Characterization of Polysaccharides Sequentially Extracted from Allium roseum Leaves and Their Hepatoprotective Effects against Cadmium Induced Toxicity in Mouse Liver
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material
2.2. Preparation of Alcohol Insoluble Solids (AIS)
2.3. Extraction and Purification of Polysaccharides from Allium roseum Leaves
2.4. Chemical Composition Analysis
2.5. Monosaccharide Composition Analysis
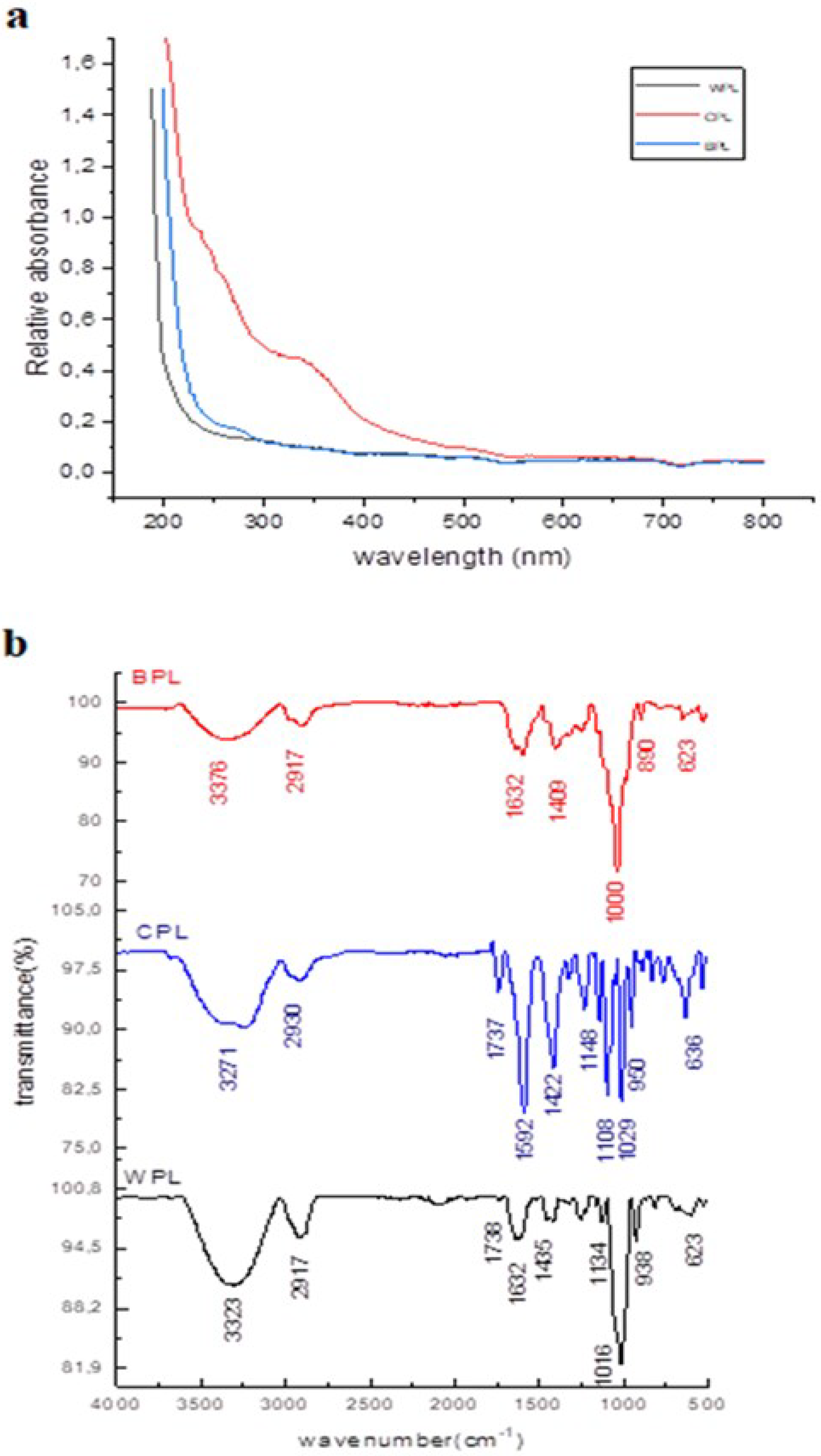
2.6. UV, FTIR and NMR Analysis
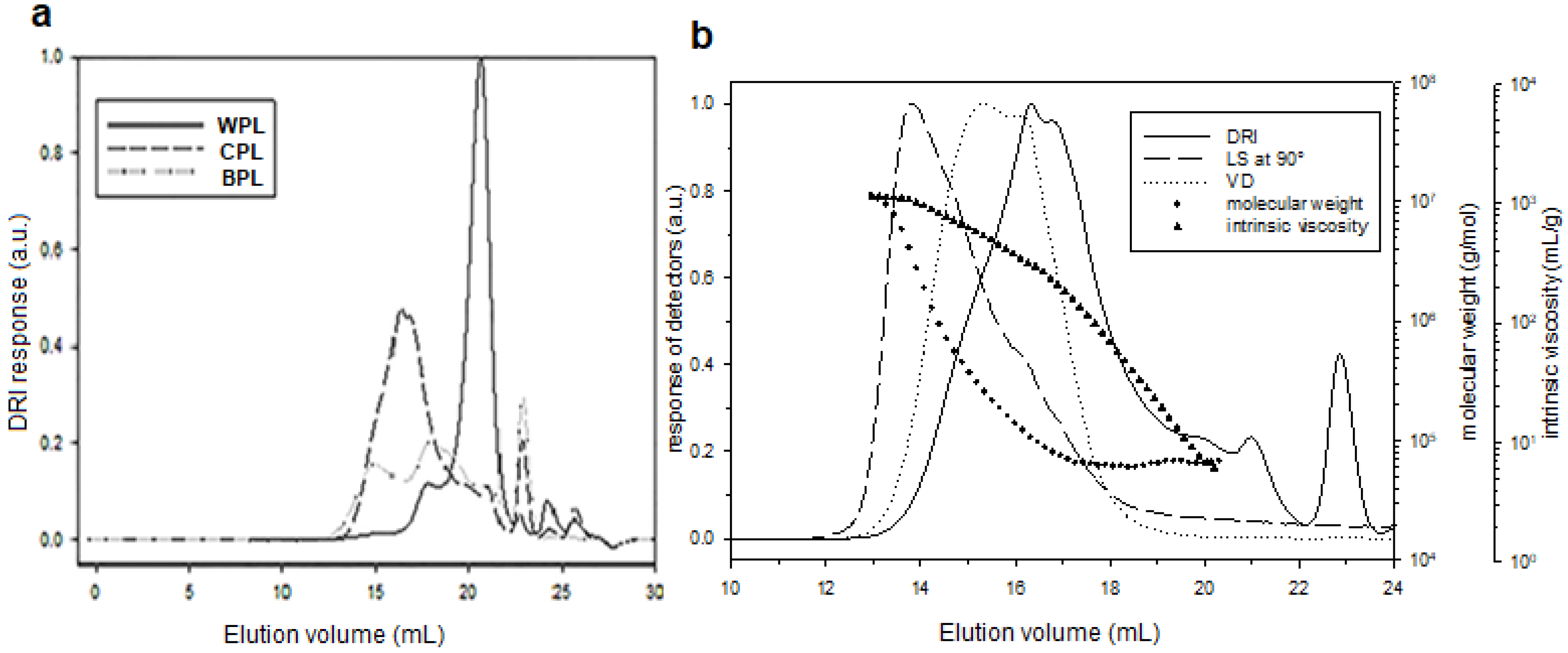
2.7. Macromolecular Characterization
2.8. In Vitro Assay of Antioxidant Activity
2.8.1. DPPH Free Radical Scavenging Activity
2.8.2. ABTS Radical Scavenging Activity
2.8.3. Ferric Reducing Activity (FRAP)
2.9. Hepatotoxicity Assay
2.9.1. Animals
2.9.2. Cadmium Exposure and Sampling
2.10. Biochemical Analysis
2.10.1. Total Protein Content
2.10.2. Superoxide Dismutase Activity
2.10.3. Catalase Activity
2.10.4. Lipid Peroxidation Measurement
2.11. Data Analysis
3. Results
3.1. Isolation, Purification and Chemical Analyses of Polysaccharides
3.2. NMR Characterization
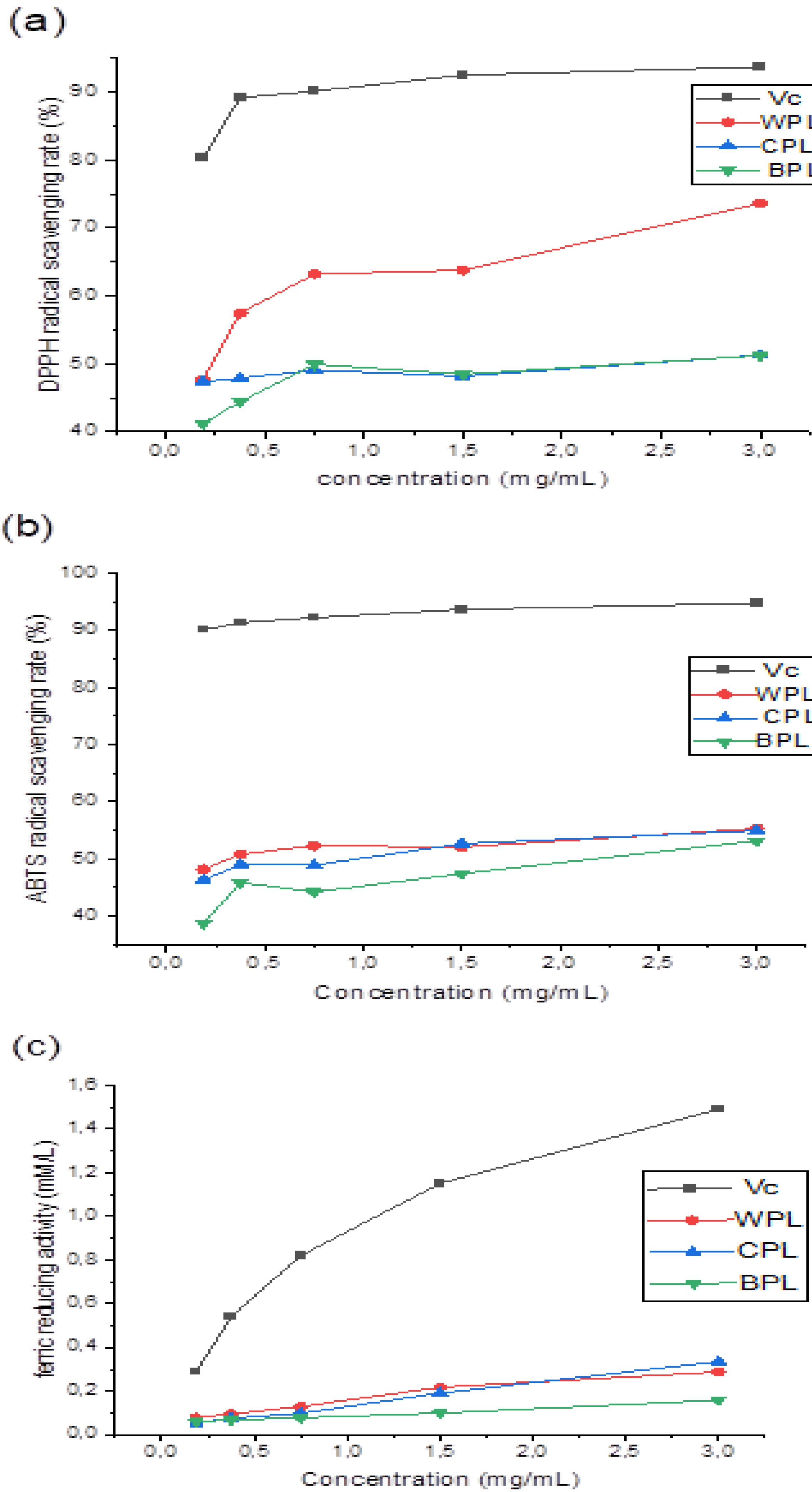
3.3. Antioxidant Activities
3.3.1. DPPH Radical Scavenging Activity
3.3.2. ABTS Radical Scavenging Activity
3.3.3. Ferric Reducing Power
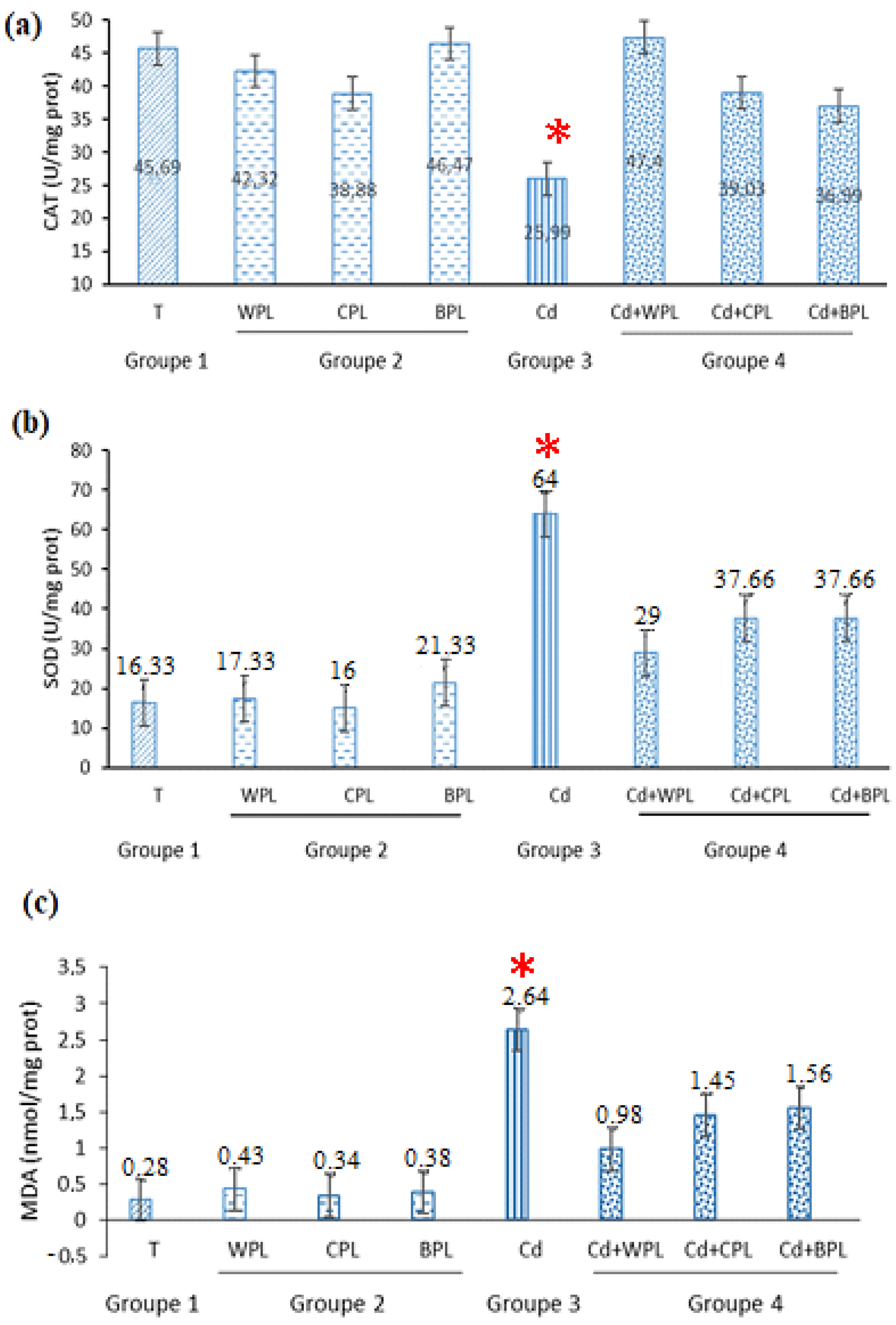
3.4. Capacity of Hepatoprotective Activities In Vivo
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kasai, H.; Kawai, K. 8-Hydroxyguanine, an oxidative DNA and RNA modification. In Modified Nucleic Acids in Biology and Medicine; Springer: Berlin/Heidelberg, Germany, 2016; pp. 147–185. [Google Scholar]
- Chen, T.; Zhou, Z.; Han, R.; Meng, R.; Wang, H.; Lu, W. Adsorption of cadmium by biochar derived from municipal sewage sludge: Impact factors and adsorption mechanism. Chemosphere 2015, 134, 286–293. [Google Scholar] [CrossRef] [PubMed]
- Oluwatoyin, J.A.; Kusemiju, V.; Adu, A.; Babalola, O. Occurrence and distribution of heavy metals in surface water, sediment and some aquatic organisms sampled from Ologe Lagoon, Agbara, Lagos, Nigeria. Int. J. Innov. Appl. Stud. 2017, 20, 601. [Google Scholar]
- Suhani, I.; Sahab, S.; Srivastava, V.; Singh, R.P. Impact of cadmium pollution on food safety and human health. Curr. Opin. Toxicol. 2021, 27, 1–7. [Google Scholar] [CrossRef]
- Souid, G.; Souayed, N.; Yaktiti, F.; Maaroufi, K. Effect of acute cadmium exposure on metal accumulation and oxidative stress biomarkers of Sparus aurata. Ecotoxicol. Environ. Saf. 2013, 89, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Rahimzadeh, M.R.; Rahimzadeh, M.R.; Kazemi, S.; Moghadamnia, A.-a. Cadmium toxicity and treatment: An update. Casp. J. Intern. Med. 2017, 8, 135. [Google Scholar]
