EPR Study of KO2 as a Source of Superoxide and •BMPO-OH/OOH Radical That Cleaves Plasmid DNA and Detects Radical Interaction with H2S and Se-Derivatives
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals, EPR Sample Preparation, and Measurement
2.2. Plasmid DNA (pDNA) Cleavage Assay
3. Results
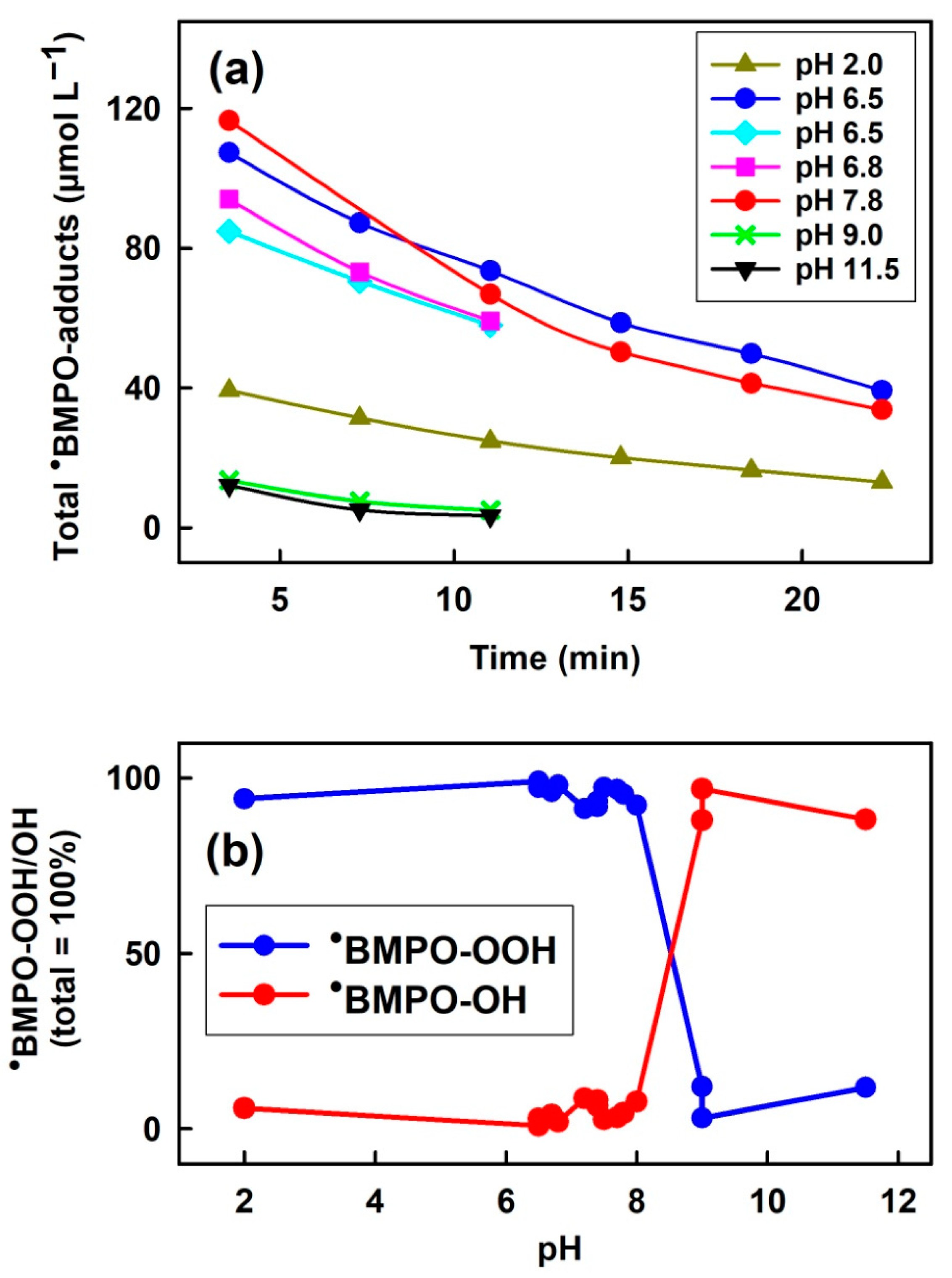
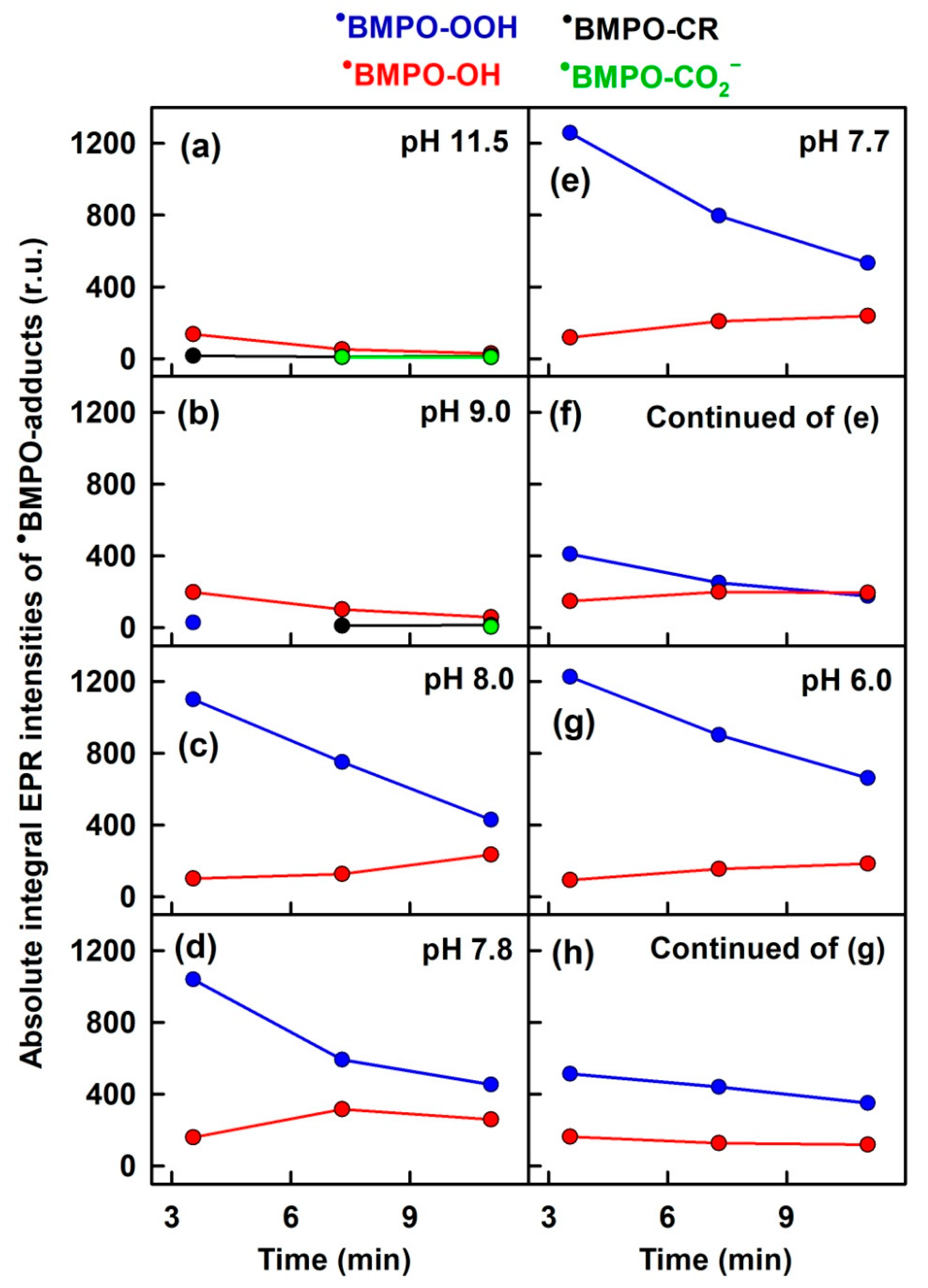
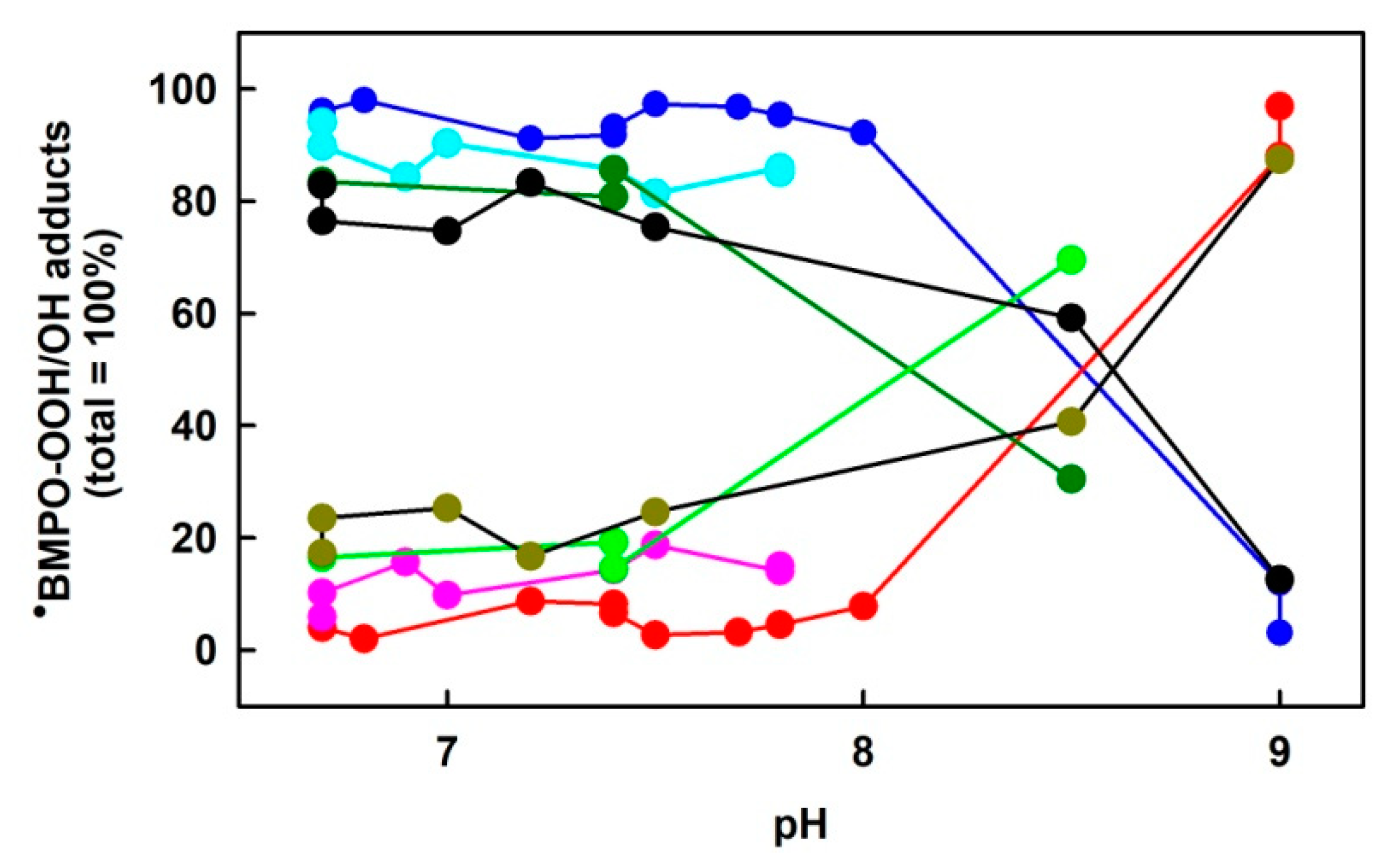
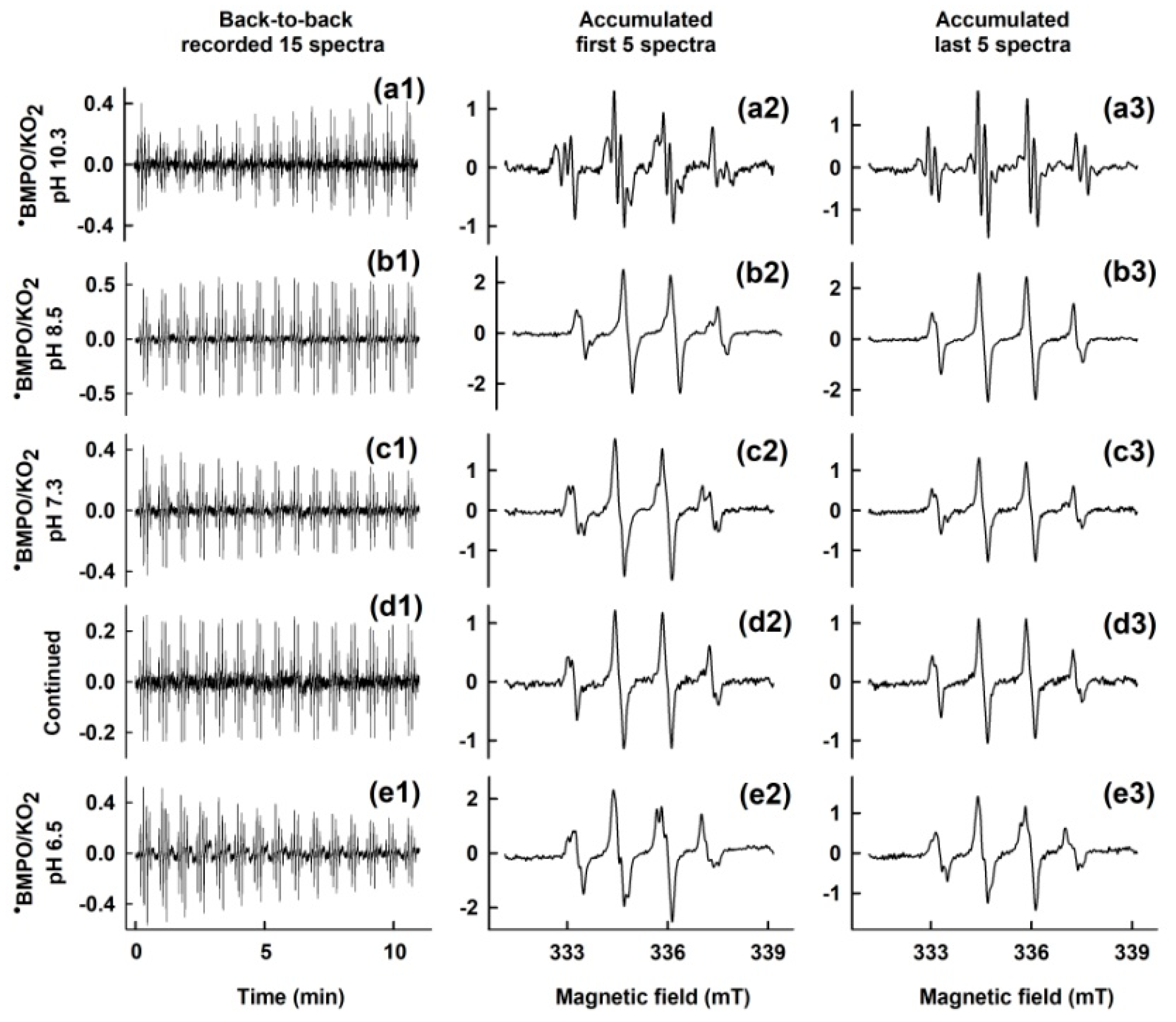
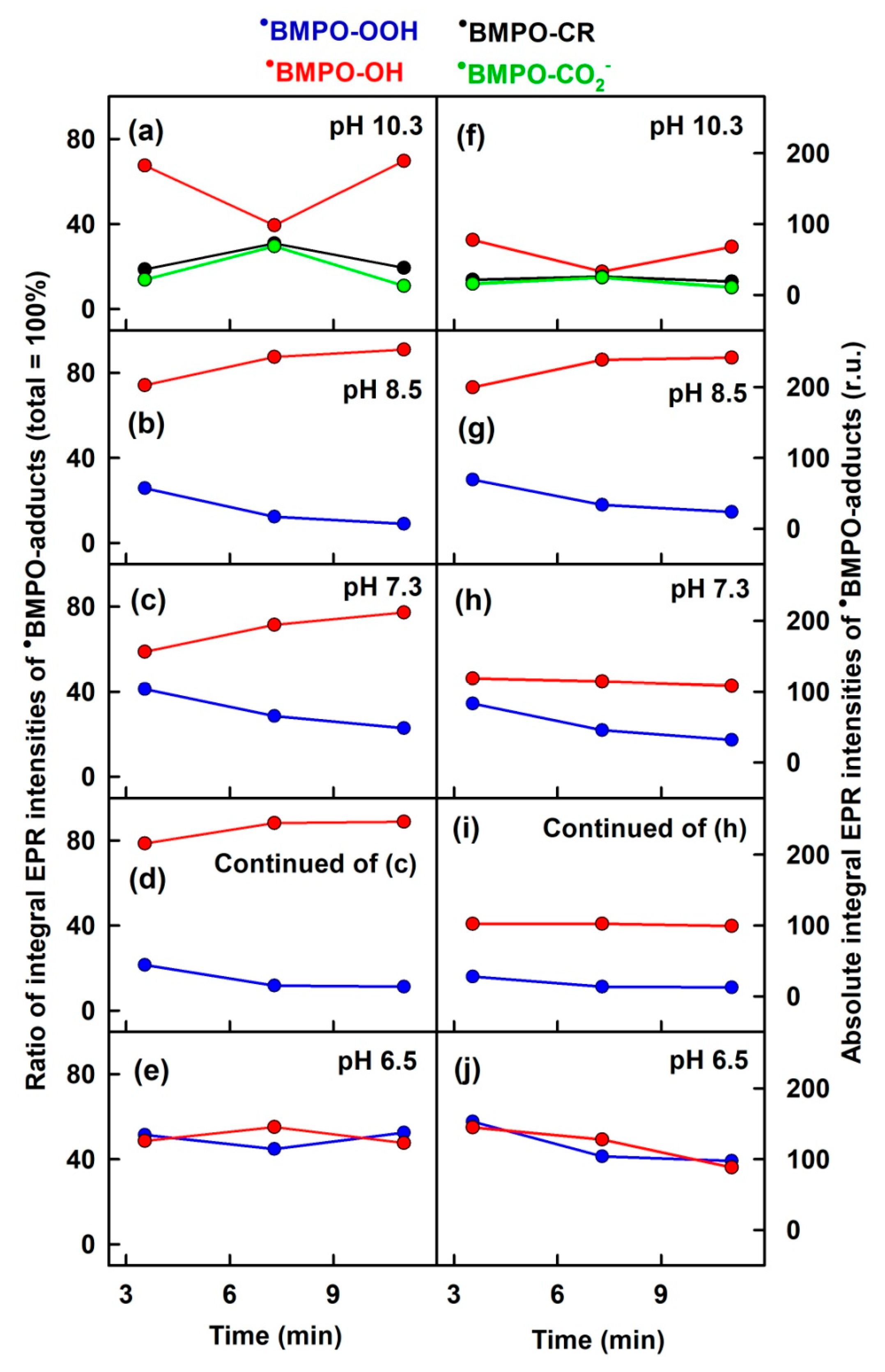
3.1. pH-Dependent Composition of •BMPO-Adducts of (BMPO+HCl)-KO2 Interaction
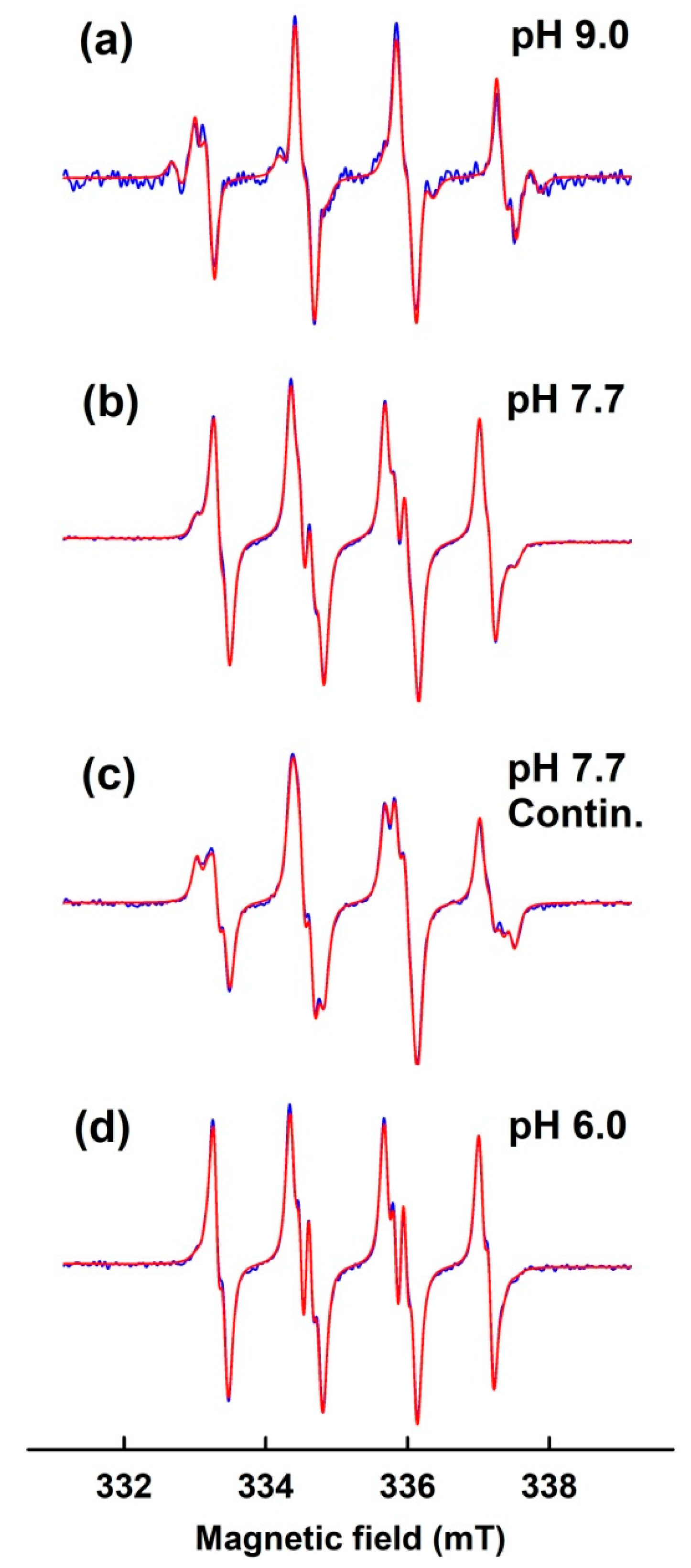
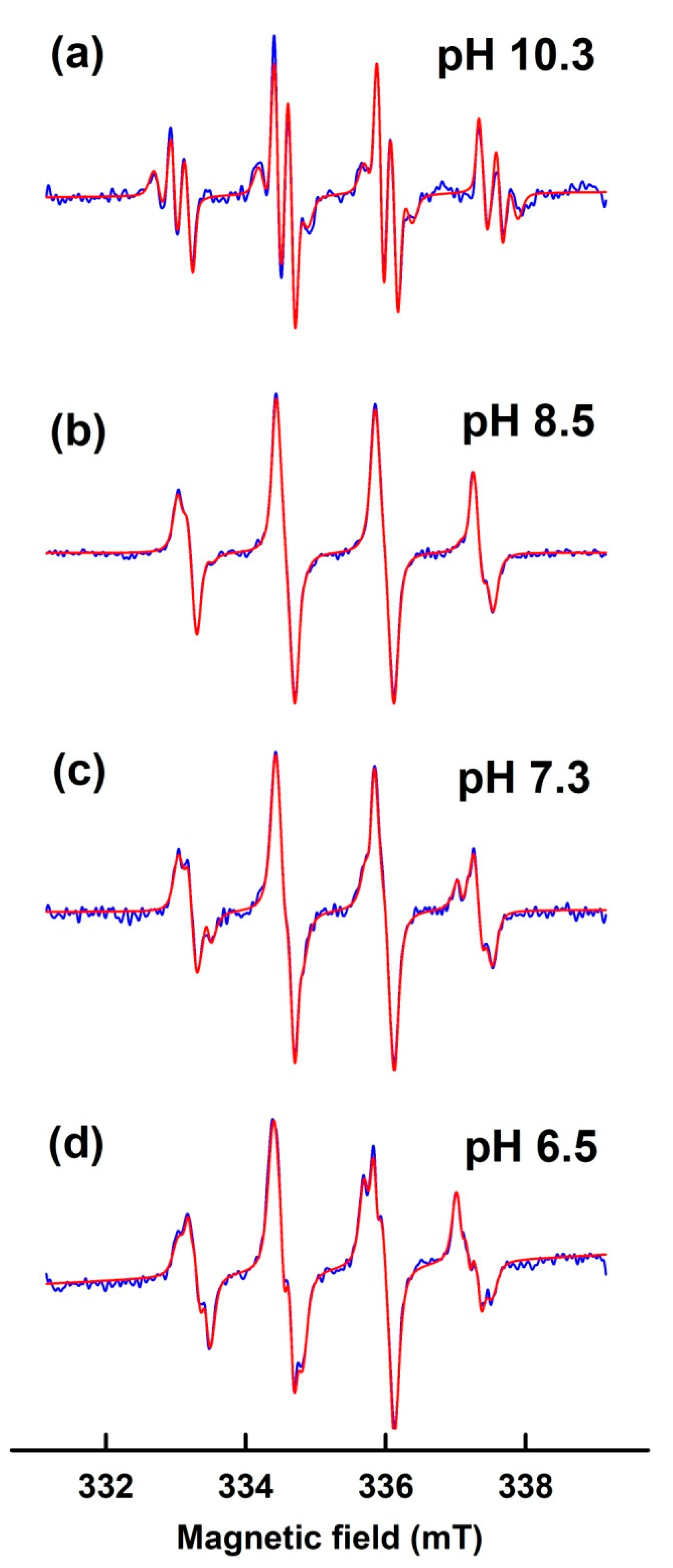
3.2. Simulation of EPR Spectra of pH-Dependent BMPO Adducts
3.3. Comparison of pH-Dependent BMPO Adduct Spectra of BMPO−KO2 Interaction
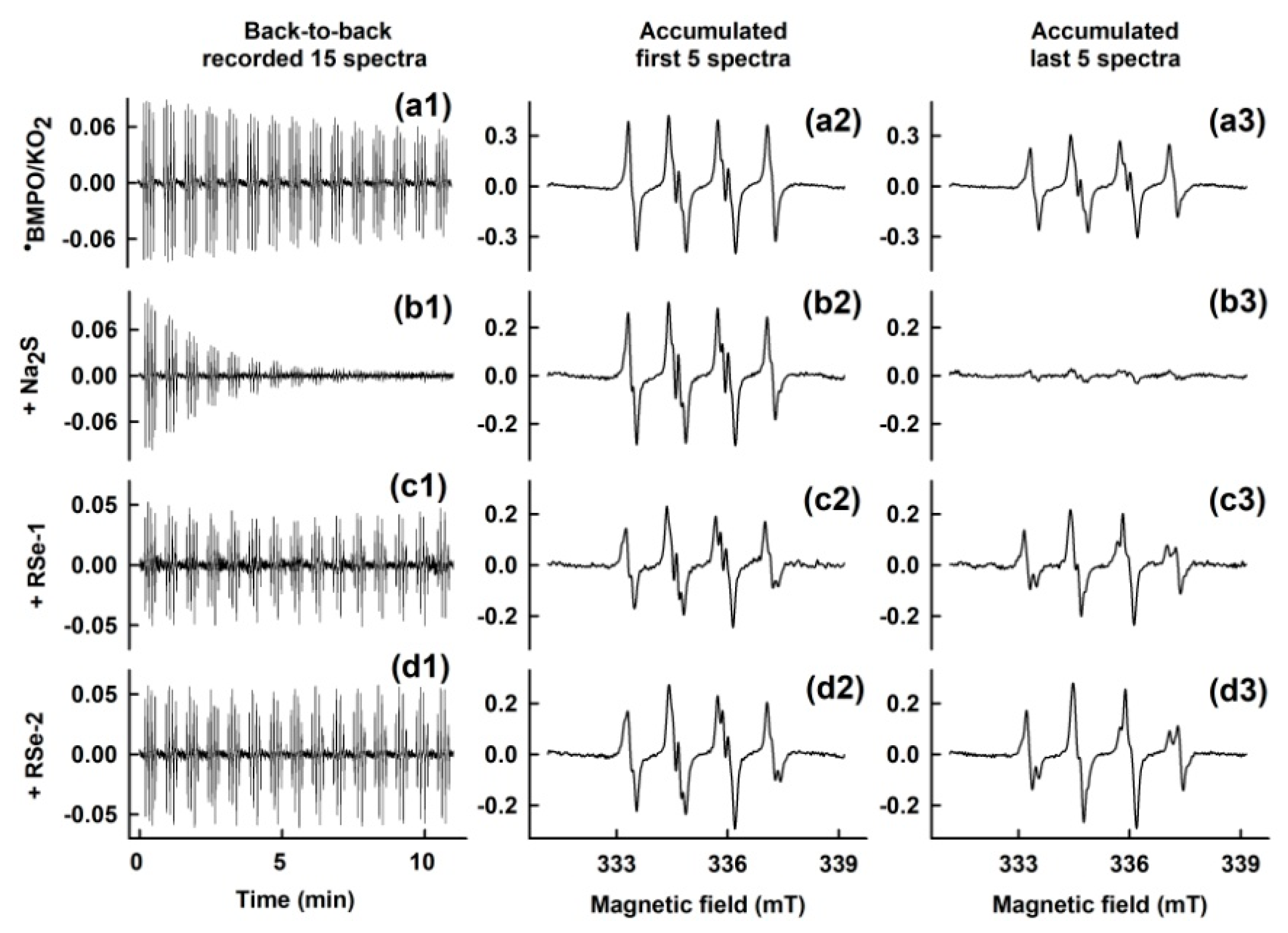
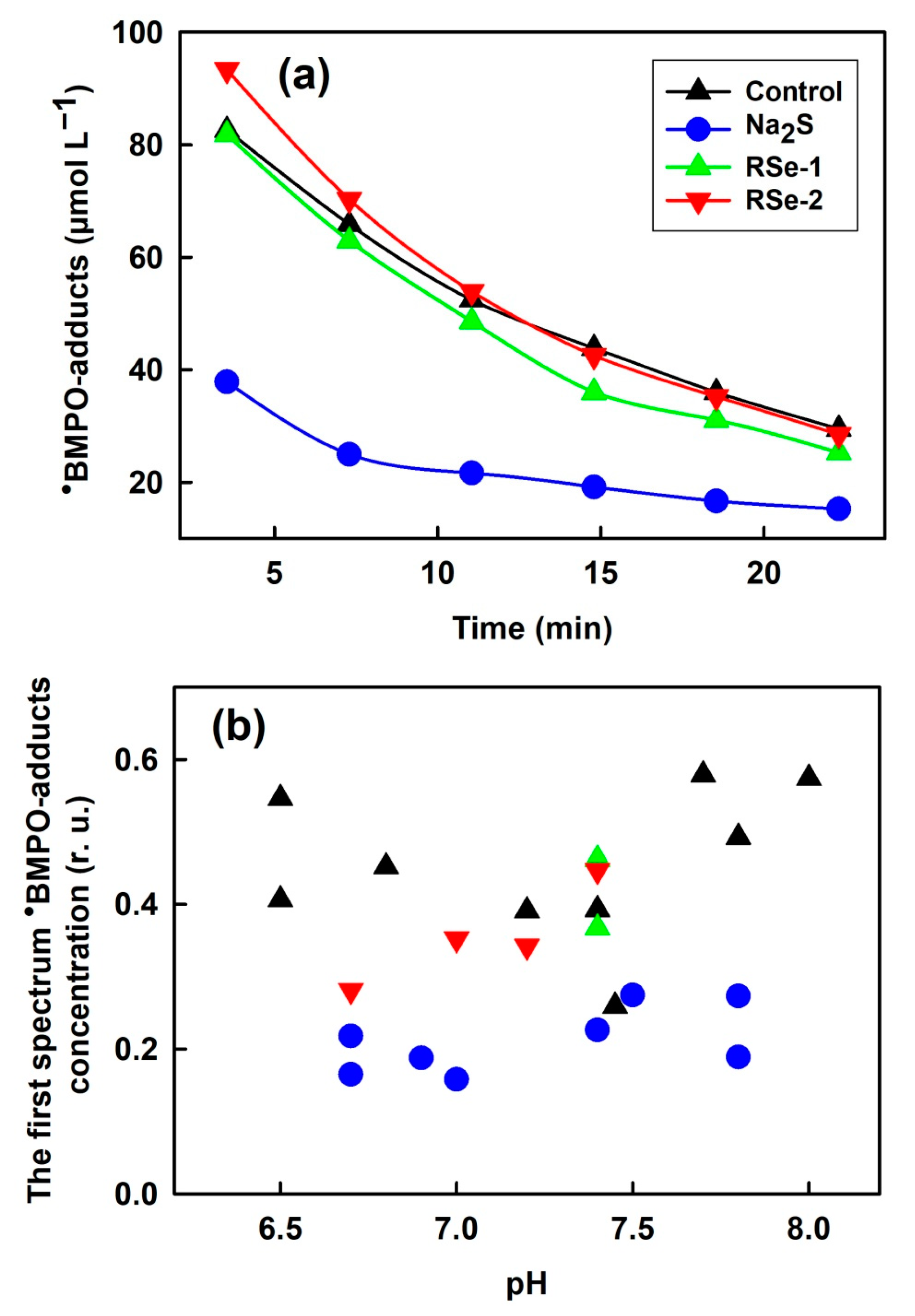
3.4. Effect of Na2S and Selenium Derivatives on •BMPO-OOH/OH Radicals
3.5. Composition of •BMPO Adducts of BMPO−KO2 Mixture after Addition of HCl
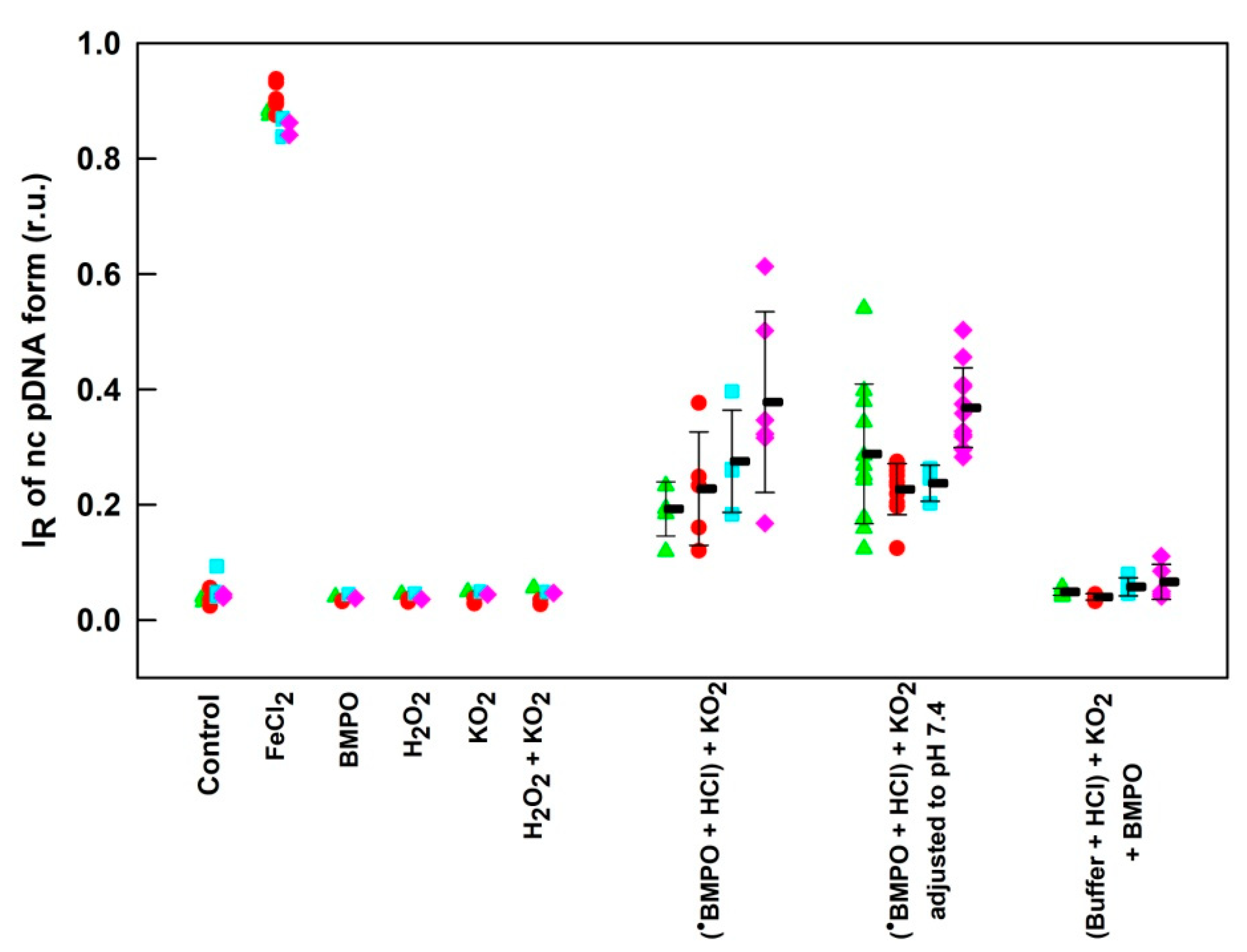
3.6. pH-Dependent Cleavage of pDNA by BMPO–KO2 Interaction
