Management of Feline Hyperthyroidism and the Need to Prevent Oxidative Stress: What Can We Learn from Human Research?
Abstract
:1. Introduction
2. Feline Hyperthyroidism
2.1. Similarities with Human Disease
2.2. Etiology
2.3. Pathogenesis
2.4. Clinical Features
2.4.1. Signalment
2.4.2. Clinical Presentation and Laboratory Abnormalities
2.4.3. Diagnosis
3. Treatment Options of Feline Hyperthyroidism
3.1. Radioiodine Ablation of the Thyroid (Radio-Iodine Therapy) and Surgical Thyroidectomy
3.2. Antithyroid Drugs
3.3. Administration of Iodine-Limited Diets
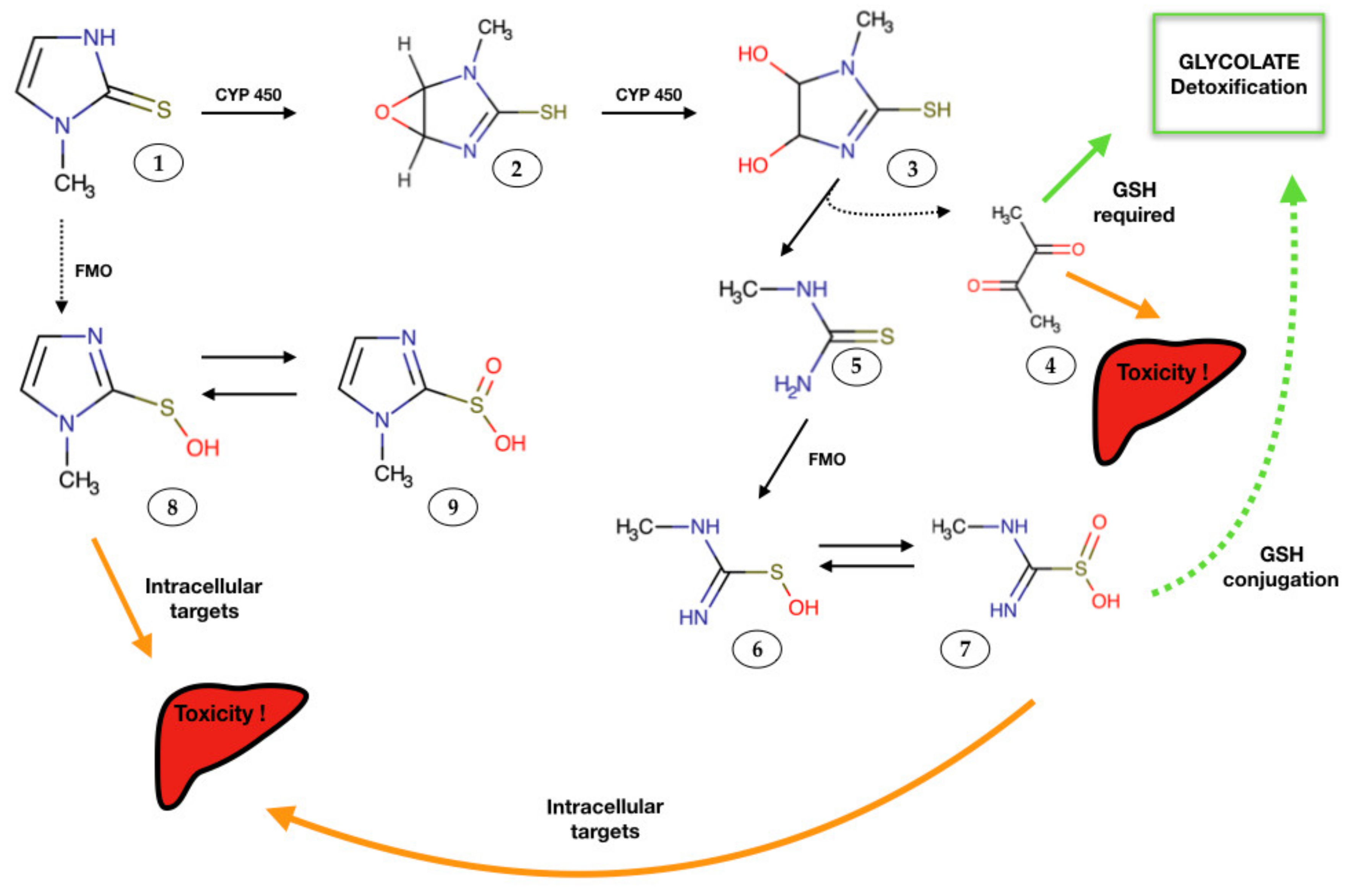
4. Mechanisms of MMI-Induced Organ Injury
5. Oxidative Stress and Thyroid Hormones
5.1. Free Radical Generation and Antioxidant Defenses
5.2. Hyperthyroidism and Redox Imbalance
5.2.1. Human Studies
5.2.2. Feline Studies
6. Nutritional Management
6.1. Dietary Scope
6.2. Synergistic Scope
6.2.1. Antioxidant Supplementation
Curcumin
Quercetin
Resveratrol
Vitamin E
6.2.2. Efficacy of Antioxidant Administration
Human Studies
Feline Studies
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
References
- Ross, D.S.; Burch, H.B.; Cooper, D.S.; Greenlee, M.C.; Laurberg, P.; Maia, A.L.; Rivkees, S.A.; Samuels, M.; Sosa, J.A.; Stan, M.N.; et al. 2016 American Thyroid Association Guidelines for Diagnosis and Management of Hyperthyroidism and Other Causes of Thyrotoxicosis. Thyroid 2016, 26, 1343–1421. [Google Scholar] [CrossRef] [PubMed]
- Stephens, M.J.; O’Neill, D.; Church, D.B.; McGreevy, P.; Thomson, P.; Brodbelt, D. Feline hyperthyroidism reported in primary-care veterinary practices in England: Prevalence, associated factors and spatial distribution. Vet. Rec. 2014, 175, 458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peterson, M. Hyperthyroidism in cats: What’s causing this epidemic of thyroid disease and can we prevent it? J. Feline Med. Surg. 2012, 14, 804–818. [Google Scholar] [CrossRef] [PubMed]
- Peterson, M.E. Animal models of disease: Feline hyperthyroidism: An animal model for toxic nodular goiter. J. Endocrinol. 2014, 223, T97–T114. [Google Scholar] [CrossRef]
- Candellone, A.; Gianella, P.; Ceccarelli, L.; Raviri, G.; Badino, P.; Roncone, S.; Kooistra, H.S.; Meineri, G. Redox unbalance in the hyperthyroid cat: A comparison with healthy and non-thyroidal diseased cats. BMC Vet. Res. 2019, 15, 136. [Google Scholar] [CrossRef] [Green Version]
- Mizutani, T.; Murakami, M.; Shirai, M.; Tanaka, M.; Nakanishi, K. Metabolism-dependent hepatotoxicity of methimazole in mice depleted of glutathione. J. Appl. Toxicol. 1999, 19, 193–198. [Google Scholar] [CrossRef]
- Ademoğlu, E.; Özbey, N.; Erbil, Y.; Tanrikulu, S.; Barbaros, U.; Yanik, B.T.; Bozbora, A.; Özarmağan, S. Determination of oxidative stress in thyroid tissue and plasma of patients with Graves’ disease. Eur. J. Intern. Med. 2006, 17, 545–550. [Google Scholar] [CrossRef]
