Variation of the Polyphenolic Composition and Antioxidant Capacity of Freshly Prepared Pomegranate Leaf Infusions over One-Day Storage
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Plant Materials
2.3. Preparation of Plant Infusion
2.4. Determination of Phenolic Content
2.5. Evaluation of In Vitro Antioxidant Capacity
2.6. Chromatographic Analysis of Phenolic Compounds
2.7. Data and Statistical Analysis
3. Results and Discussion
3.1. Effect of Storage Time on the Polyphenolic Composition of PLI during One-Day Storage
3.1.1. Change in the Levels of Total Phenols (TPs), Ortho-Diphenols (OPs), Flavonoids (TFs), and Condensed Tannins (CTs)
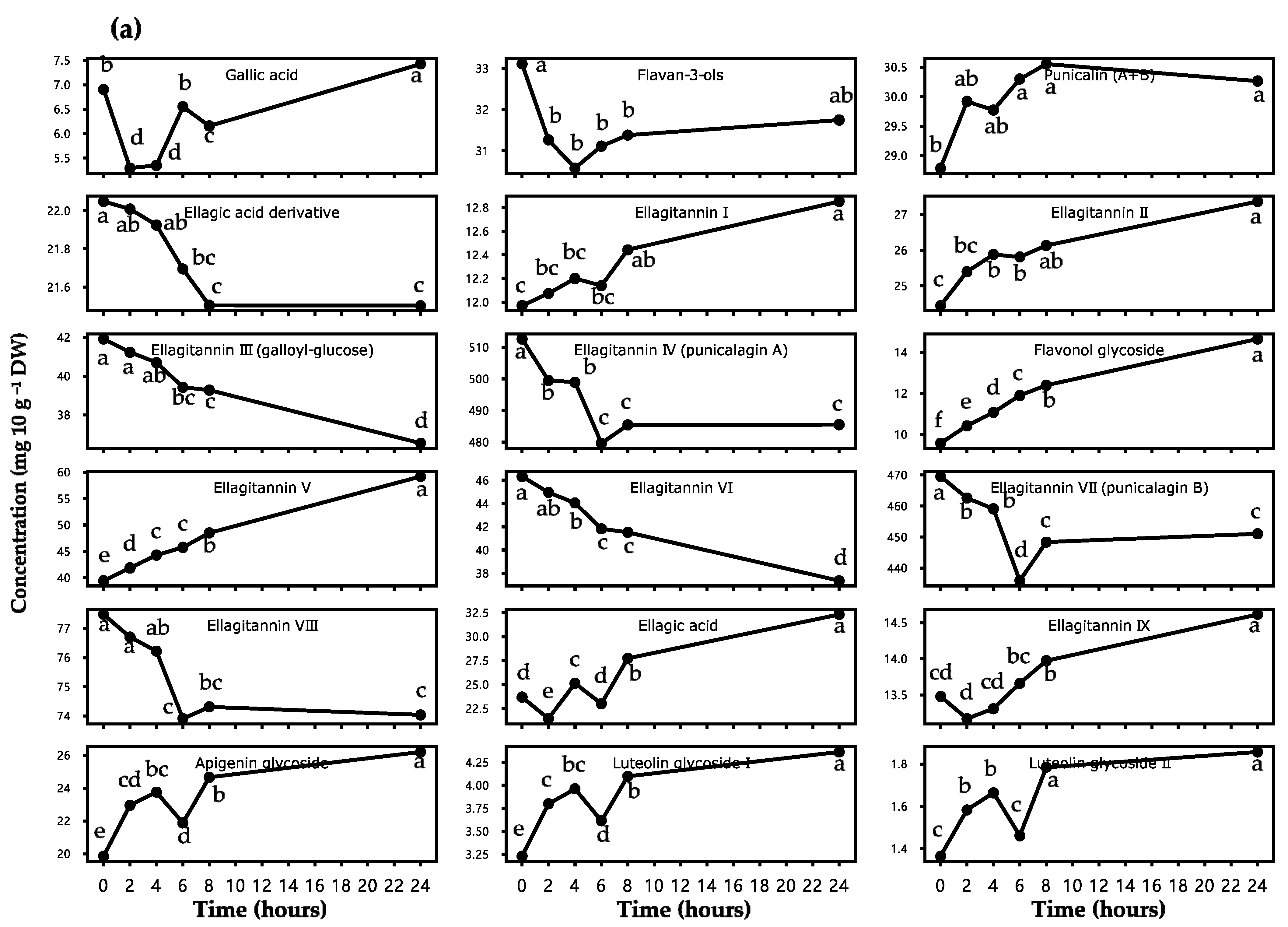
3.1.2. Change in the Polyphenolic Profiles
3.2. Effect of Storage Time on the Antioxidant Activity (AOA) of PLI during One-Day Storage
3.3. Correlation Analysis
3.3.1. Pearson Correlation Coefficient (PCC)
3.3.2. Principal Component Analysis (PCA)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khan, I.; Ahmad, S. The Impact of Natural Antioxidants on Human Health. In Functional Food Products and Sustainable Health; Ahmad, S., Al-Shabib, N.A., Eds.; Springer Singapore: Singapore, 2020; pp. 11–24. [Google Scholar]
- Granato, D.; Barba, F.J.; Kovačević, D.B.; Lorenzo, J.M.; Cruz, A.G.; Putnik, P. Functional Foods: Product Development, Technological Trends, Efficacy Testing, and Safety. Annu. Rev. Food Sci. Technol. 2020, 11, 93–118. [Google Scholar] [CrossRef]
- Poswal, F.S.; Russell, G.; Mackonochie, M.; MacLennan, E.; Adukwu, E.C.; Rolfe, V. Herbal Teas and their Health Benefits: A Scoping Review. Plant. Foods Hum. Nutr. 2019, 74, 266–276. [Google Scholar] [CrossRef]
- Van Wyk, B.-E.; Wink, M. Medicinal Plants of the World; CABI: Wallingford, UK, 2018. [Google Scholar]
- Saffarzadeh-Matin, S.; Khosrowshahi, F.M. Phenolic compounds extraction from Iranian pomegranate (Punica granatum) industrial waste applicable to pilot plant scale. Ind. Crops Prod. 2017, 108, 583–597. [Google Scholar] [CrossRef]
- Niaz, K.; Khan, F. Chapter 3-Analysis of polyphenolics. In Recent Advances in Natural Products Analysi; Sanches Silva, A., Nabavi, S.F., Saeedi, M., Nabavi, S.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 39–197. [Google Scholar]
- Wu, Q.-Q.; Liang, Y.-F.; Ma, S.-B.; Li, H.; Gao, W.-Y. Stability and stabilization of (–)-gallocatechin gallate under various experimental conditions and analyses of its epimerization, auto-oxidation, and degradation by LC-MS. J. Sci. Food Agric. 2019, 99, 5984–5993. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Singh, J.P.; Kaur, A.; Singh, N. Phenolic compounds as beneficial phytochemicals in pomegranate (Punica granatum L.) peel: A review. Food Chem. 2018, 261, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Vučić, V.; Grabež, M.; Trchounian, A.; Arsić, A. Composition and Potential Health Benefits of Pomegranate: A Review. Curr. Pharm. Des. 2019, 25, 1817–1827. [Google Scholar] [CrossRef]
