Easy-to-Use HPLC Method to Measure Intracellular Ascorbic Acid Levels in Human Peripheral Blood Mononuclear Cells and in Plasma
Abstract
1. Introduction
2. Materials and Methods
2.1. HPLC and Conditions
2.2. Sample Preparation
2.3. Method Validation
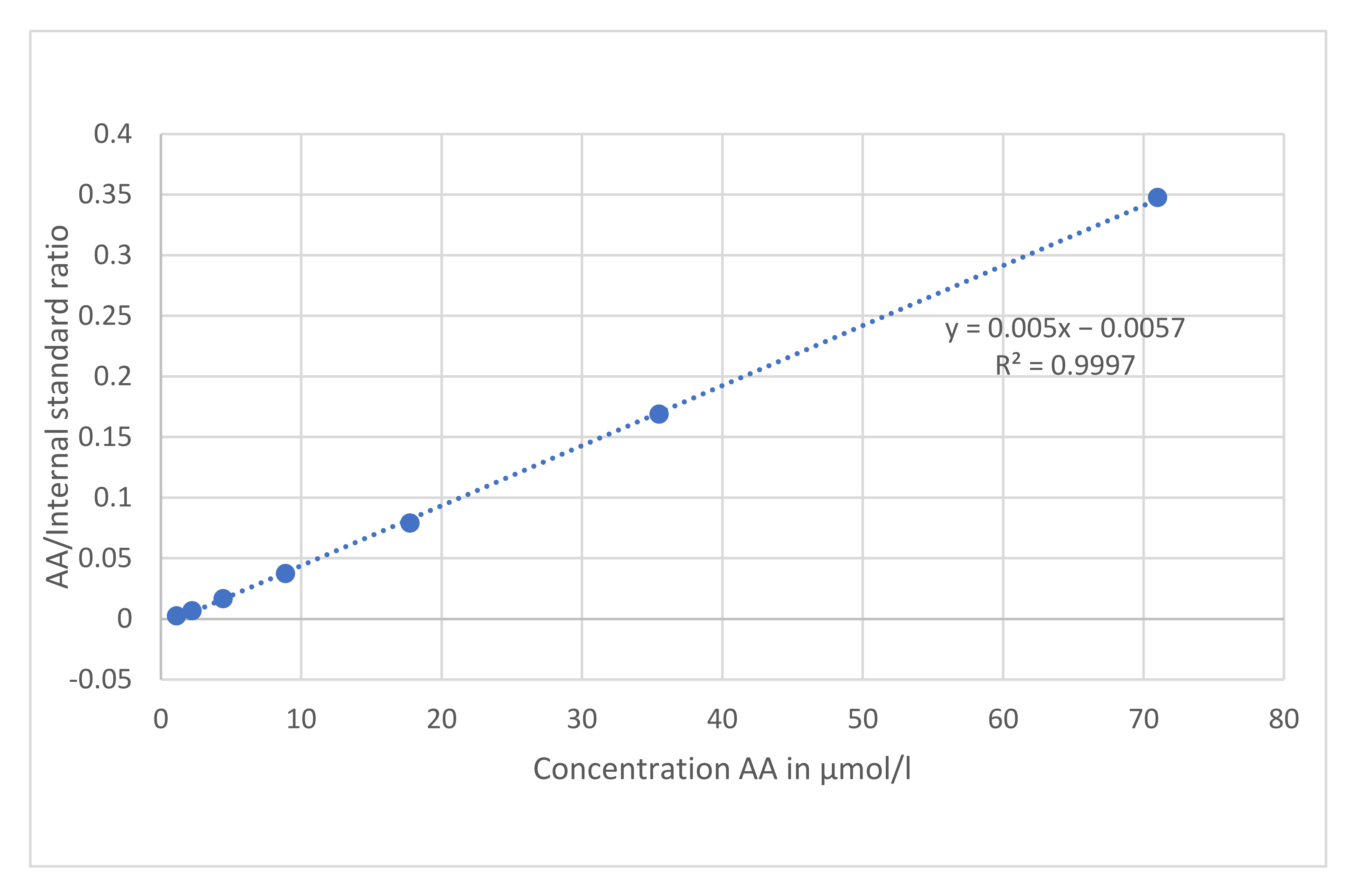
2.3.1. Linearity and Quantification Limits
2.3.2. Precision
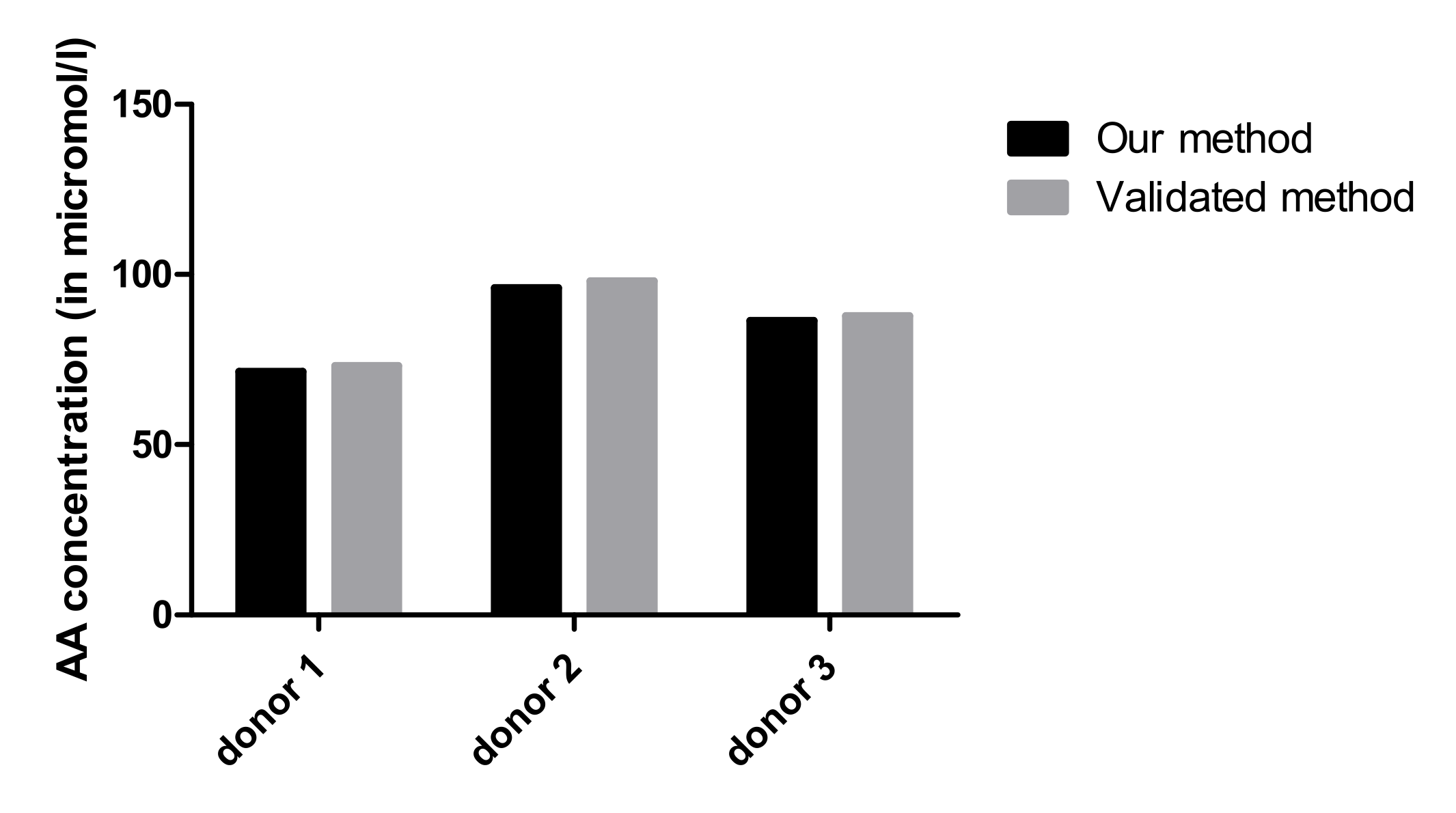
2.3.3. Accuracy
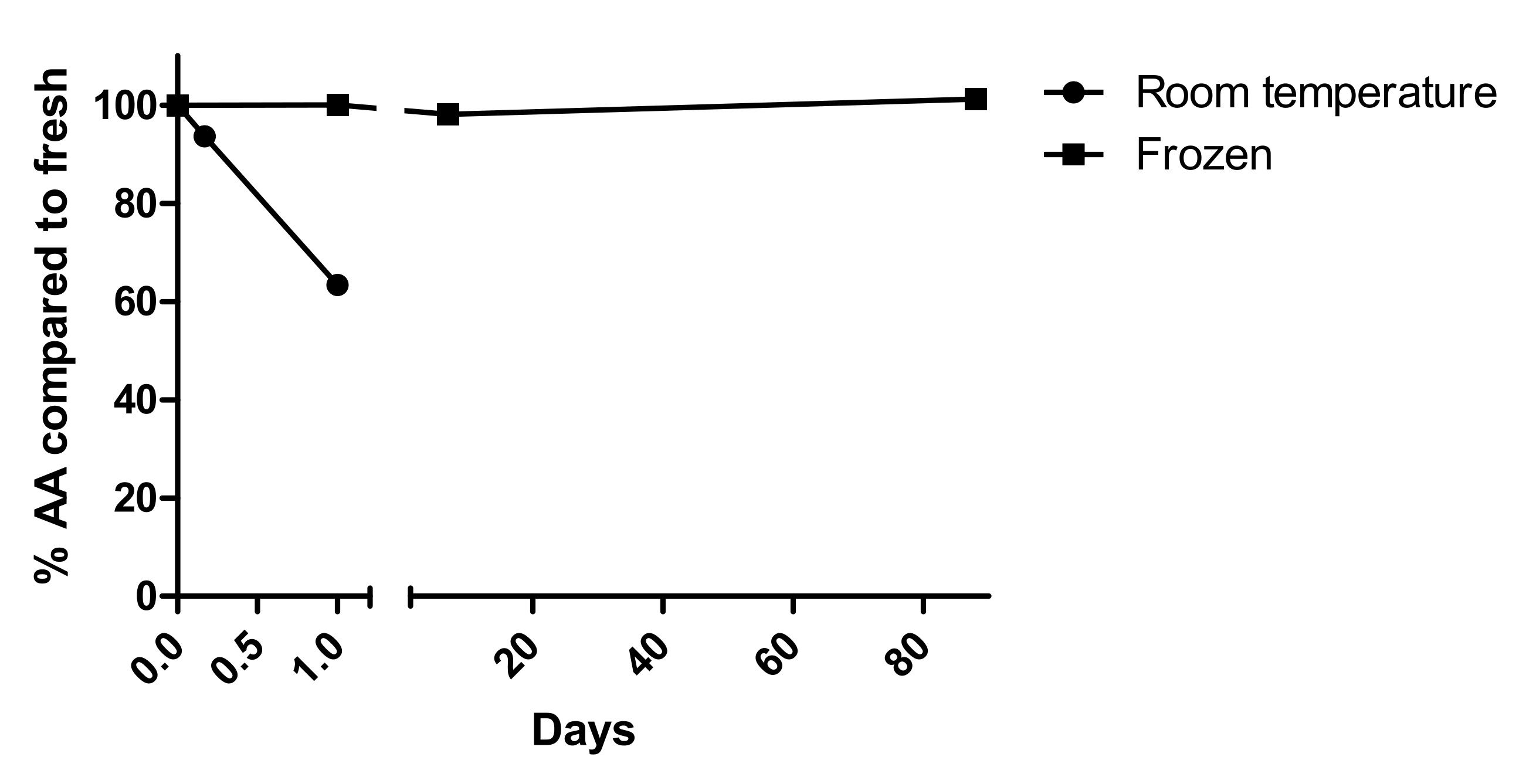
2.3.4. Sample Stability
2.3.5. Carryover
2.4. Clinical Application
2.5. Calculations and Statistics
3. Results
3.1. Sample Preparation
3.2. Method Validation
3.2.1. Chromatography
3.2.2. Linearity
3.2.3. Quantification Limits
3.2.4. Precision
3.2.5. Accuracy
3.2.6. Stability
3.2.7. Carryover
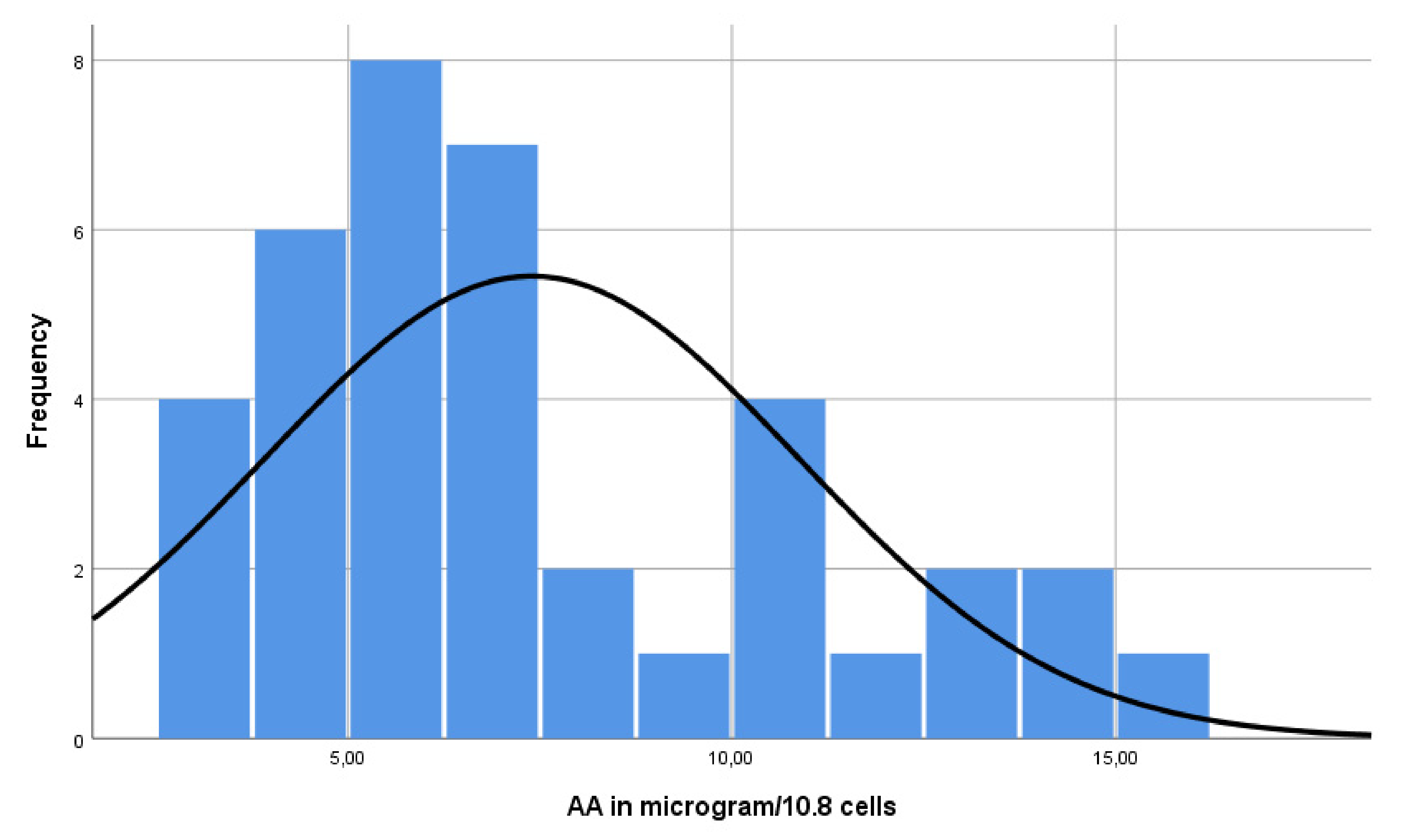
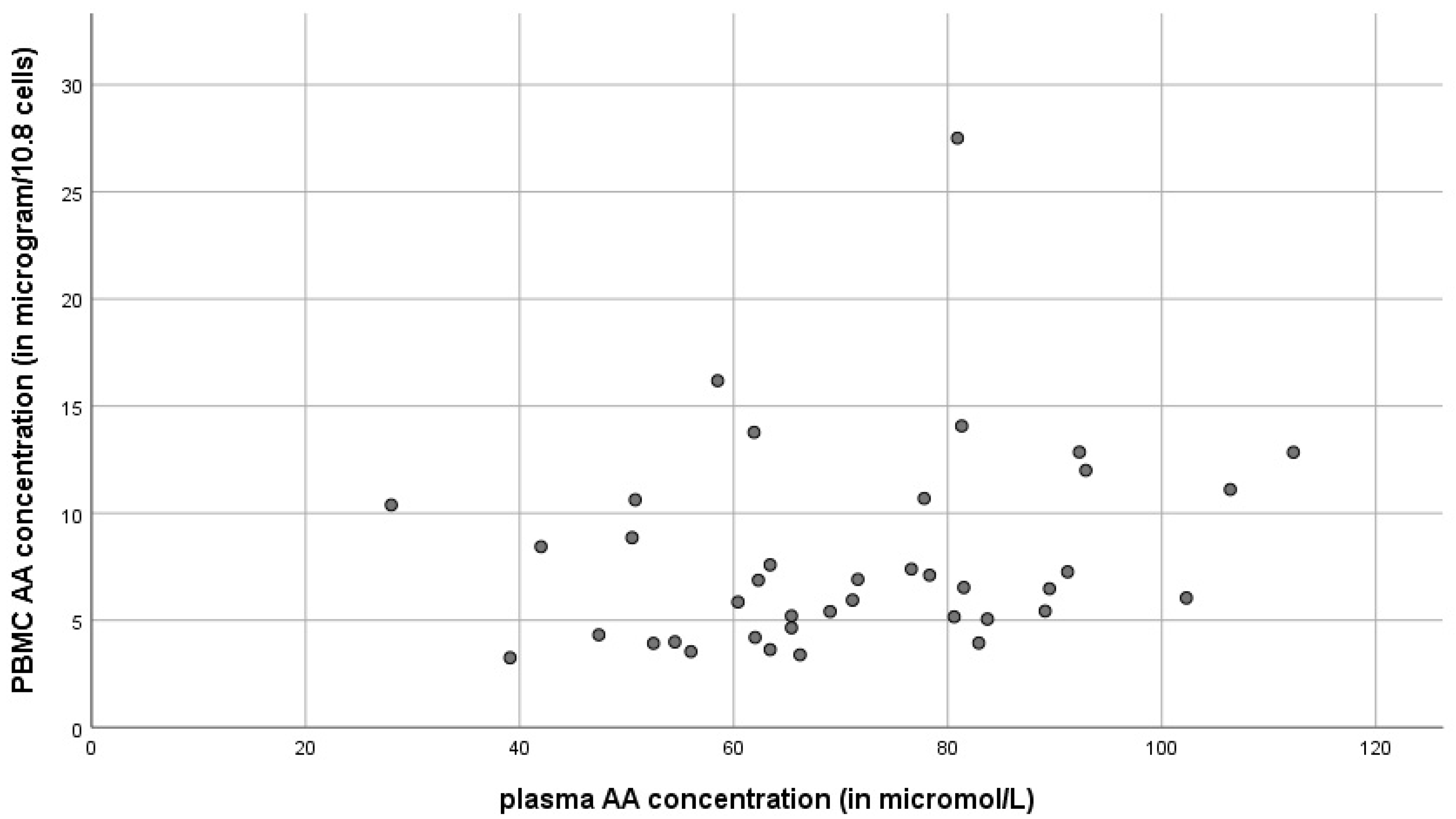
3.3. Reference Values
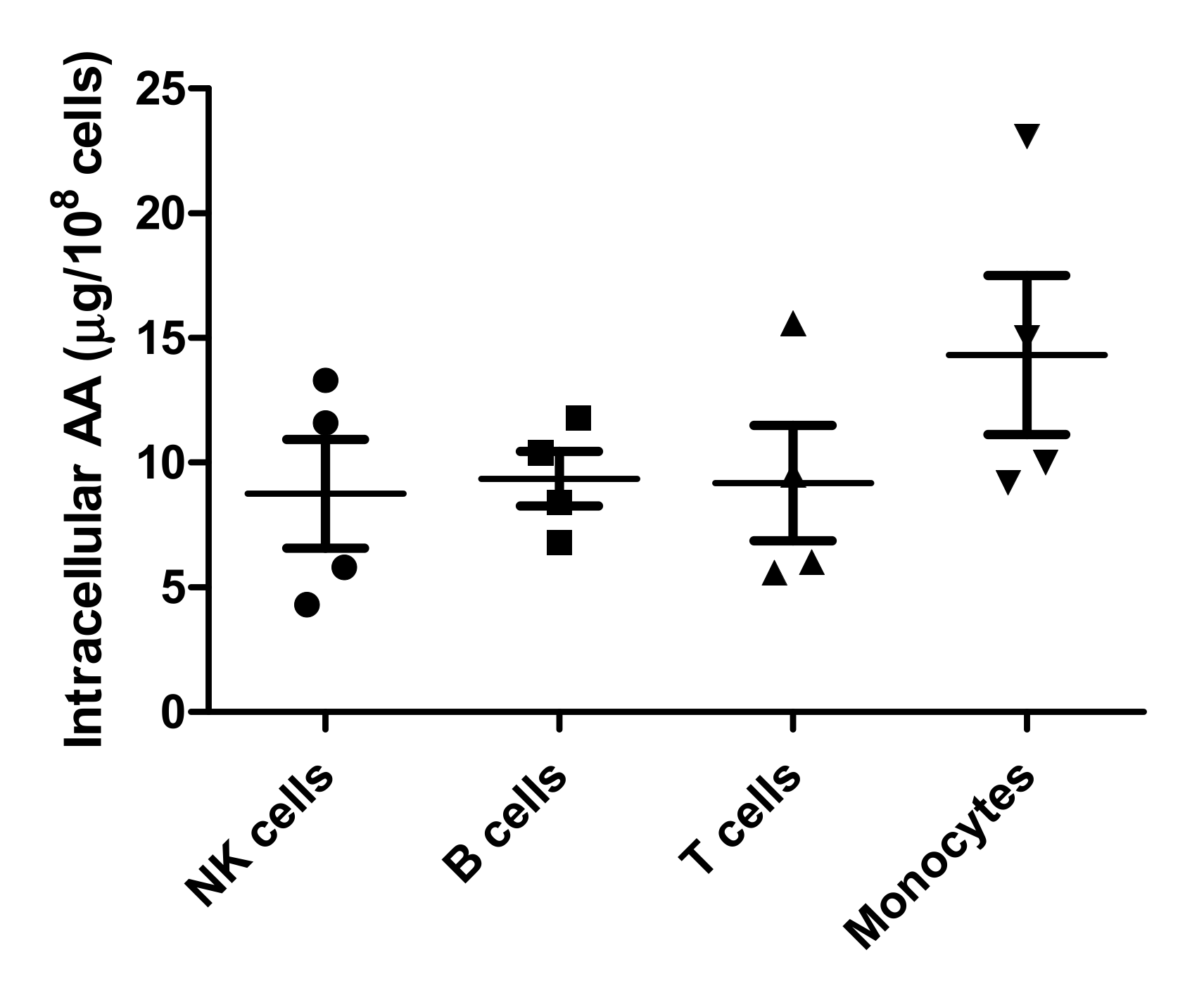
3.4. PBMC Subsets
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Packer, L.; Fuchs, J. Vitamin C in Health and Disease; Marcel Dekker Inc.: New York, NY, USA, 1997; pp. 367–379. [Google Scholar]
