-mediated transcription. Free Radic. Biol. Med. 2022, 179, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.F.; Cao, B.; Xu, M.Y.; Liu, Y.Q.; Yan, L.L.; Liu, R.; Wang, J.Y.; Lu, Q.B. Meta-Analyses of Manganese Superoxide Dismutase Activity, Gene Ala-9Val Polymorphism, and the Risk of Schizophrenia. Medicine 2015, 94, e1507. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.Y.; Chen, D.C.; Xiu, M.H.; Tan, Y.L.; Yang, F.D.; Zhang, L.Y.; Zhang, L.Y.; Haile, C.N.; Kosten, T.R. Clinical symptoms and cognitive impairment associated with male schizophrenia relate to plasma manganese superoxide dismutase activity: A case-control study. J. Psychiatr. Res. 2013, 47, 1049–1053. [Google Scholar] [CrossRef] [PubMed]
- Lang, X.; Wang, D.M.; Du, X.D.; Jia, Q.F.; Chen, D.C.; Xiu, M.; Wang, L.; Zhang, X. Elevated activity of plasma superoxide dismutase in never-treated first-episode schizophrenia patients: Associated with depressive symptoms. Schizophr. Res. 2020, 222, 291–296. [Google Scholar] [CrossRef]
- Michel, T.M.; Thome, J.; Martin, D.; Nara, K.; Zwerina, S.; Tatschner, T.; Weijers, H.G.; Koutsilieri, E. Cu, Zn- and Mn-superoxide dismutase levels in brains of patients with schizophrenic psychosis. J. Neural Transm. 2004, 111, 1191–1201. [Google Scholar] [CrossRef]
- Lerer, B.; Segman, R.H.; Hamdan, A.; Kanyas, K.; Karni, O.; Kohn, Y.; Korner, M.; Lanktree, M.; Kaadan, M.; Turetsky, N.; et al. Genome scan of Arab Israeli families maps a schizophrenia susceptibility gene to chromosome 6q23 and supports a locus at chromosome 10q24. Mol. Psychiatry 2003, 8, 488–498. [Google Scholar] [CrossRef]
- Wan, X.S.; Devalaraja, M.N.; St Clair, D.K. Molecular structure and organization of the human manganese superoxide dismutase gene. DNA Cell Biol. 1994, 13, 1127–1136. [Google Scholar] [CrossRef]
- Pourvali, K.; Abbasi, M.; Mottaghi, A. Role of Superoxide Dismutase 2 Gene Ala16Val Polymorphism and Total Antioxidant Capacity in Diabetes and its Complications. Avicenna J. Med. Biotechnol. 2016, 8, 48–56. [Google Scholar] [PubMed]
- Bresciani, G.; Cruz, I.B.; de Paz, J.A.; Cuevas, M.J.; González-Gallego, J. The MnSOD Ala16Val SNP: Relevance to human diseases and interaction with environmental factors. Free. Radic. Res. 2013, 47, 781–792. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.Y.; Chen, D.C.; Xiu, M.H.; Yang, F.D.; Tan, Y.; Luo, X.; Zuo, L.; Kosten, T.A.; Kosten, T.R. Cognitive function, plasma MnSOD activity, and MnSOD Ala-9Val polymorphism in patients with schizophrenia and normal controls. Schizophr. Bull. 2014, 40, 592–601. [Google Scholar] [CrossRef] [PubMed]
- Akyol, O.; Yanik, M.; Elyas, H.; Namli, M.; Canatan, H.; Akin, H.; Yuce, H.; Yilmaz, H.R.; Tutkun, H.; Sogut, S.; et al. Association between Ala-9Val polymorphism of Mn-SOD gene and schizophrenia. Prog. Neuropsychopharmacol. Biol. Psychiatry 2005, 29, 123–131. [Google Scholar] [CrossRef]
- Gałecki, P.; Pietras, T.; Szemraj, J.; Florkowska, K.; Florkowski, A.; Zboralski, K. unctional polymorphism of manganese superoxide dismutase (MnSOD) gene correlates with schizophrenia in Polish population. Pol. Merkur. Lek. Organ Pol. Tow. Lek. 2006, 20, 329–332. [Google Scholar]
- Ventriglia, M.; Scassellati, C.; Bonvicini, C.; Squitti, R.; Bevacqua, M.G.; Foresti, G.; Tura, G.B.; Genarelli, M. No association between Ala9Val functional polymorphism of MnSOD gene and schizophrenia in a representative Italian sample. Neurosci. Lett. 2006, 410, 208–211. [Google Scholar] [CrossRef]