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Carroll, L.; Pattison, D.I.; Davies, J.B.; Anderson, R.F.; Lopez-Alarcon, C.; Davies, M.J. Superoxide radicals react with peptide-derived tryptophan radicals with very high rate constants to give hydroperoxides as major products. Free Radic. Biol. Med. 2018, 118, 126–136. [Google Scholar] [CrossRef]
- Chiste, R.C.; Freitas, M.; Mercadante, A.Z.; Fernandes, E. Superoxide Anion Radical: Generation and Detection in Cellular and Non-Cellular Systems. Curr. Med. Chem. 2015, 22, 4234–4256. [Google Scholar] [CrossRef] [PubMed]
- Hayyan, M.; Hashim, M.A.; AlNashef, I. Superoxide Ion: Generation and Chemical Implications. Chem. Rev. 2016, 116, 3029–3085. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, H.; Joseph, J.; Zhang, H.; Karoui, H.; Kalyanaraman, B. Synthesis and biochemical applications of a solid cyclic nitrone spin trap: A relatively superior trap for detecting superoxide anions and glutathiyl radicals. Free Radic. Biol. Med. 2001, 31, 599–606. [Google Scholar] [CrossRef]
- Bézière, N.; Hardy, M.; Poulhès, F.; Karoui, H.; Tordo, P.; Ouari, O.; Frapart, Y.-M.; Rockenbauer, A.; Boucher, J.-L.; Mansuy, D.; et al. Metabolic stability of superoxide adducts derived from newly developed cyclic nitrone spin traps. Free Radic. Biol. Med. 2014, 67, 150–158. [Google Scholar] [CrossRef] [Green Version]