- Strohle, A.; Wolters, M.; Hahn, A. Micronutrients at the interface between inflammation and infection—Ascorbic acid and calciferol: Part 1, general overview with a focus on ascorbic acid. Inflamm. Allergy Drug Targets 2011, 10, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Rümelin, A.; Fauth, U.; Halmágyi, M. Determination of ascorbic acid in plasma and urine by high performance liquid chromatography with ultraviolet detection. Clin. Chem. Lab. Med. 1999, 37, 533–536. [Google Scholar] [CrossRef] [PubMed]
- Attila, S.; Vancea, S.; Kiss, I.; Donáth-Nagy, G. Quantification of Plasma and Leukocyte Vitamin C by High Performance Liquid Chromatography with Mass Spectrometric Detection. J. Anal. Chem. 2020, 75, 1168–1176. [Google Scholar] [CrossRef]
- Omaye, S.T.; Schaus, E.E.; Kutnink, M.A.; Hawkes, W.C. Measurement of vitamin C in blood components by high-performance liquid chromatography. Implication in assessing vitamin C status. Ann. N. Y. Acad. Sci. 1987, 498, 389–401. [Google Scholar] [CrossRef] [PubMed]
- Levine, M.; Conry-Cantilena, C.; Wang, Y.; Welch, R.W.; Washko, P.W.; Dhariwal, K.R.; Park, J.B.; Lazarev, A.; Graumlich, J.F.; King, J.; et al. Vitamin C pharmacokinetics in healthy volunteers: Evidence for a recommended dietary allowance. Proc. Natl. Acad. Sci. USA 1996, 93, 3704–3709. [Google Scholar] [CrossRef] [PubMed]
- Carr, A.C.; Maggini, S. Vitamin C and Immune Function. Nutrients 2017, 9, 1211. [Google Scholar] [CrossRef] [PubMed]
- van Gorkom, G.N.Y.; Klein Wolterink, R.G.J.; Van Elssen, C.; Wieten, L.; Germeraad, W.T.V.; Bos, G.M.J. Influence of Vitamin C on Lymphocytes: An Overview. Antioxidants 2018, 7, 41. [Google Scholar] [CrossRef] [PubMed]
- Denson, K.W.; Bowers, E.F. The determination of ascorbic acid in white blood cells. A comparison of W.B.C. ascorbic acid and phenolic acid excretion in elderly patients. Clin. Sci. 1961, 21, 157–162. [Google Scholar] [PubMed]
- Emadi-Konjin, P.; Verjee, Z.; Levin, A.V.; Adeli, K. Measurement of intracellular vitamin C levels in human lymphocytes by reverse phase high performance liquid chromatography (HPLC). Clin. Biochem. 2005, 38, 450–456. [Google Scholar] [CrossRef] [PubMed]
