Plant Foods Rich in Antioxidants and Human Cognition: A Systematic Review
Abstract
1. Introduction
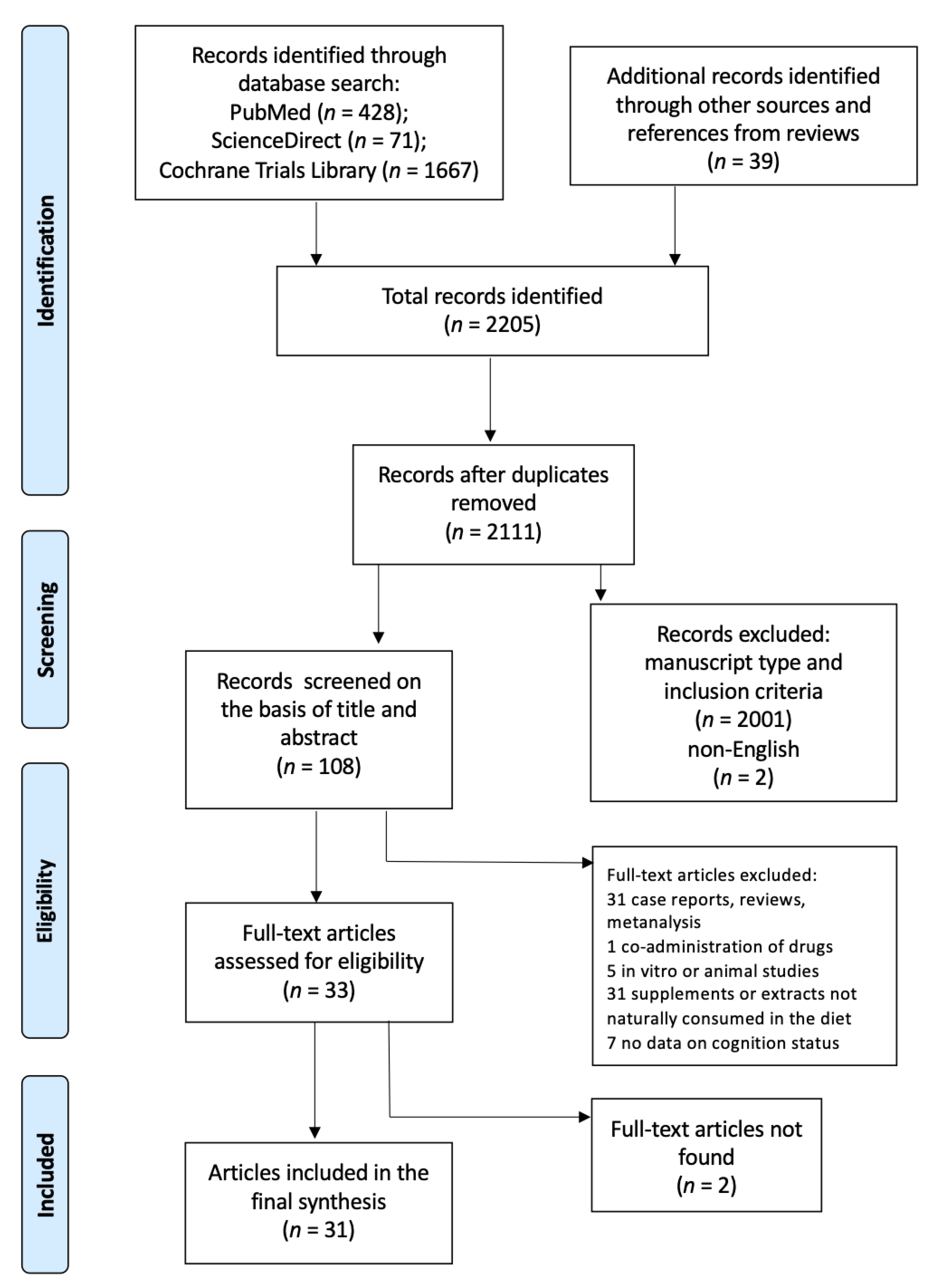
2. Materials and Methods
- (a)
- “plant-based” OR “vegan” OR “veganism” OR “vegetarian” OR “vegetarianism” OR “adventist”;
- (b)
- “neurodegeneration” OR “dementia” OR “Parkinson” OR “cognitive disease” OR “extrapyramidal” OR “extrapyramidalism” OR “neurodegenerative” OR “cognition” OR “Alzheimer”;
- (c)
- “oxidative” OR “antioxidant” OR “phytochemical” OR “ROS.”