- Shukla, R.; Kashaw, V. Extraction and wound healing potential of Nerium Indicum M, Artocarpus Heterophyllus Lam, Murraya Koenigii L, Punica Granatum L on albino rats using burn wound model. J. Drug Deliv. Ther. 2019, 9, 337–346. [Google Scholar] [CrossRef]
- Mestry, S.N.; Gawali, N.B.; Pai, S.A.; Gursahani, M.S.; Dhodi, J.B.; Munshi, R.; Juvekar, A.R. Punica granatum improves renal function in gentamicin-induced nephropathy in rats via attenuation of oxidative stress. J. Ayurveda Integr. Med. 2020, 11, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Al-Muammar, M.N.; Khan, F. Obesity: The preventive role of the pomegranate (Punica granatum). Nutrition 2012, 28, 595–604. [Google Scholar] [CrossRef]
- Rao, S.P.; Krishnamurthy, V. Ethanol Extract of Punica granatum L. Leaf in Combination with Gallic acid Ameliorates Liver Dysfunction in High-fat Diet-induced Obese Rats. Indian J. Pharm. Sci. 2019, 81, 673–680. [Google Scholar] [CrossRef]
- Yu, M.; Gouvinhas, I.; Rocha, J.; Barros, A.I.R.N.A. Phytochemical and antioxidant analysis of medicinal and food plants towards bioactive food and pharmaceutical resources. Sci. Rep. 2021, 11, 10041. [Google Scholar] [CrossRef] [PubMed]
- Trabelsi, A.; El Kaibi, M.A.; Abbassi, A.; Horchani, A.; Chekir-Ghedira, L.; Ghedira, K. Phytochemical Study and Antibacterial and Antibiotic Modulation Activity of Punica granatum (Pomegranate) Leaves. Scientifica 2020, 2020, 8271203. [Google Scholar] [CrossRef]
- Pinheiro, A.J.M.C.R.; Mendes, A.R.S.; Neves, M.D.F.d.J.; Prado, C.M.; Bittencourt-Mernak, M.I.; Santana, F.P.R.; Lago, J.H.G.; de Sá, J.C.; da Rocha, C.Q.; de Sousa, E.M.; et al. Galloyl-Hexahydroxydiphenoyl (HHDP)-Glucose Isolated From Punica granatum L. Leaves Protects Against Lipopolysaccharide (LPS)-Induced Acute Lung Injury in BALB/c Mice. Front. Immunol. 2019, 10, 1978. [Google Scholar] [CrossRef]
- Pottathil, S.; Nain, P.; Morsy, M.A.; Kaur, J.; Al-Dhubiab, B.E.; Jaiswal, S.; Nair, A.B. Mechanisms of Antidiabetic Activity of Methanolic Extract of Punica granatum Leaves in Nicotinamide/Streptozotocin-Induced Type 2 Diabetes in Rats. Plants 2020, 9, 1609. [Google Scholar] [CrossRef]
- Gouvinhas, I.; Breda, C.; Barros, A.I. Characterization and Discrimination of Commercial Portuguese Beers Based on Phenolic Composition and Antioxidant Capacity. Foods 2021, 10, 1144. [Google Scholar] [CrossRef] [PubMed]
- Dambergs, R.G.; Mercurio, M.D.; Kassara, S.; Cozzolino, D.; Smith, P.A. Rapid measurement of methyl cellulose precipitable tannins using ultraviolet spectroscopy with chemometrics: Application to red wine and inter-laboratory calibration transfer. Appl. Spectrosc. 2012, 66, 656–664. [Google Scholar] [CrossRef] [PubMed]