- Misak, A.; Brezova, V.; Grman, M.; Tomasova, L.; Chovanec, M.; Ondrias, K. •BMPO-OOH Spin-Adduct as a Model for Study of Decomposition of Organic Hydroperoxides and the Effects of Sulfide/Selenite Derivatives. An EPR Spin-Trapping Approach. Antioxidants 2020, 9, 918. [Google Scholar] [CrossRef]
- Janik, I.; Tripathi, G.N. The nature of the superoxide radical anion in water. J. Chem. Phys. 2013, 139, 014302. [Google Scholar] [CrossRef]
- Villamena, F.A.; Zweier, J.L. Superoxide radical trapping and spin adduct decay of 5-tert-butoxycarbonyl-5-methyl-1-pyrroline N-oxide (BocMPO): Kinetics and theoretical analysis. J. Am. Chem. Soc. Perkin Trans 1 2002, 1340–1344. [Google Scholar] [CrossRef]
- Tsai, P.; Ichikawa, K.; Mailer, C.; Pou, S.; Halpern, H.J.; Robinson, B.H.; Nielsen, R.; Rosen, G.M. Esters of 5-Carboxyl-5-methyl-1-pyrrolineN-Oxide: A Family of Spin Traps for Superoxide. J. Org. Chem. 2003, 68, 7811–7817. [Google Scholar] [CrossRef]
- Stolze, K.; Udilova, N.; Rosenau, T.; Hofinger, A.; Nohl, H. Synthesis and Characterization of EMPO-Derived 5,5-Disubstituted 1-Pyrroline N-Oxides as Spin Traps Forming Exceptionally Stable Superoxide Spin Adducts. Biol. Chem. 2003, 384, 493–500. [Google Scholar] [CrossRef]