- Guerra, L.N.; Molina, M.D.C.R.D.; Miler, E.A.; Moiguer, S.; Karner, M.; Burdman, J.A. Antioxidants and methimazole in the treatment of Graves’ disease: Effect on urinary malondialdehyde levels. Clin. Chim. Acta 2005, 352, 115–120. [Google Scholar] [CrossRef]
- Bednarek, J.; Wysocki, H.; Sowinski, J. Oxidation Products and Antioxidant Markers in Plasma of Patients with Graves’ Disease and Toxic Multinodular Goiter: Effect of Methimazole Treatment. Free Radic. Res. 2004, 38, 659–664. [Google Scholar] [CrossRef]
- Bianchi, G.; Solaroli, E.; Zaccheroni, V.; Grossi, G.; Bargossi, A.M.; Melchionda, N.; Marchesini, G. Oxidative stress and anti-oxidant metabolites in patients with hyperthyroidism: Effect of treatment. Horm. Metab. Res. 1999, 31, 620–624. [Google Scholar] [CrossRef]
- Candellone, A.; Badino, P.; Gianella, P.; Girolami, F.; Raviri, G.; Saettone, V.; Meineri, G. Evaluation of Antioxidant Supplementation on Redox Unbalance in Hyperthyroid Cats Treated with Methimazole: A Blinded Randomized Controlled Trial. Antioxidants 2019, 9, 15. [Google Scholar] [CrossRef] [Green Version]
- Rybus-Kalinowska, B.; Zwirska-Korczala, K.; Kalinowski, M.; Kukla, M.; Birkner, E.; Jochem, J. Activity of antioxidative enzymes and concentration of malondialdehyde as oxidative status markers in women with non-autoimmunological subclinical hyperthyroidism. Endokrynol. Polska 2009, 60, 199–202. [Google Scholar]
- Guerra, L.N.; Moiguer, S.; Karner, M.; de Molina, M.C.; Sreider, C.M.; Burdman, J.A. Antioxidants in the treatment of Graves’ disease. Life 2001, 51, 105–109. [Google Scholar] [CrossRef] [PubMed]
- Feldman, E.; Nelson, R.; Reusch, C.; Scott-Moncrieff, J.C. Feline Hyperthyroidism. In Canine and Feline Endocrinology, 4th ed.; Saunders: Philadelphia, PA, USA, 2015; pp. 137–190. [Google Scholar]
- Peterson, M.E.; Eirmann, L. Dietary Management of Feline Endocrine Disease. Vet. Clin. Small Anim. Pract. 2014, 44, 775–788. [Google Scholar] [CrossRef] [PubMed]
- Carney, H.C.; Ward, C.R.; Bailey, S.J.; Bruyette, D.; Dennis, S.; Ferguson, D.; Hinc, A.; Rucinsky, A.R. 2016 AAFP Guidelines for the Management of Feline Hyperthyroidism. J. Feline Med. Surg. 2016, 18, 400–416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turrel, J.M.; Feldman, E.C.; Nelson, R.W.; Cain, G.R. Thyroid carcinoma causing hyperthyroidism in cats: 14 cases (1981–1986). J. Am. Vet. Med. Assoc. 1988, 193, 359–364. [Google Scholar]
- Beck-Peccoz, P.; Persani, L.; Mannavola, D.; Campi, I. Pituitary tumours: TSH-secreting adenomas. Best Pract. Res. Clin. Endocrinol. Metab. 2009, 23, 597–606. [Google Scholar] [CrossRef]
