Effects of pH and Temperature on Water under Pressurized Conditions in the Extraction of Nutraceuticals from Chaga (Inonotus obliquus) Mushroom
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Preparation of Chaga Sporocarp Extracts
2.3. Total Antioxidant Activity (TAA) Analysis
2.4. Total Phenolic Content (TPC) Analysis
2.5. B-Vitamin (B1, B2, B3, B5, B6, B9, and B12) Analysis by Ultra-High-Pressure Liquid Chromatography-Mass Spectrometry (UHPLC-MS)
2.6. Fat-Soluble Vitamins (A, E, D3, K1, K2, E) Analysis by UHPLC-HRAM-MS/MS
2.7. Phenolic Compounds Analysis by UHPLC-HRAM-MS/MS
2.8. Extraction and Analysis of Minerals by Inductively Coupled Plasma-Mass Spectrometry (ICP-MS)
2.9. Statistical Analysis
3. Results and Discussion
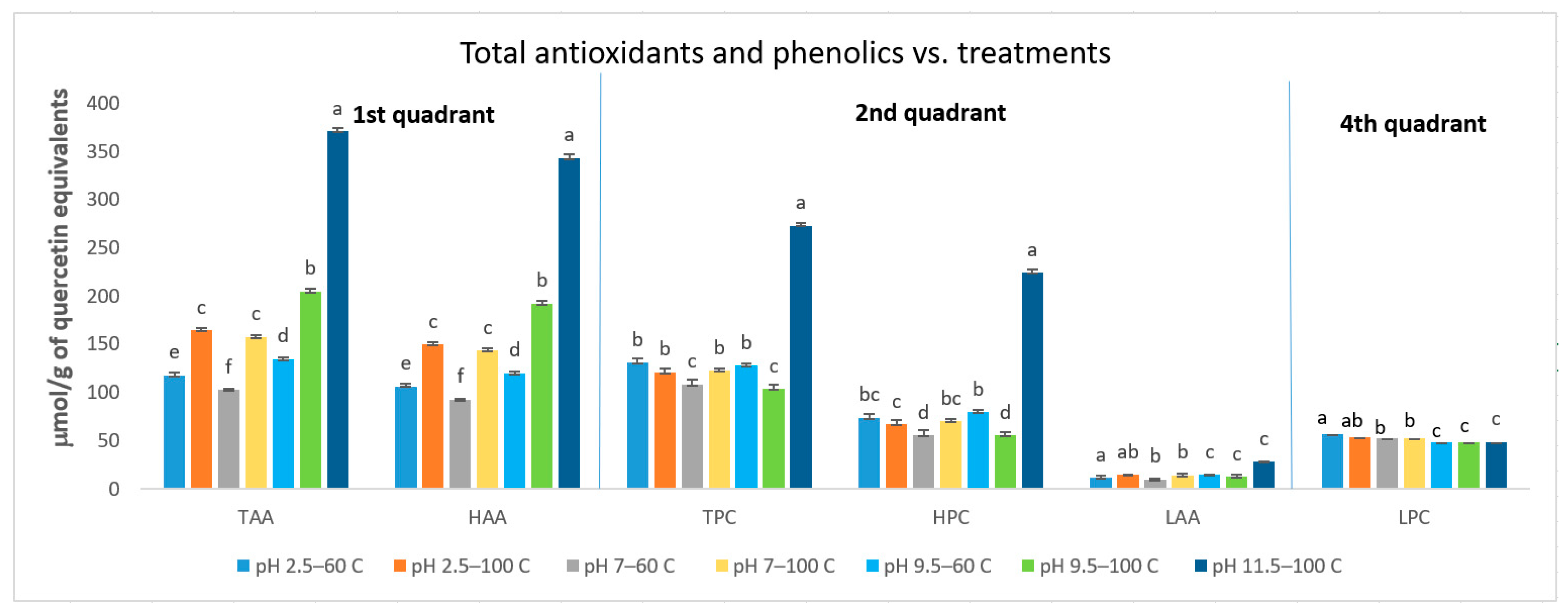
3.1. Total Polyphenols Content (TPC) and Total Antioxidant Activity (TAA)
3.2. Chaga Extracts Composition
3.2.1. Minerals
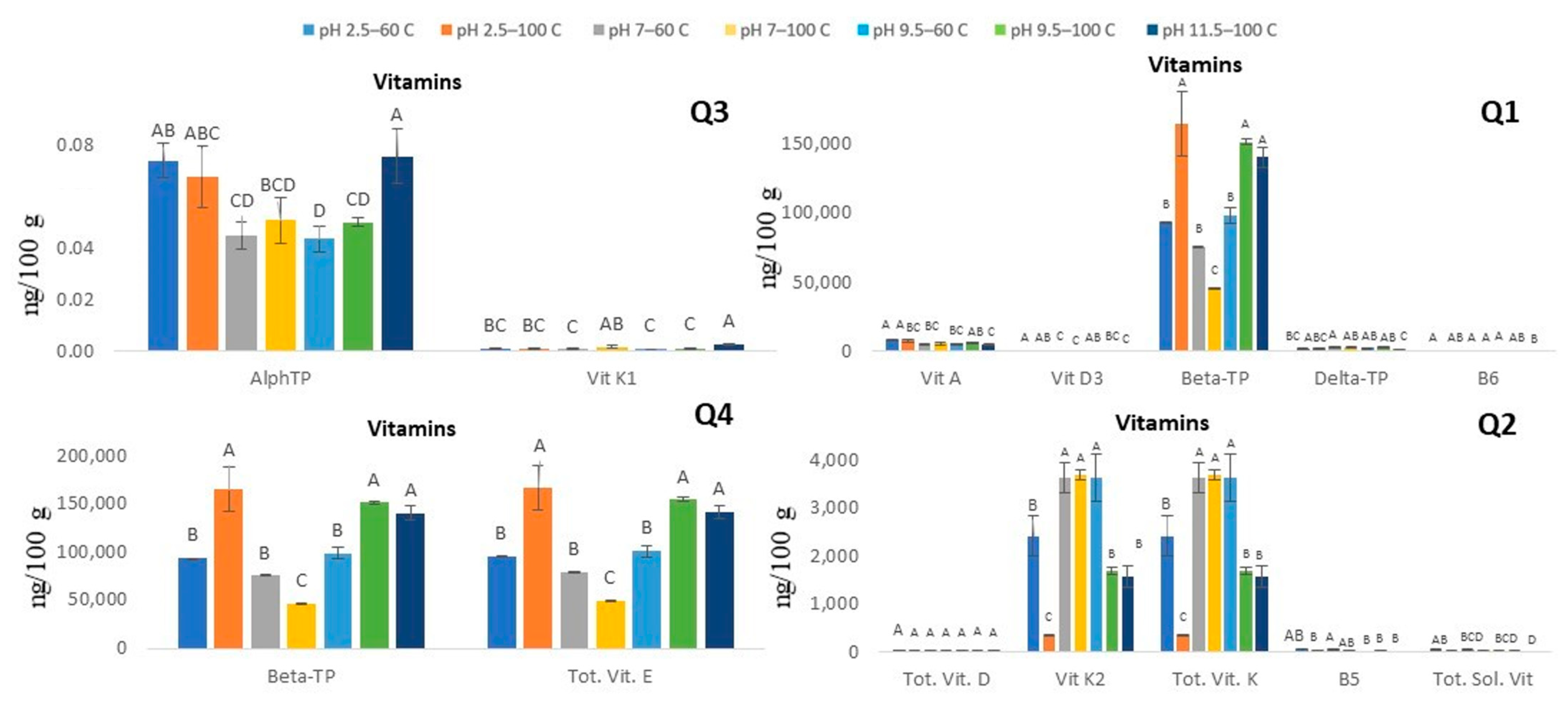
3.2.2. Vitamins (Water- and Fat-Soluble)
3.2.3. Phenolics
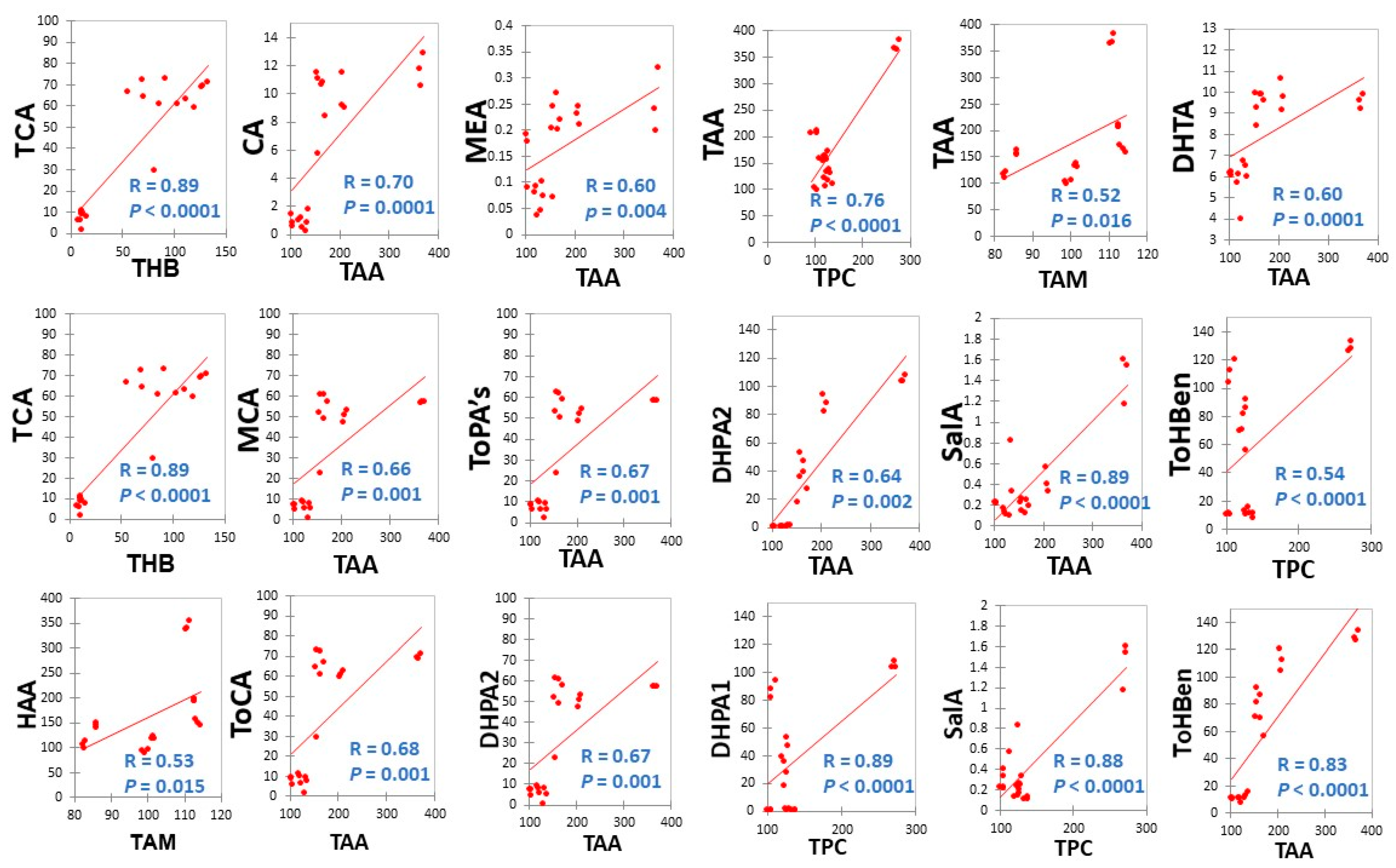
3.2.4. Correlations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ismail, A.; Marjan, Z.; Foong, C. Total antioxidant activity and phenolic content in selected vegetables. Food Chem. 2004, 87, 581–586. [Google Scholar] [CrossRef]
- Valverde, E.M.; Hernández-Pérez, T.; Paredes-López, O. Edible Mushrooms: Improving Human Health and Promoting Quality Life. Int. J. Microbiol. 2015, 2015, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Lindequist, U.; Niedermeyer, T.H.J.; Jülich, W.