N-acetyl-L-cysteine Improves the Developmental Competence of Bovine Oocytes and Embryos Cultured In Vitro by Attenuating Oxidative Damage and Apoptosis
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Oocyte Collection and In Vitro Maturation
2.3. In Vitro Fertilization (IVF) and In Vitro Culture (IVC)
2.4. Total RNA Extraction and Real-Time RT-PCR
2.5. Measurement of Intracellular ROS, GSH Levels and Mitochondrial Membrane Potential
2.6. Cell Proliferation Assay
2.7. Total Antioxidant Capability Measurement
2.8. Apoptosis Assays and Total Blastocyst Cell Counting
2.9. Blastocyst Outgrowth Assay
2.10. Statistical Analyses
3. Results
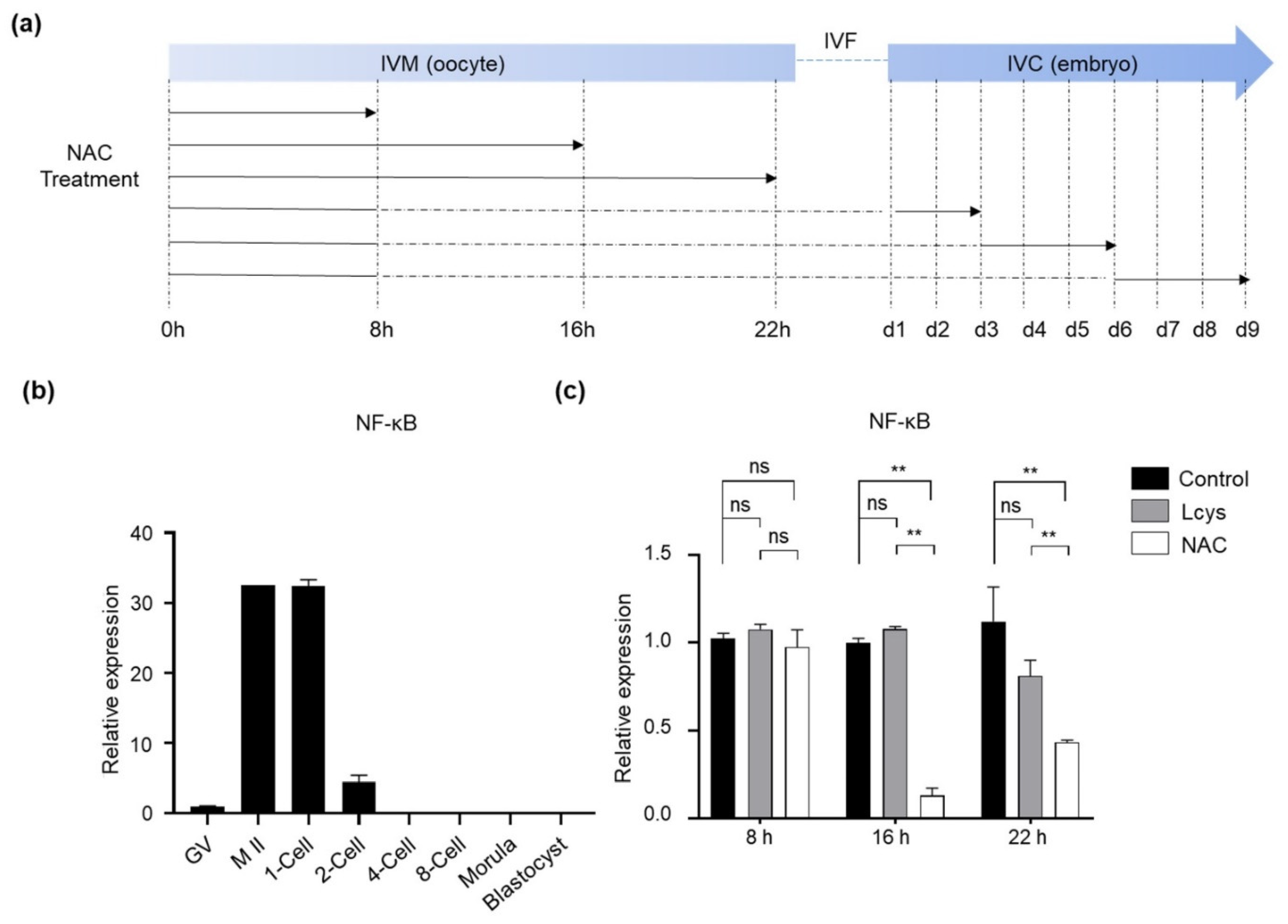
3.1. Effects of NAC Supplementation in IVM Medium on the Expression of NFKB1 Gene in Bovine Oocytes
3.2. Effects of NAC Supplementation in IVM Medium on Oocyte Maturation and Embryo Development
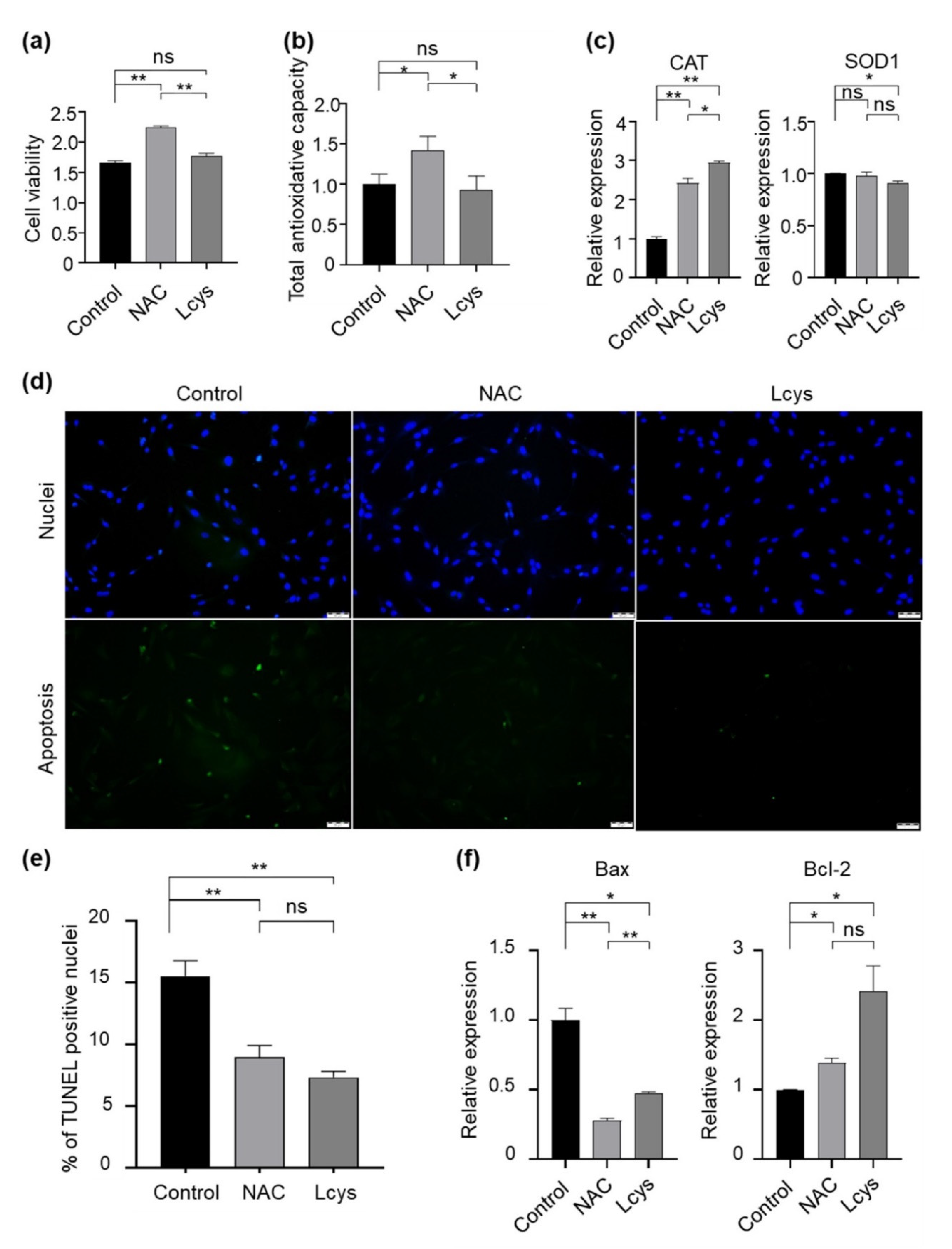
3.3. Effects of NAC Supplementation in IVM Medium on Bovine Oocyte Quality
3.4. Effects of NAC Supplementation in IVM Medium on Bovine Cumulus Cells Quality
3.5. Effects of NAC Supplementation in IVC Medium on Bovine Embryo Development
3.6. Effects of NAC Supplementation in IVC Medium on Bovine Embryo Quality
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wongsrikeao, P.; Otoi, T.; Karja, N.W.K.; Agung, B.; Nii, M.; Nagai, T. Effects of ovary storage time and temperature on DNA fragmentation and development of porcine oocytes. J. Reprod. Dev. 2005, 51, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Khazaei, M.; Aghaz, F. Reactive Oxygen Species Generation and Use of Antioxidants during In Vitro Maturation of Oocytes. Int. J. Fertil. Steril. 2017, 11, 63–70. [Google Scholar] [CrossRef]
- Harman, D. Free radicals in aging. Mol. Cell. Biochem. 1988, 84, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.-F.; Qi, S.-T.; Xian, Y.-X.; Huang, L.; Sun, X.-F.; Wang, W.-H. Protective effect of antioxidants on the pre-maturation aging of mouse oocytes. Sci. Rep. 2017, 7, 1–10. [Google Scholar]
- Torres-Osorio, V.; Urrego, R.; Echeverri-Zuluaga, J.J.; López-Herrera, A. Oxidative stress and antioxidant use during in vitro mammal embryo production. Review. Rev. Mex. Cienc. Pecu. 2019, 10, 433–459. [Google Scholar] [CrossRef]