- -
- Observational or intervention study design.
- -
- Sufficient definition and data of the food source or dietary antioxidant intake.
- -
- Data on cognition assessments of normal subjects and subjects affected by the main neurodegenerative diseases compromising cognition.
- -
- Case reports, reviews, metanalyses.
- -
- In vitro or animal studies.
- -
- Co-administration of drugs affecting CNS.
- -
- Supplements not consumed in the form of food.
- -
- Insufficient data on cognition measurements.
3. Results
3.1. Nuts
3.2. Fruit and Vegetables
3.2.1. Grapes (From Different Cultivars)
3.2.2. Berries
3.2.3. Cherries
3.2.4. Pomegranate
3.2.5. Oranges
3.2.6. Apples
3.2.7. Onion
3.2.8. Rosemary
3.2.9. Mixed Plant Sources
3.2.10. Flavonoids in Food
3.3. Caffeinate Foods
3.3.1. Chocolate
3.3.2. Tea and Coffee
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Harrison, S.L.; Birdi, R.; Smart, C.O.; Brittain, K.; Rutjes, A.W.; Siervo, M.; Stephan, B. Dietary Interventions for Maintaining Cognitive Function in Cognitively Healthy People in Mid Life. Cochrane Database Syst. Rev. 2015. [Google Scholar] [CrossRef]
- Vauzour, D.; Camprubi-Robles, M.; Miquel-Kergoat, S.; Andres-Lacueva, C.; Bánáti, D.; Barberger-Gateau, P.; Bowman, G.L.; Caberlotto, L.; Clarke, R.; Hogervorst, E.; et al. Nutrition for the Ageing Brain: Towards Evidence for an Optimal Diet. Ageing Res. Rev. 2017, 35, 222–240. [Google Scholar] [CrossRef] [PubMed]
- Pistollato, F.; Iglesias, R.C.; Ruiz, R.; Aparicio, S.; Crespo, J.; Lopez, L.D.; Manna, P.P.; Giampieri, F.; Battino, M. Nutritional Patterns Associated with the Maintenance of Neurocognitive Functions and the Risk of Dementia and Alzheimer’s Disease: A Focus on Human Studies. Pharmacol. Res. 2018, 131, 32–43. [Google Scholar] [CrossRef]
- Rajaram, S.; Jones, J.; Lee, G.J. Plant-Based Dietary Patterns, Plant Foods, and Age-Related Cognitive Decline. Adv. Nutr. 2019, 10 (Suppl. S4), S422–S436. [Google Scholar] [CrossRef]
- Krajcovicová-Kudlácková, M.; Simoncic, R.; Béderová, A.; Klvanová, J.; Brtková, A.; Grancicová, E. Lipid and Antioxidant Blood Levels in Vegetarians. Food Nahrung 1996, 40, 17–20. [Google Scholar] [CrossRef] [PubMed]
- Dewell, A.; Weidner, G.; Sumner, M.D.; Chi, C.S.; Ornish, D. A Very-Low-Fat Vegan Diet Increases Intake of Protective Dietary Factors and Decreases Intake of Pathogenic Dietary Factors. J. Am. Diet. Assoc. 2008, 108, 347–356. [Google Scholar] [CrossRef]
- Milgram, N.W.; Siwak-Tapp, C.T.; Araujo, J.; Head, E. Neuroprotective Effects of Cognitive Enrichment. Ageing Res. Rev. 2006, 5, 354–369. [Google Scholar] [CrossRef]
- Fattoretti, P.; Malavolta, M.; Fabbietti, P.; Papa, R.; Giacconi, R.; Costarelli, L.; Galeazzi, R.; Paoloni, C.; Postacchini, D.; Lattanzio, F.; et al. Oxidative Stress in Elderly with Different Cognitive Status: My Mind Project. J. Alzheimers Dis. 2018, 63, 1405–1414. [Google Scholar] [CrossRef]
- Abate, G.; Vezzoli, M.; Sandri, M.; Rungratanawanich, W.; Memo, M.; Uberti, D. Mitochondria and Cellular Redox State on the Route from Ageing to Alzheimer’s Disease. Mech. Ageing Dev. 2020, 192, 111385. [Google Scholar] [CrossRef] [PubMed]