- Mena, P.; García-Viguera, C.; Navarro-Rico, J.; Moreno, D.A.; Bartual, J.; Saura, D.; Martí, N. Phytochemical characterisation for industrial use of pomegranate (Punica granatum L.) cultivars grown in Spain. J. Sci. Food Agric. 2011, 91, 1893–1906. [Google Scholar] [CrossRef]
- Fraga-Corral, M.; García-Oliveira, P.; Pereira, A.G.; Lourenço-Lopes, C.; Jimenez-Lopez, C.; Prieto, M.A.; Simal-Gandara, J. Technological Application of Tannin-Based Extracts. Molecules 2020, 25, 614. [Google Scholar] [CrossRef]
- Zeng, L.; Ma, M.; Li, C.; Luo, L. Stability of tea polyphenols solution with different pH at different temperatures. Int. J. Food Prop. 2017, 20, 1–18. [Google Scholar] [CrossRef]
- Das, A.K.; Islam, M.N.; Faruk, M.; Ashaduzzaman, M.; Dungani, R.; Rosamah, E.; Hartati, S.; Rumidatul, A. Hardwood Tannin: Sources, Utilizations, and Prospects. In Tannins-Structural Properties, Biological Properties and Current Knowledge; Aires, A., Ed.; IntechOpen: London, UK, 2019; pp. 1–18. [Google Scholar]
- Dai, Q.; He, Y.; Ho, C.-T.; Wang, J.; Wang, S.; Yang, Y.; Gao, L.; Xia, T. Effect of interaction of epigallocatechin gallate and flavonols on color alteration of simulative green tea infusion after thermal treatment. J. Food Sci. Technol. 2017, 54, 2919–2928. [Google Scholar] [CrossRef]
- Toda, K.; Ueyama, M.; Tanaka, S.; Tsukayama, I.; Mega, T.; Konoike, Y.; Tamenobu, A.; Bastian, F.; Akai, I.; Ito, H.; et al. Ellagitannins from Punica granatum leaves suppress microsomal prostaglandin E synthase-1 expression and induce lung cancer cells to undergo apoptosis. Biosci. Biotechnol. Biochem. 2020, 84, 757–763. [Google Scholar] [CrossRef]
- Swilam, N.; Nematallah, K.A. Polyphenols profile of pomegranate leaves and their role in green synthesis of silver nanoparticles. Sci. Rep. 2020, 10, 14851. [Google Scholar] [CrossRef] [PubMed]
- Villalba, K.J.O.; Barka, F.V.; Pasos, C.V.; Rodríguez, P.E. Food ellagitannins: Structure, metabolomic fate, and biological properties. In Tannins-Structural Properties, Biological Properties and Current Knowledge; Aires, A., Ed.; IntechOpen: London, UK, 2019; pp. 26–46. [Google Scholar]
- Ito, H.; Li, P.; Koreishi, M.; Nagatomo, A.; Nishida, N.; Yoshida, T. Ellagitannin oligomers and a neolignan from pomegranate arils and their inhibitory effects on the formation of advanced glycation end products. Food Chem. 2014, 152, 323–330. [Google Scholar] [CrossRef]
- Fellah, B.; Rocchetti, G.; Senizza, B.; Giuberti, G.; Bannour, M.; Ferchichi, A.; Lucini, L. Untargeted metabolomics reveals changes in phenolic profile following in vitro large intestine fermentation of non-edible parts of Punica granatum L. Food Res. Int. 2020, 128, 108807. [Google Scholar] [CrossRef]