- Kohler, B.; Stengel, C.; Neiger, R. Dietary hyperthyroidism in dogs. J. Small Anim. Pract. 2012, 53, 182–184. [Google Scholar] [CrossRef] [PubMed]
- Gordon, J.M.; Ehrhart, E.J.; Sisson, D.D.; Jones, M.A. Juvenile Hyperthyroidism in a Cat. J. Am. Anim. Hosp. Assoc. 2003, 39, 67–71. [Google Scholar] [CrossRef]
- Peterson, M.; Kintzer, P.P.; Cavanagh, P.G.; Fox, P.R.; Ferguson, D.C.; Johnson, G.F.; Becker, D.V. Feline hyperthyroidism: Pretreatment clinical and laboratory evaluation of 131 cases. J. Am. Vet. Med. Assoc. 1983, 183, 103–110. [Google Scholar] [PubMed]
- Rijnberk, A.; Kooistra, H. Thyroids. In Clinical Endocrinology of Dogs and Cats, 2nd ed.; Schlutersche: Hannover, Germany, 2010; pp. 55–92. [Google Scholar]
- Taylor, S.; Sparkes, A.; Briscoe, K.; Carter, J.; Sala, S.C.; E Jepson, R.; Reynolds, B.S.; Scansen, B. ISFM Consensus Guidelines on the Diagnosis and Management of Hypertension in Cats. J. Feline Med. Surg. 2017, 19, 288–303. [Google Scholar] [CrossRef] [PubMed]
- Peterson, M.; Varela, F.; Rishniw, M.; Polzin, D. Evaluation of Serum Symmetric Dimethylarginine Concentration as a Marker for Masked Chronic Kidney Disease in Cats with Hyperthyroidism. J. Vet. Intern. Med. 2018, 32, 295–304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Branter, E.; Drescher, N.; Padilla, M.; Trepanier, L. Antioxidant Status in Hyperthyroid Cats before and after Radioiodine Treatment. J. Vet. Intern. Med. 2012, 26, 582–588. [Google Scholar] [CrossRef] [PubMed]
- Peterson, M.; Melián, C.; Nichols, R. Measurement of serum concentrations of free thyroxine, total thyroxine, and total triiodothyronine in cats with hyperthyroidism and cats with nonthyroidal disease. J. Am. Vet. Med. Assoc. 2001, 218, 529–536. [Google Scholar] [CrossRef]
- Peterson, M.E.; Guterl, J.; Nichols, R.; Rishniw, M. Evaluation of Serum Thyroid-Stimulating Hormone Concentration as a Diagnostic Test for Hyperthyroidism in Cats. J. Vet. Intern. Med. 2015, 29, 1327–1334. [Google Scholar] [CrossRef] [Green Version]
- Daminet, S.; Kooistra, H.S.; Fracassi, F.; Graham, P.A.; Hibbert, A.; Lloret, A.; Mooney, C.T.; Neiger, R.; Rosenberg, D.; Syme, H.M.; et al. Best practice for the pharmacological management of hyperthyroid cats with antithyroid drugs. J. Small Anim. Pract. 2013, 55, 4–13. [Google Scholar] [CrossRef] [Green Version]
- Hill, K.; Gieseg, M.; Bridges, J.; Chambers, J. The pharmacokinetics of methimazole in a novel lipophilic formulation administered transdermally to healthy cats. N. Z. Vet. J. 2014, 62, 208–213. [Google Scholar] [CrossRef]
- Boretti, F.S.; Sieber-Ruckstuhl, N.S.; Schäfer, S.; Gerber, B.; Baumgartner, C.; Riond, B.; Hofmann-Lehmann, R.; Reusch, C.E. Transdermal application of methimazole in hyperthyroid cats: A long-term follow-up study. J. Feline Med. Surg. 2014, 16, 453–459. [Google Scholar] [CrossRef]