- Committee for Medicinal Products for Human Use. Guideline on Bioanalytical Method Validation; European Medicines Agency: London, UK, 2011; pp. 4–10. [Google Scholar]
- Simiele, M.; D’Avolio, A.; Baietto, L.; Siccardi, M.; Sciandra, M.; Agati, S.; Cusato, J.; Bonora, S.; Di Perri, G. Evaluation of the mean corpuscular volume of peripheral blood mononuclear cells of HIV patients by a coulter counter to determine intracellular drug concentrations. Antimicrob. Agents Chemother. 2011, 55, 2976–2978. [Google Scholar] [CrossRef] [PubMed]
- Nováková, L.; Solichová, D.; Pavlovicová, S.; Solich, P. Hydrophilic interaction liquid chromatography method for the determination of ascorbic acid. J. Sep. Sci. 2008, 31, 1634–1644. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.; Hamernyik, P.; Hutchinson, M.; Raisys, V.A.; Labbé, R.F. Ascorbic acid in lymphocytes: Cell preparation and liquid-chromatographic assay. Clin. Chem. 1982, 28, 2165–2169. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, T. Comparison of ascorbic acid concentrations in granulocytes and lymphocytes. Tohoku J. Exp. Med. 1984, 142, 117–118. [Google Scholar] [CrossRef] [PubMed]