- Macierzyński, J.; Sójka, M.; Kosmala, M.; Karlińska, E. Transformation of Oligomeric Ellagitannins, Typical for Rubus and Fragaria Genus, during Strong Acid Hydrolysis. J. Agric. Food Chem. 2020, 68, 8212–8222. [Google Scholar] [CrossRef]
- Sójka, M.; Janowski, M.; Grzelak-Błaszczyk, K. Stability and transformations of raspberry (Rubus idaeus L.) ellagitannins in aqueous solutions. Eur. Food Res. Technol. 2019, 245, 1113–1122. [Google Scholar] [CrossRef]
- Yang, X.; Tomás-Barberán, F.A. Tea Is a Significant Dietary Source of Ellagitannins and Ellagic Acid. J. Agric. Food Chem. 2019, 67, 5394–5404. [Google Scholar] [CrossRef] [PubMed]
- Shahat, A.A.; Marzouk, M.S. 13-Tannins and Related Compounds from Medicinal Plants of Africa. In Medicinal Plant Research in Africa; Kuete, V., Ed.; Elsevier: Oxford, UK, 2013; pp. 479–555. [Google Scholar]
- García-Villalba, R.; Espín, J.C.; Aaby, K.; Alasalvar, C.; Heinonen, M.; Jacobs, G.; Voorspoels, S.; Koivumäki, T.; Kroon, P.A.; Pelvan, E.; et al. Validated Method for the Characterization and Quantification of Extractable and Nonextractable Ellagitannins after Acid Hydrolysis in Pomegranate Fruits, Juices, and Extracts. J. Agric. Food Chem. 2015, 63, 6555–6566. [Google Scholar] [CrossRef]
- Wang, C.; Shi, L.; Fan, L.; Ding, Y.; Zhao, S.; Liu, Y.; Ma, C. Optimization of extraction and enrichment of phenolics from pomegranate (Punica granatum L.) leaves. Ind. Crops Prod. 2013, 42, 587–594. [Google Scholar] [CrossRef]
- Kaneria, M.J.; Bapodara, M.B.; Chanda, S.V. Effect of Extraction Techniques and Solvents on Antioxidant Activity of Pomegranate (Punica granatum L.) Leaf and Stem. Food Anal. Methods 2012, 5, 396–404. [Google Scholar] [CrossRef]
- Uysal, S.; Zengin, G.; Aktumsek, A.; Karatas, S. Chemical and biological approaches on nine fruit tree leaves collected from the Mediterranean region of Turkey. J. Funct. Foods 2016, 22, 518–532. [Google Scholar] [CrossRef]
- Akkawi, M.; Abu-Lafi, S.; Abu-Remeleh, Q. Phytochemical screening of pomegranate juice, peels, leaves and membranes water extracts and their effect on β-hematin formation, a comparative study. Pharm. Pharmacol. Int. J. 2019, 7, 193–200. [Google Scholar] [CrossRef]
- Viswanatha, G.L.; Venkataranganna, M.V.; Prasad, N.B.L.; Ashok, G. Evaluation of anti-epileptic activity of leaf extracts of Punica granatum on experimental models of epilepsy in mice. J. Intercult. Ethnopharmacol. 2016, 5, 415–421. [Google Scholar] [CrossRef]
- Orgil, O.; Schwartz, E.; Baruch, L.; Matityahu, I.; Mahajna, J.; Amir, R. The antioxidative and anti-proliferative potential of non-edible organs of the pomegranate fruit and tree. LWT 2014, 58, 571–577. [Google Scholar] [CrossRef]
