Hypochlorite-Modified LDL Induces Arrhythmia and Contractile Dysfunction in Cardiomyocytes
Abstract
1. Introduction
2. Materials and Methods
2.1. Isolation and Modification of Native LDL
2.2. Cell Culture
2.3. Isolation of Primary GPV Cardiomyocytes
2.4. Electrophysiological Recordings and Analysis
2.5. Incubation of Right Atrial Appendages (RAAs) with HOCl-LDL
2.6. Immunoblot Experiments
2.7. qPCR
2.8. Measurement of Reactive Oxygen Species (ROS) and Reactive Nitrogen Species (RNS)
2.9. Scavenger Receptor Silencing by siRNA
2.10. Cell Shortening and CaT Measurements
2.11. Immunohistochemistry
2.12. Statistical Analysis
3. Results
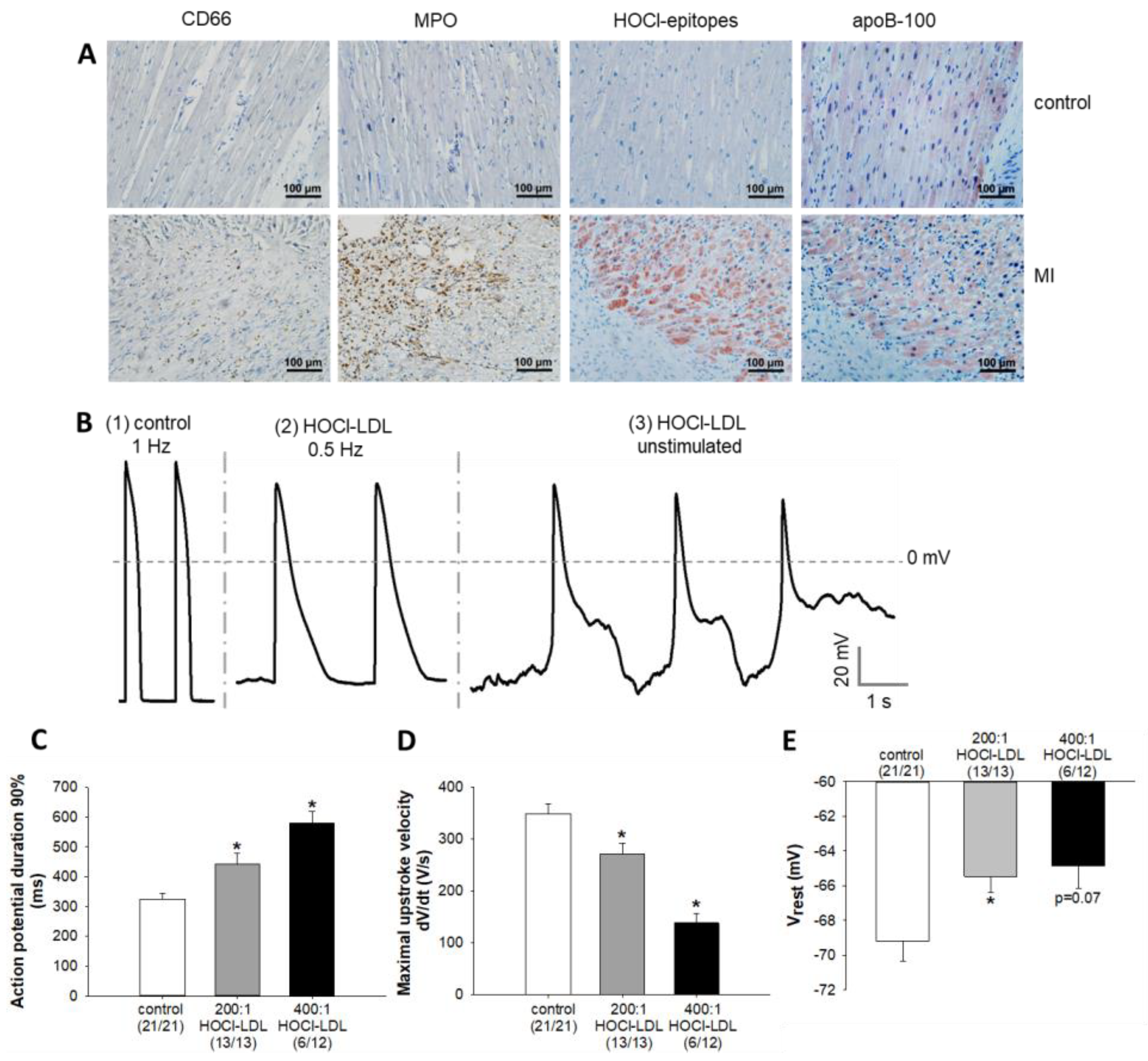
3.1. Neutrophils, MPO, apoB-100, and HOCl-Modified Epitopes Accumulate in the Infarcted Myocardium
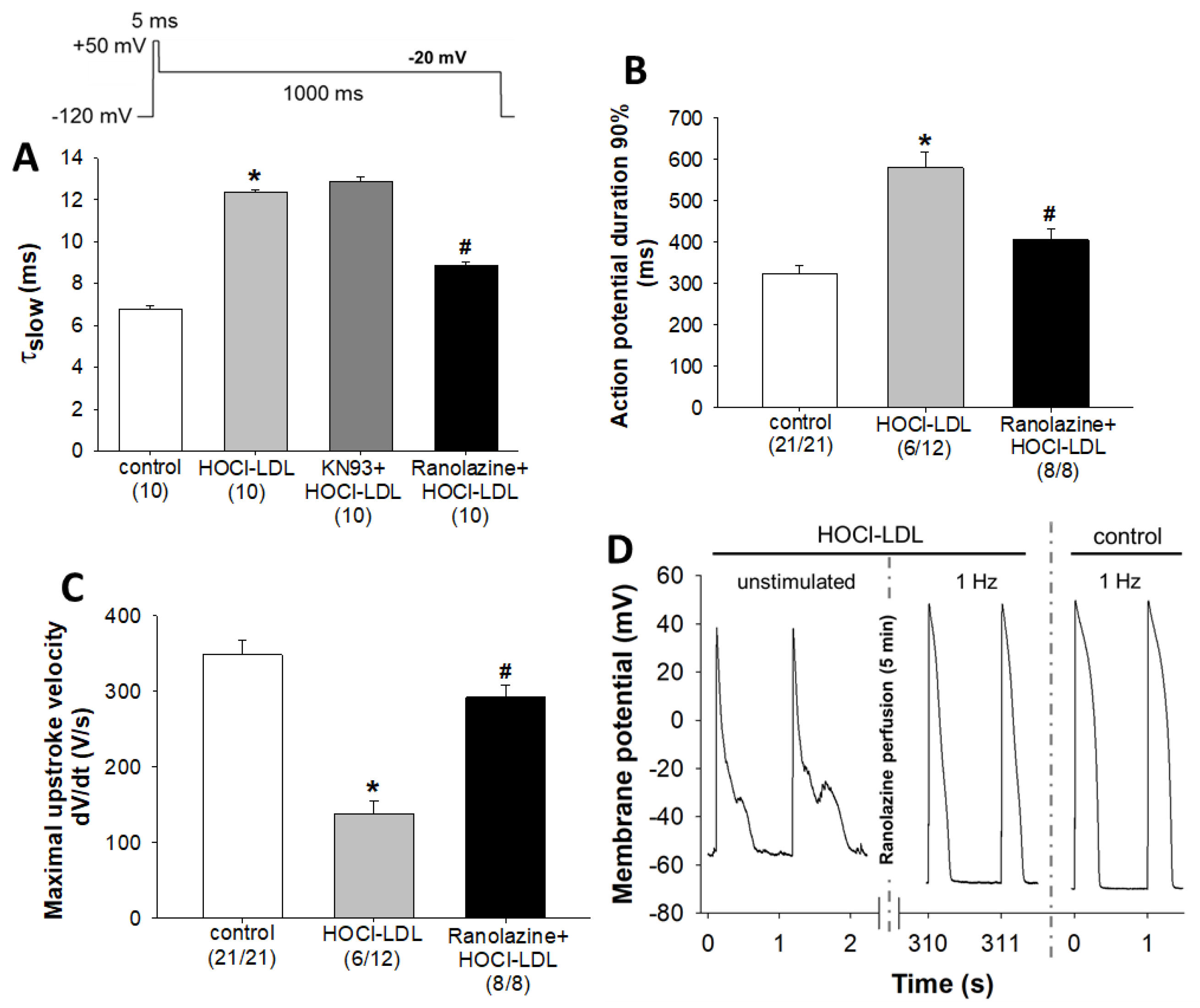
3.2. Alteration of Action Potential Parameters in Response to HOCl-LDL
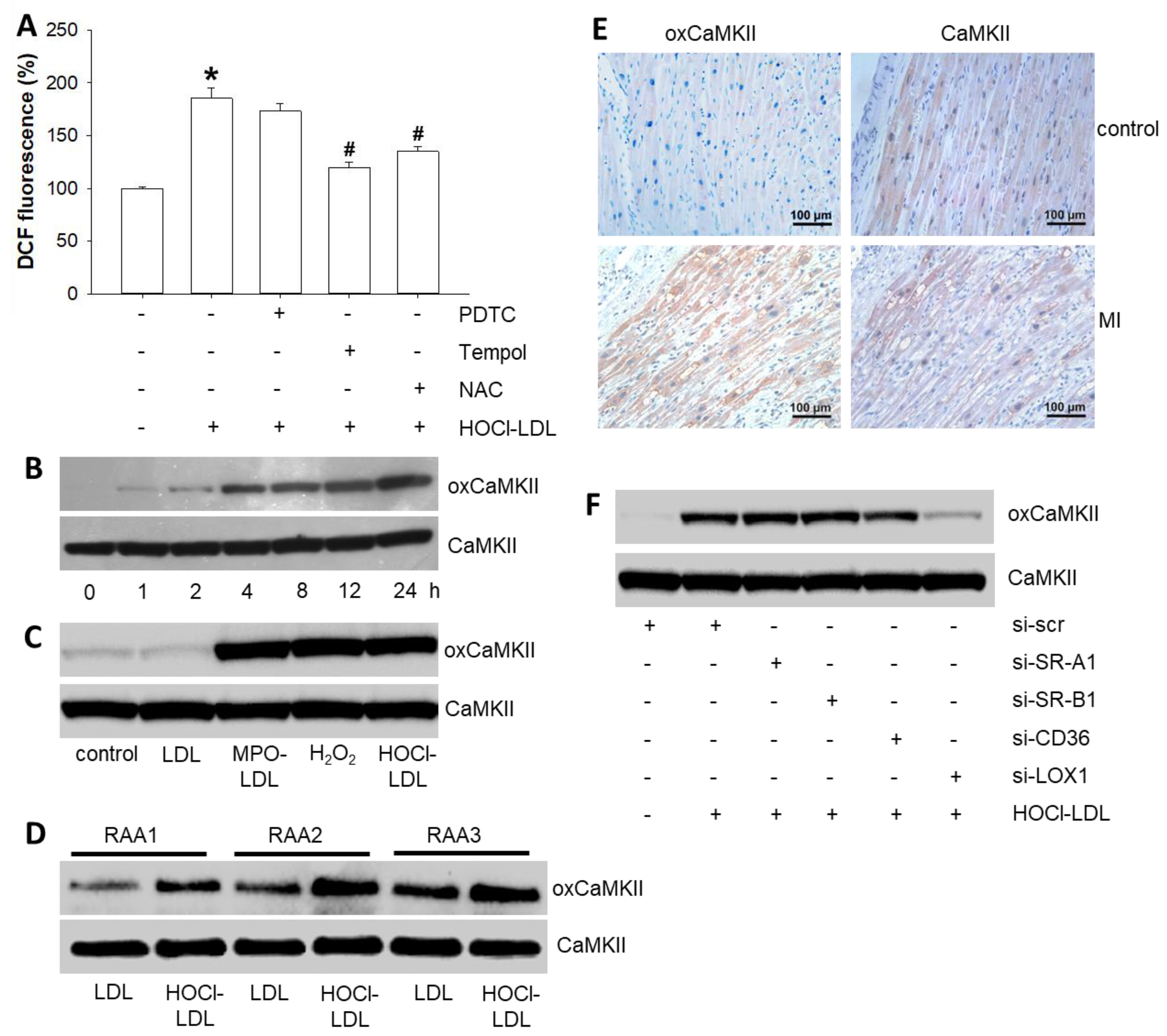
3.3. HOCl-LDL Raises Intracellular Superoxide Levels and, in turn, Oxidizes CaMKII via LOX-1 Signaling
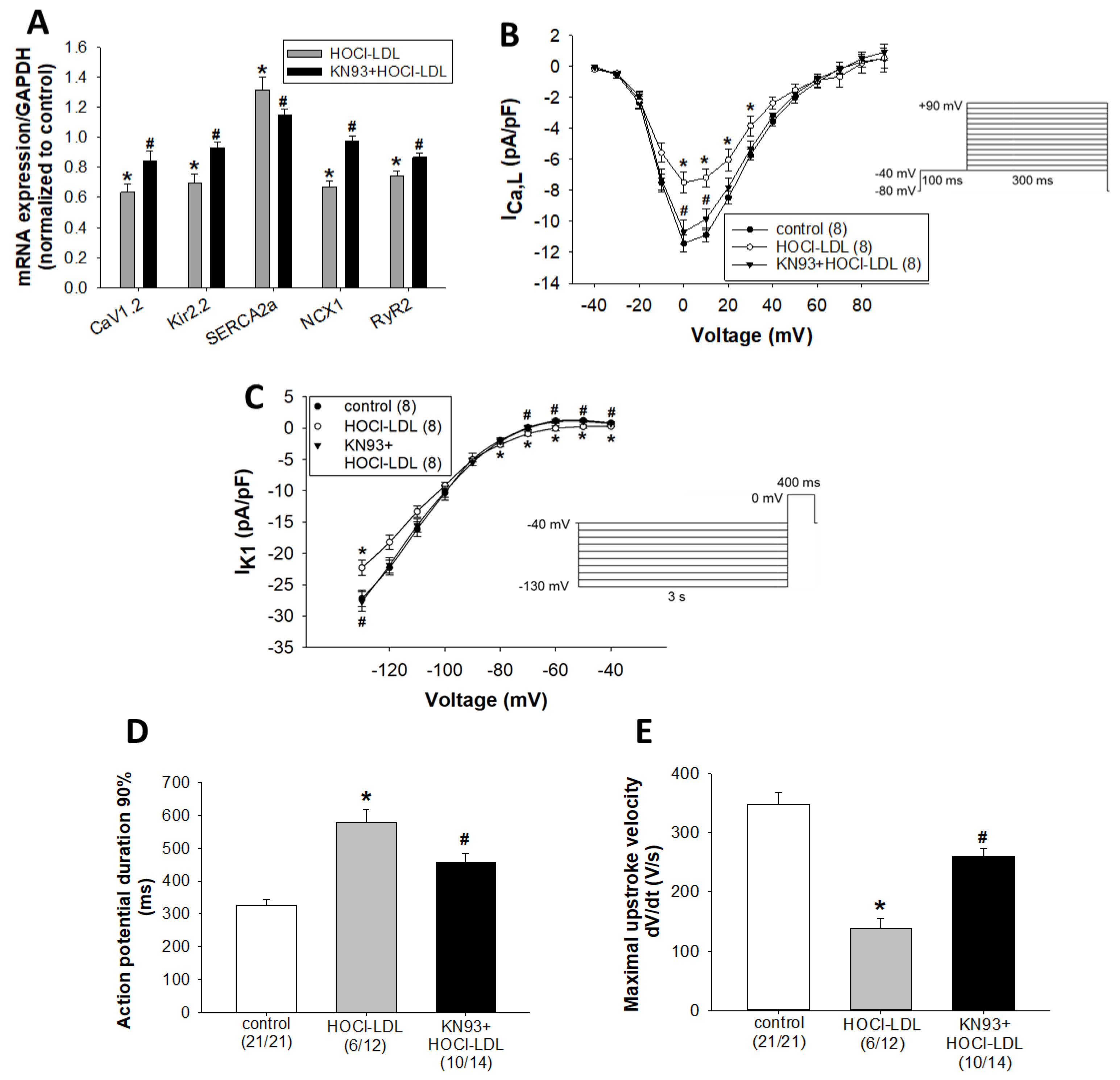
3.4. HOCl-LDL Modulates the Expression and Function of Ion Channels via CaMKII Oxidation
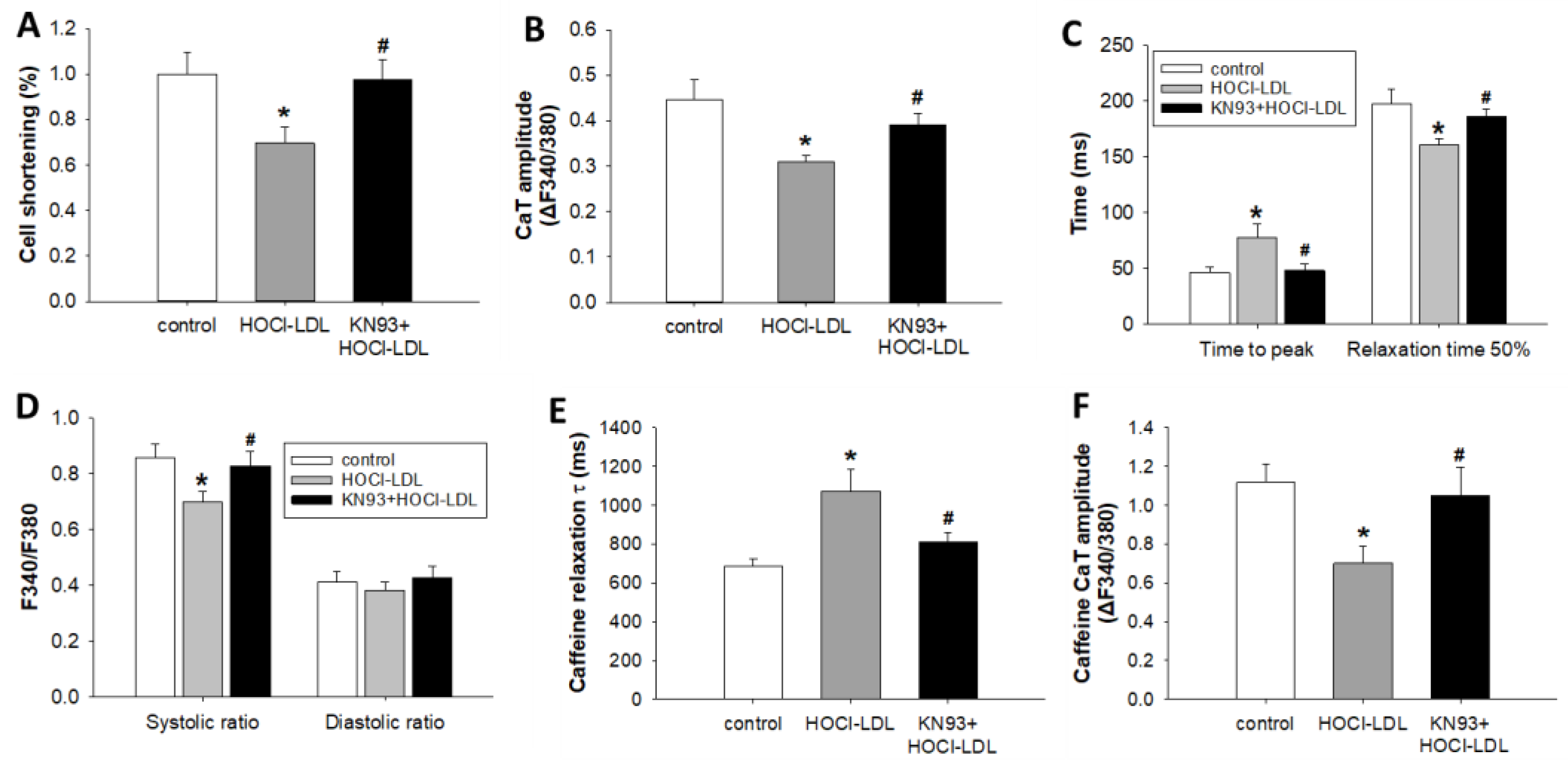
3.5. HOCl-LDL Modulates Ca2+ Homeostasis and Contractility via CaMKII Oxidation
3.6. HOCl-LDL Induces Arrhythmia via INaL Activation
4. Discussion and Conclusions
5. Limitations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Steven, S.; Frenis, K.; Oelze, M.; Kalinovic, S.; Kuntic, M.; Bayo Jimenez, M.T.; Vujacic-Mirski, K.; Helmstadter, J.; Kroller-Schon, S.; Munzel, T.; et al. Vascular Inflammation and Oxidative Stress: Major Triggers for Cardiovascular Disease. Oxid. Med. Cell Longev. 2019, 2019, 7092151. [Google Scholar] [CrossRef] [PubMed]
- Pignatelli, P.; Menichelli, D.; Pastori, D.; Violi, F. Oxidative stress and cardiovascular disease: New insights. Kardiol. Pol. 2018, 76, 713–722. [Google Scholar] [CrossRef] [PubMed]
- El Kazzi, M.; Rayner, B.S.; Chami, B.; Dennis, J.M.; Thomas, S.R.; Witting, P.K. Neutrophil-Mediated Cardiac Damage After Acute Myocardial Infarction: Significance of Defining a New Target Cell Type for Developing Cardioprotective Drugs. Antioxid. Redox. Signal. 2020, 33, 689–712. [Google Scholar] [CrossRef]
- Silvestre-Roig, C.; Braster, Q.; Ortega-Gomez, A.; Soehnlein, O. Neutrophils as regulators of cardiovascular inflammation. Nat. Rev. Cardiol. 2020, 17, 327–340. [Google Scholar] [CrossRef] [PubMed]
- Schindhelm, R.K.; van der Zwan, L.P.; Teerlink, T.; Scheffer, P.G. Myeloperoxidase: A useful biomarker for cardiovascular disease risk stratification? Clin. Chem. 2009, 55, 1462–1470. [Google Scholar] [CrossRef] [PubMed]
- Kamanna, V.S.; Ganji, S.H.; Kashyap, M.L. Myeloperoxidase and Atherosclerosis. Curr. Cardiovasc. Risk Rep. 2013, 7, 102–107. [Google Scholar] [CrossRef]
- Kaya, M.G.; Yalcin, R.; Okyay, K.; Poyraz, F.; Bayraktar, N.; Pasaoglu, H.; Boyaci, B.; Cengel, A. Potential role of plasma myeloperoxidase level in predicting long-term outcome of acute myocardial infarction. Tex. Heart Inst. J. 2012, 39, 500–506. [Google Scholar]
