Targeted Ablation of Primary Cilia in Differentiated Dopaminergic Neurons Reduces Striatal Dopamine and Responsiveness to Metabolic Stress
Abstract
:1. Introduction
2. Materials and Methods
2.1. Mice
2.2. Brain Dissection and Tissue Preparation for Histology and Western Blot
2.3. Immunohistochemistry
2.4. Analysis of Primary Cilia Frequency and Length and TH and DAT Immunoreactivity
2.5. HPLC Analysis of Dopamine Content
2.6. Western Blot Analysis
2.7. Electrophysiological Multi-Electrode Array (MEA) Recordings
2.8. MPTP Treatment
2.9. Stereological Analysis
2.10. Statistical Analysis
3. Results
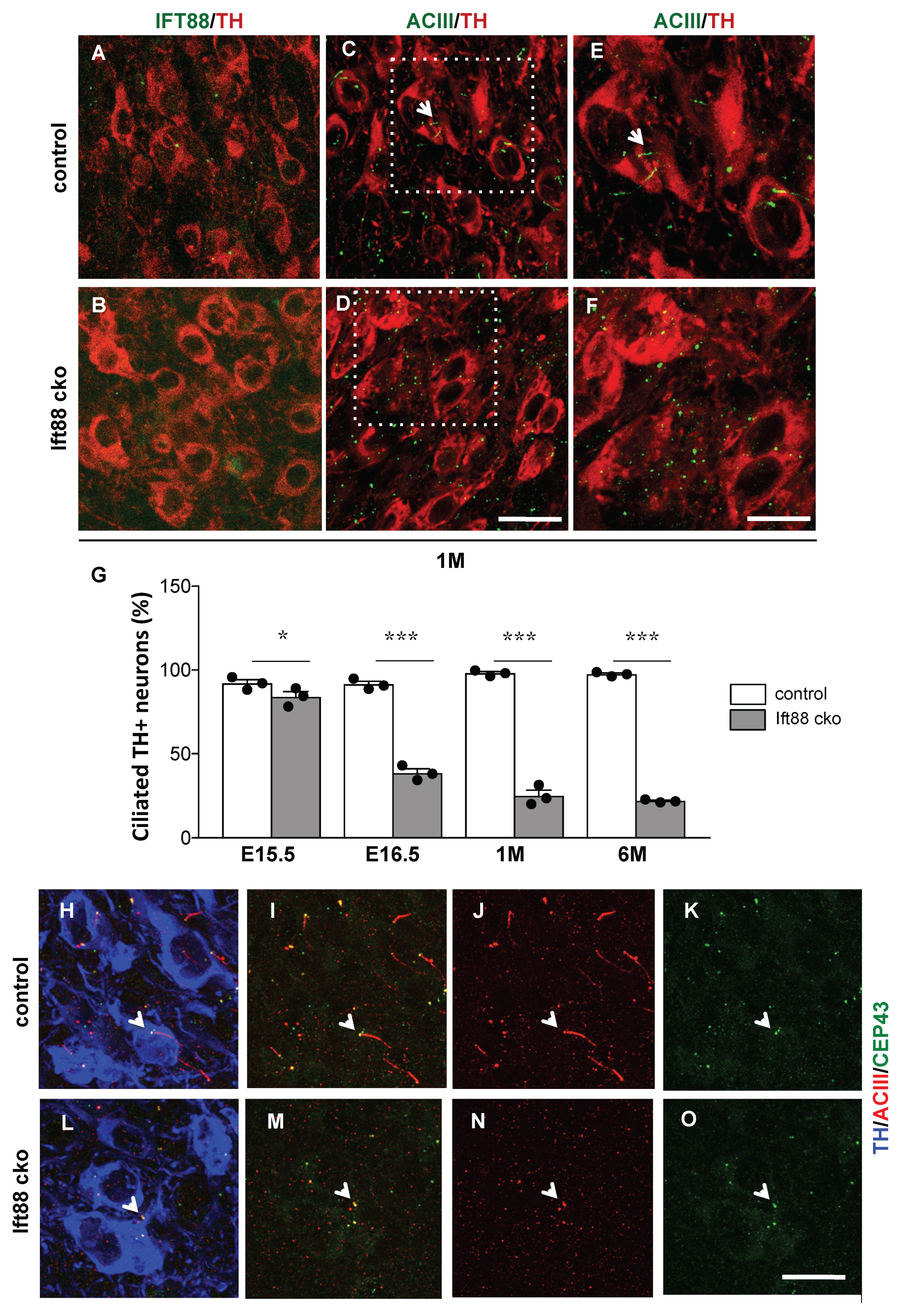
3.1. Generation of Mutant Mice Lacking Primary Cilia in Differentiated DA Neurons
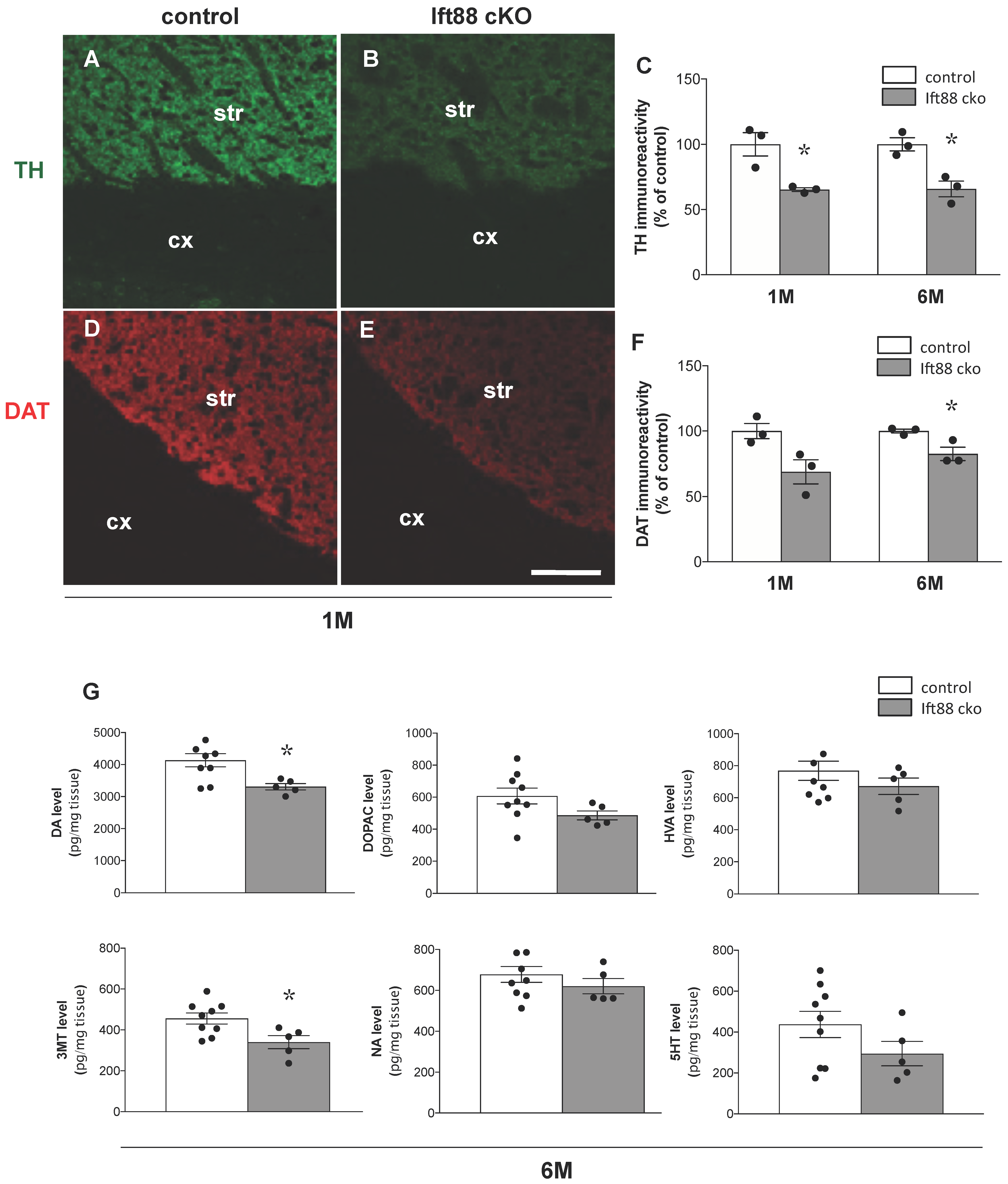
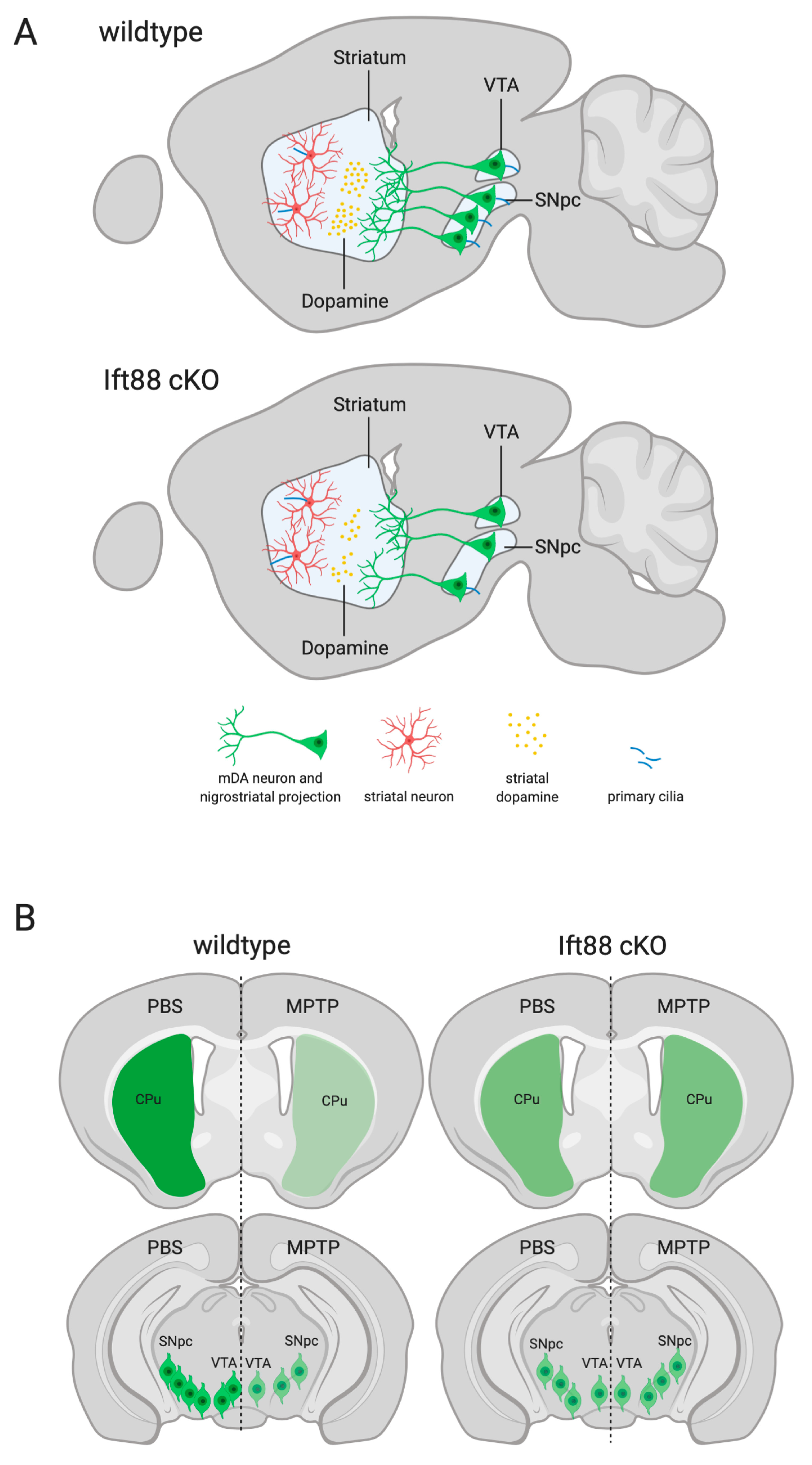
3.2. Loss of Primary Cilia in Differentiated DA Neurons Reduces Nigrostriatal DA Projections and Striatal Dopamine Content
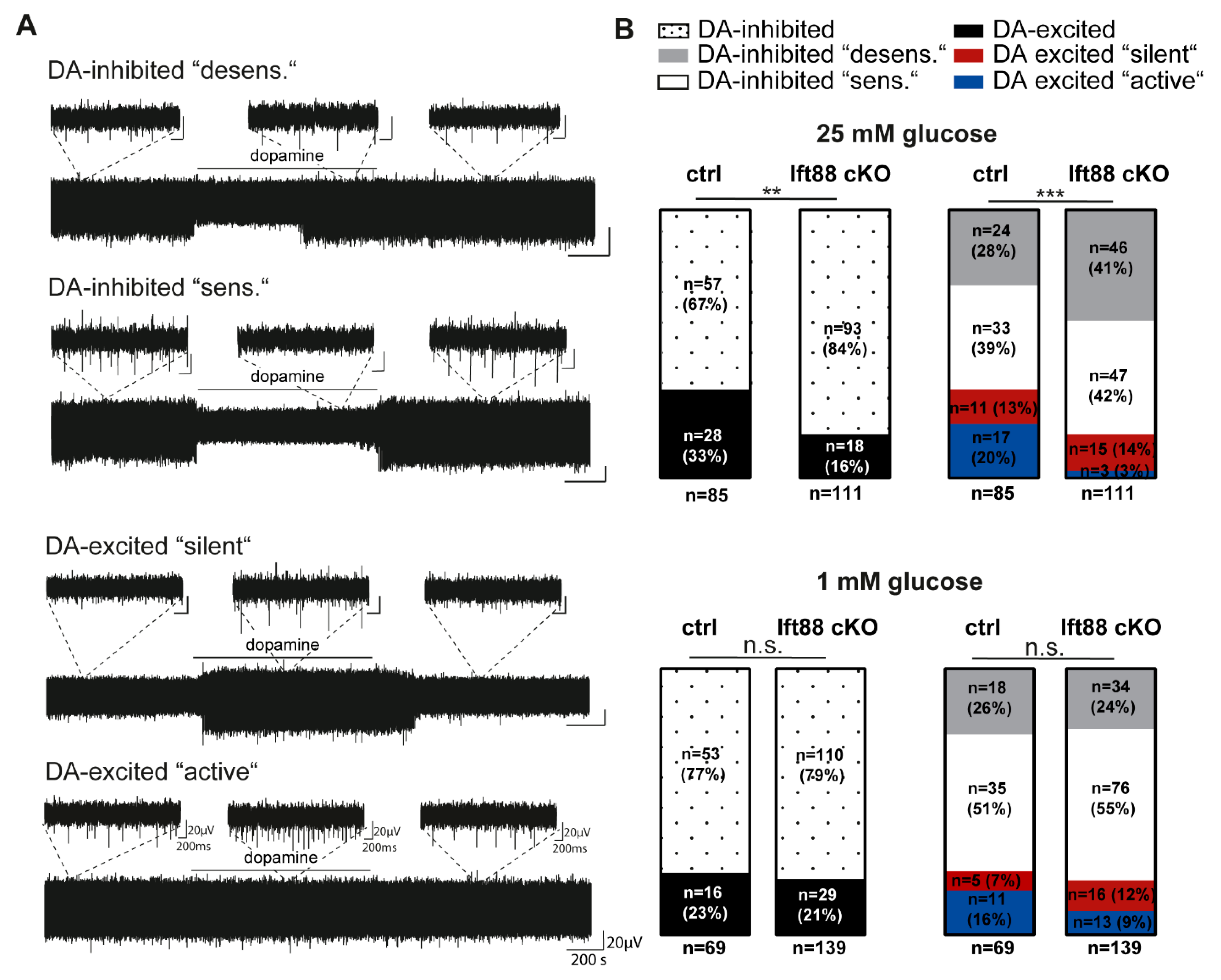
3.3. Loss of Primary Cilia in Differentiated DA Neurons Reduces the Number of Dopamine-Excited SN Neurons
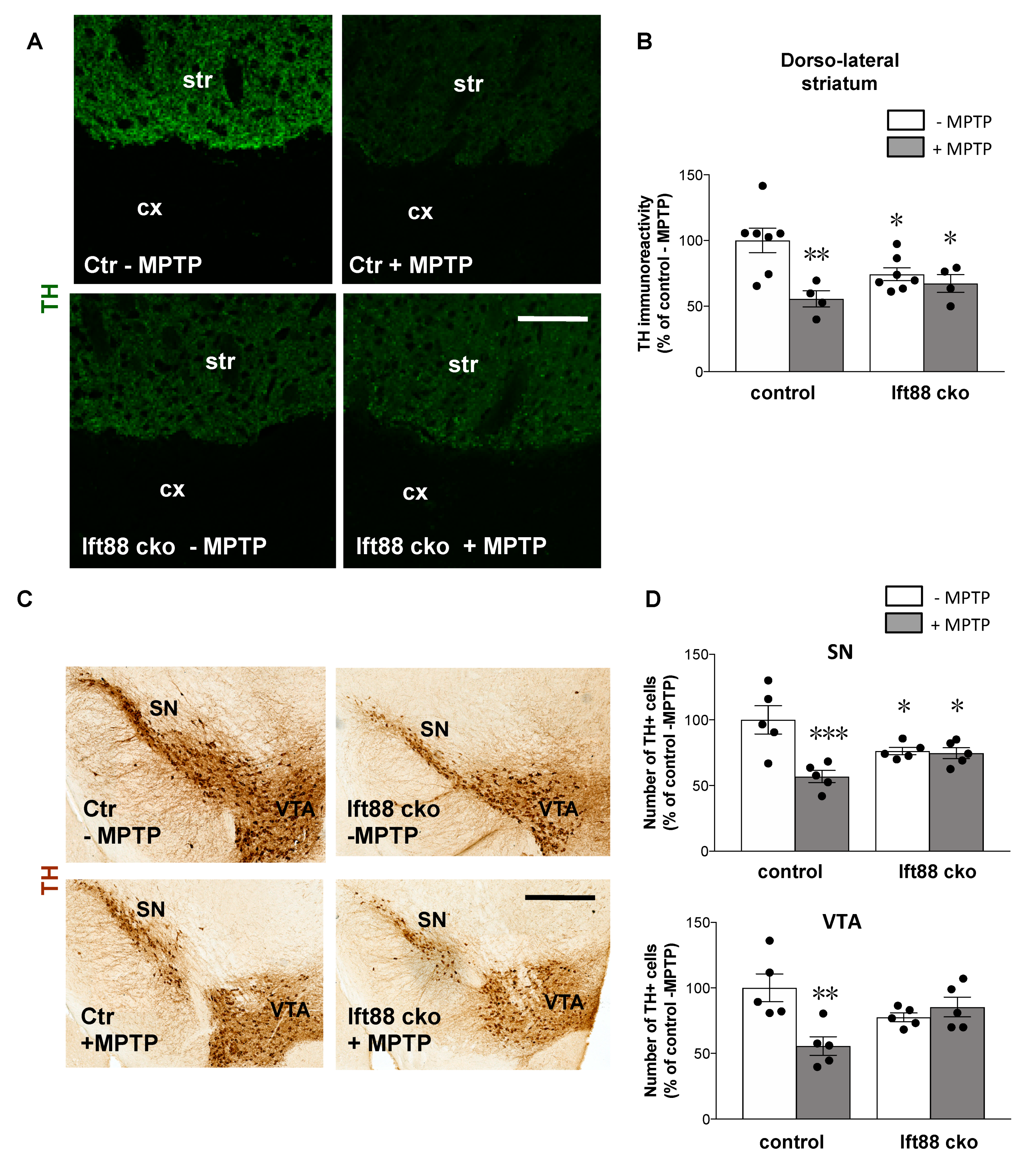
3.4. Loss of Primary Cilia in Differentiated DA Neurons of Ift88 cKO Mutants Renders Them Insensitive to the Neurotoxin MPTP
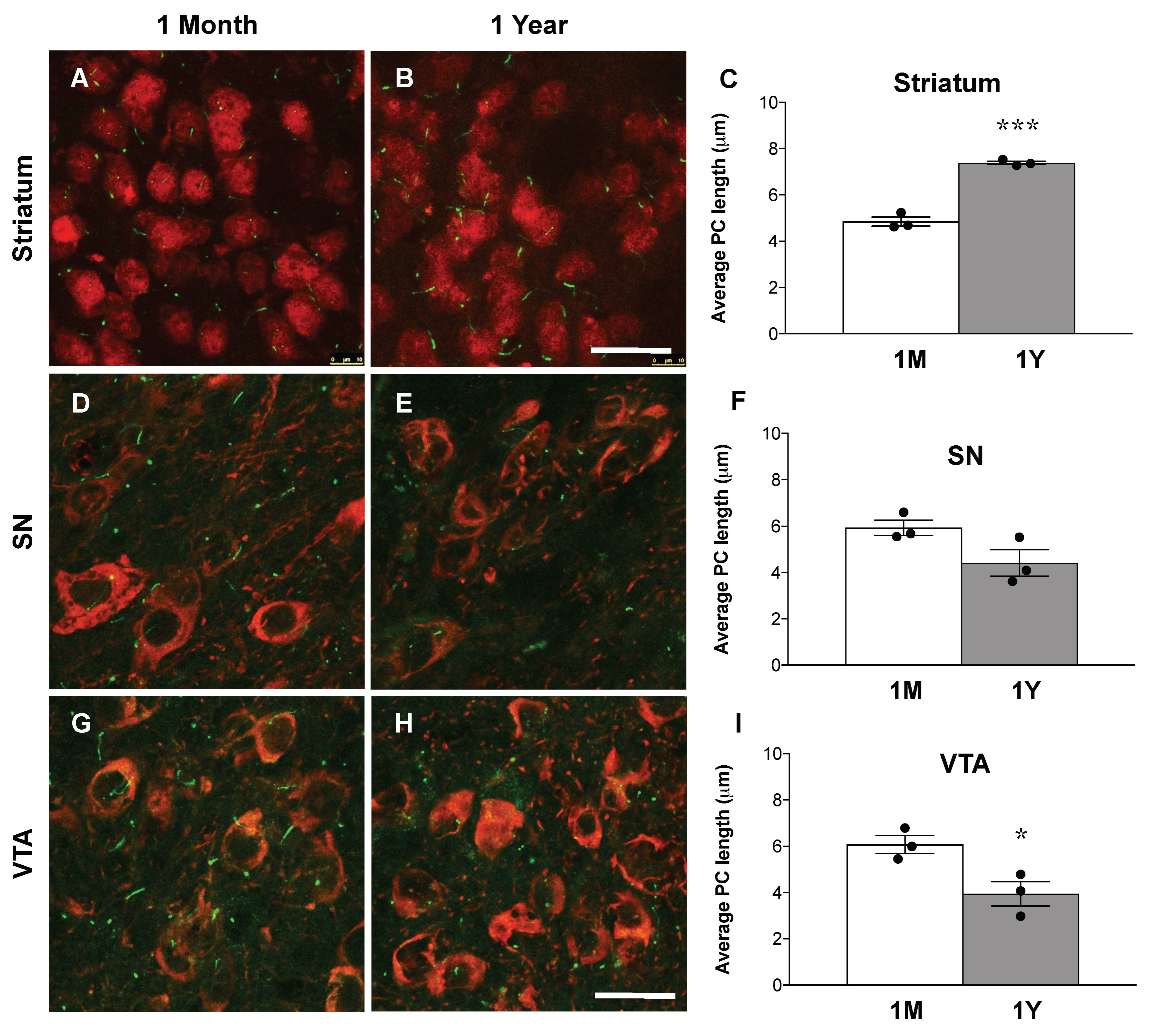
3.5. Loss of Primary Cilia in DA Neurons Results in Increased Primary Cilia Length in the Striatum of Ift88 Conditional Knock-Out Mice
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Reiter, J.F.; Leroux, M.R. Genes and molecular pathways underpinning ciliopathies. Nat. Rev. Mol. Cell Biol. 2017, 18, 533–547. [Google Scholar] [CrossRef]
- Sterpka, A.; Chen, X. Neuronal and astrocytic primary cilia in the mature brain. Pharmacol. Res. 2018, 137, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Kiesel, P.; Viar, G.A.; Tsoy, N.; Maraspini, R.; Gorilak, P.; Varga, V.; Honigmann, A.; Pigino, G. The molecular structure of mammalian primary cilia revealed by cryo-electron tomography. Nat. Struct. Mol. Biol. 2020, 27, 1115–1124. [Google Scholar] [CrossRef] [PubMed]