- Kaderides, K.; Mourtzinos, I.; Goula, A.M. Stability of pomegranate peel polyphenols encapsulated in orange juice industry by-product and their incorporation in cookies. Food Chem. 2020, 310, 125849. [Google Scholar] [CrossRef]
- Manzoor, A.; Dar, I.H.; Bhat, S.A.; Ahmad, S. Flavonoids: Health Benefits and Their Potential Use in Food Systems. In Functional Food Products and Sustainable Health; Springer: Berlin/Heidelberg, Germany, 2020; pp. 235–256. [Google Scholar]
- Moser, P.; Telis, V.R.N.; de Andrade Neves, N.; García-Romero, E.; Gómez-Alonso, S.; Hermosín-Gutiérrez, I. Storage stability of phenolic compounds in powdered BRS Violeta grape juice microencapsulated with protein and maltodextrin blends. Food Chem. 2017, 214, 308–318. [Google Scholar] [CrossRef] [PubMed]
- Krook, M.; Kreinberg, A.; Hagerman, A.; Gonzalez, J.; Halvorson, J. Tannin (Polyphenol) Stability in Aqueous Solutions. In Proceedings of the Soil Science Society of America Annual Meeting, Pittsburgh, PA, USA, 1–5 November 2009. [Google Scholar]
- Xu, P.; Chen, L.; Wang, Y. Effect of storage time on antioxidant activity and inhibition on α-amylase and α-glucosidase of white tea. Food Sci. Nutr. 2019, 7, 636–644. [Google Scholar] [CrossRef]
- Granato, D.; Shahidi, F.; Wrolstad, R.; Kilmartin, P.; Melton, L.D.; Hidalgo, F.J.; Miyashita, K.; Camp, J.v.; Alasalvar, C.; Ismail, A.B.; et al. Antioxidant activity, total phenolics and flavonoids contents: Should we ban in vitro screening methods? Food Chem. 2018, 264, 471–475. [Google Scholar] [CrossRef] [PubMed]
- Kaewnarin, K.; Niamsup, H.; Shank, L.; Rakariyatham, N. Antioxidant and antiglycation activities of some edible and medicinal plants. Chiang Mai J. Sci 2014, 41, 105–116. [Google Scholar]
- Gil, M.I.; Tomás-Barberán, F.A.; Hess-Pierce, B.; Holcroft, D.M.; Kader, A.A. Antioxidant Activity of Pomegranate Juice and Its Relationship with Phenolic Composition and Processing. J. Agric. Food Chem. 2000, 48, 4581–4589. [Google Scholar] [CrossRef]
- Saratale, R.G.; Shin, H.S.; Kumar, G.; Benelli, G.; Kim, D.-S.; Saratale, G.D. Exploiting antidiabetic activity of silver nanoparticles synthesized using Punica granatum leaves and anticancer potential against human liver cancer cells (HepG2). Artif. Cells Nanomed. Biotechnol. 2018, 46, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Eddebbagh, M.; Messaoudi, M.; Abourriche, A.; Berrada, M.; Attaleb, M.; Benbacer, L.; Bennamara, A. Correlation of the cytotoxic and antioxidant activities of moroccan pomegranate (Punica granatum) with phenolic and flavonoid contents. J. Pharm. Pharmacol. 2016, 4, 511–519. [Google Scholar] [CrossRef][Green Version]
- Apak, R.; Capanoglu, E.; Shahidi, F. Measurement of Antioxidant Activity and Capacity; Wiley Online Library: Hoboken, NJ, USA, 2017. [Google Scholar]