- Klebanoff, S.J.; Kettle, A.J.; Rosen, H.; Winterbourn, C.C.; Nauseef, W.M. Myeloperoxidase: A front-line defender against phagocytosed microorganisms. J. Leukoc. Biol. 2013, 93, 185–198. [Google Scholar] [CrossRef]
- Davies, M.J.; Hawkins, C.L. The Role of Myeloperoxidase in Biomolecule Modification, Chronic Inflammation, and Disease. Antioxid. Redox Signal. 2020, 32, 957–981. [Google Scholar] [CrossRef]
- Malle, E.; Marsche, G.; Arnhold, J.; Davies, M.J. Modification of low-density lipoprotein by myeloperoxidase-derived oxidants and reagent hypochlorous acid. Biochim. Biophys. Acta 2006, 1761, 392–415. [Google Scholar] [CrossRef]
- Marsche, G.; Weigle, B.; Sattler, W.; Malle, E. Soluble RAGE blocks scavenger receptor CD36-mediated uptake of hypochlorite-modified low-density lipoprotein. FASEB J. 2007, 21, 3075–3082. [Google Scholar] [CrossRef]
- Marsche, G.; Zimmermann, R.; Horiuchi, S.; Tandon, N.N.; Sattler, W.; Malle, E. Class B scavenger receptors CD36 and SR-BI are receptors for hypochlorite-modified low density lipoprotein. J. Biol. Chem. 2003, 278, 47562–47570. [Google Scholar] [CrossRef] [PubMed]
- Storey, B.C.; Staplin, N.; Haynes, R.; Reith, C.; Emberson, J.; Herrington, W.G.; Wheeler, D.C.; Walker, R.; Fellstrom, B.; Wanner, C.; et al. Lowering LDL cholesterol reduces cardiovascular risk independently of presence of inflammation. Kidney Int. 2018, 93, 1000–1007. [Google Scholar] [CrossRef]
- Gao, S.; Liu, J. Association between circulating oxidized low-density lipoprotein and atherosclerotic cardiovascular disease. Chronic Dis. Transl. Med. 2017, 3, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Boren, J.; Veniant, M.M.; Young, S.G. Apo B100-containing lipoproteins are secreted by the heart. J. Clin. Investig. 1998, 101, 1197–1202. [Google Scholar] [CrossRef]
- Nielsen, L.B.; Veniant, M.; Boren, J.; Raabe, M.; Wong, J.S.; Tam, C.; Flynn, L.; Vanni-Reyes, T.; Gunn, M.D.; Goldberg, I.J.; et al. Genes for apolipoprotein B and microsomal triglyceride transfer protein are expressed in the heart: Evidence that the heart has the capacity to synthesize and secrete lipoproteins. Circulation 1998, 98, 13–16. [Google Scholar] [CrossRef] [PubMed]
- Zani, I.A.; Stephen, S.L.; Mughal, N.A.; Russell, D.; Homer-Vanniasinkam, S.; Wheatcroft, S.B.; Ponnambalam, S. Scavenger receptor structure and function in health and disease. Cells 2015, 4, 178–201. [Google Scholar] [CrossRef] [PubMed]
- Malle, E.; Ibovnik, A.; Leis, H.J.; Kostner, G.M.; Verhallen, P.F.; Sattler, W. Lysine modification of LDL or lipoprotein(a) by 4-hydroxynonenal or malondialdehyde decreases platelet serotonin secretion without affecting platelet aggregability and eicosanoid formation. Arterioscler. Thromb. Vasc. Biol. 1995, 15, 377–384. [Google Scholar] [CrossRef]
- Marsche, G.; Furtmuller, P.G.; Obinger, C.; Sattler, W.; Malle, E. Hypochlorite-modified high-density lipoprotein acts as a sink for myeloperoxidase in vitro. Cardiovasc. Res. 2008, 79, 187–194. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Scheruebel, S.; Koyani, C.N.; Hallstrom, S.; Lang, P.; Platzer, D.; Machler, H.; Lohner, K.; Malle, E.; Zorn-Pauly, K.; Pelzmann, B. I(f) blocking potency of ivabradine is preserved under elevated endotoxin levels in human atrial myocytes. J. Mol. Cell. Cardiol. 2014, 72, 64–73. [Google Scholar] [CrossRef]