- Čolak, E.; Ignjatović, S.; Radosavljević, A.; Žorić, L. The association of enzymatic and non-enzymatic antioxidant defense parameters with inflammatory markers in patients with exudative form of age-related macular degeneration. J. Clin. Biochem. Nutr. 2017, 60, 100–107. [Google Scholar] [CrossRef]
- De Flora, S.; Bennicelli, C.; Camoirano, A.; Serra, D.; Romano, M.; Rossi, G.A.; Morelli, A.; De Flora, A. In vivo effects of N-acetylcysteine on glutathione metabolism and on the biotransformation of carcinogenic and/or mutagenic compounds. Carcinogenesis 1985, 6, 1735–1745. [Google Scholar] [CrossRef]
- Li, Z.; Gu, R.; Lu, X.; Zhao, S.; Feng, Y.; Sun, Y. Preincubation with glutathione ethyl ester improves the developmental competence of vitrified mouse oocytes. J. Assist. Reprod. Genet. 2018, 35, 1169–1178. [Google Scholar] [CrossRef]
- de Matos, D.G.; Furnus, C.C.; Moses, D.F.; Baldassarre, H. Effect of cysteamine on glutathione level and developmental capacity of bovine oocyte matured in vitro. Mol. Reprod. Dev. 1995, 42, 432–436. [Google Scholar] [CrossRef] [PubMed]
- Furnus, C.C.; de Matos, D.G.; Picco, S.; Garcia, P.P.; Inda, A.M.; Mattioli, G.; Errecalde, A.L. Metabolic requirements associated with GSH synthesis during in vitro maturation of cattle oocytes. Anim. Reprod. Sci. 2008, 109, 88–99. [Google Scholar] [CrossRef]
- Caamaño, J.N.; Ryoo, Z.Y.; Thomas, J.A.; Youngs, C.R. beta-mercaptoethanol enhances blastocyst formation rate of bovine in vitro-matured/in vitro-fertilized embryos. Biol. Reprod. 1996, 55, 1179–1184. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Takahashi, M.; Nagai, T.; Hamano, S.; Kuwayama, M.; Okamura, N.; Okano, A. Effect of thiol compounds on in vitro development and intracellular glutathione content of bovine embryos. Biol. Reprod. 1993, 49, 228–232. [Google Scholar] [CrossRef]
- Krattenmacher, F.; Heermann, T.; Calvet, A.; Krawczyk, B.; Noll, T. Effect of manufacturing temperature and storage duration on stability of chemically defined media measured with LC-MS/MS. J. Chem. Technol. Biotechnol. 2019, 94, 1144–1155. [Google Scholar] [CrossRef]
