Redox Biomarker Baseline Levels in Cattle Tissues and Their Relationships with Meat Quality
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Sample Collection
2.3. Blood Fractionation
2.4. Tissue Homogenization and the Measurement of the Total Protein Concentration
2.5. Redox Biomarkers Determination
2.5.1. Reduced form of Glutathione (GSH)
2.5.2. Catalase (CAT)
2.5.3. Total Antioxidant Activity (TAC)
2.5.4. Thiobarbituric Acid Reactive Substances (TBARS)
2.5.5. Protein Carbonyls
2.6. Meat Quality Assessment
- Hardness 1: The maximum force during the first compression cycle, representing the hardness of the sample experienced during the first bite.
- Hardness 2: The maximum force during the second compression cycle, representing the hardness of the sample experienced during the second bite.
- Cohesiveness: The ratio of the total work in the second compression cycle to the total work in the first, indicating the degree of the sample’s resistance to deformation during the first compression that was retained during the second compression.
- Springiness: The ratio of the sample height in the second compression to the original height detected in the first compression, indicating how well a sample can return to its original form after being compressed and deformed during the first cycle.
- Chewiness: The outcome of the multiplication of Hardness 1 * Cohesiveness * Springiness, indicating the energy needed to chew the sample to the point where it can be swallowed.
2.7. Statistical Analysis
3. Results
3.1. Reduced Form of Glutathione
3.2. Catalase Activity
3.3. Total Antioxidant Capacity Levels
3.4. Lipid Peroxidation Levels
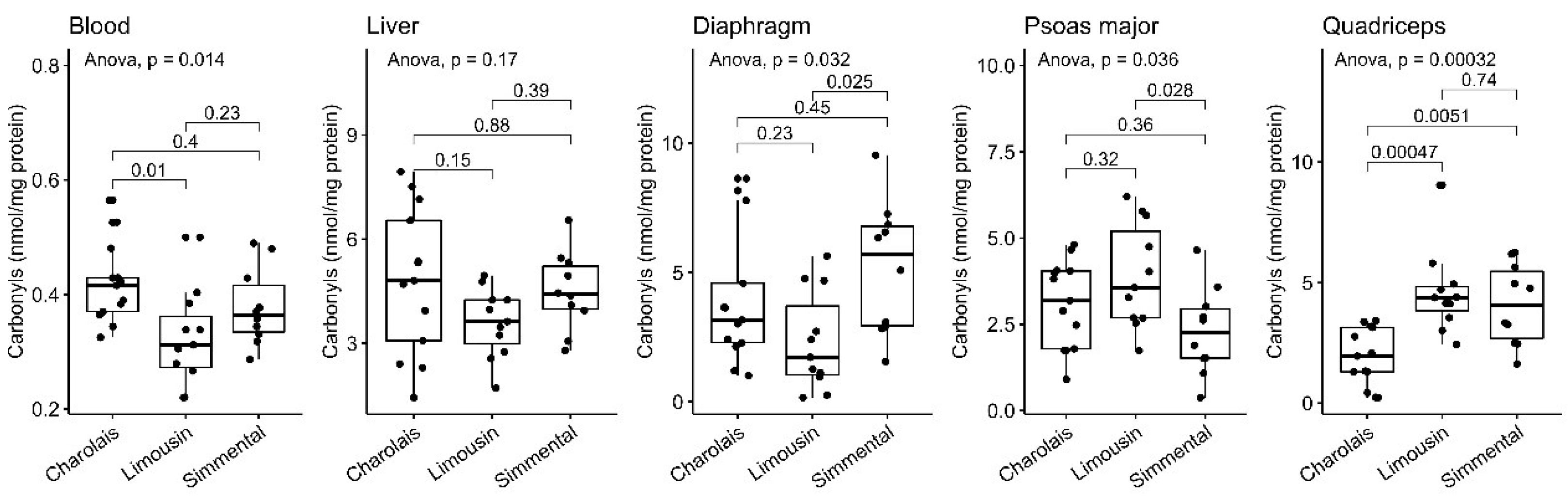
3.5. Protein Carbonyl Content
3.6. Effect of the Redox Status on the Meat Quality Parameters
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| LIM | Limousin cattle breed |
| CHA | Charolais cattle breed |
| SIM | Simmental cattle breed |
| GSH | reduced glutathione |
| CAT | catalase |
| TAC | total antioxidant activity |
| TBARS | thiobarbituric acid reactive substances |
| CARBs | protein carbonyls |
References
- Frona, D.; Szenderák, J.; Harangi-Rákos, M. The Challenge of Feeding the World. Sustainability 2019, 11, 5816. [Google Scholar] [CrossRef]
- Gaughan, J.B.; Sejian, V.; Mader, T.L.; Dunshea, F.R. Adaptation strategies: Ruminants. Anim. Front. 2019, 9, 47–53. [Google Scholar] [CrossRef]
- Davis, T.C.; White, R.R. Breeding animals to feed people: The many roles of animal reproduction in ensuring global food security. Theriogenology 2020, 150, 27–33. [Google Scholar] [CrossRef]
- Henry, B.K.; Eckard, R.J.; Beauchemin, K.A. Review: Adaptation of ruminant livestock production systems to climate changes. Animal 2018, 12, s445–s456. [Google Scholar] [CrossRef] [PubMed]
- Kimman, T.; Hoek, M.; de Jong, M.C.M. Assessing and controlling health risks from animal husbandry. NJAS Wageningen J. Life Sci. 2013, 66, 7–14. [Google Scholar] [CrossRef]
- Cronin, G.M.; Rault, J.L.; Glatz, P.C. Lessons learned from past experience with intensive livestock management systems. Rev. Sci. Tech. 2014, 33, 139–151. [Google Scholar] [CrossRef]
- WHO. Livestock and Human and Animal Health; WHO: Geneva, Switzerland, 2005. [Google Scholar]
- Oltenacu, P.A.; Algers, B. Selection for increased production and the welfare of dairy cows: Are new breeding goals needed? Ambio 2005, 34, 311–315. [Google Scholar] [CrossRef]
- Puppel, K.; Kapusta, A.; Kuczyńska, B. The etiology of oxidative stress in the various species of animals, a review. J. Sci. Food Agric. 2015, 95, 2179–2184. [Google Scholar] [CrossRef] [PubMed]
- Sampels, S. Oxidation and Antioxidants in Fish and Meat from Farm to Fork. In Food Industry; IntechOpen: London, UK, 2013. [Google Scholar]
- Shahidi, F.; Zhong, Y. Lipid oxidation and improving the oxidative stability. Chem. Soc. Rev. 2010, 39, 4067–4079. [Google Scholar] [CrossRef]
- Domínguez, R.; Pateiro, M.; Gagaoua, M.; Barba, F.J.; Zhang, W.; Lorenzo, J.M. A Comprehensive Review on Lipid Oxidation in Meat and Meat Products. Antioxidants 2019, 8, 429. [Google Scholar] [CrossRef]
- Soladoye, O.P.; Juárez, M.L.; Aalhus, J.L.; Shand, P.; Estévez, M. Protein Oxidation in Processed Meat: Mechanisms and Potential Implications on Human Health. Compr. Rev. Food Sci. Food Saf. 2015, 14, 106–122. [Google Scholar] [CrossRef]