- Liu, T.; Li, B.; Zhou, X.; Chen, H. A Study on the Time–Effect and Dose–Effect Relationships of Polysaccharide from Opuntia dillenii against Cadmium-Induced Liver Injury in Mice. Foods 2022, 11, 1340. [Google Scholar] [CrossRef]
- Liu, D.; Yang, J.; Wang, L. Cadmium induces ultrastructural changes in the hepatopancreas of the freshwater crab Sinopotamon henanense. Micron 2013, 47, 24–32. [Google Scholar] [CrossRef]
- Okutu Jackson, B.; Onitsha Enebrayi, N. ameliorative effect of allium sativum and justicia carnea extracts co-administration on acute cadmium chloride-induced changes on liver function parameters of albino rats. World J. Pharm. Life Sci. 2022, 4, 11–24. [Google Scholar]
- Othman, M.S.; Nada, A.; Zaki, H.S.; Abdel Moneim, A.E. Effect of Physalis peruviana L. on cadmium-induced testicular toxicity in rats. Biol. Trace Elem. Res. 2014, 159, 278–287. [Google Scholar] [CrossRef]
- Al-Olayan, E.M.; El-Khadragy, M.F.; Metwally, D.M.; Abdel Moneim, A.E. Protective effects of pomegranate (Punica granatum) juice on testes against carbon tetrachloride intoxication in rats. BMC Complementary Altern. Med. 2014, 14, 164. [Google Scholar] [CrossRef]
- Mohanta, B.; Sen, D.J.; Mahanti, B.; Nayak, A.K. Antioxidant potential of herbal polysaccharides: An overview on recent researches. Sens. Inter. 2022, 3, 100158. [Google Scholar] [CrossRef]
- Yang, L.; Li, L.; Wu, X.; Cai, W.; Lin, Q.; Zhu, D.; Liu, H. The effect of natural soluble polysaccharides on the type 2 diabetes through modulating gut microbiota: A review. Curr. Med. Chem. 2021, 28, 5368–5385. [Google Scholar] [CrossRef] [PubMed]
- El-Ghoul, Y.; Alminderej, F.M.; Alsubaie, F.M.; Alrasheed, R.; Almousa, N.H. Recent Advances in Functional Polymer Materials for Energy, Water, and Biomedical Applications: A Review. Polymers 2021, 13, 4327. [Google Scholar] [CrossRef] [PubMed]
- Alminderej, F.M.; Ammar, C.; El-Ghoul, Y. Functionalization, characterization and microbiological performance of new biocompatible cellulosic dressing grafted chitosan and Suaeda fruticosa polysaccharide extract. Cellulose 2021, 28, 9821–9835. [Google Scholar] [CrossRef]
- Hamden, Z.; El-Ghoul, Y.; Alminderej, F.M.; Saleh, S.M.; Majdoub, H. High-Quality Bioethanol and Vinegar Production from Saudi Arabia Dates: Characterization and Evaluation of Their Value and Antioxidant Efficiency. Antioxidants 2022, 11, 1155. [Google Scholar] [CrossRef]