- Pae, C.U.; Kim, T.S.; Patkar, A.A.; Kim, J.J.; Lee, C.U.; Lee, S.J.; Jun, T.Y.; Lee, C.; Paik, I.H. Manganese superoxide dismutase (MnSOD: Ala-9Val) gene polymorphism may not be associated with schizophrenia and tardive dyskinesia. Psychiatry Res. 2007, 153, 77–81. [Google Scholar] [CrossRef]
- Tan, B.L.; Norhaizan, M.E. Effect of High-Fat Diets on Oxidative Stress, Cellular Inflammatory Response and Cognitive Function. Nutrients 2019, 11, 2579. [Google Scholar] [CrossRef]
- Mecocci, P.; Boccardi, V.; Cecchetti, R.; Bastiani, P.; Scamosci, M.; Ruggiero, C.; Baroni, M. A Long Journey into Aging, Brain Aging, and Alzheimer’s Disease Following the Oxidative Stress Tracks. J. Alzheimer’s Dis. JAD 2018, 62, 1319–1335. [Google Scholar] [CrossRef]
- Lin, M.T.; Beal, M.F. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature 2006, 443, 787–795. [Google Scholar] [CrossRef]
- Trist, B.G.; Hare, D.J.; Double, K.L. Oxidative stress in the aging substantia nigra and the etiology of Parkinson’s disease. Aging Cell 2019, 18, e13031. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Wei, X.; Yang, X.; Huang, Z.; Chang, Z.; Xie, F.; Yang, Q.; Ding, C.; Xiang, W.; Yang, H.; et al. Plasma Lipoprotein-associated Phospholipase A2 and Superoxide Dismutase are Independent Predicators of Cognitive Impairment in Cerebral Small Vessel Disease Patients: Diagnosis and Assessment. Aging Dis. 2019, 10, 834–846. [Google Scholar] [CrossRef]
- Massaad, C.A.; Washington, T.M.; Pautler, R.G.; Klann, E. Overexpression of SOD-2 reduces hippocampal superoxide and prevents memory deficits in a mouse model of Alzheimer’s disease. Proc. Natl. Acad. Sci. USA 2009, 106, 13576–13581. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.L.; Li, X.H.; Dun, X.P.; Jing, X.K.; Yang, K.; Li, Y.K. Grape Seed Proanthocyanidin Extract Ameliorates Streptozotocin-induced Cognitive and Synaptic Plasticity Deficits by Inhibiting Oxidative Stress and Preserving AKT and ERK Activities. Curr. Med. Sci. 2020, 40, 434–443. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Liencres, C.; Tas, C.; Brown, E.C.; Erdin, S.; Onur, E.; Cubukcoglu, Z.; Aydemir, O.; Esen-Danaci, A.; Brune, M. Oxidative stress in schizophrenia: A case-control study on the effects on social cognition and neurocognition. BMC Psychiatry 2014, 14, 268. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Cengotitabengoa, M.; Mac-Dowell, K.S.; Leza, J.C.; Mico, J.A.; Fernandez, M.; Echevarria, E.; Sanjuan, J.; Elorza, J.; Gonzalez-Pinto, A. Cognitive impairment is related to oxidative stress and chemokine levels in first psychotic episodes. Schizophr. Res. 2012, 137, 66–72. [Google Scholar] [CrossRef]
- Wu, J.Q.; Chen, D.C.; Tan, Y.L.; Tan, S.P.; Wang, Z.R.; Xiu, M.H.; Yang, F.D.; Zhang, X.Y. Cognition impairment in schizophrenia patients with tardive dyskinesia: Association with plasma superoxide dismutase activity. Schizophr. Res. 2014, 152, 210–216. [Google Scholar] [CrossRef]
- Wei, C.; Sun, Y.; Chen, N.; Chen, S.; Xiu, M.; Zhang, X. Interaction of oxidative stress and BDNF on executive dysfunction in patients with chronic schizophrenia. Psychoneuroendocrinology 2020, 111, 104473. [Google Scholar] [CrossRef]