- Tsai, P.; Marra, J.M.; Pou, S.; Bowman, M.K.; Rosen, G.M. Is There Stereoselectivity in Spin Trapping Superoxide by 5-tert-Butoxycarbonyl-5-methyl-1-pyrrolineN-Oxide? J. Org. Chem. 2005, 70, 7093–7097. [Google Scholar] [CrossRef]
- Spasojević, I. Free radicals and antioxidants at a glance using EPR spectroscopy. Crit. Rev. Clin. Lab. Sci. 2011, 48, 114–142. [Google Scholar] [CrossRef]
- Abbas, K.; Hardy, M.; Poulhès, F.; Karoui, H.; Tordo, P.; Ouari, O.; Peyrot, F. Detection of superoxide production in stimulated and unstimulated living cells using new cyclic nitrone spin traps. Free Radic. Biol. Med. 2014, 71, 281–290. [Google Scholar] [CrossRef]
- Chang, J.; Taylor, R.D.; Davidson, R.A.; Sharmah, A.; Guo, T. Electron Paramagnetic Resonance Spectroscopy Investigation of Radical Production by Gold Nanoparticles in Aqueous Solutions Under X-ray Irradiation. J. Phys. Chem. A 2016, 120, 2815–2823. [Google Scholar] [CrossRef]
- Suzen, S.; Gurer-Orhan, H.; Saso, L. Detection of Reactive Oxygen and Nitrogen Species by Electron Paramagnetic Resonance (EPR) Technique. Molecules 2017, 22, 181. [Google Scholar] [CrossRef] [PubMed]
- Olson, K.R. Hydrogen sulfide, reactive sulfur species and coping with reactive oxygen species. Free Radic. Biol. Med. 2019, 140, 74–83. [Google Scholar] [CrossRef]