- Papuc, C.; Goran, G.V.; Predescu, C.N.; Nicorescu, V. Mechanisms of Oxidative Processes in Meat and Toxicity Induced by Postprandial Degradation Products: A Review. Compr. Rev. Food Sci. Food Saf. 2017, 16, 96–123. [Google Scholar] [CrossRef]
- Birben, E.; Sahiner, U.M.; Sackesen, C.; Erzurum, S.; Kalayci, O. Oxidative stress and antioxidant defense. World Allergy Organ. J. 2012, 5, 9–19. [Google Scholar] [CrossRef]
- Piszcz, P.; Tomaszewska, M.; Głód, B.K. Estimation of the total antioxidant potential in the meat samples using thin-layer chromatography. Open Chem. 2020, 18, 50–57. [Google Scholar] [CrossRef]
- Halliwell, B.; Gutteridge, J.M.C. Antioxidant defences synthesized in vivo. In Free Radicals in Biology and Medicine; Oxford University Press: Oxford, UK, 2015; ISBN 9780198717478. [Google Scholar]
- Pradhan, A.A.; Rhee, K.S.; Hernández, P. Stability of catalase and its potential role in lipid oxidation in meat. Meat Sci. 2000, 54, 385–390. [Google Scholar] [CrossRef]
- Hosseindoust, A.; Oh, S.M.; Ko, H.S.; Jeon, S.M.; Ha, S.H.; Jang, A.; Son, J.S.; Kim, G.Y.; Kang, H.K.; Kim, J.S. Muscle Antioxidant Activity and Meat Quality Are Altered by Supplementation of Astaxanthin in Broilers Exposed to High Temperature. Antioxidants 2020, 9, 1032. [Google Scholar] [CrossRef]
- Bourne, N.; Wathes, D.C.; Lawrence, K.E.; McGowan, M.; Laven, R.A. The effect of parenteral supplementation of vitamin E with selenium on the health and productivity of dairy cattle in the UK. Vet. J. 2008, 177, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Politis, I. Reevaluation of vitamin E supplementation of dairy cows: Bioavailability, animal health and milk quality. Animal 2012, 6, 1427–1434. [Google Scholar] [CrossRef] [PubMed]
- Amaral, G.P.; Mizdal, C.R.; Stefanello, S.T.; Mendez, A.S.L.; Puntel, R.L.; de Campos, M.M.A.; Soares, F.A.A.; Fachinetto, R. Antibacterial and antioxidant effects of Rosmarinus officinalis L. extract and its fractions. J. Tradit. Complement. Med. 2019, 9, 383–392. [Google Scholar] [CrossRef] [PubMed]
- Vossen, E.; Ntawubizi, M.; Raes, K.; Smet, K.; Huyghebaert, G.; Arnouts, S.; De Smet, S. Effect of dietary antioxidant supplementation on the oxidative status of plasma in broilers. J. Anim. Physiol. Anim. Nutr. 2011, 95, 198–205. [Google Scholar] [CrossRef]
- Renerre, M.; Poncet, K.; Mercier, Y.; Gatellier, P.; Métro, B. Influence of dietary fat and vitamin E on antioxidant status of muscles of turkey. J. Agric. Food Chem. 1999, 47, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Bouwstra, R.J.; Nielen, M.; Stegeman, J.A.; Dobbelaar, P.; Newbold, J.R.; Jansen, E.H.J.M.; van Werven, T. Vitamin E supplementation during the dry period in dairy cattle. Part I: Adverse effect on incidence of mastitis postpartum in a double-blind randomized field trial. J. Dairy Sci. 2010, 93, 5684–5695. [Google Scholar] [CrossRef]
- Rizzo, A.; Pantaleo, M.; Mutinati, M.; Minoia, G.; Trisolini, C.; Ceci, E.; Sciorsci, R.L. Blood and milk oxidative status after administration of different antioxidants during early postpartum in dairy cows. Res. Vet. Sci. 2013, 95, 1171–1174. [Google Scholar] [CrossRef] [PubMed]
- Abuelo, A.; Hernández, J.; Benedito, J.L.; Castillo, C. The importance of the oxidative status of dairy cattle in the periparturient period: Revisiting antioxidant supplementation. J. Anim. Physiol. Anim. Nutr. 2015, 99, 1003–1016. [Google Scholar] [CrossRef]
- Bouwstra, R.J.; Nielen, M.; Newbold, J.R.; Jansen, E.H.J.M.; Jelinek, H.F.; van Werven, T. Vitamin E supplementation during the dry period in dairy cattle. Part II: Oxidative stress following vitamin E supplementation may increase clinical mastitis incidence postpartum. J. Dairy Sci. 2010, 93, 5696–5706. [Google Scholar] [CrossRef] [PubMed]
- Veskoukis, A.; Kerasioti, E.; Priftis, A.; Kouka, P.; Spanidis, Y.; Makri, S.; Kouretas, D. A battery of translational biomarkers for the assessment of the in vitro and in vivo antioxidant action of plant polyphenolic compounds: The biomarker issue. Curr. Opin. Toxicol. 2019, 13, 99–109. [Google Scholar] [CrossRef]
- Council, E. Parliament European Parliament and Council. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex:32013R1308 (accessed on 27 April 2021).