- Saha, D.; Paul, S. Evaluation of antioxidant and free radical scavenging activities of different fractions of Pterospermum suberifolium leaf extract. Thai J. Pharm. Sci. 2014, 38, 1–56. [Google Scholar]
- Athmouni, K.; Belhaj, D.; El Feki, A.; Ayadi, H. Optimization, antioxidant properties and GC–MS analysis of Periploca angustifolia polysaccharides and chelation therapy on cadmium-induced toxicity in human HepG2 cells line and rat liver. Int. J. Biol. Macromol. 2018, 108, 853–862. [Google Scholar] [CrossRef]
- Athmouni, K.; Belhaj, D.; Chawech, R.; Jarraya, R.; El Feki, A.; Ayadi, H. Characterization of polysaccharides isolated from Periploca angustifolia and its antioxidant activity and renoprotective potential against cadmium induced toxicity in HEK293 cells and rat kidney. Int. J. Biol. Macromol. 2019, 125, 730–742. [Google Scholar] [CrossRef]
- Watson, R.R.; Preedy, V.R. Bioactive Food as Dietary Interventions for Cardiovascular Disease: Bioactive Foods in Chronic Disease States; Academic Press: Cambridge, MA, USA, 2012. [Google Scholar]
- Najjaa, H.; Neffati, M.; Zouari, S.; Ammar, E. Essential oil composition and antibacterial activity of different extracts of Allium roseum L., a North African endemic species. Comptes Rendus Chim. 2007, 10, 820–826. [Google Scholar] [CrossRef]
- El-Demerdash, F.; Yousef, M.I.; Abou El-Naga, N. Biochemical study on the hypoglycemic effects of onion and garlic in alloxan-induced diabetic rats. Food Chem. Toxicol. 2005, 43, 57–63. [Google Scholar] [CrossRef]
- Najjaa, H.; Zerria, K.; Fattouch, S.; Ammar, E.; Neffati, M. Antioxidant and antimicrobial activities of Allium roseum L.“Lazoul”, a wild edible endemic species in North Africa. Int. J. Food Prop. 2011, 14, 371–380. [Google Scholar] [CrossRef]
- Zouari, S.; Najjaa, H.; Neffati, M.; Ammar, E. A new essential oil chemotype of Allium roseum analyzed by an apolar column. Int. J. Food Prop. 2012, 15, 385–397. [Google Scholar] [CrossRef]
- Rouis-Soussi, L.S.; Boughelleb-M’Hamdi, N.; El Ayeb-Zakhama, A.; Flamini, G.; Jannet, H.B.; Harzallah-Skhiri, F. Phytochemicals, antioxidant and antifungal activities of Allium roseum var. grandiflorum subvar. typicum Regel. S. Afr. J. Bot. 2014, 91, 63–70. [Google Scholar] [CrossRef]
- Souid, S.; Najjaa, H.; Riahi-Chebbi, I.; Haoues, M.; Neffati, M.; Arnault, I.; Auger, J.; Karoui, H.; Essafi, M.; Essafi-Benkhadir, K. Allium roseum L. extract exerts potent suppressive activities on chronic myeloid leukemia K562 cell viability through the inhibition of BCR-ABL, PI3K/Akt, and ERK1/2 pathways and the abrogation of VEGF secretion. Nutr. Cancer 2017, 69, 117–130. [Google Scholar] [CrossRef] [PubMed]