- Walter, F. Rapid Oxidation of Cysteine to Cystine in Aqueous Solutions—Implications for Spent Media Analysis. Xell AG 2019, 6. [Google Scholar]
- Sameem, B.; Khan, F.; Niaz, K. Chapter 2.6-l-Cysteine. In Nonvitamin and Nonmineral Nutritional Supplements; Nabavi, S.M., Silva, A.S., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 53–58. [Google Scholar] [CrossRef]
- Grinberg, L.; Fibach, E.; Amer, J.; Atlas, D. N-acetylcysteine amide, a novel cell-permeating thiol, restores cellular glutathione and protects human red blood cells from oxidative stress. Free Radic. Biol. Med. 2005, 38, 136–145. [Google Scholar] [CrossRef]
- Samuni, Y.; Goldstein, S.; Dean, O.M.; Berk, M. The chemistry and biological activities of N-acetylcysteine. Biochim. Biophys. Acta (BBA) Gen. Subj. 2013, 1830, 4117–4129. [Google Scholar] [CrossRef] [PubMed]
- Shih, W.-L.; Chang, C.-D.; Chen, H.-T.; Fan, K.-K. Antioxidant activity and leukemia initiation prevention in vitro and in vivo by N-acetyl-L-cysteine. Oncol. Lett. 2018, 16, 2046–2052. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.-Y. N-acetylcysteine, reactive oxygen species and beyond. Cancer Biol. Ther. 2010, 9, 109–110. [Google Scholar] [CrossRef]
- Cotgreave, I.A. N-Acetylcystei ne: Pharmacological Considerations and Experimental and Clinical Applications. In Advances in Pharmacology; Sies, H., Ed.; Academic Press: Cambridge, MA, USA, 1996; Volume 38, pp. 205–227. [Google Scholar]
- Hu, J.J.; Wong, N.-K.; Lu, M.-Y.; Chen, X.; Ye, S.; Zhao, A.Q.; Gao, P.; Yi-Tsun Kao, R.; Shen, J.; Yang, D. HKOCl-3: A fluorescent hypochlorous acid probe for live-cell and imaging and quantitative application in flow cytometry and a 96-well microplate assay. Chem. Sci. 2016, 7, 2094–2099. [Google Scholar] [CrossRef]
- Badawy, A.; Baker El Nashar, A.; El Totongy, M. Clomiphene citrate plus N-acetyl cysteine versus clomiphene citrate for augmenting ovulation in the management of unexplained infertility: A randomized double-blind controlled trial. Fertil. Steril. 2006, 86, 647–650. [Google Scholar] [CrossRef] [PubMed]
- Bobb, A.J.; Arfsten, D.P.; Jederberg, W.W. N-acetyl-L-Cysteine as prophylaxis against sulfur mustard. Mil. Med. 2005, 170, 52–56. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Keilty, D.; Zhang, Z.F.; Chian, R.C. Mitochondria in oocyte aging: Current understanding. Facts Views Vis. ObGyn 2017, 9, 29–38. [Google Scholar] [PubMed]