-D. The pharmacological potential of mushrooms. eCAM 2005, 2, 285–299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duru, K.C.; Kovaleva, E.G.; Danilova, I.G.; van der Bijl, P. The pharmacological potential and possible molecular mechanisms of action of Inonotus obliquus from preclinical studies. Phytother. Res. 2019, 33, 1966–1980. [Google Scholar] [CrossRef] [PubMed]
- Rhee, S.J.; Cho, S.Y.; Kim, K.M.; Cha, D.-S.; Park, H.-J. A comparative study of analytical methods for alkali-soluble β-glucan in medicinal mushroom, Chaga (Inonotus obliquus). LWT Food Sci. Technol. 2008, 41, 545–549. [Google Scholar] [CrossRef]
- Agnestisia, R.; Ono, A.; Nakamura, L.; Chino, R.; Nodera, K.; Aiso-Sanada, H.; Nezu, I.; Ishiguri, F.; Suzuki, T.; Yokota, S. The complete mitochondrial genome sequence of the medicinal fungus Inonotus obliquus (Hymenochaetaceae, Basidiomycota). mtDNA 2019, 4, 3504–3506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lyle, K.L. Complete Guide to Edible Wild Plants, Mushrooms. In Fruits, and Nuts: How to Find, Identify, and Cook Them: Falcon Guides, 2nd ed.; Lyons Press: Guilford, CT, USA, 2010; pp. 58–59. [Google Scholar]
- Lee, M.-W.; Hur, H.; Chang, K.-C.; Lee, T.-S.; Ka, K.-H.; Jankovsky, L. Introduction to Distribution and Ecology of Sterile Conks of Inonotus obliquus. Mycobio 2008, 36, 199–202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, H.S.; Kim, E.J.; Kim, S.H. Ethanol extract of Innotus obliquus (Chaga mushroom) induces G1 cell cycle arrest in HT-29 human colon cancer cells. Nutr. Res. Pract. 