- Chaouch, M.A.; Hafsa, J.; Rihouey, C.; Le Cerf, D.; Majdoub, H. Effect of extraction conditions on the antioxidant and antiglycation capacity of carbohydrates from Opuntia robusta cladodes. Int. J. Food Sci. Technol. 2016, 51, 929–937. [Google Scholar] [CrossRef]
- Kratchanova, M.; Gocheva, M.; Pavlova, E.; Yanakieva, I.; Nedelcheva, D.; Kussovski, V.; Slavov, A. Characteristics of pectic polysaccharides from leek obtained through consecutive extraction with various reaction agents. Bulg. Chem. Commun. 2008, 40, 561–567. [Google Scholar]
- Sevag, M.; Lackman, D.B.; Smolens, J. The Isolation of the Components of Streptoeoeeal Nueleoproteins in serologieally Active Form. J. Biol. Chem. 1938, 124, 425–436. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.t.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Bitter, T. A modified uronic acid carbazole reaction. Anal. Biochem. 1962, 4, 330–334. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Bartolozzi, F.; Bertazza, G.; Bassi, D.; Cristoferi, G. Simultaneous determination of soluble sugars and organic acids as their trimethylsilyl derivatives in apricot fruits by gas-liquid chromatography. J. Chromatogr. A 1997, 758, 99–107. [Google Scholar] [CrossRef]
- Zimm, B.H. The scattering of light and the radial distribution function of high polymer solutions. J. Chem. Phys. 1948, 16, 1093–1099. [Google Scholar] [CrossRef]
- Hafsa, J.; Chaouch, M.A.; Charfeddine, B.; Rihouey, C.; Limem, K.; Le Cerf, D.; Rouatbi, S.; Majdoub, H. Effect of ultrasonic degradation of hyaluronic acid extracted from rooster comb on antioxidant and antiglycation activities. Pharm. Biol. 2017, 55, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Hafsa, J.; Hammi, K.M.; Le Cerf, D.; Limem, K.; Majdoub, H.; Charfeddine, B. Characterization, antioxidant and antiglycation properties of polysaccharides extracted from the medicinal halophyte Carpobrotus edulis L. Int. J. Biol. Macromol. 2018, 107, 833–842. [Google Scholar] [CrossRef]
- Di, T.; Chen, G.; Sun, Y.; Ou, S.; Zeng, X.; Ye, H. Antioxidant and immunostimulating activities in vitro of sulfated polysaccharides isolated from Gracilaria rubra. J. Funct. Foods 2017, 28, 64–75. [Google Scholar] [CrossRef]
- Ahmad, M.M. Characterization and antioxidant activities of polysaccharides extracted from flageolet bean pods waste. Curr. Res. Green. Sustain. Chem. 2021, 4, 100154. [Google Scholar] [CrossRef]
- Varoni, M.V.; Gadau, S.D.; Pasciu, V.; Baralla, E.; Serra, E.; Palomba, D.; Demontis, M.P. Investigation of the effects of Lycium barbarum polysaccharides against cadmium induced damage in testis. Exp. Mol. Pathol. 2017, 103, 26–32. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Marklund, S.; Marklund, G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur. J. Biochem. 1974, 47, 469–474. [Google Scholar] [CrossRef]