- Liu, J.; Liu, M.; Ye, X.; Liu, K.; Huang, J.; Wang, L.; Ji, G.; Liu, N.; Tang, X.; Baltz, J.M.; et al. Delay in oocyte aging in mice by the antioxidant N-acetyl-L-cysteine (NAC). Hum. Reprod. 2012, 27, 1411–1420. [Google Scholar] [CrossRef]
- Hu, X.; Cheng, L.; Wang, X.; Luo, G.; Zhao, T.; Tian, J.; An, L. N-acetyl-l-cysteine protects porcine oocytes undergoing meiotic resumption from heat stress. Reprod. Toxicol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Whitaker, B.D.; Knight, J.W. Effects of N-acetyl-cysteine and N-acetyl-cysteine-amide supplementation on in vitro matured porcine oocytes. Reprod. Domest. Anim. 2010, 45, 755–759. [Google Scholar] [CrossRef]
- Ali, A.A.; Bilodeau, J.F.; Sirard, M.A. Antioxidant requirements for bovine oocytes varies during in vitro maturation, fertilization and development. Theriogenology 2003, 59, 939–949. [Google Scholar] [CrossRef]
- Sun, W.-S.; Jang, H.; Kwon, H.J.; Kim, K.Y.; Ahn, S.B.; Hwang, S.; Lee, S.G.; Lee, J.H.; Hwang, I.-S.; Lee, J.-W. The protective effect of Leucosporidium-derived ice-binding protein (LeIBP) on bovine oocytes and embryos during vitrification. Theriogenology 2020, 151, 137–143. [Google Scholar] [CrossRef]
- Chen, Z.; Lei, L.; Wen, D.; Yang, L. Melatonin attenuates palmitic acid-induced mouse granulosa cells apoptosis via endoplasmic reticulum stress. J. Ovarian Res. 2019, 12, 43. [Google Scholar] [CrossRef]
- Huang, Z.; Pang, Y.; Hao, H.; Du, W.; Zhao, X.; Zhu, H. Effects of epigallocatechin-3-gallate on bovine oocytes matured in vitro. Asian-Australas. J. Anim. Sci. 2018, 31, 1420–1430. [Google Scholar] [CrossRef]
- Liang, S.; Yuan, B.; Jin, Y.X.; Zhang, J.B.; Bang, J.K.; Kim, N.H. Effects of antifreeze glycoprotein 8 (AFGP8) supplementation during vitrification on the in vitro developmental capacity of expanded bovine blastocysts. Reprod. Fertil. Dev. 2017, 29, 2140–2148. [Google Scholar] [CrossRef]
- Nishikimi, A.; Mukai, J.; Yamada, M. Nuclear Translocation of Nuclear Factor Kappa B in Early 1-Cell Mouse Embryos2. Biol. Reprod. 1999, 60, 1536–1541. [Google Scholar] [CrossRef][Green Version]