- Hughes, J.W.; Cho, J.H.; Conway, H.E.; Di Gruccio, M.R.; Ng, X.W.; Roseman, H.F.; Abreu, D.; Urano, F.; Piston, D.W. Primary cilia control glucose homeostasis via islet paracrine interactions. Proc. Natl. Acad. Sci. USA 2020, 117, 8912–8923. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mannal, N.; Kleiner, K.; Fauler, M.; Dougalis, A.; Poetschke, C.; Liss, B. Multi-Electrode Array Analysis Identifies Complex Dopamine Responses and Glucose Sensing Properties of Substantia Nigra Neurons in Mouse Brain Slices. Front. Synaptic Neurosci. 2021, 13, 635050. [Google Scholar] [CrossRef] [PubMed]
- Saller, C.F.; Chiodo, L.A. Glucose suppresses basal firing and haloperidol-induced increases in the firing rate of central dopaminergic neurons. Science 1980, 210, 1269–1271. [Google Scholar] [CrossRef]
- Sportelli, C.; Urso, D.; Jenner, P.; Chaudhuri, K.R. Metformin as a Potential Neuroprotective Agent in Prodromal Parkinson’s Disease-Viewpoint. Front. Neurol. 2020, 11, 556. [Google Scholar] [CrossRef]
- Hassan, A.; Kandel, R.S.; Mishra, R.; Gautam, J.; Alaref, A.; Jahan, N. Diabetes Mellitus and Parkinson’s Disease: Shared Pathophysiological Links and Possible Therapeutic Implications. Cureus 2020, 12, e9853. [Google Scholar] [CrossRef] [PubMed]
- Domire, J.S.; Green, J.A.; Lee, K.G.; Johnson, A.D.; Askwith, C.C.; Mykytyn, K. Dopamine receptor 1 localizes to neuronal cilia in a dynamic process that requires the Bardet-Biedl syndrome proteins. Cell Mol. Life Sci. 2011, 68, 2951–2960. [Google Scholar] [CrossRef] [Green Version]
- Leaf, A.; von Zastrow, M. Dopamine receptors reveal an essential role of IFT-B, KIF17, and Rab23 in delivering specific receptors to primary cilia. eLife 2015, 4, e06996. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez-Reyes, L.E.; Verbitsky, M.; Blesa, J.; Jackson-Lewis, V.; Paredes, D.; Tillack, K.; Phani, S.; Kramer, E.R.; Przedborski, S.; Kottmann, A.H. Sonic hedgehog maintains cellular and neurochemical homeostasis in the adult nigrostriatal circuit. Neuron 2012, 75, 306–319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karam, A.; Tebbe, L.; Weber, C.; Messaddeq, N.; Morle, L.; Kessler, P.; Wolfrum, U.; Trottier, Y. A novel function of Huntingtin in the cilium and retinal ciliopathy in Huntington’s disease mice. Neurobiol. Dis. 2015, 80, 15–28. [Google Scholar] [CrossRef]