- Evans, R.M.; Currie, L.; Campbell, A. The distribution of ascorbic acid between various cellular components of blood, in normal individuals, and its relation to the plasma concentration. Br. J. Nutr. 1982, 47, 473–482. [Google Scholar] [CrossRef] [PubMed]
- Gibson, S.L.; Moore, F.M.; Goldberg, A. Measurement of leucocyte ascorbic acid. Br. Med. J. 1966, 1, 1152–1153. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Evans, R.M.; Currie, L.; Campbell, A. Effect of platelets on apparent leucocyte ascorbic acid content. Ann. Clin. Biochem. 1980, 17, 252–255. [Google Scholar] [CrossRef] [PubMed]
- Loh, H.S.; Wilson, C.W. Relationship between leucocyte and plasma ascorbic acid concentrations. Br. Med. J. 1971, 3, 733–735. [Google Scholar] [CrossRef] [PubMed]
- Burkin, C.A.; Ashwood, E.R. Tietz Textbook of Clinical Chemistry; WB Saunders: Philadelphia, PA, USA, 1998; pp. 1311–1312. [Google Scholar]
- Huijskens, M.J.; Wodzig, W.K.; Walczak, M.; Germeraad, W.T.; Bos, G.M. Ascorbic acid serum levels are reduced in patients with hematological malignancies. Results Immunol. 2016, 6, 8–10. [Google Scholar] [CrossRef] [PubMed]