- Yn, R.; Murthy, S.; Krishna, D.R.; Prabhakar, M.C. Role of free radicals and antioxidants in tuberculosis patients. Indian J. Tuberc 2004, 51, 213–218. [Google Scholar]
- Veskoukis, A.S.; Kyparos, A.; Paschalis, V.; Nikolaidis, M.G. Spectrophotometric assays for measuring redox biomarkers in blood. Biomark. Biochem. Indic. Expo. Response Susceptibility Chem. 2016, 21, 208–217. [Google Scholar] [CrossRef]
- Aebi, H.B.T.-M. E. [13] Catalase in vitro. In Oxygen Radicals in Biological Systems; Academic Press: Cambridge, MA, USA, 1984; Volume 105, pp. 121–126. ISBN 0076-6879. [Google Scholar]
- Janaszewska, A.; Bartosz, G. Assay of total antioxidant capacity: Comparison of four methods as applied to human blood plasma. Scand. J. Clin. Lab. Investig. 2002, 62, 231–236. [Google Scholar] [CrossRef]
- Keles, M.S.; Taysi, S.; Sen, N.; Aksoy, H.; Akçay, F. Effect of corticosteroid therapy on serum and CSF malondialdehyde and antioxidant proteins in multiple sclerosis. Can. J. Neurol. Sci. Le J. Can. Sci. Neurol. 2001, 28, 141–143. [Google Scholar] [CrossRef]
- Spanidis, Y.; Goutzourelas, N.; Stagos, D.; Mpesios, A.; Priftis, A.; Bar-Or, D.; Spandidos, D.A.; Tsatsakis, A.M.; Leon, G.; Kouretas, D. Variations in oxidative stress markers in elite basketball players at the beginning and end of a season. Exp. Ther. Med. 2016, 11, 147–153. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Patsoukis, N.; Zervoudakis, G.; Panagopoulos, N.T.; Georgiou, C.D.; Angelatou, F.; Matsokis, N.A. Thiol redox state (TRS) and oxidative stress in the mouse hippocampus after pentylenetetrazol-induced epileptic seizure. Neurosci. Lett. 2004, 357, 83–86. [Google Scholar] [CrossRef] [PubMed]
- AMSA. AMSA Meat Color Measurement Guidelines; AMSA, Ed.; AMSA, 201 West Springfield Avenue, Suite 1202: Champaign, IL, USA, 2012; ISBN 61820 800-517-2672.
- Trinh, T. On The Texture Profile Analysis Test. In Proceedings of the Chemeca 2012, Wellington, New Zealand, 23–26 September 2012. [Google Scholar]
- Peleg, M. The instrumental texture profile analysis revisited. J. Texture Stud. 2019, 50, 362–368. [Google Scholar] [CrossRef]
- Li, S.; Tan, H.-Y.; Wang, N.; Zhang, Z.-J.; Lao, L.; Wong, C.-W.; Feng, Y. The Role of Oxidative Stress and Antioxidants in Liver Diseases. Int. J. Mol. Sci. 2015, 16, 26087–26124. [Google Scholar] [CrossRef] [PubMed]
- Mello, T.; Zanieri, F.; Ceni, E.; Galli, A. Oxidative Stress in the Healthy and Wounded Hepatocyte: A Cellular Organelles Perspective. Oxid. Med. Cell. Longev. 2016, 2016, 8327410. [Google Scholar] [CrossRef] [PubMed]