2015, 9, 111–116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, Y.; Teng, C.; Yu, S.; Wang, X.; Liang, J.; Bai, X.; Dong, L.; Song, T.; Yu, M.; Qu, J. Inonotus obliquus polysaccharide regulates gut microbiota of chronic pancreatitis in mice. AMB Express 2017, 7, 39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kozarski, M.; Klaus, A.; Jakovljevic, D.; Todorovic, N.; Vunduk, J.; Petrović, P.; Niksic, M.; Vrvic, M.M.; van Griensven, L. Antioxidants of Edible Mushrooms. Molecules 2015, 20, 19489–19525. [Google Scholar] [CrossRef] [Green Version]
- Cui, Y.; Kim, D.-S.; Park, K.-C. Antioxidant effect of Inonotus obliquus. J. Ethnopharmacol. 2005, 96, 79–85. [Google Scholar] [CrossRef]
- Jayachandran, M.; Xiao, J.; Xu, B. A Critical Review on Health Promoting Benefits of Edible Mushrooms through Gut Microbiota. Int. J. Mol. Sci. 2017, 18, 1934. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.-J.; Li, Y.; Zhou, T.; Xu, D.-P.; Zhang, P.; Li, S.; Li, H.-B. Bioactivities and Health Benefits of Mushrooms Mainly from China. Molecules 2016, 21, 938. [Google Scholar] [CrossRef] [Green Version]
- Wolfe, D.; Beaumier, P.; Saad, R. Chaga: King of the Medicinal Mushrooms; North Atlantic Books: Berkeley, CA, USA, 2012; pp. 153–154. [Google Scholar]
- Shashkina, M.Y.; Shashkin, P.N.; Sergeev, A.V. Chemical and medicobiological properties of Chaga (review). Pharm. Chem. J. 2006, 40, 560–568. [Google Scholar] [CrossRef]
- Zheng, W.; Zhang, M.; Zhao, Y.; Miao, K.; Pan, S.; Cao, F.; Dai, Y. Analysis of antioxidant metabolites by solvent extraction from sclerotia of Inonotus obliquus (Chaga). Phytochem. Anal. 2011, 22, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Sharpe, E.; Farragher-Gnadt, A.; Igbanugo, M.; Huber, T.; Michelotti, J.; Milenkowic, A.; Ludlam, S.; Walker, M.; Hanes, D.; Bradley, R.; et al. Comparison of antioxidant activity and extraction techniques for commercially and laboratory prepared extracts from six mushroom species. J. Sci. Food Agric. 2021, 4, 100130. [Google Scholar]