- Corvino, A.; Frecentese, F.; Magli, E.; Perissutti, E.; Santagada, V.; Scognamiglio, A.; Caliendo, G.; Fiorino, F.; Severino, B. Trends in H2S-Donors Chemistry and Their Effects in Cardiovascular Diseases. Antioxidants 2021, 10, 429. [Google Scholar] [CrossRef] [PubMed]
- Di Leo, I.; Sancineto, L.; Messina, F.; Santi, C. Organoselenium compounds, an overview on the biological activities beyond antioxidant properties. In Proceedings of the 20th International Electronic Conference on Synthetic Organic Chemistry (ECSOC), online, 1–30 November 2016. [Google Scholar]
- Ali, W.; Spengler, G.; Kincses, A.; Nové, M.; Battistelli, C.; Latacz, G.; Starek, M.; Dąbrowska, M.; Honkisz-Orzechowska, E.; Romanelli, A.; et al. Discovery of phenylselenoether-hydantoin hybrids as ABCB1 efflux pump modulating agents with cytotoxic and antiproliferative actions in resistant T-lymphoma. Eur. J. Med. Chem. 2020, 200, 112435. [Google Scholar] [CrossRef] [PubMed]
- Stoll, S.; Schweiger, A. EasySpin, a comprehensive software package for spectral simulation and analysis in EPR. J. Magn. Reson. 2006, 178, 42–55. [Google Scholar] [CrossRef]
- Nagai, F.; Ushiyama, K.; Kano, I. DNA cleavage by metabolites of butylated hydroxytoluene. Arch. Toxicol. 1993, 67, 552–557. [Google Scholar] [CrossRef] [PubMed]