- Margaritelis, N.V.; Veskoukis, A.S.; Paschalis, V.; Vrabas, I.S.; Dipla, K.; Zafeiridis, A.; Kyparos, A.; Nikolaidis, M.G. Blood reflects tissue oxidative stress: A systematic review. Biomark. Biochem. Indic. Expo. Response Susceptibility Chem. 2015, 20, 97–108. [Google Scholar] [CrossRef]
- Alzaid, F.; Patel, V.B.; Preedy, V.R. Biomarkers of Oxidative Stress in Blood BT—General Methods in Biomarker Research and their Applications; Preedy, V.R., Patel, V.B., Eds.; Springer: Dordrecht, The Netherlands, 2015; pp. 567–594. ISBN 978-94-007-7696-8. [Google Scholar]
- Forman, H.J.; Zhang, H.; Rinna, A. Glutathione: Overview of its protective roles, measurement, and biosynthesis. Mol. Aspects Med. 2009, 30, 1–12. [Google Scholar] [CrossRef]
- Kurutas, E.B. The importance of antioxidants which play the role in cellular response against oxidative/nitrosative stress: Current state. Nutr. J. 2016, 15, 71. [Google Scholar] [CrossRef]
- Kusano, C.; Ferrari, B. Total antioxidant capacity: A biomarker in biomedical and nutritional studies. J. Cell Mol. Biol. 2008, 7, 1–15. [Google Scholar]
- Estévez, M. Protein carbonyls in meat systems: A review. Meat Sci. 2011, 89, 259–279. [Google Scholar] [CrossRef]
- Solarczyk, P.; Gołębiewski, M.; Slósarz, J.; Łukasiewicz, M.; Przysucha, T.; Puppel, K. Effect of Breed on the Level of the Nutritional and Health-Promoting Quality of Semimembranosus Muscle in Purebred and Crossbred Bulls. Animals 2020, 10, 1822. [Google Scholar] [CrossRef]
- Waritthitham, A.; Lambertz, C.; Langholz, H.-J.; Wicke, M.; Gauly, M. Assessment of beef production from Brahman×Thai native and Charolais×Thai native crossbred bulls slaughtered at different weights. II: Meat quality. Meat Sci. 2010, 85, 196–200. [Google Scholar] [CrossRef]
- Xie, X.; Meng, Q.; Cui, Z.; Ren, L. Effect of Cattle Breed on Meat Quality, Muscle Fiber Characteristics, Lipid Oxidation and Fatty Acids in China. Asian Australas J. Anim. Sci. 2012, 25, 824–831. [Google Scholar] [CrossRef] [PubMed]
- Skaperda, Z.; Veskoukis, S.A.; Kouretas, D. Farm animal welfare, productivity and meat quality: Interrelation with redox status regulation and antioxidant supplementation as a nutritional intervention (Review). World Acad. Sci. J. 2019, 1, 177–183. [Google Scholar] [CrossRef][Green Version]
- Amaral, A.B.; Silva, M.V.d.; Lannes, S.C.d.S. Lipid oxidation in meat: Mechanisms and protective factors a review. Food Sci. Technol. 2018, 38, 1–15. [Google Scholar] [CrossRef]
- Ribeiro, D.; Freitas, M.; Silva, A.M.S.; Carvalho, F.; Fernandes, E. Antioxidant and pro-oxidant activities of carotenoids and their oxidation products. Food Chem. Toxicol. 2018, 120, 681–699. [Google Scholar] [CrossRef] [PubMed]