- Wolkart, G.; Schrammel, A.; Koyani, C.N.; Scherubel, S.; Zorn-Pauly, K.; Malle, E.; Pelzmann, B.; Andra, M.; Ortner, A.; Mayer, B. Cardioprotective effects of 5-hydroxymethylfurfural mediated by inhibition of L-type Ca(2+) currents. Br. J. Pharmacol. 2017, 174, 3640–3653. [Google Scholar] [CrossRef] [PubMed]
- Koyani, C.N.; Trummer, C.; Shrestha, N.; Scheruebel, S.; Bourgeois, B.; Plastira, I.; Kickmaier, S.; Sourij, H.; Rainer, P.P.; Madl, T.; et al. Saxagliptin but Not Sitagliptin Inhibits CaMKII and PKC via DPP9 Inhibition in Cardiomyocytes. Front Physiol. 2018, 9, 1622. [Google Scholar] [CrossRef] [PubMed]
- Zorn-Pauly, K.; Schaffer, P.; Pelzmann, B.; Bernhart, E.; Wei, G.; Lang, P.; Ledinski, G.; Greilberger, J.; Koidl, B.; Jurgens, G. Oxidized LDL induces ventricular myocyte damage and abnormal electrical activity—Role of lipid hydroperoxides. Cardiovasc. Res. 2005, 66, 74–83. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wagner, S.; Ruff, H.M.; Weber, S.L.; Bellmann, S.; Sowa, T.; Schulte, T.; Anderson, M.E.; Grandi, E.; Bers, D.M.; Backs, J.; et al. Reactive oxygen species-activated Ca/calmodulin kinase IIdelta is required for late I(Na) augmentation leading to cellular Na and Ca overload. Circ. Res. 2011, 108, 555–565. [Google Scholar] [CrossRef] [PubMed]
- Sossalla, S.; Maurer, U.; Schotola, H.; Hartmann, N.; Didie, M.; Zimmermann, W.H.; Jacobshagen, C.; Wagner, S.; Maier, L.S. Diastolic dysfunction and arrhythmias caused by overexpression of CaMKIIdelta(C) can be reversed by inhibition of late Na(+) current. Basic Res. Cardiol. 2011, 106, 263–272. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Koyani, C.N.; Windischhofer, W.; Rossmann, C.; Jin, G.; Kickmaier, S.; Heinzel, F.R.; Groschner, K.; Alavian-Ghavanini, A.; Sattler, W.; Malle, E. 15-deoxy-Delta(1)(2),(1)(4)-PGJ(2) promotes inflammation and apoptosis in cardiomyocytes via the DP2/MAPK/TNFalpha axis. Int. J. Cardiol. 2014, 173, 472–480. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hazell, L.J.; Arnold, L.; Flowers, D.; Waeg, G.; Malle, E.; Stocker, R. Presence of hypochlorite-modified proteins in human atherosclerotic lesions. J. Clin. Investig. 1996, 97, 1535–1544. [Google Scholar] [CrossRef] [PubMed]
- Malle, E.; Hazell, L.; Stocker, R.; Sattler, W.; Esterbauer, H.; Waeg, G. Immunologic detection and measurement of hypochlorite-modified LDL with specific monoclonal antibodies. Arterioscler. Thromb. Vasc. Biol. 1995, 15, 982–989. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, J.; Marsh, L.M.; Pieper, M.; Stacher, E.; Ghanim, B.; Kovacs, G.; Konig, P.; Wilkens, H.; Haitchi, H.M.; Hoefler, G.; et al. Compartment-specific expression of collagens and their processing enzymes in intrapulmonary arteries of IPAH patients. American journal of physiology. Lung Cell. Mol. Physiol. 2015, 308, L1002–L1013. [Google Scholar] [CrossRef]