- Clairbone, A. Catalase Activity in: RA Greenwald; CRC Press: Boca Raton, FL, USA, 1985; pp. 283–484. [Google Scholar]
- Niehaus Jr, W.; Samuelsson, B. Formation of malonaldehyde from phospholipid arachidonate during microsomal lipid peroxidation. Eur. J. Biochem. 1968, 6, 126–130. [Google Scholar] [CrossRef]
- Wang, N.; Zhang, X.; Wang, S.; Guo, Q.; Li, Z.; Liu, H.; Wang, C. Structural characterisation and immunomodulatory activity of polysaccharides from white asparagus skin. Carbohydr. Polym. 2020, 227, 115314. [Google Scholar] [CrossRef] [PubMed]
- Košťálová, Z.; Hromádková, Z. Structural characterisation of polysaccharides from roasted hazelnut skins. Food Chem. 2019, 286, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Rozi, P.; Abuduwaili, A.; Mutailifu, P.; Gao, Y.; Rakhmanberdieva, R.; Aisa, H.A.; Yili, A. Sequential extraction, characterization and antioxidant activity of polysaccharides from Fritillaria pallidiflora Schrenk. Int. J. Biol. Macromol. 2019, 131, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Trigui, I.; Yaich, H.; Sila, A.; Cheikh-Rouhou, S.; Bougatef, A.; Blecker, C.; Attia, H.; Ayadi, M. Physicochemical properties of water-soluble polysaccharides from black cumin seeds. Int. J. Biol. Macromol. 2018, 117, 937–946. [Google Scholar] [CrossRef] [PubMed]
- Hannuksela, T.; du Penhoat, C.H. NMR structural determination of dissolved O-acetylated galactoglucomannan isolated from spruce thermomechanical pulp. Carbohydr. Res. 2004, 339, 301–312. [Google Scholar] [CrossRef]
- Pu, X.; Ma, X.; Liu, L.; Ren, J.; Li, H.; Li, X.; Yu, S.; Zhang, W.; Fan, W. Structural characterization and antioxidant activity in vitro of polysaccharides from angelica and astragalus. Carbohydr. Polym. 2016, 137, 154–164. [Google Scholar] [CrossRef]
- Habibi, Y.; Heyraud, A.; Mahrouz, M.; Vignon, M. Structural features of pectic polysaccharides from the skin of Opuntia ficus-indica prickly pear fruits. Carbohydr. Res. 2004, 339, 1119–1127. [Google Scholar] [CrossRef]
- Muhidinov, Z.K.; Bobokalonov, J.T.; Ismoilov, I.B.; Strahan, G.D.; Chau, H.K.; Hotchkiss, A.T.; Liu, L. Characterization of two types of polysaccharides from Eremurus hissaricus roots growing in Tajikistan. Food Hydrocoll. 2020, 105, 105768. [Google Scholar] [CrossRef]
- Habibi, Y.; Mahrouz, M.; Vignon, M.R. Arabinan-rich polysaccharides isolated and characterized from the endosperm of the seed of Opuntia ficus-indica prickly pear fruits. Carbohydr. Polym. 2005, 60, 319–329. [Google Scholar] [CrossRef]