- da Silva, A.D.; da Silva, A.S.; Baldissera, M.D.; Schwertz, C.I.; Bottari, N.B.; Carmo, G.M.; Machado, G.; Lucca, N.J.; Henker, L.C.; Piva, M.M.; et al. Oxidative stress in dairy cows naturally infected with the lungworm Dictyocaulus viviparus (Nematoda: Trichostrongyloidea). J. Helminthol. 2017, 91, 462–469. [Google Scholar] [CrossRef] [PubMed]
- Glombowsky, P.; Bottari, N.B.; Klauck, V.; Fávero, J.F.; Soldá, N.M.; Baldissera, M.D.; Perin, G.; Morsch, V.M.; Schetinger, M.R.C.; Stefani, L.M.; et al. Oxidative stress in dairy cows seropositives for Neospora caninum. Comp. Immunol. Microbiol. Infect. Dis. 2017, 54, 34–37. [Google Scholar] [CrossRef] [PubMed]
- Fidan, A.F.; Cingi, C.C.; Karafakioglu, Y.S.; Utuk, A.E.; Pekkaya, S.; Piskin, F.C. The Levels of Antioxidant Activity, Malondialdehyde and Nitric Oxide in Cows Naturally Infected with Neospora caninum. J. Anim. Vet. Adv. 2010, 9, 1707–1711. [Google Scholar] [CrossRef]
- Li, Y.; Ding, H.Y.; Wang, X.C.; Feng, S.B.; Li, X.B.; Wang, Z.; Liu, G.W.; Li, X.W. An association between the level of oxidative stress and the concentrations of NEFA and BHBA in the plasma of ketotic dairy cows. J. Anim. Physiol. Anim. Nutr. 2016, 100, 844–851. [Google Scholar] [CrossRef]
- Kuhn, M.J.; Mavangira, V.; Gandy, J.C.; Sordillo, L.M. Production of 15-F2t-isoprostane as an assessment of oxidative stress in dairy cows at different stages of lactation. J. Dairy Sci. 2018, 101, 9287–9295. [Google Scholar] [CrossRef] [PubMed]
- Hanschke, N.; Kankofer, M.; Ruda, L.; Höltershinken, M.; Meyer, U.; Frank, J.; Dänicke, S.; Rehage, J. The effect of conjugated linoleic acid supplements on oxidative and antioxidative status of dairy cows. J. Dairy Sci. 2016, 99, 8090–8102. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F. Indicators for evaluation of lipid oxidation and off-flavor development in food. In Food Flavors: Formation, Analysis and Packaging Influences; Contis, E.T., Ho, C.-T., Mussinan, C.J., Parliment, T.H., Shahidi, F., Spanier, A.M.B.T.-D., Eds.; Elsevier: Amsterdam, The Netherlands, 1998; Volume 40, pp. 55–68. ISBN 0167-4501. [Google Scholar]
- Henchion, M.M.; McCarthy, M.; Resconi, V.C. Beef quality attributes: A systematic review of consumer perspectives. Meat Sci. 2017, 128, 1–7. [Google Scholar] [CrossRef]
- Suman, S.P.; Joseph, P. Myoglobin chemistry and meat color. Annu. Rev. Food Sci. Technol. 2013, 4, 79–99. [Google Scholar] [CrossRef]