- Cai, Y.; Zhang, J.; Chen, N.G.; Shi, Z.; Qiu, J.; He, C.; Chen, M. Recent Advances in Anticancer Activities and Drug Delivery Systems of Tannins. Med. Res. Rev. 2017, 37, 665–701. [Google Scholar] [CrossRef] [PubMed]
- Negrão-Murakami, A.N.; Nunes, G.L.; Pinto, S.S.; Murakami, F.S.; Amante, E.R.; Petrus, J.C.C.; Prudêncio, E.S.; Amboni, R.D.M.C. Influence of DE-value of maltodextrin on the physicochemical properties, antioxidant activity, and storage stability of spray dried concentrated mate (Ilex paraguariensis A. St. Hil.). LWT 2017, 79, 561–567. [Google Scholar] [CrossRef]
- Rocha-Parra, D.F.; Lanari, M.C.; Zamora, M.C.; Chirife, J. Influence of storage conditions on phenolic compounds stability, antioxidant capacity and colour of freeze-dried encapsulated red wine. LWT 2016, 70, 162–170. [Google Scholar] [CrossRef]
- Lakshminarayanashastry Viswanatha, G.; Venkatanarasappa Venkataranganna, M.; Lingeswara Prasad, N.B. Methanolic leaf extract of Punica granatum attenuates ischemia-reperfusion brain injury in Wistar rats: Potential antioxidant and anti-inflammatory mechanisms. Iran. J. Basic Med. Sci. 2019, 22, 187–196. [Google Scholar] [CrossRef]
- Acquadro, S.; Civra, A.; Cagliero, C.; Marengo, A.; Rittà, M.; Francese, R.; Sanna, C.; Bertea, C.; Sgorbini, B.; Lembo, D. Punica granatum Leaf Ethanolic Extract and Ellagic Acid as Inhibitors of Zika Virus Infection. Planta Med. 2020, 86, 1363–1374. [Google Scholar] [CrossRef] [PubMed]
- Moghaddam, G.; Sharifzadeh, M.; Hassanzadeh, G.; Khanavi, M.; Hajimahmoodi, M. Anti-ulcerogenic activity of the pomegranate peel (Punica granatum) methanol extract. Food Nutr. Sci. 2013, 4, 43. [Google Scholar] [CrossRef]


| Spectrophotometric Analysis 1 | Storage Time (Hours) 2 | Degradation | p-Value (2–8 h) | p-Value (0–24 h) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 2 | 4 | 6 | 8 | 24 | |||||
| Phenolic content | Total phenols (mg GA g−1 DW) | 133.47 ± 3.40 a | 132.02 ± 5.01 a | 132.49 ± 2.35 a | 132.34 ± 0.46 a | 129.67 ± 4.50 a | 131.40 ± 1.60 a | 1.55% | 0.43 | 0.80 |
| Ortho-diphenols (mg GA g−1 DW) | 244.25 ± 3.59 a | 238.63 ± 2.32 a | 240.01 ± 2.14 a | 237.24 ± 3.25 a | 240.42 ± 0.63 a | 239.91 ± 3.57 a | 1.78% | 0.70 | 0.12 | |
| Flavonoids (mg RU g−1 DW) | 55.84 ± 0.45 a | 54.86 ± 0.73 ab | 54.46 ± 0.54 abc | 53.67 ± 0.78 bc | 53.81 ± 0.22 bc | 53.30 ± 0.11 c | 4.54% | 0.61 | 0.00 | |
| Tannins (mg EC g−1 DW) | 124.20 ± 3.67 a | 110.60 ± 3.50 b | 109.35 ± 1.11 bc | 107.37 ± 2.90 bc | 105.68 ± 3.42 bc | 102.15 ± 1.24 c | 17.75% | 0.27 | 0.00 | |
| Antioxidant capacity (mmol Trolox g−1 DW) | ABTS scavenging | 1.80 ± 0.00 a | 1.72 ± 0.02 b | 1.71 ± 0.01 b | 1.69 ± 0.04 b | 1.68 ± 0.04 b | 1.66 ± 0.03 b | 7.93% | 0.63 | 0.00 |
| DPPH scavenging | 1.69 ± 0.01 a | 1.63 ± 0.04 ab | 1.60 ± 0.03 ab | 1.54 ± 0.03 b | 1.56 ± 0.03 b | 1.59 ± 0.06 b | 5.92% | 0.14 | 0.00 | |