- Hammerschmidt, S.; Wahn, H. The effect of the oxidant hypochlorous acid on the L-type calcium current in isolated ventricular cardiomyocytes. J. Mol. Cell Cardiol. 1998, 30, 1855–1867. [Google Scholar] [CrossRef]
- Mollenhauer, M.; Friedrichs, K.; Lange, M.; Gesenberg, J.; Remane, L.; Kerkenpass, C.; Krause, J.; Schneider, J.; Ravekes, T.; Maass, M.; et al. Myeloperoxidase Mediates Postischemic Arrhythmogenic Ventricular Remodeling. Circ. Res. 2017, 121, 56–70. [Google Scholar] [CrossRef]
- Resch, U.; Semlitsch, M.; Hammer, A.; Susani-Etzerodt, H.; Walczak, H.; Sattler, W.; Malle, E. Hypochlorite-modified low-density lipoprotein induces the apoptotic machinery in Jurkat T-cell lines. Biochem. Biophys. Res. Commun. 2011, 410, 895–900. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Erickson, J.R.; Joiner, M.L.; Guan, X.; Kutschke, W.; Yang, J.; Oddis, C.V.; Bartlett, R.K.; Lowe, J.S.; O’Donnell, S.E.; Aykin-Burns, N.; et al. A dynamic pathway for calcium-independent activation of CaMKII by methionine oxidation. Cell 2008, 133, 462–474. [Google Scholar] [CrossRef] [PubMed]
- Erickson, J.R.; He, B.J.; Grumbach, I.M.; Anderson, M.E. CaMKII in the cardiovascular system: Sensing redox states. Physiol. Rev. 2011, 91, 889–915. [Google Scholar] [CrossRef] [PubMed]
- Ibarra, J.; Morley, G.E.; Delmar, M. Dynamics of the inward rectifier K+ current during the action potential of guinea pig ventricular myocytes. Biophys. J. 1991, 60, 1534–1539. [Google Scholar] [CrossRef][Green Version]
- Dhamoon, A.S.; Jalife, J. The inward rectifier current (IK1) controls cardiac excitability and is involved in arrhythmogenesis. Heart Rhythm. 2005, 2, 316–324. [Google Scholar] [CrossRef]
- Koyani, C.N.; Kolesnik, E.; Wolkart, G.; Shrestha, N.; Scheruebel, S.; Trummer, C.; Zorn-Pauly, K.; Hammer, A.; Lang, P.; Reicher, H.; et al. Dipeptidyl peptidase-4 independent cardiac dysfunction links saxagliptin to heart failure. Biochem. Pharmacol. 2017, 145, 64–80. [Google Scholar] [CrossRef]
- Antzelevitch, C.; Nesterenko, V.; Shryock, J.C.; Rajamani, S.; Song, Y.; Belardinelli, L. The role of late I Na in development of cardiac arrhythmias. Handb. Exp. Pharmacol. 2014, 221, 137–168. [Google Scholar] [CrossRef]
- Makielski, J.C.; Valdivia, C.R. Ranolazine and late cardiac sodium current--a therapeutic target for angina, arrhythmia and more? Br. J. Pharmacol. 2006, 148, 4–6. [Google Scholar] [CrossRef]
- Alrabadi, N.; Chami, B.; Kim, H.B.; Maw, A.M.; Dennis, J.M.; Witting, P.K. Hypochlorous acid generated in the heart following acute ischaemic injury promotes myocardial damage: A new target for therapeutic development. Trends Cell Molecul. Biol. 2014, 9, 1–17. [Google Scholar]
- Malle, E.; Waeg, G.; Schreiber, R.; Grone, E.F.; Sattler, W.; Grone, H.J. Immunohistochemical evidence for the myeloperoxidase/H2O2/halide system in human atherosclerotic lesions: Colocalization of myeloperoxidase and hypochlorite-modified proteins. Eur. J. Biochem. 2000, 267, 4495–4503. [Google Scholar] [CrossRef]