- Keryer, G.; Pineda, J.R.; Liot, G.; Kim, J.; Dietrich, P.; Benstaali, C.; Smith, K.; Cordelieres, F.P.; Spassky, N.; Ferrante, R.J.; et al. Ciliogenesis is regulated by a huntingtin-HAP1-PCM1 pathway and is altered in Huntington disease. J. Clin. Investig. 2011, 121, 4372–4382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, J.; Higginbotham, H.; Li, J.; Nichols, J.; Hirt, J.; Ghukasyan, V.; Anton, E.S. Developmental disruptions underlying brain abnormalities in ciliopathies. Nat. Commun. 2015, 6, 7857. [Google Scholar] [CrossRef] [Green Version]
- Guemez-Gamboa, A.; Coufal, N.G.; Gleeson, J.G. Primary cilia in the developing and mature brain. Neuron 2014, 82, 511–521. [Google Scholar] [CrossRef] [Green Version]
- Mustafa, R.; Kreiner, G.; Kaminska, K.; Wood, A.J.; Kirsch, J.; Tucker, K.L.; Parlato, R. Targeted Depletion of Primary Cilia in Dopaminoceptive Neurons in a Preclinical Mouse Model of Huntington’s Disease. Front. Cell Neurosci. 2019, 13, 565. [Google Scholar] [CrossRef]
- Miyoshi, K.; Kasahara, K.; Murakami, S.; Takeshima, M.; Kumamoto, N.; Sato, A.; Miyazaki, I.; Matsuzaki, S.; Sasaoka, T.; Katayama, T.; et al. Lack of dopaminergic inputs elongates the primary cilia of striatal neurons. PLoS ONE 2014, 9, e97918. [Google Scholar] [CrossRef] [PubMed]
- Dhekne, H.S.; Yanatori, I.; Gomez, R.C.; Tonelli, F.; Diez, F.; Schule, B.; Steger, M.; Alessi, D.R.; Pfeffer, S.R. A pathway for Parkinson’s Disease LRRK2 kinase to block primary cilia and Sonic hedgehog signaling in the brain. eLife 2018, 7, e40202. [Google Scholar] [CrossRef] [PubMed]
- Steger, M.; Diez, F.; Dhekne, H.S.; Lis, P.; Nirujogi, R.S.; Karayel, O.; Tonelli, F.; Martinez, T.N.; Lorentzen, E.; Pfeffer, S.R.; et al. Systematic proteomic analysis of LRRK2-mediated Rab GTPase phosphorylation establishes a connection to ciliogenesis. eLife 2017, 6, e31012. [Google Scholar] [CrossRef] [Green Version]
- Jiang, J.Y.; Falcone, J.L.; Curci, S.; Hofer, A.M. Direct visualization of cAMP signaling in primary cilia reveals up-regulation of ciliary GPCR activity following Hedgehog activation. Proc. Natl. Acad. Sci. USA 2019, 116, 12066–12071. [Google Scholar] [CrossRef] [Green Version]