- Heidari, R.; Niknahad, H.; Jamshidzadeh, A.; Abdoli, N. Factors affecting drug-induced liver injury: Antithyroid drugs as instances. Clin. Mol. Hepatol. 2014, 20, 237–248. [Google Scholar] [CrossRef]
- Niknahad, H.; Jamshidzadeh, A.; Heidari, R.; Hosseini, Z.; Mobini, K.; Khodaei, F.; Ommati, M.M.; Abdoli, N.; Keshavarz, N.; Bazyari, M.; et al. Paradoxical effect of methimazole on liver mitochondria: In vitro and in vivo. Toxicol. Lett. 2016, 259, 108–115. [Google Scholar] [CrossRef]
- Heidari, R.; Niknahad, H.; Jamshidzadeh, A.; Eghbal, M.A.; Abdoli, N. An Overview on the Proposed Mechanisms of Antithyroid Drugs-Induced Liver Injury. Adv. Pharm. Bull. 2015, 5, 1–11. [Google Scholar] [CrossRef]
- Kim, H.; Lee, T.H.; Hwang, Y.S.; Bang, M.A.; Kim, K.H.; Suh, J.M.; Chung, H.K.; Yu, D.Y.; Lee, K.K.; Kwon, O.; et al. Methimazole as an antioxidant and immunomodulator in thyroid cells: Mechanisms involving interferon-gamma signaling and H(2)O(2) scavenging. Mol. Pharmacol. 2001, 60, 972–980. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Higuchi, S.; Ide, M.; Nishikawa, S.; Fukami, T.; Nakajima, M.; Yokoi, T. Th2 cytokine-mediated methimazole-induced acute liver injury in mice. J. Appl. Toxicol. 2012, 32, 823–833. [Google Scholar] [CrossRef]
- Hui, T.; Bruyette, D.; Moore, G.; Scott-Moncrieff, J. Effect of Feeding an Iodine-Restricted Diet in Cats with Spontaneous Hyperthyroidism. J. Vet. Intern. Med. 2015, 29, 1063–1068. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- AAFCO (Association of American Feed Control Officials). 2014 Official Publication; AAFCO: Champaign, IL, USA, 2014. [Google Scholar]
- Vogt, A.H.; Rodan, I.; Brown, M.; Brown, S.; Buffington, C.A.; Forman, M.J.; Neilson, J.; Sparkes, A. AAFP-AAHA: Feline life stage guidelines. J Feline Med. Surg. 2010, 12, 43–54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Melendez, L.M.; Yamka, R.M.; Forrester, S.D. Titration of dietary iodine for reducing serum thyroxine concentrations in newly diagnosed hyperthyroid cats. J. Vet. Intern. Med. 2011, 29, 3. [Google Scholar]
- Yu, S.; Wedeking, K.J.; Burris, P.A.; Dru Forrester, S.; Locniskar, M.F. Controlled level of dietary iodine normalizes serum total thyroxine in cats with naturally occurring hyperthyroidism. J. Vet. Intern. Med. 2011, 25, 683–684. [Google Scholar]
- Patrick, L. Iodine: Deficiency and therapeutic considerations. Altern. Med. Rev. 2008, 13, 116–127. [Google Scholar]
- Hibbert, A.; Gruffydd-Jones, T.; Barrett, E.L.; Day, M.J.; Harvey, A.M. Feline thyroid carcinoma: Diagnosis and response to high-dose radioactive iodine treatment. J. Feline Med. Surg. 2009, 11, 116–124. [Google Scholar] [CrossRef]