- Grman, M.; Misak, A.; Kurakova, L.; Brezova, V.; Cacanyiova, S.; Berenyiova, A.; Balis, P.; Tomasova, L.; Kharma, A.; Domínguez-Álvarez, E.; et al. Products of Sulfide/Selenite Interaction Possess Antioxidant Properties, Scavenge Superoxide-Derived Radicals, React with DNA, and Modulate Blood Pressure and Tension of Isolated Thoracic Aorta. Oxid. Med. Cell. Longev. 2019, 2019, 9847650. [Google Scholar] [CrossRef] [Green Version]
- Bielski, B.H.J.; Cabelli, D.E.; Arudi, R.L.; Ross, A.B. Reactivity of HO2/O2− Radicals in Aqueous Solution. J. Phys. Chem. Ref. Data 1985, 14, 1041–1100. [Google Scholar] [CrossRef]
- Galbács, Z.M.; Csányi, L.J. Alkali-induced decomposition of hydrogen peroxide. J. Chem. Soc. Dalton Trans. 1983, 2353–2357. [Google Scholar] [CrossRef]
- Csányi, L.J.; Nagy, L.; Galbács, Z.M.; Horváth, I. Alkali-Induced Generation of Superoxide and Hydroxyl Radicals from Aqueous Hydrogen Peroxide Solution. Z. Phys. Chem. 1983, 138, 107–116. [Google Scholar] [CrossRef]
- Dong, D.; Vandegrift, G.F. Alkaline Peroxide Processing of Low-Enriched Uranium Targets for 99Mo Production—Decomposition of Hydrogen Peroxide. Nucl. Sci. Eng. 1997, 126, 213–223. [Google Scholar] [CrossRef]
- Miller, C.M.; Valentine, R.L. Mechanistic studies of surface catalyzed H2O2 decomposition and contaminant degradation in the presence of sand. Water Res. 1999, 33, 2805–2816. [Google Scholar] [CrossRef]