- Lucarelli, M.; Di Pietro, C.; La Sala, G.; Fiorenza, M.T.; Marazziti, D.; Canterini, S. Anomalies in Dopamine Transporter Expression and Primary Cilium Distribution in the Dorsal Striatum of a Mouse Model of Niemann-Pick C1 Disease. Front. Cell Neurosci. 2019, 13, 226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bae, J.E.; Kang, G.M.; Min, S.H.; Jo, D.S.; Jung, Y.K.; Kim, K.; Kim, M.S.; Cho, D.H. Primary cilia mediate mitochondrial stress responses to promote dopamine neuron survival in a Parkinson’s disease model. Cell Death Dis. 2019, 10, 952. [Google Scholar] [CrossRef] [PubMed]
- Burkhalter, M.D.; Sridhar, A.; Sampaio, P.; Jacinto, R.; Burczyk, M.S.; Donow, C.; Angenendt, M.; Hempel, M.; Walther, P.; Pennekamp, P.; et al. Imbalanced mitochondrial function provokes heterotaxy via aberrant ciliogenesis. J. Clin. Investig. 2019, 129, 2841–2855. [Google Scholar] [CrossRef] [PubMed]
- Chikamori, M.; Kimura, H.; Inagi, R.; Zhou, J.; Nangaku, M.; Fujii, T. Intracellular calcium response of primary cilia of tubular cells to modulated shear stress under oxidative stress. Biomicrofluidics 2020, 14, 044102. [Google Scholar] [CrossRef]
- Han, S.J.; Jang, H.S.; Kim, J.I.; Lipschutz, J.H.; Park, K.M. Unilateral nephrectomy elongates primary cilia in the remaining kidney via reactive oxygen species. Sci. Rep. 2016, 6, 22281. [Google Scholar] [CrossRef] [Green Version]
- Gazea, M.; Tasouri, E.; Tolve, M.; Bosch, V.; Kabanova, A.; Gojak, C.; Kurtulmus, B.; Novikov, O.; Spatz, J.; Pereira, G.; et al. Primary cilia are critical for Sonic hedgehog-mediated dopaminergic neurogenesis in the embryonic midbrain. Dev. Biol. 2016, 409, 55–71. [Google Scholar] [CrossRef] [Green Version]
- Gazea, M.; Tasouri, E.; Heigl, T.; Bosch, V.; Tucker, K.L.; Blaess, S. Definition of a critical spatiotemporal window within which primary cilia control midbrain dopaminergic neurogenesis. Neurogenesis 2016, 3, e1248206. [Google Scholar] [CrossRef] [Green Version]
- Parlato, R.; Rieker, C.; Turiault, M.; Tronche, F.; Schutz, G. Survival of DA neurons is independent of CREM upregulation in absence of CREB. Genesis 2006, 44, 454–464. [Google Scholar] [CrossRef]
- Rieker, C.; Engblom, D.; Kreiner, G.; Domanskyi, A.; Schober, A.; Stotz, S.; Neumann, M.; Yuan, X.; Grummt, I.; Schutz, G.; et al. Nucleolar disruption in dopaminergic neurons leads to oxidative damage and parkinsonism through repression of mammalian target of rapamycin signaling. J. Neurosci. 2011, 31, 453–460. [Google Scholar] [CrossRef] [Green Version]