- Mancini, A.; Di Segni, C.; Raimondo, S.; Olivieri, G.; Silvestrini, A.; Meucci, E.; Curro’, D. Thyroid Hormones, Oxidative Stress, and Inflammation. Mediat. Inflamm. 2016, 2016, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Villanueva, I.; Alva-Sánchez, C.; Pacheco-Rosado, J. The Role of Thyroid Hormones as Inductors of Oxidative Stress and Neurodegeneration. Oxidative Med. Cell. Longev. 2013, 2013, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Marcocci, C.; Leo, M.; Altea, M.A. Oxidative Stress in Graves’ Disease. Eur. Thyroid. J. 2012, 1, 80–87. [Google Scholar] [CrossRef] [Green Version]
- Kesar, V.; Jain, A. Thyroid Hormones and Oxidative Stress. Indian J. Med Biochem. 2017, 21, 58–61. [Google Scholar] [CrossRef]
- Venditti, P.; Balestrieri, M.; Di Meo, S.; De Leo, T. Effect of thyroid state on lipid peroxidation, antioxidant defences, and susceptibility to oxidative stress in rat tissues. J. Endocrinol. 1997, 155, 151–157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mureşan, A.; Dunce, I.; Petrulea, M. Oxidative Stress and Antioxidant Status in Hypo- and Hyperthyroidism. In Antioxidant Enzyme; IntechOpen: London, UK, 2012. [Google Scholar]
- Cetinkaya, A.; Kurutas, E.B.; Buyukbese, M.A.; Kantarceken, B.; Bulbuloglu, E. Levels of Malondialdehyde and Superoxide Dismutase in Subclinical Hyperthyroidism. Mediat. Inflamm. 2005, 2005, 57–59. [Google Scholar] [CrossRef] [PubMed]
- Aslan, M.; Cosar, N.; Celik, H.; Aksoy, N.; Dulger, A.C.; Begenik, H.; Soyoral, Y.U.; Kucukoglu, M.E.; Selek, Ş. Evaluation of oxidative status in patients with hyperthyroidism. Endocrine 2011, 40, 285–289. [Google Scholar] [CrossRef]
- Komosinska-Vassev, K.; Olczyk, K.; Kucharz, E.J.; Marcisz, C.; Winsz-Szczotka, K.; Kotulska, A. Free radical activity and antioxidant defense mechanisms in patients with hyperthyroidism due to Graves’ disease during therapy. Clin. Chim. Acta 2000, 300, 107–117. [Google Scholar] [CrossRef]
- Abalovich, M.; Llesuy, S.; Gutierrez, S.; Repetto, M. Peripheral parameters of oxidative stress in Graves’ disease: The effects of methimazole and 131 iodine treatments. Clin. Endocrinol. 2003, 59, 321–327. [Google Scholar] [CrossRef]
- Viviano, K.; Lavergne, S.; Goodman, L.; VanderWielen, B.; Grundahl, L.; Padilla, M.; Trepanier, L. Glutathione, Cysteine, and Ascorbate Concentrations in Clinically Ill Dogs and Cats. J. Vet. Intern. Med. 2009, 23, 250–257. [Google Scholar] [CrossRef]
- Laflamme, D.P.; Hannah, S.S. Discrepancy between use of lean body mass or nitrogen balance to determine protein requirements for adult cats. J. Feline Med. Surg. 2013, 15, 691–697. [Google Scholar] [CrossRef]
- International Renal Interest Society (IRIS). CKD Staging in Cats. 2015. Available online: http://www.iris-kidney.com/pdf/003-5559.001-iris-website-staging-of-ckd-pdf_220116-final.pdf#page=7 (accessed on 12 May 2021).