- Hunt, M.C.; Hedrick, H.B. Profile of fiber types and related properties of five bovine muscles. J. Food Sci. 1977, 42, 513–517. [Google Scholar] [CrossRef]
- Hu, H.; Wu, C.; Ding, Y.; Zhang, X.; Yang, M.; Wen, A.; Yin, Z. Comparative analysis of meat sensory quality, antioxidant status, growth hormone and orexin between Anqingliubai and Yorkshire pigs. J. Appl. Anim. Res. 2019, 47, 357–361. [Google Scholar] [CrossRef]
- Kazemi, S.; Ngadi, M.O.; Gariépy, C. Protein Denaturation in Pork Longissimus Muscle of Different Quality Groups. Food Bioprocess Technol. 2011, 4, 102–106. [Google Scholar] [CrossRef]
- Faustman, C.; Sun, Q.; Mancini, R.; Suman, S.P. Myoglobin and lipid oxidation interactions: Mechanistic bases and control. Meat Sci. 2010, 86, 86–94. [Google Scholar] [CrossRef]
- Ramanathan, R.; Mancini, R.A. Role of mitochondria in beef color: A review. Meat Muscle Biol. 2018, 2, 309–320. [Google Scholar] [CrossRef]
- Malheiros, J.M.; Braga, C.P.; Grove, R.A.; Ribeiro, F.A.; Calkins, C.R.; Adamec, J.; Chardulo, L.A.L. Influence of oxidative damage to proteins on meat tenderness using a proteomics approach. Meat Sci. 2019, 148, 64–71. [Google Scholar] [CrossRef]
- Laville, E.; Sayd, T.; Morzel, M.; Blinet, S.; Chambon, C.; Lepetit, J.; Renand, G.; Hocquette, J.F. Proteome changes during meat aging in tough and tender beef suggest the importance of apoptosis and protein solubility for beef aging and tenderization. J. Agric. Food Chem. 2009, 57, 10755–10764. [Google Scholar] [CrossRef] [PubMed]
- Arshad, M.S.; Anjum, F.M.; Khan, M.I.; Shahid, M.; Akhtar, S.; Sohaib, M. Wheat germ oil enrichment in broiler feed with α-lipoic acid to enhance the antioxidant potential and lipid stability of meat. Lipids Health Dis. 2013, 12, 164. [Google Scholar] [CrossRef] [PubMed]
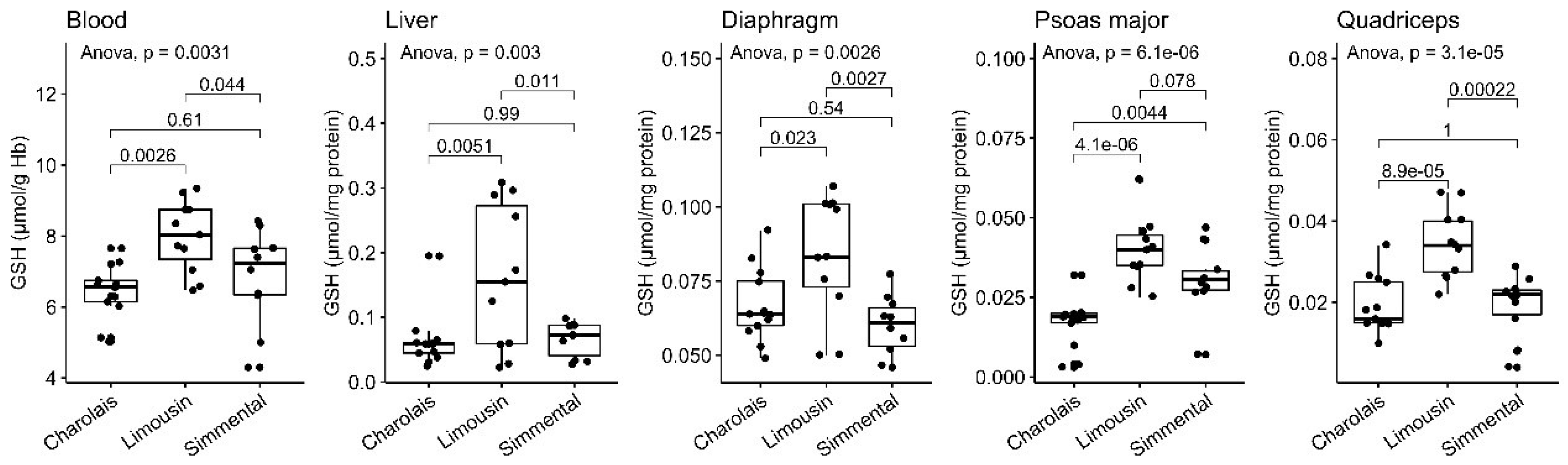
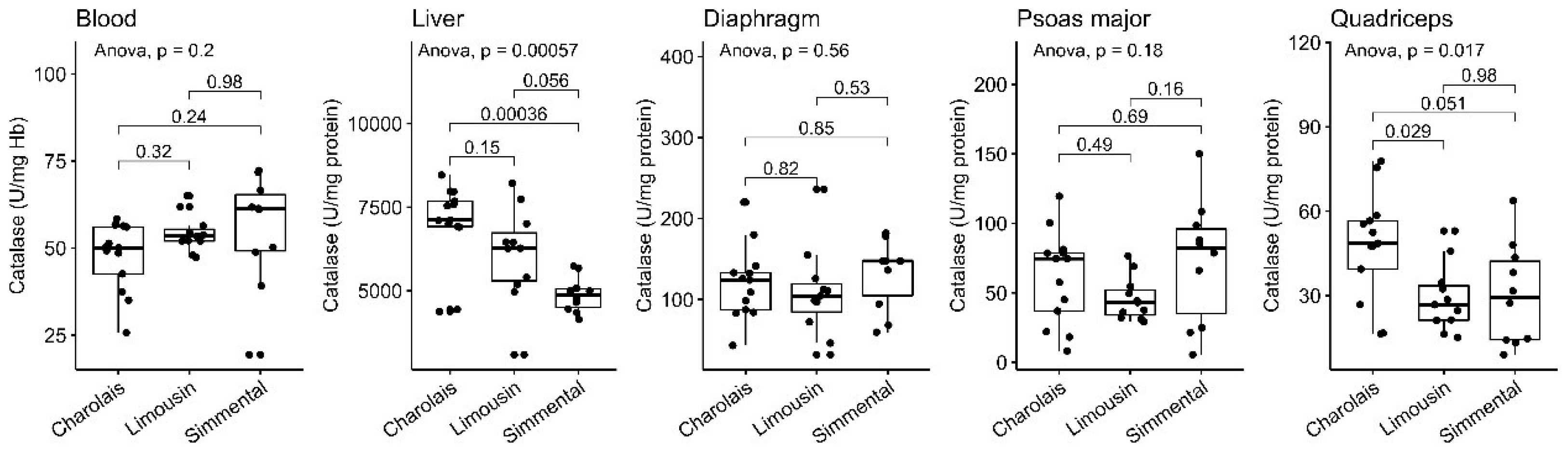
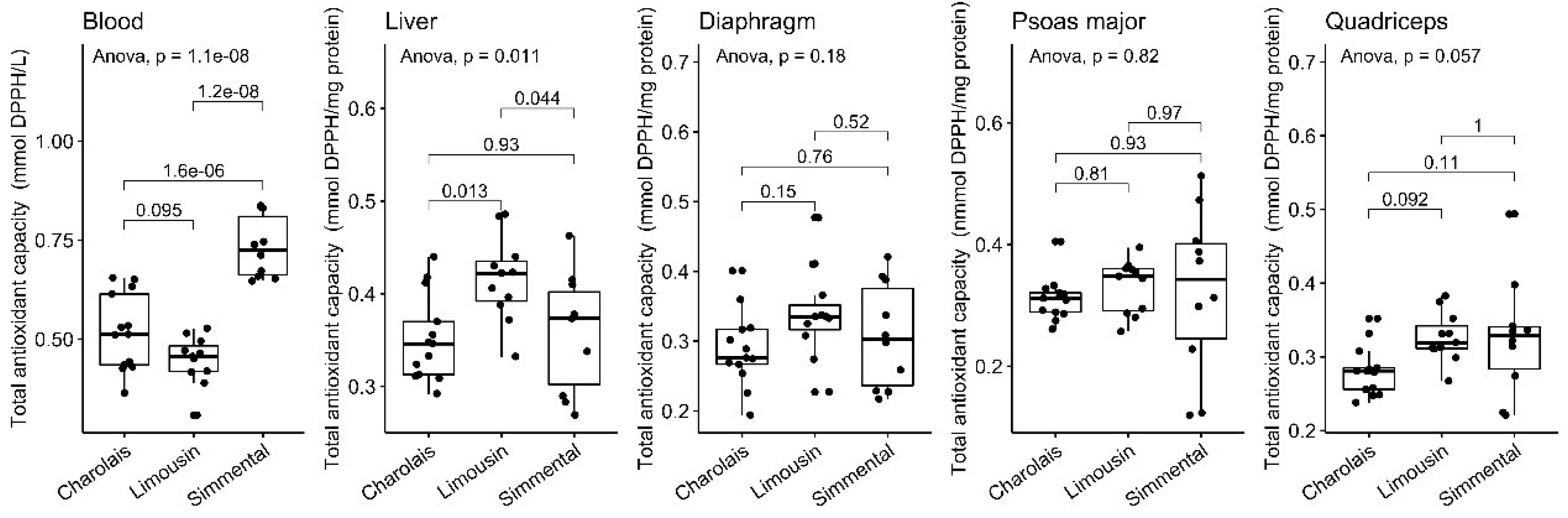
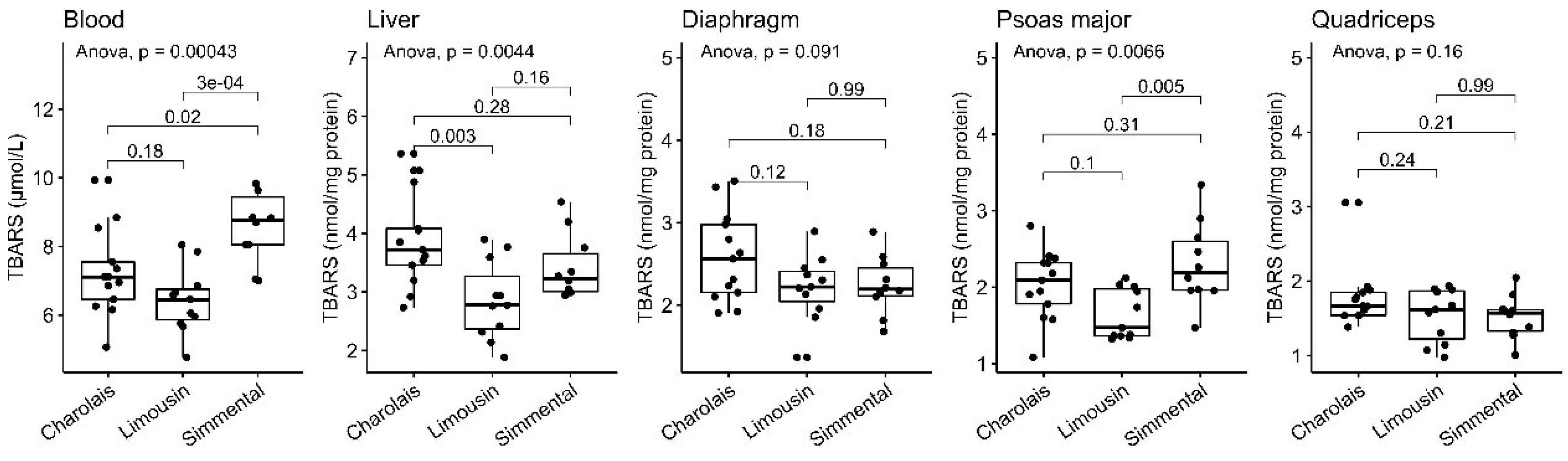
| n | Blood a | Diaphragm b | Liver b | Psoas Major b | Quadriceps b | |
|---|---|---|---|---|---|---|
| Charolais | ||||||
| GSH | 13 | 6.4 (±0.76) | 0.067 (±0.012) | 0.063 (±0.042) | 0.017 (±0.0075) | 0.019 (±0.0067) |
| Catalase | 13 | 48 (±9.8) | 120 (±45) | 7000 (±1200) | 61 (±33) | 48 (±19) |
| TBARS | 13 | 7.2 (±1.3) | 2.6 (±0.54) | 3.9 (±0.81) | 2.0 (±0.45) | 1.8 (±0.41) |
| TAC | 13 | 0.52 (±0.097) | 0.29 (±0.054) | 0.35 (±0.046) | 0.31 (±0.035) | 0.28 (±0.034) |
| Carbonyls | 13 | 0.42 (±0.070) | 4.0 (±2.6) | 4.8 (±2.1) | 3.1 (±1.3) | 1.9 (±1.2) |
| Limousin | ||||||
| GSH | 11 | 8.0 (±1.0) | 0.084 (±0.020) | 0.16 (±0.11) | 0.040 (±0.010) | 0.034 (±0.0084) |