- Haycraft, C.J.; Zhang, Q.; Song, B.; Jackson, W.S.; Detloff, P.J.; Serra, R.; Yoder, B.K. Intraflagellar transport is essential for endochondral bone formation. Development 2007, 134, 307–316. [Google Scholar] [CrossRef] [Green Version]
- Paxinos, H.; Watson, C.R.R.; Koutcherov, Y.; Wang, H. Atlas of the Developing Brain; Elsevier: Amsterdam, The Netherlands, 2007. [Google Scholar]
- Franklin, P. The Mouse Brain in Stereotaxic Coordinates, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2008. [Google Scholar]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [Green Version]
- Parker, A.K.; Le, M.M.; Smith, T.S.; Hoang-Minh, L.B.; Atkinson, E.W.; Ugartemendia, G.; Semple-Rowland, S.; Coleman, J.E.; Sarkisian, M.R. Neonatal seizures induced by pentylenetetrazol or kainic acid disrupt primary cilia growth on developing mouse cortical neurons. Exp. Neurol. 2016, 282, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Sikora, M.; Tokarski, K.; Bobula, B.; Zajdel, J.; Jastrzębska, K.; Cieślak, P.E.; Zygmunt, M.; Sowa, J.; Smutek, M.; Kamińska, K.; et al. NMDA Receptors on Dopaminoceptive Neurons Are Essential for Drug-Induced Conditioned Place Preference. eNeuro 2016, 3, ENEURO.0084-15.2016. [Google Scholar] [CrossRef] [Green Version]
- Przedborski, S.; Jackson-Lewis, V.; Naini, A.B.; Jakowec, M.; Petzinger, G.; Miller, R.; Akram, M. The parkinsonian toxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP): A technical review of its utility and safety. J. Neurochem. 2001, 76, 1265–1274. [Google Scholar] [CrossRef] [PubMed]
- Schober, A.; Peterziel, H.; von Bartheld, C.S.; Simon, H.; Krieglstein, K.; Unsicker, K. GDNF applied to the MPTP-lesioned nigrostriatal system requires TGF-beta for its neuroprotective action. Neurobiol. Dis. 2007, 25, 378–391. [Google Scholar] [CrossRef]
- Herculano-Houzel, S.; von Bartheld, C.S.; Miller, D.J.; Kaas, J.H. How to count cells: The advantages and disadvantages of the isotropic fractionator compared with stereology. Cell Tissue Res. 2015, 360, 29–42. [Google Scholar] [CrossRef] [Green Version]