- Huijskens, M.J.; Walczak, M.; Koller, N.; Briede, J.J.; Senden-Gijsbers, B.L.; Schnijderberg, M.C.; Bos, G.M.J.; Germeraad, W.T.V. Technical advance: Ascorbic acid induces development of double-positive T cells from human hematopoietic stem cells in the absence of stromal cells. J. Leukoc. Biol. 2014, 96, 1165–1175. [Google Scholar] [CrossRef] [PubMed]
- Huijskens, M.J.; Walczak, M.; Sarkar, S.; Atrafi, F.; Senden-Gijsbers, B.L.; Tilanus, M.G.; Bos, G.M.J.; Wieten, L.; Germeraad, W.T.V. Ascorbic acid promotes proliferation of natural killer cell populations in culture systems applicable for natural killer cell therapy. Cytotherapy 2015, 17, 613–620. [Google Scholar] [CrossRef] [PubMed]

| Ascorbic Acid | QC1 | QC2 | QC3 | QC4 | ||||
|---|---|---|---|---|---|---|---|---|
| Intra-Run | Inter-Run | Intra-Run | Inter-Run | Intra-Run | Inter-Run | Intra-Run | Inter-Run | |
| Target (µM) | 11 | 20 | 35 | 50 | ||||
| Number | 5 | 6 | 5 | 6 | 5 | 6 | 5 | 6 |
| Mean (µM) | 9.5 | 9.0 | 17.1 | 17.0 | 30.0 | 29.9 | 43.1 | 43.4 |
| SD | 0.73 | 0.1 | 0.19 | 0.2 | 0.27 | 0.7 | 0.25 | 0.7 |