- Alpha Scientists in Reproductive Medicine and ESHRE Special Interest Group of Embryology. The Istanbul consensus workshop on embryo assessment: Proceedings of an expert meeting. Hum. Reprod. 2011, 26, 1270–1283. [Google Scholar] [CrossRef]
- Ufer, C.; Wang, C. The roles of glutathione peroxidases during embryo development. Front. Mol. Neurosci. 2011, 4. [Google Scholar] [CrossRef]
- Peng, Z.-F.; Shi, S.-L.; Jin, H.-X.; Yao, G.-D.; Wang, E.-Y.; Yang, H.-Y.; Song, W.-Y.; Sun, Y.-P. Impact of oxygen concentrations on fertilization, cleavage, implantation, and pregnancy rates of in vitro generated human embryos. Int. J. Clin. Exp. Med. 2015, 8, 6179–6185. [Google Scholar] [PubMed]
- Combelles, C.M.H.; Gupta, S.; Agarwal, A. Could oxidative stress influence the in-vitro maturation of oocytes? Reprod. Biomed. Online 2009, 18, 864–880. [Google Scholar] [CrossRef]
- Tatemoto, H.; Sakurai, N.; Muto, N. Protection of Porcine Oocytes Against Apoptotic Cell Death Caused by Oxidative Stress During In Vitro Maturation: Role of Cumulus Cells1. Biol. Reprod. 2000, 63, 805–810. [Google Scholar] [CrossRef] [PubMed]
- de Matos, D.G.; Furnus, C.C. The importance of having high glutathione (GSH) level after bovine in vitro maturation on embryo development: Effect of β-mercaptoethanol, cysteine and cystine. Theriogenology 2000, 53, 761–771. [Google Scholar] [CrossRef]
- Bae, H.; Jeong, C.H.; Cheng, W.N.; Hong, K.; Seo, H.G.; Han, S.G. Oxidative stress-induced inflammatory responses and effects of N-acetylcysteine in bovine mammary alveolar cells. J. Dairy Res. 2017, 84, 418–425. [Google Scholar] [CrossRef] [PubMed]
- Riegger, J.; Joos, H.; Palm, H.G.; Friemert, B.; Reichel, H.; Ignatius, A.; Brenner, R.E. Antioxidative therapy in an ex vivo human cartilage trauma-model: Attenuation of trauma-induced cell loss and ECM-destructive enzymes by N-acetyl cysteine. Osteoarthr. Cartil. 2016, 24, 2171–2180. [Google Scholar] [CrossRef]
- Jung, W.W. Protective effect of apigenin against oxidative stress-induced damage in osteoblastic cells. Int. J. Mol. Med. 2014, 33, 1327–1334. [Google Scholar] [CrossRef] [PubMed]