- FEDIAF. FEDIAF Nutritional Guidelines for Complete and Complementary Pet Food for Cats and Dogs. 2020. Available online: http://www.fediaf.org/self-regulation/nutrition/ (accessed on 11 March 2020).
- Venditti, P.; Di Stefano, L.; Di Meo, S. Vitamin E management of oxidative damage-linked dysfunctions of hyperthyroid tissues. Cell. Mol. Life Sci. 2012, 70, 3125–3144. [Google Scholar] [CrossRef] [PubMed]
- Pistollato, F.; Masias, M.; Agudo, P.; Giampieri, F.; Battino, M. Effects of phytochemicals on thyroid function and their possible role in thyroid disease. Ann. N. Y. Acad. Sci. 2018, 1443, 3–19. [Google Scholar] [CrossRef] [PubMed]
- Panda, S.; Kar, A. Annona squamosa seed extract in the regulation of hyperthyroidism and lipid-peroxidation in mice: Possible involvement of quercetin. Phytomedicine 2007, 14, 799–805. [Google Scholar] [CrossRef] [PubMed]
- Kocaadam, B.; Şanlier, N. Curcumin, an active component of turmeric (Curcuma longa), and its effects on health. Crit. Rev. Food Sci. Nutr. 2017, 57, 2889–2895. [Google Scholar] [CrossRef] [PubMed]
- Lund, J.C.; Pantuso, T. Combination Effects of Quercetin, Resveratrol and Curcumin on In Vitro Intestinal Absorption. J. Restor. Med. 2014, 3, 112–120. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, A.; Lisboa, P.; Oliveira, K.; Lima, L.; Barros, I.; Carvalho, D. Inhibition of thyroid type 1 deiodinase activity by flavonoids. Food Chem. Toxicol. 2002, 40, 913–917. [Google Scholar] [CrossRef]
- Jena, S.; Dandapat, J.; Chainy, G.B. Curcumin differentially regulates the expression of superoxide dismutase in cerebral cortex and cerebellum of L-thyroxine (T4)-induced hyperthyroid rat brain. Neurol. Sci. 2013, 34, 505–510. [Google Scholar] [CrossRef]
- Jena, S.; Chainy, G.B.N. Regulation of expression of antioxidant enzymes by vitamin E and curcumin in l-thyroxine-induced oxidative stress in rat renal cortex. Mol. Biol. Rep. 2010, 38, 1047–1054. [Google Scholar] [CrossRef]
- Subudhi, U.; Das, K.; Paital, B.; Bhanja, S.; Chainy, G. Alleviation of enhanced oxidative stress and oxygen consumption of l-thyroxine induced hyperthyroid rat liver mitochondria by vitamin E and curcumin. Chem. Interactions 2008, 173, 105–114. [Google Scholar] [CrossRef]
- Subudhi, U.; Chainy, G.B. Expression of hepatic antioxidant genes in l-thyroxine-induced hyperthyroid rats: Regulation by vitamin E and curcumin. Chem. Interactions 2010, 183, 304–316. [Google Scholar] [CrossRef]
- Giuliani, C.; Bucci, I.; Di Santo, S.; Rossi, C.; Grassadonia, A.; Mariotti, M.; Piantelli, M.; Monaco, F.; Napolitano, G. Resveratrol Inhibits Sodium/Iodide Symporter Gene Expression and Function in Rat Thyroid Cells. PLoS ONE 2014, 9, e107936. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.J.; Youn, Y.-K.; Hong, M.-K.; Kim, L.S. Antiproliferation and Redifferentiation in Thyroid Cancer Cell Lines by Polyphenol Phytochemicals. J. Korean Med Sci. 2011, 26, 893–899. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giuliani, C.; Iezzi, M.; Ciolli, L.; Hysi, A.; Bucci, I.; Di Santo, S.; Rossi, C.; Zucchelli, M.; Napolitano, G. Resveratrol has anti-thyroid effects both in vitro and in vivo. Food Chem. Toxicol. 2017, 107, 237–247. [Google Scholar] [CrossRef] [PubMed]
| Side-Effects | Dose Range (Total mg/day) | Administration | Frequency (%) | Time to Onset (Days) | Recommendation | Monitoring/Comments |
|---|---|---|---|---|---|---|
| Hepatopathy | 5 to 15 5 | Oral Transdermal | 2.6 4 | 15 to 60 14 | / | Liver enzymes function |