- Ma, X.; Peterson, R.; Turnbull, J. Adenylyl cyclase type 3, a marker of primary cilia, is reduced in primary cell culture and in lumbar spinal cord in situ in G93A SOD1 mice. BMC Neurosci. 2011, 12, 71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bishop, G.A.; Berbari, N.F.; Lewis, J.; Mykytyn, K. Type III adenylyl cyclase localizes to primary cilia throughout the adult mouse brain. J. Comp. Neurol. 2007, 505, 562–571. [Google Scholar] [CrossRef]
- Acquaviva, C.; Chevrier, V.; Chauvin, J.P.; Fournier, G.; Birnbaum, D.; Rosnet, O. The centrosomal FOP protein is required for cell cycle progression and survival. Cell Cycle 2009, 8, 1217–1227. [Google Scholar] [CrossRef] [Green Version]
- Ford, C.P. The role of D2-autoreceptors in regulating dopamine neuron activity and transmission. Neuroscience 2014, 282, 13–22. [Google Scholar] [CrossRef] [Green Version]
- Berretta, N.; Bernardi, G.; Mercuri, N.B. Firing properties and functional connectivity of substantia nigra pars compacta neurones recorded with a multi-electrode array in vitro. J. Physiol. 2010, 588 Pt 10, 1719–1735. [Google Scholar] [CrossRef]
- Tomagra, G.; Picollo, F.; Battiato, A.; Picconi, B.; de Marchis, S.; Pasquarelli, A.; Olivero, P.; Marcantoni, A.; Calabresi, P.; Carbone, E.; et al. Quantal Release of Dopamine and Action Potential Firing Detected in Midbrain Neurons by Multifunctional Diamond-Based Microarrays. Front. Neurosci. 2019, 13, 288. [Google Scholar] [CrossRef] [Green Version]
- Ou, Y.; Ruan, Y.; Cheng, M.; Moser, J.J.; Rattner, J.B.; van der Hoorn, F.A. Adenylate cyclase regulates elongation of mammalian primary cilia. Exp. Cell Res. 2009, 315, 2802–2817. [Google Scholar] [CrossRef] [Green Version]
- Besschetnova, T.Y.; Kolpakova-Hart, E.; Guan, Y.; Zhou, J.; Olsen, B.R.; Shah, J.V. Identification of signaling pathways regulating primary cilium length and flow-mediated adaptation. Curr. Biol. 2010, 20, 182–187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Machado, V.; Zoller, T.; Attaai, A.; Spittau, B. Microglia-Mediated Neuroinflammation and Neurotrophic Factor-Induced Protection in the MPTP Mouse Model of Parkinson’s Disease-Lessons from Transgenic Mice. Int. J. Mol. Sci. 2016, 17, 151. [Google Scholar] [CrossRef]
- Alvarez-Satta, M.; Moreno-Cugnon, L.; Matheu, A. Primary cilium and brain aging: Role in neural stem cells, neurodegenerative diseases and glioblastoma. Ageing Res. Rev. 2019, 52, 53–63. [Google Scholar] [CrossRef]