- Delporte, C.; Boudjeltia, K.Z.; Noyon, C.; Furtmuller, P.G.; Nuyens, V.; Slomianny, M.C.; Madhoun, P.; Desmet, J.M.; Raynal, P.; Dufour, D.; et al. Impact of myeloperoxidase-LDL interactions on enzyme activity and subsequent posttranslational oxidative modifications of apoB-100. J. Lipid Res. 2014, 55, 747–757. [Google Scholar] [CrossRef]
- Vasilyev, N.; Williams, T.; Brennan, M.L.; Unzek, S.; Zhou, X.; Heinecke, J.W.; Spitz, D.R.; Topol, E.J.; Hazen, S.L.; Penn, M.S. Myeloperoxidase-generated oxidants modulate left ventricular remodeling but not infarct size after myocardial infarction. Circulation 2005, 112, 2812–2820. [Google Scholar] [CrossRef]
- Kalasz, J.; Pasztor, E.T.; Fagyas, M.; Balogh, A.; Toth, A.; Csato, V.; Edes, I.; Papp, Z.; Borbely, A. Myeloperoxidase impairs the contractile function in isolated human cardiomyocytes. Free Radic. Biol. Med. 2015, 84, 116–127. [Google Scholar] [CrossRef] [PubMed]
- Rudolph, V.; Andrie, R.P.; Rudolph, T.K.; Friedrichs, K.; Klinke, A.; Hirsch-Hoffmann, B.; Schwoerer, A.P.; Lau, D.; Fu, X.; Klingel, K.; et al. Myeloperoxidase acts as a profibrotic mediator of atrial fibrillation. Nat. Med. 2010, 16, 470–474. [Google Scholar] [CrossRef] [PubMed]
- Ndrepepa, G. Myeloperoxidase—A bridge linking inflammation and oxidative stress with cardiovascular disease. Clin. Chim. Acta. 2019, 493, 36–51. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.S.; Kim, H.B.; Szuchman-Sapir, A.; McMahon, A.; Dennis, J.M.; Witting, P.K. Neutrophils recruited to the myocardium after acute experimental myocardial infarct generate hypochlorous acid that oxidizes cardiac myoglobin. Arch. Biochem. Biophys. 2016, 612, 103–114. [Google Scholar] [CrossRef]
- Reyes, L.; Hawkins, C.L.; Rayner, B.S. Characterization of the cellular effects of myeloperoxidase-derived oxidants on H9c2 cardiac myoblasts. Arch. Biochem. Biophys. 2019, 665, 132–142. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Williams, V.; Liu, L.; Chen, H.; Sawamura, T.; Antakli, T.; Mehta, J.L. LOX-1 inhibition in myocardial ischemia-reperfusion injury: Modulation of MMP-1 and inflammation. Am. J. Physiol. Heart Circ. Physiol. 2002, 283, H1795–H1801. [Google Scholar] [CrossRef] [PubMed]
- Kataoka, K.; Hasegawa, K.; Sawamura, T.; Fujita, M.; Yanazume, T.; Iwai-Kanai, E.; Kawamura, T.; Hirai, T.; Kita, T.; Nohara, R. LOX-1 pathway affects the extent of myocardial ischemia-reperfusion injury. Biochem. Biophys. Res. Commun. 2003, 300, 656–660. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, Q.; Feng, N.; Granger, J.M.; Anderson, M.E. Myocardial death and dysfunction after ischemia-reperfusion injury require CaMKIIdelta oxidation. Sci. Rep. 2019, 9, 9291. [Google Scholar] [CrossRef] [PubMed]
- Fearon, I.M. OxLDL enhances L-type Ca2+ currents via lysophosphatidylcholine-induced mitochondrial reactive oxygen species (ROS) production. Cardiovasc. Res. 2006, 69, 855–864. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Massaeli, H.; Pierce, G.N. The action of oxidized low density lipoprotein on calcium transients in isolated rabbit cardiomyocytes. J. Biol. Chem. 1993, 268, 4145–4151. [Google Scholar] [CrossRef]