- Cvjetko Bubalo, M.; Vidović, S.; Radojčić Redovniković, I.; Jokić, S. New perspective in extraction of plant biologically active compounds by green solvents. Food Bioprod. Process. 2018, 109, 52–73. [Google Scholar] [CrossRef]
- Vuong, Q.V.; Roach, P.D. Caffeine in Green Tea: Its Removal and Isolation. Sep. Purif. Rev. 2014, 43, 155–174. [Google Scholar] [CrossRef]
- Géry, A.; Dubreule, C.; André, V.; Rioult, J.-P.; Bouchart, V.; Heutte, N.; Eldin de Pécoulas, P.; Krivomaz, T.; Garon, D. Chaga (Inonotus obliquus), a Future Potential Medicinal Fungus in Oncology? A Chemical Study and a Comparison of the Cytotoxicity Against Human Lung Adenocarcinoma Cells (A549) and Human Bronchial Epithelial Cells (BEAS-2B). Integr. Cancer Ther. 2018, 17, 832–843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thaipong, K.; Boonprakob, U.; Cisneros-Zevallos, L.; Byrne, D. Hydrophilic and lipophilic antioxidant activities of guava fruits. Southeast Asian J. Trop Med. 2005, 36, 254–257. [Google Scholar]
- Thomas, R.; Bernards, M.; Drake, E.; Guglielmo, C. Changes in the antioxidant activities of seven herb- and spice-based marinating sauces after cooking. J. Food Compos. Anal. 2010, 23, 244–252. [Google Scholar] [CrossRef]
- Akhavan, H.; Barzegar, M.; Weidlich, H.; Zimmermann, B.F. Phenolic Compounds and Antioxidant Activity of Juices from Ten Iranian Pomegranate Cultivars Depend on Extraction. J. Chem. 2015, 2015, 7. [Google Scholar] [CrossRef]