| FRAP assay | 2.23 ± 0.01 a | 2.21 ± 0.01 a | 2.09 ± 0.01 b | 2.06 ± 0.01 bc | 2.03 ± 0.02 c | 2.03 ± 0.03 c | 9.29% | 0.00 | 0.00 | |
| Peak NO. 1 | Retention Time (min) | Identified Compounds 2 | Decrease/Increase Percentage (%) | p-Value 3 |
|---|---|---|---|---|
| 1 | 10.98 | Gallic acid | 7.59 | *** |
| 2 | 11.12 | Flavan-3-ols | 4.11 | *** |
| 3 | 12.73 | Punicalin A and B | 5.14 | * |
| 4 | 13.38 | Ellagic acid derivatives | 2.48 | *** |
| 5 | 15.25 | Ellagitannin I | 7.37 | *** |
| 6 | 16.31 | Flavanone glycoside I | - | - |
| 7 | 16.54 | Ellagitannin II | 11.99 | *** |
| 8 | 17.24 | Ellagitannin III (galloyl-glucose) | 12.82 | *** |
| 9 | 19.01 | Ellagitannin IV (punicalagin A) | 5.29 | *** |
| 10 | 20.14 | Flavonol glycoside | 52.98 | *** |
| 11 | 20.37 | Ellagitannin V | 50.08 | *** |
| 12 | 21.30 | Ellagitannin VI | 19.36 | *** |
| 13 | 22.56 | Ellagitannin VII (punicalagin B) | 3.93 | *** |
| 14 | 23.33 | Ellagitannin VIII | 4.45 | *** |
| 15 | 24.35 | Ellagic acid | 36.39 | *** |
| 16 | 24.90 | Ellagitannin IX | 8.41 | *** |
| 17 | 25.10 | Flavanone glycoside II | - | - |
| 18 | 26.44 | Apigenin glycoside | 31.98 | *** |
| 19 | 27.13 | Luteolin glycoside I | 35.09 | *** |
| 20 | 28.38 | Luteolin glycoside II | 36.03 | *** |
| GA 2 | FO | PU | EAD | ET-I | ET-II | ET-III | ET- IV | FG | ET-V | ET- VI | ET- VII | ET- VIII | EA | ET- IX | AG | LG-I | LG- II | ABTS | DPPH | FRAP | TPs | OPs | TFs | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FO | 0.62 | |||||||||||||||||||||||
| PU | −0.06 | −0.68 | ||||||||||||||||||||||
| EAD | −0.48 | 0.23 | −0.81 | |||||||||||||||||||||
| ET-I | 0.50 | −0.14 | 0.60 | −0.84 * | ||||||||||||||||||||
| ET-II | 0.30 | −0.44 | 0.76 | −0.82 * | 0.94 **,1 | |||||||||||||||||||
| ET-III | −0.58 | 0.20 | −0.69 | 0.89 * | −0.95** | −0.95 ** | ||||||||||||||||||
| ET-IV | −0.21 | 0.54 | −0.93 ** | 0.85 * | −0.58 | −0.74 | 0.76 | |||||||||||||||||
| FG | 0.51 | −0.26 | 0.73 | −0.90 * | 0.96 ** | 0.97 ** | −0.99 *** | −0.77 | ||||||||||||||||
| ET-V | 0.55 | −0.17 | 0.63 | −0.85 * | 0.98 *** | 0.96 ** | −0.99 *** | −0.66 | 0.99 *** | |||||||||||||||
| ET-VI | −0.55 | 0.24 | −0.72 | 0.91 * | −0.94 ** | −0.95 ** | 0.99 *** | 0.79 | −0.99 *** | −0.98 *** | ||||||||||||||
| ET-VII | −0.22 | 0.49 | −0.80 | 0.73 | −0.37 | −0.54 | 0.61 | 0.95 ** | −0.60 | −0.47 | 0.64 | |||||||||||||
| ET-VIII | −0.40 | 0.40 | −0.87 * | 0.93 ** | −0.69 | −0.79 | 0.85 * | 0.97 ** | −0.85 * | −0.76 | 0.87 * | 0.92 ** | ||||||||||||
| EA | 0.60 | 0.04 | 0.40 | −0.77 | 0.95 ** | 0.82 * | −0.86 * | −0.41 | 0.87 * | 0.91 * | −0.85 * | −0.22 | −0.57 | |||||||||||
| ET-IX | 0.78 | 0.15 | 0.47 | −0.86 * | 0.92 ** | 0.79 | −0.93 ** | −0.57 | 0.91 * | 0.93 ** | −0.92 * | −0.43 | −0.72 | 0.93 ** | ||||||||||