- Hamatani, T.; Falco, G.; Carter, M.G.; Akutsu, H.; Stagg, C.A.; Sharov, A.A.; Dudekula, D.B.; VanBuren, V.; Ko, M.S.H. Age-associated alteration of gene expression patterns in mouse oocytes. Hum. Mol. Genet. 2004, 13, 2263–2278. [Google Scholar] [CrossRef] [PubMed]
- Paciolla, M.; Boni, R.; Fusco, F.; Pescatore, A.; Poeta, L.; Ursini, M.V.; Lioi, M.B.; Miano, M.G. Nuclear factor-kappa-B-inhibitor alpha (NFKBIA) is a developmental marker of NF-κB/p65 activation during in vitro oocyte maturation and early embryogenesis. Hum. Reprod. 2011, 26, 1191–1201. [Google Scholar] [CrossRef]
- Patel, O.V.; Bettegowda, A.; Ireland, J.J.; Coussens, P.M.; Lonergan, P.; Smith, G.W. Functional genomics studies of oocyte competence: Evidence that reduced transcript abundance for follistatin is associated with poor developmental competence of bovine oocytes. Reprod. Camb. Engl. 2007, 133, 95–106. [Google Scholar] [CrossRef] [PubMed]
- Lilienbaum, A.; Sage, J.; Mémet, S.; Rassoulzadegan, M.; Cuzin, F.; Israël, A. NF-kappa B is developmentally regulated during spermatogenesis in mice. Dev. Dyn. Off. Publ. Am. Assoc. Anat. 2000, 219, 333–340. [Google Scholar]
- Tomek, W.; Smiljakovic, T. Activation of Akt (protein kinase B) stimulates metaphase I to metaphase II transition in bovine oocytes. Reprod. Camb. Engl. 2005, 130, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, Y.-N.; Zhu, J.-J.; Liu, X.-X.; You, H.; Gong, M.-Y.; Zou, M.; Cheng, W.-H.; Zhu, J.-H. N-acetylcysteine negatively regulates Notch3 and its malignant signaling. Oncotarget 2016, 7, 30855. [Google Scholar] [CrossRef] [PubMed]
- Uyar, A.; Torrealday, S.; Seli, E. Cumulus and granulosa cell markers of oocyte and embryo quality. Fertil. Steril. 2013, 99, 979–997. [Google Scholar] [CrossRef]
- Lourenço, B.; Sousa, A.P.; Almeida-Santos, T.; Ramalho-Santos, J. Relation of cumulus cell status with single oocyte maturity, fertilization capability and patient age. J. Reprod. Infertil. 2014, 15, 15–21. [Google Scholar]
- von Mengden, L.; Klamt, F.; Smitz, J. Redox Biology of Human Cumulus Cells: Basic Concepts, Impact on Oocyte Quality, and Potential Clinical Use. Antioxid Redox Signal 2020, 32, 522–535. [Google Scholar] [CrossRef]