- Ramadan, M.; Mörsel, J.-T. Direct isocratic normal-phase HPLC assay of fat-soluble vitamins and β-carotene in oilseeds. Eur. Food Res. Technol. 2002, 214, 521–527. [Google Scholar] [CrossRef]
- Chaturvedi, V.K.; Agarwal, S.; Gupta, K.K.; Ramteke, P.W.; Singh, M.P. Medicinal mushroom: Boon for therapeutic applications. Biotech 2018, 8, 334. [Google Scholar] [CrossRef]
- Hwang, A.; Yang, S.; Kim, J.; Lim, T.; Cho, H.; Hwang, K. Effects of non-traditional extraction methods on extracting bioactive compounds from Chaga mushroom (Inonotus obliquus) compared with hot water extraction. LWT 2019, 110, 80–84. [Google Scholar] [CrossRef] [Green Version]
- Seo, H.-K.; Lee, S.-C. Antioxidant Activity of Subcritical Water Extracts from Chaga Mushroom (Inonotus obliquus). Sep. Sci. Technol. 2010, 45, 198–203. [Google Scholar] [CrossRef]
- Kabtni, S.; Sdouga, D.; Bettaib Rebey, I.; Save, M.; Trifi-Farah, N.; Fauconnier, M.; Marghali, S. Influence of climate variation on phenolic composition and antioxidant capacity of Medicago minima populations. Sci. Rep. 2020, 10, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Rathee, S.; Rathee, D.; Rathee, D.; Kumar, V.; Rathee, P. Mushrooms as therapeutic agents. Rev. Bras. Farmacogn. 2012, 22, 459–474. [Google Scholar] [CrossRef]
- Institute of Medicine (US) Panel on Micronutrients. Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc; National Academies Press (US): Washington, DC, USA, 2001; pp. 150–237. Available online: https://www.ncbi.nlm.nih.gov/books/NBK222322/ (accessed on 1 July 2021).