- Paiatto, L.N.; Silva, F.G.D.; Yamada, A.T.; Tamashiro, W.; Simioni, P.U. Adoptive transfer of dendritic cells expressing CD11c reduces the immunological response associated with experimental colitis in BALB/c mice. PLoS ONE 2018, 13, e0196994. [Google Scholar] [CrossRef]
- Chien, Y.L.; Hwu, H.G.; Hwang, T.J.; Hsieh, M.H.; Liu, C.C.; Lin-Shiau, S.Y.; Lin, C.M. Clinical implications of oxidative stress in schizophrenia: Acute relapse and chronic stable phase. Prog. Neuropsychopharmacol. Biol. Psychiatry 2020, 99, 109868. [Google Scholar] [CrossRef]
- Talarowska, M.; Orzechowska, A.; Szemraj, J.; Su, K.P.; Maes, M.; Gałecki, P. Manganese superoxide dismutase gene expression and cognitive functions in recurrent depressive disorder. Neuropsychobiology 2014, 70, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Buckley, P.E.; Evans, D. First-episode schizophrenia. A window of opportunity for optimizing care and outcomes. Postgrad. Med. 2006, spec, 5–19. [Google Scholar]
- Kay, S.R.; Fiszbein, A.; Opler, L.A. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull 1987, 13. [Google Scholar] [CrossRef] [PubMed]
- Randolph, C.; Tierney, M.C.; Mohr, E.; Chase, T.N. The Repeatable Battery for the Assessment of Neuropsychological Status (RBANS): Preliminary clinical validity. J. Clin. Exp. Neuropsychol. 1998, 20, 310–319. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.-H.; Tan, Y.-L.; Zhang, W.-F.; Wang, Z.-R.; Yang, G.-G.; Shi, C.; Zhang, X.-Y.; Zhou, D.-F. Repeatable battery for the assessment of neuropsychological status as a screening test in Chinese: Reliability and validity. Chin. Ment. Health J. 2008, 12, 865–869. [Google Scholar]
- Wu, J.Q.; Chen, D.C.; Tan, Y.L.; Tan, S.; Wang, Z.; Yang, F.; Soares, J.C.; Zhang, X.Y. Association of altered CuZn superoxide dismutase and cognitive impairment in schizophrenia patients with tardive dyskinesia. J. Psychiatr. Res. 2014, 58, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Zhang, X.Y.; Wang, H.; Tang, W.; Xia, Y.; Zhang, F.; Liu, J.; Fu, Y.; Chen, Y.; Liu, L.; et al. Elevated plasma superoxide dismutase in first-episode and drug naive patients with schizophrenia: Inverse association with positive symptoms. Prog. Neuropsychopharmacol Biol. Psychiatry 2012, 36, 34–38. [Google Scholar] [CrossRef]
- Oyanagui, Y. Reevaluation of assay methods and establishment of kit for superoxide dismutase activity. Anal. Biochem. 1984, 142, 290–296. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Rao, W.-W.; Yu, Q.; Yu, Y.; Kou, C.; Tan, Y.-L.; Chen, D.-C.; Zuo, L.; Luo, X.; Soares, J.C. Association of the manganese superoxide dismutase gene Ala–9Val polymorphism with age of smoking initiation in male schizophrenia smokers. Am. J. Med. Genet. Part B Neuropsychiatr. Genet. 2016, 171, 243–249. [Google Scholar] [CrossRef]
- Hori, H.; Ohmori, O.; Shinkai, T.; Kojima, H.; Okano, C.; Suzuki, T.; Nakamura, J. Manganese superoxide dismutase gene polymorphism and schizophrenia: Relation to tardive dyskinesia. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2000, 23, 170–177. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, X.; Hou, G.; Sha, W.; Reynolds, G.P. The increased activity of plasma manganese superoxide dismutase in tardive dyskinesia is unrelated to the Ala-9Val polymorphism. J. Psychiatr. Res. 2002, 36, 317–324. [Google Scholar] [CrossRef]