- Loft, S.; Poulsen, H.E. Cancer risk and oxidative DNA damage in man. J. Mol. Med. 1996, 74, 297–312. [Google Scholar] [CrossRef]
- Miura, T. The peroxidase activity of ADM-Fe3+ cooperates with lipid peroxidation: The participation of hydroperoxide and hydroxyl radicals in the damage to proteins and DNA. Chem. Biol. Interact. 2015, 236, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; Rusling, J.F. Oxidation Chemistry of DNA and p53 Tumor Suppressor Gene. ChemistryOpen 2019, 8, 252–265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
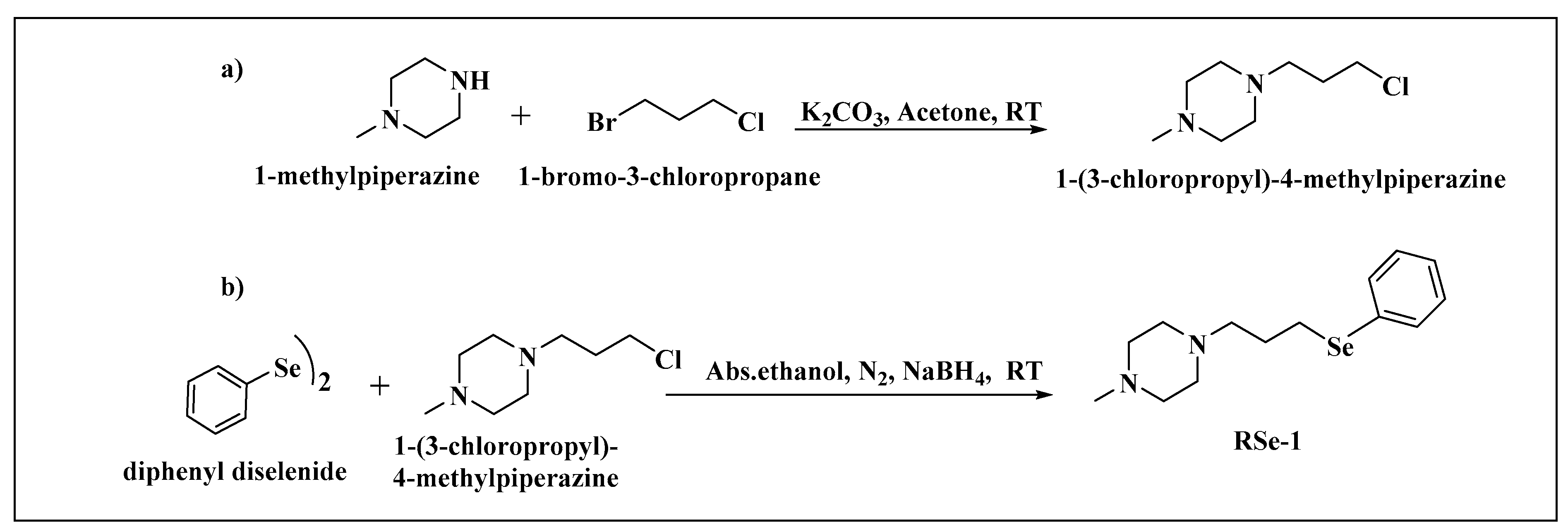
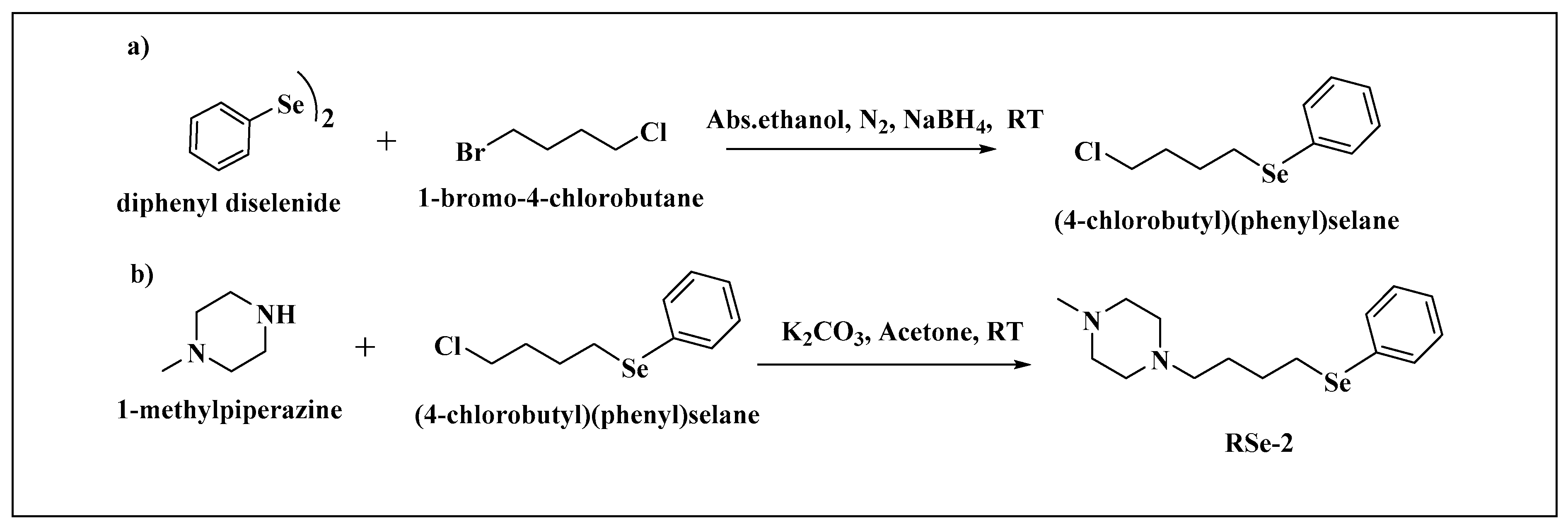




| BMPO-Adduct | aN, mT | aHβ, mT | aHγ, mT |
|---|---|---|---|
| •BMPO-OH(1) | 1.433 ± 0.003 | 1.521 ± 0.005 | 0.074 ± 0.004 |
| •BMPO-OH(2) | 1.421 ± 0.004 | 1.264 ± 0.003 | 0.065 ± 0.002 |
| •BMPO-OOH(1) | 1.341 ± 0.003 | 1.198 ± 0.004 | – |
| •BMPO-OOH(2) | 1.340 ± 0.001 | 0.967 ± 0.006 | – |
| •BMPO-CR | 1.515 ± 0.008 | 2.077 ± 0.024 | – |
| •BMPO-CO2− | 1.490 ± 0.003 | 1.710 ± 0.012 | – |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Misak, A.; Brezova, V.; Chovanec, M.; Luspai, K.; Nasim, M.J.; Grman, M.; Tomasova, L.; Jacob, C.; Ondrias, K. EPR Study of KO2 as