- Schluter, K.D.; Wolf, A.; Weber, M.; Schreckenberg, R.; Schulz, R. Oxidized low-density lipoprotein (oxLDL) affects load-free cell shortening of cardiomyocytes in a proprotein convertase subtilisin/kexin 9 (PCSK9)-dependent way. Basic Res. Cardiol. 2017, 112, 63. [Google Scholar] [CrossRef] [PubMed]
- Ho, H.T.; Liu, B.; Snyder, J.S.; Lou, Q.; Brundage, E.A.; Velez-Cortes, F.; Wang, H.; Ziolo, M.T.; Anderson, M.E.; Sen, C.K.; et al. Ryanodine receptor phosphorylation by oxidized CaMKII contributes to the cardiotoxic effects of cardiac glycosides. Cardiovasc. Res. 2014, 101, 165–174. [Google Scholar] [CrossRef][Green Version]
- Kho, C.; Lee, A.; Hajjar, R.J. Altered sarcoplasmic reticulum calcium cycling—Targets for heart failure therapy. Nat. Rev. Cardiol. 2012, 9, 717–733. [Google Scholar] [CrossRef]
- Talukder, M.A.; Zweier, J.L.; Periasamy, M. Targeting calcium transport in ischaemic heart disease. Cardiovasc. Res. 2009, 84, 345–352. [Google Scholar] [CrossRef]
- Curran, J.; Tang, L.; Roof, S.R.; Velmurugan, S.; Millard, A.; Shonts, S.; Wang, H.; Santiago, D.; Ahmad, U.; Perryman, M.; et al. Nitric oxide-dependent activation of CaMKII increases diastolic sarcoplasmic reticulum calcium release in cardiac myocytes in response to adrenergic stimulation. PLoS ONE 2014, 9, e87495. [Google Scholar] [CrossRef]
- Wang, Q.; Quick, A.P.; Cao, S.; Reynolds, J.; Chiang, D.Y.; Beavers, D.; Li, N.; Wang, G.; Rodney, G.G.; Anderson, M.E.; et al. Oxidized CaMKII (Ca(2+)/Calmodulin-Dependent Protein Kinase II) Is Essential for Ventricular Arrhythmia in a Mouse Model of Duchenne Muscular Dystrophy. Circ. Arrhythm. Electrophysiol. 2018, 11, e005682. [Google Scholar] [CrossRef]
- Anderson, M.E. Oxidant stress promotes disease by activating CaMKII. J. Mol. Cell. Cardiol. 2015, 89, 160–167. [Google Scholar] [CrossRef]
- Li, J.; Marionneau, C.; Zhang, R.; Shah, V.; Hell, J.W.; Nerbonne, J.M.; Anderson, M.E. Calmodulin kinase II inhibition shortens action potential duration by upregulation of K+ currents. Circ. Res. 2006, 99, 1092–1099. [Google Scholar] [CrossRef] [PubMed]
- Wagner, S.; Hacker, E.; Grandi, E.; Weber, S.L.; Dybkova, N.; Sossalla, S.; Sowa, T.; Fabritz, L.; Kirchhof, P.; Bers, D.M.; et al. Ca/calmodulin kinase II differentially modulates potassium currents. Circ. Arrhythm. Electrophysiol. 2009, 2, 285–294. [Google Scholar] [CrossRef] [PubMed]
- Wong, M.H.; Samal, A.B.; Lee, M.; Vlach, J.; Novikov, N.; Niedziela-Majka, A.; Feng, J.Y.; Koltun, D.O.; Brendza, K.M.; Kwon, H.J.; et al. The KN-93 Molecule Inhibits Calcium/Calmodulin-Dependent Protein Kinase II (CaMKII) Activity by Binding to Ca(2+)/CaM. J. Mol. Biol. 2019, 431, 1440–1459. [Google Scholar] [CrossRef] [PubMed]
- Sommese, L.; Valverde, C.A.; Blanco, P.; Castro, M.C.; Rueda, O.V.; Kaetzel, M.; Dedman, J.; Anderson, M.E.; Mattiazzi, A.; Palomeque, J. Ryanodine receptor phosphorylation by CaMKII promotes spontaneous Ca(2+) release events in a rodent model of early stage diabetes: The arrhythmogenic substrate. Int. J. Cardiol. 2016, 202, 394–406. [Google Scholar] [CrossRef] [PubMed]