- World Health Organization. Vitamin and Mineral Requirements in Human Nutrition; Report of a Joint FAO/WHO Expert Consultation, Bangkok, Thailand, 21–30 September 1998; Sun Fung: Hong Kong, China, 2005. [Google Scholar]
- Tao, S.; Michael Bolger, P. Dietary arsenic intakes in the United States: FDA Total Diet Study, September 1991–December 1996. Food Addit. Contam. 1999, 16, 465–472. [Google Scholar] [CrossRef]
- Plaza, M.; Turner, C. Pressurized hot water extraction of bioactives. Trac. Trends Anal. Chem. 2015, 71, 39–54. [Google Scholar] [CrossRef] [Green Version]
- Carr, A.G.; Mammucari, R.; Foster, N.R. A review of subcritical water as a solvent and its utilisation for the processing of hydrophobic organic compounds. Chem. Eng. J. 2011, 172, 1–17. [Google Scholar] [CrossRef]
- Jiang, Q. Natural forms of vitamin E: Metabolism, antioxidant, and anti-inflammatory activities and their role in disease prevention and therapy. Free Radic. Biol. Med. 2014, 72, 76–90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jäpelt, R.B.; Jakobsen, J. Vitamin D in plants: A review of occurrence; analysis, and biosynthesis. Front. Plant Sci. 2013, 4, 136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Zhang, J.; Wu, L.; Zhao, Y.; Li, T.; Li, J.; Wang, Y.; Liu, H. A mini-review of chemical composition and nutritional value of edible wild-grown mushroom from China. Food Chem. 2014, 151, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Yu, Y.; Chen, J.; Guo, B.; Yang, L.; Ding, W. Evaluation of the Antibacterial Effects and Mechanism of Action of Protocatechualdehyde against Ralstonia solanacearum. Molecules 2016, 21, 754. [Google Scholar] [CrossRef] [Green Version]
- Filho, A.; Rodrigues, P.; Benjamin, S.; Paim, R.; Holanda, M.; Silva, J.; Milo, T.S.; Vieira, I.G.P.; Queiroz, M.G.R.; Guedes, M.I.F. Hypolipidemic activity of P-methoxycinnamic diester (PCO-C) isolated from Copernicia prunífera against Triton WR-1339 and hyperlipidemic diet in mice. Environ. Toxicol. Pharmacol. 2017, 56, 198–203. [Google Scholar] [CrossRef]
- Freitas, C.; Vieira, Í.; Sousa, P.; Muniz, C.; Gonzaga, M.; Guedes, M. Carnauba wax p-methoxycinnamic diesters: Characterisation, antioxidant activity and simulated gastrointestinal digestion followed by in vitro bioaccessibility. Food Chem. 2016, 196, 1293–1300. [Google Scholar] [CrossRef] [Green Version]
- Adisakwattana, S.; Roengsamran, S.; Hsu, W.; Yibchok-anun, S. Mechanisms of antihyperglycemic effect of p-methoxycinnamic acid in normal and streptozotocin-induced diabetic rats. Life Sci. 2005, 78, 406–412. [Google Scholar] [CrossRef]
- Ferreres, F.; Grosso, C.; Gil-Izquierdo, A.; Valentão, P.; Andrade, P. Ellagic Acid and Derivatives from Cochlospermum angolensis Welw. Extracts: HPLC-DAD-ESI/MSnProfiling, Quantification and in vitro Anti-depressant, Anti-cholinesterase and Antioxidant Activities. Phytochem. Anal. 2013, 24, 534–540. [Google Scholar] [CrossRef]