- Hitzeroth, A.; Niehaus, D.J.; Koen, L.; Botes, W.C.; Deleuze, J.F.; Warnich, L. Association between the MnSOD Ala-9Val polymorphism and development of schizophrenia and abnormal involuntary movements in the Xhosa population. Prog. Neuropsychopharmacol. Biol. Psychiatry 2007, 31, 664–672. [Google Scholar] [CrossRef] [PubMed]
- Man, L.; Lv, X.; Du, X.D.; Yin, G.; Zhu, X.; Zhang, Y.; Soares, J.C.; Yang, X.N.; Chen, X.; Zhang, X.Y. Cognitive impairments and low BDNF serum levels in first-episode drug-naive patients with schizophrenia. Psychiatry Res. 2018, 263, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Xiu, M.H.; Wang, D.; Chen, S.; Du, X.D.; Chen, D.C.; Chen, N.; Wang, Y.C.; Yin, G.; Zhang, Y.; Tan, Y.L.; et al. Interleukin-3, symptoms and cognitive deficits in first-episode drug-naive and chronic medicated schizophrenia. Psychiatry Res. 2018, 263, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.Y.; Du, X.; Yin, G.; Zhang, Y.; Chen, D.; Xiu, M.; Wang, C.; Zhang, R.; Cassidy, R.M.; Ning, Y.; et al. Prevalence and Clinical Correlates of and Cognitive Function at the Time of Suicide Attempts in First-Episode and Drug-Naive Patients With Schizophrenia. J. Clin. Psychiatry 2018, 79. [Google Scholar] [CrossRef]
- Piotrowski, P.; Kotowicz, K.; Rymaszewska, J.; Beszlej, J.A.; Plichta, P.; Samochowiec, J.; Kalinowska, S.; Trzesniowska-Drukala, B.; Misiak, B. Allostatic load index and its clinical correlates at various stages of psychosis. Schizophr. Res. 2019, 210, 73–80. [Google Scholar] [CrossRef]
- Reid, M.A.; Salibi, N.; White, D.M.; Gawne, T.J.; Denney, T.S.; Lahti, A.C. 7T Proton Magnetic Resonance Spectroscopy of the Anterior Cingulate Cortex in First-Episode Schizophrenia. Schizophr. Bull. 2019, 45, 180–189. [Google Scholar]
- Lindenberger, U.; Nagel, I.E.; Chicherio, C.; Li, S.C.; Heekeren, H.R.; Bäckman, L. Age-related decline in brain resources modulates genetic effects on cognitive functioning. Front. Neurosci. 2008, 2, 234–244. [Google Scholar]
- Tucker-Drob, E.M.; Briley, D.A. Continuity of genetic and environmental influences on cognition across the life span: A meta-analysis of longitudinal twin and adoption studies. Psychol. Bull. 2014, 140, 949–979. [Google Scholar] [CrossRef]
- Papenberg, G.; Lindenberger, U.; Bäckman, L. Aging-related magnification of genetic effects on cognitive and brain integrity. Trends Cogn. Sci. 2015, 19, 506–514. [Google Scholar]
- Gamarra, D.; Elcoroaristizabal, X.; Fernández-Martínez, M.; de Pancorbo, M.M. Association of the C47T Polymorphism in SOD2 with Amnestic Mild Cognitive Impairment and Alzheimer’s Disease in Carriers of the APOEε4 Allele. Dis. Markers 2015, 2015, 746329. [Google Scholar] [CrossRef] [PubMed]
- Xiu, M.H.; Li, Z.; Chen, D.C.; Chen, S.; Curbo, M.E.; Wu, H.E.; Tong, Y.S.; Tan, S.P.; Zhang, X.Y. Interrelationships Between BDNF, Superoxide Dismutase, and Cognitive Impairment in Drug-Naive First-Episode Patients With Schizophrenia. Schizophr. Bull. 2020, 46, 1498–1510. [Google Scholar] [CrossRef] [PubMed]