- Makielski, J.C. Late sodium current: A mechanism for angina, heart failure, and arrhythmia. Trends Cardiovasc. Med. 2015, 26, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Christensen, M.D.; Dun, W.; Boyden, P.A.; Anderson, M.E.; Mohler, P.J.; Hund, T.J. Oxidized calmodulin kinase II regulates conduction following myocardial infarction: A computational analysis. PLoS Comput. Biol. 2009, 5, e1000583. [Google Scholar] [CrossRef]
- Song, Y.; Shryock, J.C.; Wagner, S.; Maier, L.S.; Belardinelli, L. Blocking late sodium current reduces hydrogen peroxide-induced arrhythmogenic activity and contractile dysfunction. J. Pharmacol. Exp. Ther. 2006, 318, 214–222. [Google Scholar] [CrossRef]
- Pezhouman, A.; Madahian, S.; Stepanyan, H.; Ghukasyan, H.; Qu, Z.; Belardinelli, L.; Karagueuzian, H.S. Selective inhibition of late sodium current suppresses ventricular tachycardia and fibrillation in intact rat hearts. Heart Rhythm. Off. J. Heart Rhythm. Soc. 2014, 11, 492–501. [Google Scholar] [CrossRef]
- Davies, M.J. Myeloperoxidase: Mechanisms, reactions and inhibition as a therapeutic strategy in inflammatory diseases. Pharmacol. Ther. 2021, 218, 107685. [Google Scholar] [CrossRef]
- Maiocchi, S.L.; Ku, J.; Thai, T.; Chan, E.; Rees, M.D.; Thomas, S.R. Myeloperoxidase: A versatile mediator of endothelial dysfunction and therapeutic target during cardiovascular disease. Pharmacol. Ther. 2021, 221, 107711. [Google Scholar] [CrossRef]
- Szuchman-Sapir, A.J.; Pattison, D.I.; Ellis, N.A.; Hawkins, C.L.; Davies, M.J.; Witting, P.K. Hypochlorous acid oxidizes methionine and tryptophan residues in myoglobin. Free Radic. Biol. Med. 2008, 45, 789–798. [Google Scholar] [CrossRef] [PubMed]
- Ramachandra, C.J.A.; Kp, M.M.J.; Chua, J.; Hernandez-Resendiz, S.; Liehn, E.A.; Knoll, R.; Gan, L.M.; Michaelsson, E.; Jonsson, M.K.B.; Ryden-Markinhuhta, K.; et al. Inhibiting cardiac myeloperoxidase alleviates the relaxation defect in hypertrophic cardiomyocytes. Cardiovasc. Res. 2021. [Google Scholar] [CrossRef] [PubMed]
- Thukkani, A.K.; Albert, C.J.; Wildsmith, K.R.; Messner, M.C.; Martinson, B.D.; Hsu, F.F.; Ford, D.A. Myeloperoxidase-derived reactive chlorinating species from human monocytes target plasmalogens in low density lipoprotein. J. Biol. Chem. 2003, 278, 36365–36372. [Google Scholar] [CrossRef]
- Marsche, G.; Heller, R.; Fauler, G.; Kovacevic, A.; Nuszkowski, A.; Graier, W.; Sattler, W.; Malle, E. 2-chlorohexadecanal derived from hypochlorite-modified high-density lipoprotein-associated plasmalogen is a natural inhibitor of endothelial nitric oxide biosynthesis. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 2302–2306. [Google Scholar] [CrossRef]
- Palladino, E.N.D.; Hartman, C.L.; Albert, C.J.; Ford, D.A. The chlorinated lipidome originating from myeloperoxidase-derived HOCl targeting plasmalogens: Metabolism, clearance, and biological properties. Arch. Biochem. Biophys. 2018, 641, 31–38. [Google Scholar] [CrossRef] [PubMed]
| Gene | Species (Accession ID) | Forward Primer (5′-3′) | Reverse Primer (5′-3′) |
|---|---|---|---|
| GAPDH | H (NM_002046.7) | Hs_GAPDH_1_SG (Qiagen) | |