- Mirończuk-Chodakowska, I.; Socha, K.; Zujko, M.; Terlikowska, K.; Borawska, M.; Witkowska, A. Copper, Manganese, Selenium and Zinc in Wild-Growing Edible Mushrooms from the Eastern Territory of “Green Lungs of Poland”: Nutritional and Toxicological Implications. Int. J. Environ. Res. Public Health 2019, 16, 3614. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| pH Treatment and Temperature ** | ||||||||
|---|---|---|---|---|---|---|---|---|
| Classification of Minerals | pH 2.5 at 60 °C | pH 7 at 60 °C | pH 9.5 at 60 °C | pH 2.5 at 100 °C | pH 7 at 100 °C | pH 9.5 at 100 °C | pH 11.5 at 100 °C | RDAs * |
| Antioxidant minerals | ||||||||
| 55Mn | 9.85 ± 0.04 a | 0.53 ± ND b | 0.38 ± 0.01 c | 0.34 ± 0.01 d | 0.28 ± 0.14 e | 0.11 ± 0.08 f | ND *** | 2 (mg) |
| 63Cu | 2.10 ± 0.15 f | 3.84 ± 0.25 d | 4.24 ± 0.08 c | 4.27 ± 0.19 c | 3.45 ± 0.31 e | 4.38 ± 0.78 b | 4.82 ± 0.04 a | 1 (mg) |
| 64Zn | 67.37 ± 1.29 g | 91.86 ± 4.90 e | 93.20 ± 2.53 d | 105.87 ± 3.05 a | 79.75 ± 0.42 f | 104.75 ± 0.37 b | 103.05 ± 2.84 c | 10 (mg) |
| 80Se | 3.46 ± 0.07 ab | 3.05 ± 1.76 c | 3.63 ± 0.42 a | 3.37 ± 0.93 b | 2.42 ± 0.18 d | 3.51 ± 0.46 ab | 3.04 ± 0.11 c | 55 (µg) |
| Macrominerals | ||||||||
| 23Na | 1439.35 ± 5.91 c | 1406.28 ± 5.16 d | 1414.55 ± 11.03 cd | 1612.63 ± 1.21 a | 1228.21 ± 0.68 e | 1583.92 ± 2.11 ab | 1580.22 ± 23.89 b | 800 (mg) |
| 24Mg | 787.45 ± 2.15 d | 1028.89 ± 1.58 b | 1036.58 ± 1.35b | 1155.43 ± 10.34 a | 891.44 ± 1.24 c | 1159.06 ± 7.20 a | 1161.53 ± 6.87 a | 375 (mg) |
| 31P | 896.61± 0.60 a | 339.63 ± ND*** c | 335.85 ± 15.82 c | 370.48 ± 2.40 b | 290.58 ± 0.46 d | 378.80 ± 9.31 b | 335.65 ± 3.00 c | 700 (mg) |
| 39K | 2004.07 ± 19.96 a | 940.47 ± 4.41 e | 1026.71 ± 9.75 d | 1853.77 ± 27.08 b | 902.02 ± 7.62 e | 1211.62 ± 2.28 c | 899.74 ± 4.81 e | 2000 (mg) |
| 44Ca | 480.10 ± 7.83 d | 660.32 ± 3.67 b | 671.57 ± 3.90 b | 745.19 ± 6.58 a | 577.58 ± 5.58 c | 756.34 ± 11.04 a | 750.47 ± 3.73 a | 800 (mg) |
| Trace Minerals | ||||||||
| 11B | 64.25 ± 17.44 a | 11.25 ± 2.57 c | 8.97 ± 3.86 d | 12.81 ± 5.68 bc | 10.21 ± 0.36 d | 15.26 ± 11.78 b | 13.84 ± ND b | |
| 52Cr | 3.94 ± 0.49 | ND | ND | ND | ND | ND | ND | 40 (µg) |
| Toxic Minerals | ||||||||
| 75As | 1.63 ± 0.04 a | 0.94 ± 0.01 d | 0.97 ± 0.02 c | 1.03 ± 0.01 b | 0.81 ± 0.03 e | 1.04 ± 0.09 b | 1.04 ± 0.01 b | |
| Compounds | Formula | MW (g/mol) | m/z [M − H]− | RT (min) | Amount Recovered (µg/mL PA eq.) | Structure |
|---|---|---|---|---|---|---|
| Hydroxybenzoic acids | ||||||
| Gallic acid_1 Gallic acid_2 Gallic acid_3 | C7H6O5 | 170.12 | 169.0142 | 11.12 13.35 14.74 | 0.26 0.20 0.11 | 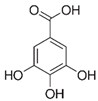 |
| Protocatechuic acid | C7H6O4 | 154.12 | 153.0193 | 16.90 | 32.21 | 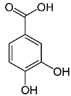 |
| Salicylic acid | C7H6O3 | 138.122 | 137.0244 | 29.71 | 1.44 | 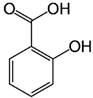 |
| Vanillic acid_1 Vanillic acid_2 Vanillic acid_3 Vanillic acid_4 | C8H8O4 | 168.148 | 167.0350 | 19.48 | 0.47 0.45 0.33 0.18 | 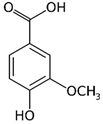 |
| 2,3-Dihydroxybenzaldehyde DHBA1 | C7H6O3 | 138.12 | 137.0244 | 18.28 | 104.85 | 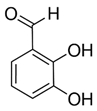 |
| 2,5-Dihydroxyterephthalic acid (DHTA) | C8H6O6 | 198.13 | 197.0092 | 16.00 | 9.83 | 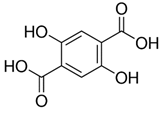 |