| CV (%) | 7.7 | 1.1 | 1.1 | 1.0 | 0.9 | 2.5 | 0.6 | 1.6 |
| TI (%) | 7.8 | 1.1 | 2.7 | 1.7 | ||||
| Extraction Number | PBMC AA (in µg/108 Cells) |
|---|---|
| 1 | 11.31 |
| 2 | 11.79 |
| 3 | 9.80 |
| 4 | 11.16 |
| 5 | 9.31 |
| 6 | 10.75 |
| 7 | 10.61 |
| 8 | 10.97 |
| 9 | 9.71 |
| 10 | 9.89 |
| MEAN | 10.53 |
| SD | 0.81 |
| CV (%) | 7.72 |
| Ascorbic Acid | QC1 | QC2 | QC3 | QC4 | ||||
|---|---|---|---|---|---|---|---|---|
| Intra-Run | Inter-Run | Intra-Run | Inter-Run | Intra-Run | Inter-Run | Intra-Run | Inter-Run | |
| Target (µM) | 11 | 20 | 35 | 50 | ||||
| Number | 5 | 6 | 5 | 6 | 5 | 6 | 5 | 6 |
| Mean (µM) | 9.5 | 9.0 | 17.1 | 17.0 | 30.0 | 29.9 | 43.1 | 43.4 |
| Accuracy (%) | 86 | 82 | 86 | 85 | 86 | 85 | 86 | 87 |
| Deviation (%) | 14 | 18 | 14 | 15 | 14 | 15 | 14 | 13 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
van Gorkom, G.; Gijsbers, B.; Ververs, E.-J.; El Molla, A.; Sarodnik, C.; Riess, C.; Wodzig, W.; Bos, G.; Van Elssen, C. Easy-to-Use HPLC Method to Measure Intracellular Ascorbic Acid Levels in Human Peripheral Blood Mononuclear Cells and in Plasma. Antioxidants 2022, 11, 134. https://doi.org/10.3390/antiox11010134