- Zhang, X.; Bi, C.; Shi, H.; Li, X. Structural studies of a mannoglucan from Cremastra appendiculata (Orchidaceae) by chemical and enzymatic methods. Carbohydr.Polym. 2021, 272, 118524. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, L.; Lin, C. Isolation, structural characterization and antioxidant activity of a neutral polysaccharide from Sisal waste. Food Hydrocoll. 2014, 39, 10–18. [Google Scholar] [CrossRef]
- Amamou, S.; Lazreg, H.; Hafsa, J.; Majdoub, H.; Rihouey, C.; Le Cerf, D.; Achour, L. Effect of extraction condition on the antioxidant, antiglycation and α-amylase inhibitory activities of Opuntia macrorhiza fruit peels polysaccharides. LWT 2020, 127, 109411. [Google Scholar] [CrossRef]
- Du, X.; Mu, H.; Zhou, S.; Zhang, Y.; Zhu, X. Chemical analysis and antioxidant activity of polysaccharides extracted from Inonotus obliquus sclerotia. Int. J. Biol. Macromol. 2013, 62, 691–696. [Google Scholar] [CrossRef] [PubMed]
- Bede, D.; Zaixiang, L. Dietary Polysaccharides from Allium Species: A Critical Review in Dietary Polysaccharides from Allium Species: Extraction, Characterization, Bioactivity, And Potential Utilization. Acta Sci. Agric. 2020, 4, 1–15. [Google Scholar] [CrossRef]
- Yang, Z.; He, Y.; Wang, H.; Zhang, Q. Protective effect of melatonin against chronic cadmium-induced hepatotoxicity by suppressing oxidative stress, inflammation, and apoptosis in mice. Ecotoxicol. Environ. Saf. 2021, 228, 112947. [Google Scholar] [CrossRef]
- Belhaj, D.; Athmouni, K.; Ahmed, M.B.; Aoiadni, N.; El Feki, A.; Zhou, J.L.; Ayadi, H. Polysaccharides from Phormidium versicolor (NCC466) protecting HepG2 human hepatocellular carcinoma cells and rat liver tissues from cadmium toxicity: Evidence from in vitro and in vivo tests. Int. J. Biol. Macromol. 2018, 113, 813–820. [Google Scholar] [CrossRef]
- He, N.; Yang, X.; Jiao, Y.; Tian, L.; Zhao, Y. Characterisation of antioxidant and antiproliferative acidic polysaccharides from Chinese wolfberry fruits. Food Chem. 2012, 133, 978–989. [Google Scholar] [CrossRef]
- Zhu, D.Y.; Ma, Y.L.; Wang, C.H.; Wang, H.; Ren, Y.F.; Zhang, J.G.; Thakur, K.; Wei, Z.J. Insights into physicochemical and functional properties of polysaccharides sequentially extracted from onion (Allium cepa L.). Int. J. Biol. Macromol. 2017, 105, 1192–1201. [Google Scholar] [CrossRef]
- Liu, H.; Fan, H.; Zhang, J.; Zhang, S.; Zhao, W.; Liu, T.; Wang, D. Isolation, purification, structural characteristic and antioxidative property of polysaccharides from A. cepa L. var. agrogatum Don. Food Sci. Hum. Wellness 2020, 9, 71–79. [Google Scholar] [CrossRef]