| Hydroxycinnamic acids | ||||||
| o-Coumaric acid p-Coumaric acid | C9H8O3 | 164.16 | 163.0401 | 18.11 22.91 | 0.22 0.24 | 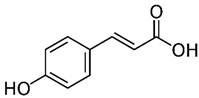 |
| Caffeic acid | C9H8O4 | 180.16 | 179.0349 | 19.99 | 11.72 | 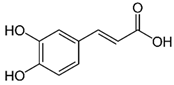 |
| 4-methoxycinnamic acid | C10H10O3 | 178.187 | 177.0557 | 21.11 | 0.57 | 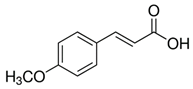 |
| Hispidin | C13H10O5 | 246.22 | 245.0455 | 18.81 | 0.45 | 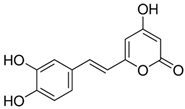 |
| Ferrulic acid (trans) Ferrulic acid_1 (cis) | C10H10O4 | 194.18 | 193.0506 | 21.90 17.10 | 0.40 0.18 | 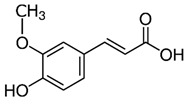 |
| Flavonoid derivatives | ||||||
| Isorhamnetin | C16H12O7 | 316.26 | 315.0510 | 23.34 | 0.47 | 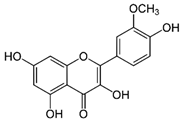 |
| Myricetin_1 Myricetin_2 | C15H10O8 | 318.24 | 317.0303 | 22.41 30.10 | 0.30 1.32 | 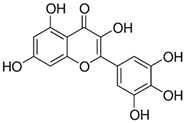 |
| Quercetin | C15H10O7 | 302.236 | 301.0354 | 30.27 | 0.54 | 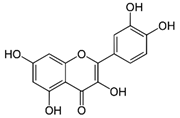 |
| Other phenolic acids | ||||||
| Syringic acid | C9H10O5 | 198.174 | 197.0455 | 17.30 | 1.14 |  |
| Ellagic acid | C14H6O8 | 302.197 | 300.9989 | 22.61 | 0.25 | 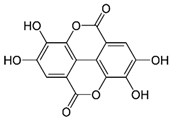 |
| Hispolon | C12H12O4 | 220.224 | 219.0663 | 18.72 | 0.03 | 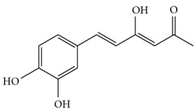 |
| 3,4-Dihydroxybenzalacetone(DHBA2) | C10H10O3 | 178.187 | 177.0557 | 19.03 | 57.17 | 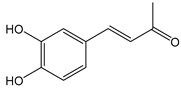 |
| 3-O-Methylellagic acid | C15H8O8 | 316.22 | 315.0146 | 25.55 | 0.25 |  |
| Total cinnamates | 69.65 | |||||
| Total Benzoate | 129.20 | |||||
| Total flavonoids | 1.80 | |||||
| Total other phenolics | 58.24 | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abu-Reidah, I.M.; Critch, A.L.; Manful, C.F.; Rajakaruna, A.; Vidal, N.P.; Pham, T.H.; Cheema, M.; Thomas, R. Effects of pH and Temperature on Water under Pressurized Conditions in the Extraction of Nutraceuticals from Chaga (Inonotus obliquus) Mushroom. Antioxidants 2021, 10, 1322. https://doi.org/10.3390/antiox10081322
Abu-Reidah IM, Critch AL, Manful CF, Rajakaruna A, Vidal NP, Pham TH, Cheema M, Thomas R. Effects of pH and Temperature on Water under Pressurized Conditions in the Extraction of Nutraceuticals from Chaga (Inonotus obliquus) Mushroom. Antioxidants. 2021; 10(8):1322. https://doi.org/10.3390/antiox10081322
Chicago/Turabian StyleAbu-Reidah, Ibrahim M., Amber L. Critch, Charles F. Manful, Amanda Rajakaruna, Natalia P. Vidal, Thu H. Pham, Mumtaz Cheema, and Raymond Thomas. 2021. "Effects of pH and Temperature on Water under Pressurized Conditions in the Extraction of Nutraceuticals from Chaga (Inonotus obliquus) Mushroom" Antioxidants 10, no. 8: 1322. https://doi.org/10.3390/antiox10081322
APA StyleAbu-Reidah, I. M., Critch, A. L., Manful, C. F., Rajakaruna, A., Vidal, N. P., Pham, T. H., Cheema, M., & Thomas, R. (2021). Effects of pH and Temperature on Water under Pressurized Conditions in the Extraction of Nutraceuticals from Chaga (Inonotus obliquus) Mushroom. Antioxidants, 10(8), 1322. https://doi.org/10.3390/antiox10081322