| Bleeding diathesis | 5 to 15 | Oral | 2.5 | 15 to 50 | / | In most cases associated with thrombo- cytopenia |
| Marked thrombocytopenia | 5 to 20 10 | Oral Transdermal | 2.8 8 | 14 to 90 14 to 28 | Discontinue treatment | Platelet counts 2 weeks later |
| Agranulocytosis neutropenia | 5 to 20 5 to 10 | Oral Transdermal | 2.7 7 | 26 to 95 Within the first 4 weeks | / | cell blood count two weeks later |
| Myasthenia gravis | Unknown | Oral | 4 cases reported | 60 to 120 | Discontinue treatment, administer steroids | |
| Anaemia | 2.5 to 5 | Oral | 1 case report | After 3 years of treatment | Discontinue treatment if anaemia is severe, no re- challenge | cell blood count 2 weeks later |
| Gastro-intestinal upset or lethargy | 2.5 to 15 | Oral | Vomiting, nausea, 9.3 Anorexia, 8.9 Unspecified GI upset, 23 Lethargy, 10.5 | 7 to 60 1 to 78 Within the first 4 weeks 1 to 60 | Continue treatment Lower dosage if no improvement | |
| Generalized peripheral lymphadenopathy | 10 | Oral | 1 case report | Within 2 weeks | Discontinue treatment | |
| Antinuclear antibodies | 2.5 to 20 | Oral | 23 | 10 to 870 | Not routinely measured | |
| Mild haematological abnormalities | 2.5 to 25 | Oral | 16.4 | 10 to 490 | Continue treatment, unless associated with clinical signs | |
| Positive DAT (Direct Antiglobulin Test) | 10 to 15 | Oral | 1.9 | 45 to 60 | Continue treatment if no haemolytic anaemia | |
| Dermatological reactions (i.e., facial excoriation) | 5 to 15 5 to 10 | Oral Transdermal | 4 8 | 6 to 40 Within the first 4 weeks | Ideally, discontinuation of treatment is recommended |
| Treatment | Advantages | Disadvantages |
|---|---|---|
| Radioactive Iodine |
|
|
| Oral or transdermal medication |
|
|
| Surgical thyroidectomy |
|
|
| Diet |
|
|
| Nutritional Recommendations for FHT [14,15,16,54,55] | Restricted-Iodine Commercial Diet | Feline Senior Commercial Diet 1 | Feline Senior Commercial Diet II | Nutritional Recommendations FEDIAF [56] | |
|---|---|---|---|---|---|
| Kcal ME/100 g DMB | ↑↑ caloric density/100 g DMB | 450 | 412 | 390 | 100 kcal/kg |
| Protein % MER and/or g/100 g DMB | 40% MER | 34.07 g/100 g DMB 30.2% MER | 30.4 g/100 DMB | 39.1 g/100 DMB 40% MER | 25 g/100 g DMB |
| Protein (g/100 kcal ME) | 12 | 7.58 | 7.37 | 10 | 6.25 |
| Lipid % MER and/or g/100 g DMB | 40-50% MER | 24.97 g/100 g DMB 49.9% MER | 15.2 g/100 g DMB 33.2% MER | 10.8 g/100 g DMB 24.9% MER | 9 |
| Lipid (g/100 kcal ME) | / | 5.55 | 3.7 | 2.76 | 2.25 |
| NFE % MER and/or g/100 g DMB | <15% MER | 33.65 g/100 g DMB 29.6% MER | 36.8 g/100 g DMB 35.7% MER | 30.3 g/100 g DMB 31% MER | / |
| NFE (g/100 kcal EM) | / | 7.48 | 8.9 | 7.74 | / |
| Phos % | Depends on IRIS CKD stage | 0.6 | 0.59 | 0.86 | 0.5 |
| Phos (mg/100 kcal ME) | 125–250 But depends on IRIS CKD stage | 134.12 | 133.49 | 219.9 | 125 |
| Species | Organ | Investigated Aspect | Results | Reference |
|---|---|---|---|---|
| rat | cerebral cortex and cerebellum | lipid peroxidation and SOD activity | ↓ to control values of elevated levels of lipid peroxidation ↑ activity of SOD and of the translated products of SOD1 and SOD2 | [63] |
| rat | renal cortex | modulation of antioxidant enzymes (i.e., reduced GSH and ascorbic acid, SOD, CAT) and OS parameters (i.e., lipid peroxidation and protein carbonylation) | restored control values levels of OS parameters and small antioxidant molecules ↑ SOD and CAT | [64] |
| rat | liver | lipid peroxidation and antioxidant genes expression | alleviation of lipid peroxidation and up-regulation of antioxidant genes | [65,66,67] |