- Lee, J.-B.; Miyake, S.; Umetsu, R.; Hayashi, K.; Chijimatsu, T.; Hayashi, T. Anti-influenza A virus effects of fructan from Welsh onion (Allium fistulosum L.). Food Chem. 2012, 134, 2164–2168. [Google Scholar] [CrossRef]
- Rao, R.S.P.; Muralikrishna, G. Water soluble feruloyl arabinoxylans from rice and ragi: Changes upon malting and their consequence on antioxidant activity. Phytochemistry 2006, 67, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Gan, D.; Wang, M.; Zhang, Z.; Jiang, C.; Zeng, X. Optimization of extraction, preliminary characterization and hepatoprotective effects of polysaccharides from Stachys floridana Schuttl. ex Benth. Carbohydr. Polym. 2012, 87, 1390–1398. [Google Scholar] [CrossRef]
- Wang, S.; Shi, X. Molecular mechanisms of metal toxicity and carcinogenesis. Mol. Cell. Biochem. 2001, 222, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Wang, C.; Shi, Q.; Ma, C. Preparation of different molecular weight polysaccharides from Porphyridium cruentum and their antioxidant activities. Int. J. Biol. Macromol. 2009, 45, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Wang, J.; Jin, W.; Zhang, H.; Zhang, Q. Degradation of Laminaria japonica fucoidan by hydrogen peroxide and antioxidant activities of the degradation products of different molecular weights. Carbohydr. Polym. 2012, 87, 153–159. [Google Scholar] [CrossRef] [PubMed]

| Samples | WPL | CPL | BPL |
|---|---|---|---|
| Yield (%) | 4.03 | 2.80 | 3.02 |
| Neutral sugar (%) | 65.40 ± 0.22 | 70.94 ± 0.29 | 81.35 ± 0.32 |
| Uronic acid (%) | 34.64 ± 0.35 | 29.27 ± 0.20 | 18.62 ± 0.24 |
| Protein (%) | n.d b | n.d b | n.d b |
| Neutral monosaccharide composition (mol %) | |||
| Rhamnose | 13.12 | 10.78 | 13.31 |
| Galactose | 6.91 | 7.67 | 3.98 |
| Glucose | 41.78 | 41.94 | 50.37 |
| Mannose | 32.87 | 30.80 | 27.03 |
| xylose | 3.31 | 4.61 | 4.28 |
| Samples | WPL | CPL | BPL |
|---|---|---|---|
| Mw (g/mol) | 600,000 ± 5000 | 240,000 ± 10,000 | 660,000 ± 20,000 |
| Mn (g/mol) | 40,000 ± 3000 | 95,000 ± 6000 | 160,000 ± 10,000 |
| [ղ] (mL/g) | 11.5 ± 1 | 257 ± 9 | 62.1 ± 0.6 |
| ÐIpb = Mw/Mn | 15 | 2.5 | 4.1 |
| RH (nm) | 5.6 ± 0.3 | 18± 1 | 26 ± 2 |
| WPL: | |||||||
|---|---|---|---|---|---|---|---|
| Glycosyl Residues | Chemical Shifts, δ (ppm) | ||||||
| H1/C1 | H2/C2 | H3/C3 | H4/C4 | H5/C5 | H6/C6 | ||
| M | -β-D-Manp | 4.50/96.75 | 4.08/69.67 | 3.75/72.44 | 3.85/74.45 | 3.50/74.45 | 3.88/63.35 |
| G | -β-D-Glcp | 4.47/98.17 | 3.41/72.45 | 3.54/74.45 | 3.69/80.67 | 3.45/72.45 | 3.66/61.67 |