| M (NM_001289726.1) | Mm_Gapdh_3_SG (Qiagen) | ||
| GP (NM_001172951.1) | GTGAAGCAGGCATCAGAGGGC | GGCTCAGGTGGGGTCCACTTAC | |
| CaV1.2 | H (NM_199460.4) | GAGAACAGCAAGTTTGACTTTGACAA | CGAAGGTGGAGACGGTGAA |
| M (NM_009781.4) | Mm_Cacna1c_1_SG (Qiagen) | ||
| GP (NM_001172923.1) | GCGGACACAGAGGTGAGGGG | GTGGGGATGTGCTCAGGGGC | |
| NCX1 | H (NM_001112800.4) | CTGGTGGAGATGAGTGAGAAGA | GGTTGGCCAAACAGGTATTTTC |
| M (NM_011406.3) | CCCTGTTGTTGAATGAGCTTGGTGG | TGCTGGTCAGTGGCTGCTTGT | |
| GP (NM_001173019.1) | TCGCCCTCCACTGCCACTGT | TGACCTCCATGATGCCAATGCTCT | |
| SERCA2a | H (NM_001681.4) | CCGCAACTACCTGGAACCTG | CACGCAACCGAACACCCTTA |
| M (NM_001110140.3) | CACGTGCCTGGTGGAGAAGATGA | CCGGCTTGGCTTGTTTGGGG | |
| GP (XR_001199631.2) | AGGTGCTGGGCCACTTCGGT | TTCAGCCGGTAACTCGTTGGAGC | |
| RyR2 | H (NM_001035.3) | GGCGAAGACGAGATCCAGTT | CTTTGTGGATGGTTGCGGTG |
| M (NM_023868.2) | CCATGGCTGATGCGGGCGAA | GCAGGGCCCGTACTGACAGG | |
| Kir2.1 | H (NM_000891.3) | TTCAGTCACAATGCCGTGATT | GCTTTTCCGAAGATTGCCCA |
| GP (NM_001172975.1) | TGTGTCCATGCTCCCGTGCC | TGCTGAGGACGCCAGTGCTT | |
| Kir2.2 | H (NM_021012.5) | GCCCACTCAGCACCATTACA | CCTCCTCCGATGACACGATG |
| M (NM_010603.6) | GAGTCTGTGCCCACTGTGCCTG | TTGGGGTACTCAGACGCCGGG | |
| GP (NM_001173037.1) | ACGCAGACCACCATCGGCTAC | GCCACCACCATGAAGACAGCCAC | |
| Kir2.3 | H (NM_152868.3) | ACCTCAACGTGGGCTATGAC | CGTCGATCTCGTGGACAATGAT |
| GP (NM_001172710.1) | GTCTTCCCAGGTGACACGCCG | TCTTGACGAAGCGGTTGCGG | |
| SR-A1 | M (NM_031195.2) | TGAACGAGAGGATGCTGACTG | TGTCATTGAACGTGCGTCAAA |
| SR-B1 | M (NM_016741.2) | TTTGGAGTGGTAGTAAAAAGGGC | TGACATCAGGGACTCAGAGTAG |
| CD36 | M (NM_001159558.1) | AGATGACGTGGCAAAGAACAG | CCTTGGCTAGATAACGAACTCTG |
| LOX-1 | M (NM_138648.2) | CAAGATGAAGCCTGCGAATGA | ACCTGGCGTAATTGTGTCCAC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koyani, C.N.; Scheruebel, S.; Jin, G.; Kolesnik, E.; Zorn-Pauly, K.; Mächler, H.; Hoefler, G.; von Lewinski, D.; Heinzel, F.R.; Pelzmann, B.; et al. Hypochlorite-Modified LDL Induces Arrhythmia and Contractile Dysfunction in Cardiomyocytes. Antioxidants 2022, 11, 25. https://doi.org/10.3390/antiox11010025
Koyani CN, Scheruebel S, Jin G, Kolesnik E, Zorn-Pauly K, Mächler H, Hoefler G, von Lewinski D, Heinzel FR, Pelzmann B, et al. Hypochlorite-Modified LDL Induces Arrhythmia and Contractile Dysfunction in Cardiomyocytes. Antioxidants. 2022; 11(1):25. https://doi.org/10.3390/antiox11010025
Chicago/Turabian StyleKoyani, Chintan N., Susanne Scheruebel, Ge Jin, Ewald Kolesnik, Klaus Zorn-Pauly, Heinrich Mächler, Gerald Hoefler, Dirk von Lewinski, Frank R. Heinzel, Brigitte Pelzmann, and et al. 2022. "Hypochlorite-Modified LDL Induces Arrhythmia and Contractile Dysfunction in Cardiomyocytes" Antioxidants 11, no. 1: 25. https://doi.org/10.3390/antiox11010025
APA StyleKoyani, C. N., Scheruebel, S., Jin, G., Kolesnik, E., Zorn-Pauly, K., Mächler, H., Hoefler, G., von Lewinski, D., Heinzel, F. R., Pelzmann, B., & Malle, E. (2022). Hypochlorite-Modified LDL Induces Arrhythmia and Contractile Dysfunction in Cardiomyocytes. Antioxidants, 11(1), 25. https://doi.org/10.3390/antiox11010025