- Hoshino, Y. Updating the markers for oocyte quality evaluation: Intracellular temperature as a new index. Reprod. Med. Biol. 2018, 17, 434–441. [Google Scholar] [CrossRef]
- Grupen, C.G.; Armstrong, D.T. Relationship between cumulus cell apoptosis, progesterone production and porcine oocyte developmental competence: Temporal effects of follicular fluid during IVM. Reprod. Fertil. Dev. 2010, 22, 1100–1109. [Google Scholar] [CrossRef] [PubMed]
- Uhde, K.; van Tol, H.T.A.; Stout, T.A.E.; Roelen, B.A.J. Metabolomic profiles of bovine cumulus cells and cumulus-oocyte-complex-conditioned medium during maturation in vitro. Sci. Rep. 2018, 8, 9477. [Google Scholar] [CrossRef] [PubMed]
- Truong, T.; Gardner, D.K. Antioxidants improve IVF outcome and subsequent embryo development in the mouse. Hum. Reprod. 2017, 32, 2404–2413. [Google Scholar] [CrossRef] [PubMed]



| Gene | Primer Sequences (5′-3′) | Accession Number | Product Size (bp) |
|---|---|---|---|
| Bax | GGCTGGACATTGGACTTCCTTC | NM_173894 | 112 |
| TGGTCACTGTCTGCCATGTGG | |||
| Bcl-2 | TGGATGACCGAGTACCTGAA | NM_001166486.1 | 123 |
| GAGACAGCCAGGAGAAATCAAA | |||
| SOD1 | CCACGTCCATCAGTTTGGAGA | NM_174615.2 | 92 |
| CTTTTGGCCCACCGTGTTTT | |||
| CAT | CTGGGACCCAACTATCTCCA | NM_001035386.2 | 148 |
| GATGCTCGGGAGCACTAAAG | |||
| NFKB1 | TGGCGGAATTACCTTCCATAC | DQ464067 | 110 |
| CATCACTCTTGCCACAACTTTC | |||
| GAPDH | CCCAGAATATCATCCCTGCT | NM_001034034 | 185 |
| CTGCTTCACCACCTTCTTGA |
| Group | No. (%, ± SEM) of Mature/Retrieved Oocytes | No. of Oocytes Used for IVF | No. (%, ± SEM) of IVF Embryos Developed to | |||
|---|---|---|---|---|---|---|
| 2-Cell | Top-Quality 2-Cell 1 | Blastocyst | Hatched Embryo | |||
| IVM medium (Control) | 236/299 | 219 | 185 | 163 | 69 | 38 |
| (79.03 ± 0.22) b | (83.56 ± 0.40) b | (88.30 ± 0.65) b | (37.15 ± 1.99) b,2 | (54.77 ± 0.14) b | ||
| IVM + Lcys (0.6 mM) | 182/220 | 250 | 231 | 206 | 90 | 51 |
| (82.76 ± 1.08) a | (91.42 ± 1.59) a | (89.26 ± 1.48) b | (38.97 ± 1.88) ab | (57.22 ± 1.04) b | ||
| IVM + NAC (0.1 mM) | 178/231 | 233 | 192 | 166 | 72 | 40 |
| (77.10 ± 0.18) bc | (82.74 ± 1.97) b | (86.71 ± 0.97) b | (37.73 ± 0.80) ab | (55.94 ± 1.56) b | ||
| IVM + NAC (1.0 mM) | 167/201 | 246 | 211 | 195 | 88 | 53 |