- An, H.; Du, X.; Huang, X.; Qi, L.; Jia, Q.; Yin, G.; Xiao, C.; Huang, X.-F.; Ning, Y.; Cassidy, R.M. Obesity, altered oxidative stress, and clinical correlates in chronic schizophrenia patients. Transl. Psychiatry 2018, 8, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Vats, P.; Sagar, N.; Singh, T.P.; Banerjee, M. Association of Superoxide dismutases (SOD1 and SOD2) and Glutathione peroxidase 1 (GPx1) gene polymorphisms with type 2 diabetes mellitus. Free. Radic. Res. 2015, 49, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Chestkov, I.V.; Jestkova, E.M.; Ershova, E.S.; Golimbet, V.G.; Lezheiko, T.V.; Kolesina, N.Y.; Dolgikh, O.A.; Izhevskaya, V.L.; Kostyuk, G.P.; Kutsev, S.I.; et al. ROS-Induced DNA Damage Associates with Abundance of Mitochondrial DNA in White Blood Cells of the Untreated Schizophrenic Patients. Oxidative Med. Cell. Longev. 2018, 2018, 8587475. [Google Scholar]
- Li, X.; Shen, M.; Cai, H.; Liu, K.; Liu, Y.; Huang, Z.; Liang, C.; Deng, X.; Ye, J.; Zou, Q.; et al. Association between manganese superoxide dismutase (MnSOD) polymorphism and prostate cancer susceptibility: A meta-analysis. Int. J. Biol. Markers 2016, 31, e422–e430. [Google Scholar]
- Martinez-Cengotitabengoa, M.; Mico, J.A.; Arango, C.; Castro-Fornieles, J.; Graell, M.; Paya, B.; Leza, J.C.; Zorrilla, I.; Parellada, M.; Lopez, M.P.; et al. Basal low antioxidant capacity correlates with cognitive deficits in early onset psychosis. A 2-year follow-up study. Schizophr. Res. 2014, 156, 23–29. [Google Scholar] [CrossRef]
- Costantini, D.; Verhulst, S. Does high antioxidant capacity indicate low oxidative stress? Funct. Ecol. 2009, 23, 506–509. [Google Scholar] [CrossRef]
- Gianni, P.; Jan, K.J.; Douglas, M.J.; Stuart, P.M.; Tarnopolsky, M.A. Oxidative stress and the mitochondrial theory of aging in human skeletal muscle. Exp. Gerontol. 2004, 39, 1391–1400. [Google Scholar]
- Leonard, B.; Maes, M. Mechanistic explanations how cell-mediated immune activation, inflammation and oxidative and nitrosative stress pathways and their sequels and concomitants play a role in the pathophysiology of unipolar depression. Neurosci. Biobehav. Rev. 2012, 36, 764–785. [Google Scholar] [CrossRef]
- Maes, M. Depression is an inflammatory disease, but cell-mediated immune activation is the key component of depression. Prog. Neuropsychopharmacol. Biol. Psychiatry 2011, 35, 664–675. [Google Scholar] [CrossRef] [PubMed]
- Sen, A.; Nelson, T.J.; Alkon, D.L.; Hongpaisan, J. Loss in PKC Epsilon Causes Downregulation of MnSOD and BDNF Expression in Neurons of Alzheimer’s Disease Hippocampus. J. Alzheimer’s Dis. JAD 2018, 63, 1173–1189. [Google Scholar] [CrossRef] [PubMed]
- Knapp, L.T.; Klann, E. Role of reactive oxygen species in hippocampal long-term potentiation: Contributory or inhibitory? J. Neurosci. Res. 2002, 70, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Thiels, E.; Urban, N.N.; Gonzalez-Burgos, G.R.; Kanterewicz, B.I.; Barrionuevo, G.; Chu, C.T.; Oury, T.D.; Klann, E. Impairment of long-term potentiation and associative memory in mice that overexpress extracellular superoxide dismutase. J. Neurosci. Off. J. Soc. Neurosci. 2000, 20, 7631–7639. [Google Scholar] [CrossRef]
- Kishida, K.T.; Klann, E. Sources and targets of reactive oxygen species in synaptic plasticity and memory. Antioxid Redox Signal 2007, 9, 233–244. [Google Scholar] [CrossRef]
- Kowalski, M.; Bielecka-Kowalska, A.; Oszajca, K.; Eusebio, M.; Jaworski, P.; Bartkowiak, J.; Szemraj, J. Manganese superoxide dismutase (MnSOD) gene (Ala-9Val, Ile58Thr) polymorphism in patients with age-related macular degeneration (AMD). Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2010, 16, Cr190–Cr196. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).