a Source of Superoxide and •BMPO-OH/OOH Radical That Cleaves Plasmid DNA and Detects Radical Interaction with H2S and Se-Derivatives. Antioxidants 2021, 10, 1286. https://doi.org/10.3390/antiox10081286
Misak A, Brezova V, Chovanec M, Luspai K, Nasim MJ, Grman M, Tomasova L, Jacob C, Ondrias K. EPR Study of KO2 as a Source of Superoxide and •BMPO-OH/OOH Radical That Cleaves Plasmid DNA and Detects Radical Interaction with H2S and Se-Derivatives. Antioxidants. 2021; 10(8):1286. https://doi.org/10.3390/antiox10081286
Chicago/Turabian StyleMisak, Anton, Vlasta Brezova, Miroslav Chovanec, Karol Luspai, Muhammad Jawad Nasim, Marian Grman, Lenka Tomasova, Claus Jacob, and Karol Ondrias. 2021. "EPR Study of KO2 as a Source of Superoxide and •BMPO-OH/OOH Radical That Cleaves Plasmid DNA and Detects Radical Interaction with H2S and Se-Derivatives" Antioxidants 10, no. 8: 1286. https://doi.org/10.3390/antiox10081286
APA StyleMisak, A., Brezova, V., Chovanec, M., Luspai, K., Nasim, M. J., Grman, M., Tomasova, L., Jacob, C., & Ondrias, K. (2021). EPR Study of KO2 as a Source of Superoxide and •BMPO-OH/OOH Radical That Cleaves Plasmid DNA and Detects Radical Interaction with H2S and Se-Derivatives. Antioxidants, 10(8), 1286. https://doi.org/10.3390/antiox10081286