| (83.06 ± 1.85) a | (85.86 ± 0.46) b | (92.93 ± 1.38) a | (41.69 ± 0.63) a | (60.65 ± 0.44) a | ||
| IVM + NAC (10.0 mM) | 188/248 | 205 | 160 | 132 | 59 | 12 |
| (75.69 ± 0.55) c | (78.59 ± 1.03) c | (82.28 ± 1.39) c | (37.19 ± 0.26) b | (19.42 ± 1.49) c | ||
| Oocytes Treated | No. of Zygotes for Culture | No. (%, ± SEM) of Embryos Developed to | ||
|---|---|---|---|---|
| 2-Cell | Blastocyst | Hatched Embryo | ||
| Basic CR1aa (Control) | 398 | 361 (90.25 ± 0.26) a,1 | 137 (38.20 ± 0.43) c | 80 (58.44 ± 1.18) b |
| CR1aa + NAC (0~3 dpf) | 346 | 284 (81.46 ± 1.29) b | 127 (44.46 ± 0.42) b | 72 (57.20 ± 2.05) b |
| CR1aa + NAC (3~6 dpf) | 295 | 263 (87.26 ± 1.82) a | 130 (49.72 ± 0.72) a | 86 (65.85 ± 3.23) a |
| CR1aa + NAC (6~9 dpf) | 342 | 315 (90.93 ± 0.76) a | 151 (48.06 ± 1.84) a | 90 (59.44 ± 1.67) ab |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, W.-S.; Jang, H.; Park, M.-R.; Oh, K.B.; Lee, H.; Hwang, S.; Xu, L.-J.; Hwang, I.-S.; Lee, J.-W. N-acetyl-L-cysteine Improves the Developmental Competence of Bovine Oocytes and Embryos Cultured In Vitro by Attenuating Oxidative Damage and Apoptosis. Antioxidants 2021, 10, 860. https://doi.org/10.3390/antiox10060860
Sun W-S, Jang H, Park M-R, Oh KB, Lee H, Hwang S, Xu L-J, Hwang I-S, Lee J-W. N-acetyl-L-cysteine Improves the Developmental Competence of Bovine Oocytes and Embryos Cultured In Vitro by Attenuating Oxidative Damage and Apoptosis. Antioxidants. 2021; 10(6):860. https://doi.org/10.3390/antiox10060860
Chicago/Turabian StyleSun, Wu-Sheng, Hoon Jang, Mi-Ryung Park, Keon Bong Oh, Haesun Lee, Seongsoo Hwang, Li-Jie Xu, In-Sul Hwang, and Jeong-Woong Lee. 2021. "N-acetyl-L-cysteine Improves the Developmental Competence of Bovine Oocytes and Embryos Cultured In Vitro by Attenuating Oxidative Damage and Apoptosis" Antioxidants 10, no. 6: 860. https://doi.org/10.3390/antiox10060860
APA StyleSun, W.-S., Jang, H., Park, M.-R., Oh, K. B., Lee, H., Hwang, S., Xu, L.-J., Hwang, I.-S., & Lee, J.-W. (2021). N-acetyl-L-cysteine Improves the Developmental Competence of Bovine Oocytes and Embryos Cultured In Vitro by Attenuating Oxidative Damage and Apoptosis. Antioxidants, 10(6), 860. https://doi.org/10.3390/antiox10060860