| Catalase | 11 | 54 (±5.3) | 110 (±55) | 6100 (±1400) | 46 (±16) | 29 (±12) |
| TBARS | 11 | 6.4 (±0.95) | 2.2 (±0.40) | 2.9 (±0.67) | 1.6 (±0.33) | 1.5 (±0.36) |
| TAC | 11 | 0.45 (±0.062) | 0.34 (±0.066) | 0.42 (±0.045) | 0.33 (±0.044) | 0.33 (±0.034) |
| Carbonyls | 11 | 0.32 (±0.083) | 2.3 (±1.9) | 3.6 (±0.99) | 3.9 (±1.5) | 4.6 (±1.7) |
| Simmental | ||||||
| GSH | 10 | 6.8 (±1.4) | 0.060 (±0.010) | 0.066 (±0.026) | 0.031 (±0.011) | 0.019 (±0.0078) |
| Catalase | 10 | 55 (±16) | 130 (±43) | 4900 (±530) | 73 (±45) | 30 (±18) |
| TBARS | 10 | 8.6 (±1.0) | 2.2 (±0.36) | 3.4 (±0.56) | 2.3 (±0.54) | 1.5 (±0.29) |
| TAC | 10 | 0.73 (±0.078) | 0.31 (±0.075) | 0.36 (±0.064) | 0.32 (±0.13) | 0.33 (±0.080) |
| Carbonyls | 10 | 0.38 (±0.068) | 5.2 (±2.5) | 4.5 (±1.1) | 2.3 (±1.3) | 4.1 (±1.7) |
| n | Mean | Standard Deviation | Median | Min | Max | Range | Skew | Kurtosis | Standard Error | |
|---|---|---|---|---|---|---|---|---|---|---|
| L* | 34 | 39.94 | 3.661 | 40.51 | 31.33 | 48.22 | 16.89 | −0.28 | −0.17 | 0.63 |
| a* | 34 | 22.98 | 1.854 | 23.12 | 18.41 | 25.82 | 7.41 | −0.47 | −0.34 | 0.32 |
| b* | 34 | 6.27 | 2.161 | 6.64 | 1.81 | 9.60 | 7.79 | −0.26 | −1.20 | 0.37 |
| Chroma | 34 | 23.88 | 2.219 | 23.77 | 18.50 | 27.45 | 8.95 | −0.33 | −0.42 | 0.38 |
| Hue angle | 34 | 0.26 | 0.076 | 0.29 | 0.10 | 0.38 | 0.28 | −0.40 | −1.21 | 0.01 |
| pH | 33 | 5.59 | 0.182 | 5.55 | 5.42 | 6.20 | 0.78 | 1.92 | 2.97 | 0.03 |
| Hardness 1 | 33 | 1067.97 | 804.248 | 1022.23 | 225.79 | 3837.58 | 3611.79 | 2.16 | 5.02 | 140.00 |
| Hardness 2 | 33 | 859.95 | 631.769 | 784.34 | 190.94 | 2976.72 | 2785.78 | 2.10 | 4.78 | 109.98 |
| Springiness | 33 | 0.69 | 0.103 | 0.68 | 0.46 | 0.95 | 0.48 | 0.09 | −0.28 | 0.02 |
| Cohesiveness | 33 | 0.50 | 0.057 | 0.49 | 0.41 | 0.65 | 0.24 | 0.94 | 0.62 | 0.01 |
| Chewiness | 33 | 355.09 | 252.762 | 339.42 | 75.35 | 1172.40 | 1097.05 | 1.90 | 3.85 | 44.00 |
| Dependent Variable | Independent Variable | β-Coefficient | 2.5% CI | 97.5% CI | p-Value |
|---|---|---|---|---|---|
| Redness (a*) | TAC—blood | 4.7128 | 0.8395 | 8.5861 | 0.0187 |
| Redness (a*) | TAC—liver | −14.6856 | −24.0230 | −5.3483 | 0.0031 |
| Yellowness (b*) | GSH—quadriceps | −65.6359 | −127.3821 | −3.8897 | 0.0380 |
| Yellowness (b*) | TAC—blood | 7.1183 | 2.9843 | 11.2524 | 0.0014 |
| Yellowness (b*) | CAT—diaphragm | 0.0186 | 0.0046 | 0.0325 | 0.0108 |
| Lightness (L*) | CARBS—diaphragm | −0.5585 | −0.9899 | −0.1270 | 0.0129 |
| Lightness (L*) | CARBS—psoas major | 0.9592 | 0.1256 | 1.7929 | 0.0255 |