- Guadiana, S.M.; Parker, A.K.; Filho, G.F.; Sequeira, A.; Semple-Rowland, S.; Shaw, G.; Mandel, R.J.; Foster, T.C.; Kumar, A.; Sarkisian, M.R. Type 3 Adenylyl Cyclase and Somatostatin Receptor 3 Expression Persists in Aged Rat Neocortical and Hippocampal Neuronal Cilia. Front. Aging Neurosci. 2016, 8, 127. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Dong, Z. Primary cilia and kidney injury: Current research status and future perspectives. Am. J. Physiol. Renal. Physiol. 2013, 305, F1085–F1098. [Google Scholar] [CrossRef] [Green Version]
- Boehlke, C.; Kotsis, F.; Patel, V.; Braeg, S.; Voelker, H.; Bredt, S.; Beyer, T.; Janusch, H.; Hamann, C.; Godel, M.; et al. Primary cilia regulate mTORC1 activity and cell size through Lkb1. Nat. Cell Biol. 2010, 12, 1115–1122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marley, A.; von Zastrow, M. DISC1 regulates primary cilia that display specific dopamine receptors. PLoS ONE 2010, 5, e10902. [Google Scholar] [CrossRef]
- Bezard, E.; Gross, C.E.; Fournier, M.C.; Dovero, S.; Bloch, B.; Jaber, M. Absence of MPTP-induced neuronal death in mice lacking the dopamine transporter. Exp. Neurol. 1999, 155, 268–273. [Google Scholar] [CrossRef]
- Gainetdinov, R.R.; Fumagalli, F.; Jones, S.R.; Caron, M.G. Dopamine transporter is required for in vivo MPTP neurotoxicity: Evidence from mice lacking the transporter. J. Neurochem. 1997, 69, 1322–1325. [Google Scholar] [CrossRef]
- Zhou, X.; Pace, J.; Filichia, E.; Lv, T.; Davis, B.; Hoffer, B.; Selman, W.; Luo, Y. Effect of the sonic hedgehog receptor smoothened on the survival and function of dopaminergic neurons. Exp. Neurol. 2016, 283 Pt A, 235–245. [Google Scholar] [CrossRef] [Green Version]
- Lammel, S.; Hetzel, A.; Hackel, O.; Jones, I.; Liss, B.; Roeper, J. Unique properties of mesoprefrontal neurons within a dual mesocorticolimbic dopamine system. Neuron 2008, 57, 760–773. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liss, B.; Striessnig, J. The Potential of L-Type Calcium Channels as a Drug Target for Neuroprotective Therapy in Parkinson’s Disease. Annu. Rev. Pharmacol. Toxicol. 2019, 59, 263–289. [Google Scholar] [CrossRef] [PubMed]
- Zampese, E.; Surmeier, D.J. Calcium Bioenergetics, and Parkinson’s Disease. Cells 2020, 9, 2045. [Google Scholar] [CrossRef]