| A | -α-D-GalpA | 5.21/99.17 | 3.77/69.18 | 4.01/69.67 | 4.21/80.67 | 4.54/72.45 | - 163.69–163.38 |
| R | -α-L-Rhap | 5.18/92.56 | 4.01/74.45 | 3.85/69.1 | 3.48/71.85 | 3.62/68.46 | 1.24/16.30 |
| CPL: | |||||||
| Glycosyl Residues | Chemical Shifts, δ (ppm) | ||||||
| H1/C1 | H2/C2 | H3/C3 | H4/C4 | H5/C5 | H6/C6 | ||
| M | -β-D-Manp | 4.40/98.64 | 4.05/69.29 | 3.73/72.52 | 3.48/68.82 | 3.48/76.80 | 3.67/61.02 |
| G | -β-D-Glcp | 4.44/103.32 | 3.44/71.40 | 3.58/74.61 | 3.49/80.22 | 3.49/74.61 | 3.85/62.93 |
| A | -α-D-GalpA | 4.97/101.84 | 3.73/68.82 | 4.05/69.81 | 4.30/80.22 | 4.80/72.52 | - 162.81–163.17 |
| R | -α-L-Rhap | 5.01/93.52 | 4.30/76.80 | 3.58/71.4 | 3.85/69.81 | 3.56/69.29 | 1.20/16.73 |
| BPL: | |||||||
| Glycosyl Residues | Chemical Shifts, δ (ppm) | ||||||
| H1/C1 | H2/C2 | H3/C3 | H4/C4 | H5/C5 | H6/C6 | ||
| M | -β-D-Manp | 4.55/96.61 | 4.10/71.49 | 3.71/72.75 | 3.66/76.32 | 3.45/76.78 | 3.69/62.95 |
| G | -β-D-Glcp | 4.44/101.65 | 3.47/73.64 | 3.64/75.81 | 3.66/76.78 | 3.42/76.32 | 3.64/62.95 |
| A | -α-D-GalpA | 5.20/99.34 | 3.72/69.17 | 4.03/69.21 | 4.39/nd | 4.89/71.80 | - 163.20–162.8 |
| R | -α-L-Rhap | 5.16/92.57 | 4.14/76.78 | 3.64/70.92 | 3.55/69.21 | 3.40/69.17 | 1.36/nd |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teka, N.; Alminderej, F.M.; Souid, G.; El-Ghoul, Y.; Le Cerf, D.; Majdoub, H. Characterization of Polysaccharides Sequentially Extracted from Allium roseum Leaves and Their Hepatoprotective Effects against Cadmium Induced Toxicity in Mouse Liver. Antioxidants 2022, 11, 1866. https://doi.org/10.3390/antiox11101866
Teka N, Alminderej FM, Souid G, El-Ghoul Y, Le Cerf D, Majdoub H. Characterization of Polysaccharides Sequentially Extracted from Allium roseum Leaves and Their Hepatoprotective Effects against Cadmium Induced Toxicity in Mouse Liver. Antioxidants. 2022; 11(10):1866. https://doi.org/10.3390/antiox11101866
Chicago/Turabian StyleTeka, Nesrine, Fahad M. Alminderej, Ghada Souid, Yassine El-Ghoul, Didier Le Cerf, and Hatem Majdoub. 2022. "Characterization of Polysaccharides Sequentially Extracted from Allium roseum Leaves and Their Hepatoprotective Effects against Cadmium Induced Toxicity in Mouse Liver" Antioxidants 11, no. 10: 1866. https://doi.org/10.3390/antiox11101866
APA StyleTeka, N., Alminderej, F. M., Souid, G., El-Ghoul, Y., Le Cerf, D., & Majdoub, H. (2022). Characterization of Polysaccharides Sequentially Extracted from Allium roseum Leaves and Their Hepatoprotective Effects against Cadmium Induced Toxicity in Mouse Liver. Antioxidants, 11(10), 1866. https://doi.org/10.3390/antiox11101866