| AG | 0.06 | −0.50 | 0.75 | −0.73 | 0.89 * | 0.93 ** | −0.80 | −0.59 | 0.85 * | 0.85 * | −0.81 | −0.34 | −0.62 | 0.78 | 0.66 | |||||||||
| LG-I | 0.03 | −0.53 | 0.75 | −0.72 | 0.88 * | 0.93 ** | −0.79 | −0.60 | 0.84 * | 0.84 * | −0.80 | −0.35 | −0.62 | 0.76 | 0.64 | 0.99 *** | ||||||||
| LG-II | 0.06 | −0.42 | 0.69 | −0.72 | 0.88 * | 0.88 * | −0.75 | −0.51 | 0.81 | 0.82 * | −0.76 | −0.25 | −0.56 | 0.80 | 0.66 | 0.99 *** | 0.98 *** | |||||||
| ABTS | −0.08 | 0.66 | −0.95 ** | 0.84 * | −0.77 | −0.92 * | 0.84 * | 0.91 * | −0.87 * | −0.81 | 0.86 * | 0.76 | 0.88 * | −0.58 | −0.62 | −0.86 * | −0.87 * | −0.79 | ||||||
| DPPH | 0.02 | 0.72 | −0.90 * | 0.75 | −0.44 | −0.64 | 0.60 | 0.95 ** | −0.63 | −0.50 | 0.64 | 0.94 ** | 0.89 * | −0.28 | −0.37 | −0.54 | −0.55 | −0.46 | 0.86 * | |||||
| FRAP | −0.26 | 0.52 | −0.83 * | 0.91 * | −0.75 | −0.84 * | 0.82 * | 0.88 * | −0.85 * | −0.78 | 0.85 * | 0.80 | 0.92 ** | −0.68 | −0.69 | −0.74 | −0.74 | −0.71 | 0.89 * | 0.88 * | ||||
| TPs | 0.03 | 0.35 | −0.82 | 0.79 | −0.61 | −0.59 | 0.54 | 0.63 | −0.60 | −0.54 | 0.57 | 0.45 | 0.64 | −0.51 | −0.51 | −0.72 | −0.72 | −0.77 | 0.71 | 0.60 | 0.66 | |||
| OPs | 0.21 | 0.70 | −0.77 | 0.38 | −0.19 | −0.46 | 0.39 | 0.78 | −0.40 | −0.29 | 0.42 | 0.75 | 0.63 | 0.10 | −0.07 | −0.36 | −0.37 | −0.23 | 0.72 | 0.76 | 0.46 | 0.33 | ||
| TFs | −0.25 | 0.56 | −0.91 * | 0.90 * | −0.78 | −0.91 * | 0.89 * | 0.95 ** | −0.91 * | −0.83 * | 0.91 * | 0.84 * | 0.96 ** | −0.62 | −0.70 | −0.79 | −0.79 | −0.71 | 0.98 *** | 0.89 * | 0.94 ** | 0.65 | 0.68 | |
| CTs | −0.01 | 0.70 | −0.94 ** | 0.79 | −0.76 | −0.92 * | 0.81 * | 0.87 * | −0.85 * | −0.79 | 0.84 * | 0.70 | 0.84 * | −0.56 | −0.57 | −0.89 * | −0.89 * | −0.82 | 0.99 *** | 0.83 * | 0.86 * | 0.70 | 0.72 | 0.95 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, M.; Gouvinhas, I.; Barros, A. Variation of the Polyphenolic Composition and Antioxidant Capacity of Freshly Prepared Pomegranate Leaf Infusions over One-Day Storage. Antioxidants 2021, 10, 1187. https://doi.org/10.3390/antiox10081187
Yu M, Gouvinhas I, Barros A. Variation of the Polyphenolic Composition and Antioxidant Capacity of Freshly Prepared Pomegranate Leaf Infusions over One-Day Storage. Antioxidants. 2021; 10(8):1187. https://doi.org/10.3390/antiox10081187
Chicago/Turabian StyleYu, Manyou, Irene Gouvinhas, and Ana Barros. 2021. "Variation of the Polyphenolic Composition and Antioxidant Capacity of Freshly Prepared Pomegranate Leaf Infusions over One-Day Storage" Antioxidants 10, no. 8: 1187. https://doi.org/10.3390/antiox10081187
APA StyleYu, M., Gouvinhas, I., & Barros, A. (2021). Variation of the Polyphenolic Composition and Antioxidant Capacity of Freshly Prepared Pomegranate Leaf Infusions over One-Day Storage. Antioxidants, 10(8), 1187. https://doi.org/10.3390/antiox10081187