- Arellano, J.I.; Guadiana, S.M.; Breunig, J.J.; Rakic, P.; Sarkisian, M.R. Development and distribution of neuronal cilia in mouse neocortex. J. Comp. Neurol. 2012, 520, 848–873. [Google Scholar] [CrossRef] [Green Version]
- DeCaen, P.G.; Delling, M.; Vien, T.N.; Clapham, D.E. Direct recording and molecular identification of the calcium channel of primary cilia. Nature 2013, 504, 315–318. [Google Scholar] [CrossRef] [Green Version]
- Pablo, J.L.; DeCaen, P.G.; Clapham, D.E. Progress in ciliary ion channel physiology. J. Gen. Physiol. 2017, 149, 37–47. [Google Scholar] [CrossRef] [Green Version]
- Saternos, H.; Ley, S.; AbouAlaiwi, W. Primary Cilia and Calcium Signaling Interactions. Int. J. Mol. Sci. 2020, 21, 7109. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mustafa, R.; Rawas, C.; Mannal, N.; Kreiner, G.; Spittau, B.; Kamińska, K.; Yilmaz, R.; Pötschke, C.; Kirsch, J.; Liss, B.; et al. Targeted Ablation of Primary Cilia in Differentiated Dopaminergic Neurons Reduces Striatal Dopamine and Responsiveness to Metabolic Stress. Antioxidants 2021, 10, 1284. https://doi.org/10.3390/antiox10081284
Mustafa R, Rawas C, Mannal N, Kreiner G, Spittau B, Kamińska K, Yilmaz R, Pötschke C, Kirsch J, Liss B, et al. Targeted Ablation of Primary Cilia in Differentiated Dopaminergic Neurons Reduces Striatal Dopamine and Responsiveness to Metabolic Stress. Antioxidants. 2021; 10(8):1284. https://doi.org/10.3390/antiox10081284
Chicago/Turabian StyleMustafa, Rasem, Chahinaz Rawas, Nadja Mannal, Grzegorz Kreiner, Björn Spittau, Katarzyna Kamińska, Rüstem Yilmaz, Christina Pötschke, Joachim Kirsch, Birgit Liss, and et al. 2021. "Targeted Ablation of Primary Cilia in Differentiated Dopaminergic Neurons Reduces Striatal Dopamine and Responsiveness to Metabolic Stress" Antioxidants 10, no. 8: 1284. https://doi.org/10.3390/antiox10081284
APA StyleMustafa, R., Rawas, C., Mannal, N., Kreiner, G., Spittau, B., Kamińska, K., Yilmaz, R., Pötschke, C., Kirsch, J., Liss, B., Tucker, K. L., & Parlato, R. (2021). Targeted Ablation of Primary Cilia in Differentiated Dopaminergic Neurons Reduces Striatal Dopamine and Responsiveness to Metabolic Stress. Antioxidants, 10(8), 1284. https://doi.org/10.3390/antiox10081284