| Chroma | GSH—quadriceps | −65.4472 | −128.6614 | −2.2330 | 0.0429 |
| Chroma | TAC—blood | 6.2854 | 1.8618 | 10.7090 | 0.0068 |
| Chroma | TAC—liver | −16.6420 | −27.7520 | −5.5321 | 0.0046 |
| Hue angle | TAC—blood | 0.2388 | 0.0836 | 0.3940 | 0.0037 |
| Hue angle | CAT—diaphragm | 0.0007 | 0.0002 | 0.0012 | 0.0102 |
| Hue angle | CARBS—liver | 0.0153 | 0.0005 | 0.0301 | 0.0430 |
| Log_pH | GSH—psoas major | 1.0484 | 0.3271 | 1.7698 | 0.0058 |
| Log_pH | CARBS—blood | −0.1299 | −0.2568 | −0.0031 | 0.0450 |
| Log_hardness 2 | CAT—psoas major | 0.0067 | 0.0005 | 0.0129 | 0.0361 |
| Log_hardness 2 | CAT—blood | 0.0195 | 0.0011 | 0.0378 | 0.0381 |
| Log_hardness 2 | CARBS—psoas major | −0.1584 | −0.3113 | −0.0055 | 0.0428 |
| Log_hardness 1 | CAT—psoas major | 0.0070 | 0.0007 | 0.0134 | 0.0311 |
| Log_hardness 1 | CAT—blood | 0.0207 | 0.0020 | 0.0394 | 0.0309 |
| Log_hardness 1 | CARBS—psoas major | −0.1622 | −0.3189 | −0.0055 | 0.0430 |
| Springiness | GSH—quadriceps | 5.8144 | 2.5477 | 9.0812 | 0.0011 |
| Log_cohesiveness | GSH—blood | −0.0306 | −0.0604 | −0.0008 | 0.0443 |
| Log_chewiness | CARBS—diaphragm | −0.0786 | −0.1516 | −0.0055 | 0.0359 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skaperda, Z.; Argyriadou, A.; Nechalioti, P.M.; Alvanou, M.; Makri, S.; Bouroutzika, E.; Kyriazis, I.D.; Tekos, F.; Veskoukis, A.S.; Kallitsis, T.; et al. Redox Biomarker Baseline Levels in Cattle Tissues and Their Relationships with Meat Quality. Antioxidants 2021, 10, 958. https://doi.org/10.3390/antiox10060958
Skaperda Z, Argyriadou A, Nechalioti PM, Alvanou M, Makri S, Bouroutzika E, Kyriazis ID, Tekos F, Veskoukis AS, Kallitsis T, et al. Redox Biomarker Baseline Levels in Cattle Tissues and Their Relationships with Meat Quality. Antioxidants. 2021; 10(6):958. https://doi.org/10.3390/antiox10060958
Chicago/Turabian StyleSkaperda, Zoi, Angeliki Argyriadou, Paraskevi Maria Nechalioti, Maria Alvanou, Sotiria Makri, Efterpi Bouroutzika, Ioannis D. Kyriazis, Fotios Tekos, Aristidis S. Veskoukis, Theodoros Kallitsis, and et al. 2021. "Redox Biomarker Baseline Levels in Cattle Tissues and Their Relationships with Meat Quality" Antioxidants 10, no. 6: 958. https://doi.org/10.3390/antiox10060958
APA StyleSkaperda, Z., Argyriadou, A., Nechalioti, P. M., Alvanou, M., Makri, S., Bouroutzika, E., Kyriazis, I. D., Tekos, F., Veskoukis, A. S., Kallitsis, T., Mesnage, R., Arsenos, G., & Kouretas, D. (2021). Redox Biomarker Baseline Levels in Cattle Tissues and Their Relationships with Meat Quality. Antioxidants, 10(6), 958. https://doi.org/10.3390/antiox10060958