| Species | Antioxidant Compound Administered | Tested Effect | Results | Reference |
|---|---|---|---|---|
| mouse | quercetin | thyroid function, hepatic activity and peroxidation, antioxidant enzymes activity | ↓ T3 and T4 levels; ↓ hepatic activity of glucose-6- phosphatase (G-6-Pase) 5 ‘-monodeiodinase and hepatic lipid peroxidation SOD and CAT activity | [59] |
| rat | rutin (glycoside of quercetin) | thyroid function, antioxidant enzymes activity | no changes in TSH; slight ↓ T3 and T4 levels; significant ↓ hepatic DIO1 activity ↑ in hypothalamic, pituitary and brown adipose tissues DIO2 activity | [61] |
| rat | rutin | activity of SLC5A5 and TSHR genes | up-regulation | [61] |
| Model | Investigated Aspects | Results | Reference |
|---|---|---|---|
| in vitro (normal rat thyroid cells) | NIS gene expression and iodide uptake | Inhibition of both | [67] |
| in vitro (immortalized cell line of untransformed rat thyrocytes, FRTL-5) | NIS gene expression and iodide uptake | ↓ sodium/iodide symporter rna and protein expression as a function of time; ↓ cellular iodide uptake | [68] |
| in vivo (Sprague-Dawley rat) | NIS gene expression and thyroid function | Inhibition of both | [68] |
| Species | Organ | Investigated Effect | Results | References |
|---|---|---|---|---|
| rat | renal cortex | oxidative stress parameters (lipid peroxidation and protein carbonylation) and small antioxidant molecules (reduced glutathione and ascorbic acid); antioxidant enzymes activities (i.e., SOD, CAT); translated product of Cu/Zn-SOD, Mn-SOD | Restoration of oxidative stress parameters and small antioxidant molecules levels; ↓ in translated product of Cu/Zn-SOD, Mn-SOD and CAT | [64] |
| rat | liver | hepatic function; hepatic complexes I and II mediated respiration and hepatic oxidative stress (protein carbonylation) | amelioration of hepatic function; ↓ state 4 respiration of complex I; ↑ complex II respiration both at state 3 and state 4 level; ↓ protein carbonylation | [65] |
| rat | liver | antioxidant genes (AOG) expression | alleviated message levels of SOD and CAT; ameliorated GPx1 and GR mRNA levels; alleviate SOD1, CAT and GR translated products; normalization mitochondrial SOD1 | [66] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Candellone, A.; Saettone, V.; Badino, P.; Girolami, F.; Radice, E.; Bergero, D.; Odore, R.; Meineri, G. Management of Feline Hyperthyroidism and the Need to Prevent Oxidative Stress: What Can We Learn from Human Research? Antioxidants 2021, 10, 1496. https://doi.org/10.3390/antiox10091496
Candellone A, Saettone V, Badino P, Girolami F, Radice E, Bergero D, Odore R, Meineri G. Management of Feline Hyperthyroidism and the Need to Prevent Oxidative Stress: What Can We Learn from Human Research? Antioxidants. 2021; 10(9):1496. https://doi.org/10.3390/antiox10091496
Chicago/Turabian StyleCandellone, Alessia, Vittorio Saettone, Paola Badino, Flavia Girolami, Elisabetta Radice, Domenico Bergero, Rosangela Odore, and Giorgia Meineri. 2021. "Management of Feline Hyperthyroidism and the Need to Prevent Oxidative Stress: What Can We Learn from Human Research?" Antioxidants 10, no. 9: 1496. https://doi.org/10.3390/antiox10091496
APA StyleCandellone, A., Saettone, V., Badino, P., Girolami, F., Radice, E., Bergero, D., Odore, R., & Meineri, G. (2021). Management of Feline Hyperthyroidism and the Need to Prevent Oxidative Stress: What Can We Learn from Human Research? Antioxidants, 10(9), 1496. https://doi.org/10.3390/antiox10091496