van Gorkom G, Gijsbers B, Ververs E-J, El Molla A, Sarodnik C, Riess C, Wodzig W, Bos G, Van Elssen C. Easy-to-Use HPLC Method to Measure Intracellular Ascorbic Acid Levels in Human Peripheral Blood Mononuclear Cells and in Plasma. Antioxidants. 2022; 11(1):134. https://doi.org/10.3390/antiox11010134
Chicago/Turabian Stylevan Gorkom, Gwendolyn, Birgit Gijsbers, Erik-Jan Ververs, Ahmed El Molla, Cindy Sarodnik, Celine Riess, Will Wodzig, Gerard Bos, and Catharina Van Elssen. 2022. "Easy-to-Use HPLC Method to Measure Intracellular Ascorbic Acid Levels in Human Peripheral Blood Mononuclear Cells and in Plasma" Antioxidants 11, no. 1: 134. https://doi.org/10.3390/antiox11010134
APA Stylevan Gorkom, G., Gijsbers, B., Ververs, E.-J., El Molla, A., Sarodnik, C., Riess, C., Wodzig, W., Bos, G., & Van Elssen, C. (2022). Easy-to-Use HPLC Method to Measure Intracellular Ascorbic Acid Levels in Human Peripheral Blood Mononuclear Cells and in Plasma. Antioxidants, 11(1), 134. https://doi.org/10.3390/antiox11010134