- Plauth, A.; Geikowski, A.; Cichon, S.; Wowro, S.J.; Liedgens, L.; Rousseau, M.; Weidner, C.; Fuhr, L.; Kliem, M.; Jenkins, G.; et al. Hormetic Shifting of Redox Environment by Pro-Oxidative Resveratrol Protects Cells against Stress. Free Radic. Biol. Med. 2016, 99, 608–622. [Google Scholar] [CrossRef]
- Kanner, J. Polyphenols by Generating H2O2, Affect Cell Redox Signaling, Inhibit PTPs and Activate Nrf2 Axis for Adaptation and Cell Surviving: In Vitro, In Vivo and Human Health. Antioxidants 2020, 9, 797. [Google Scholar] [CrossRef]
- Rinaldi, P.; Polidori, M.C.; Metastasio, A.; Mariani, E.; Mattioli, P.; Cherubini, A.; Catani, M.; Cecchetti, R.; Senin, U.; Mecocci, P. Plasma Antioxidants Are Similarly Depleted in Mild Cognitive Impairment and in Alzheimer’s Disease. Neurobiol. Aging 2003, 24, 915–919. [Google Scholar] [CrossRef]
- Peña-Bautista, C.; Tirle, T.; López-Nogueroles, M.; Vento, M.; Baquero, M.; Cháfer-Pericás, C. Oxidative Damage of DNA as Early Marker of Alzheimer’s Disease. Int. J. Mol. Sci. 2019, 20, 6136. [Google Scholar] [CrossRef]
- Sonnen, J.A.; Larson, E.B.; Gray, S.L.; Wilson, A.; Kohama, S.G.; Crane, P.K.; Breitner, J.C.S.; Montine, T.J. Free Radical Damage to Cerebral Cortex in Alzheimer’s Disease, Microvascular Brain Injury, and Smoking. Ann. Neurol. 2009, 65, 226–229. [Google Scholar] [CrossRef]
- Sirin, F.B.; Kumbul Doğuç, D.; Vural, H.; Eren, I.; Inanli, I.; Sütçü, R.; Delibaş, N. Plasma 8-IsoPGF2α and Serum Melatonin Levels in Patients with Minimal Cognitive Impairment and Alzheimer Disease. Turk. J. Med. Sci. 2015, 45, 1073–1077. [Google Scholar] [CrossRef]
- Thome, J.; Gsell, W.; Rösler, M.; Kornhuber, J.; Frölich, L.; Hashimoto, E.; Zielke, B.; Wiesbeck, G.A.; Riederer, P. Oxidative-Stress Associated Parameters (Lactoferrin, Superoxide Dismutases) in Serum of Patients with Alzheimer’s Disease. Life Sci. 1997, 60, 13–19. [Google Scholar] [CrossRef]
- Cardoso, B.R.; Busse, A.L.; Hare, D.J.; Cominetti, C.; Horst, M.A.; McColl, G.; Magaldi, R.M.; Jacob-Filho, W.; Cozzolino, S.M.F. Pro198Leu Polymorphism Affects the Selenium Status and GPx Activity in Response to Brazil Nut Intake. Food Funct. 2016, 7, 825–833. [Google Scholar] [CrossRef]
- Mecocci, P.; Polidori, M.C.; Troiano, L.; Cherubini, A.; Cecchetti, R.; Pini, G.; Straatman, M.; Monti, D.; Stahl, W.; Sies, H.; et al. Plasma Antioxidants and Longevity: A Study on Healthy Centenarians. Free Radic. Biol. Med. 2000, 28, 1243–1248. [Google Scholar] [CrossRef]
- Bøhn, S.K.; Myhrstad, M.C.; Thoresen, M.; Holden, M.; Karlsen, A.; Tunheim, S.H.; Erlund, I.; Svendsen, M.; Seljeflot, I.; Moskaug, J.O.; et al. Blood Cell Gene Expression Associated with Cellular Stress Defense Is Modulated by Antioxidant-Rich Food in a Randomised Controlled Clinical Trial of Male Smokers. BMC Med. 2010, 8, 54. [Google Scholar] [CrossRef]
- Rutledge, G.A.; Fisher, D.R.; Miller, M.G.; Kelly, M.E.; Bielinski, D.F.; Shukitt-Hale, B. The Effects of Blueberry and Strawberry Serum Metabolites on Age-Related Oxidative and Inflammatory Signaling in Vitro. Food Funct. 2019, 10, 7707–7713. [Google Scholar] [CrossRef]
- Stringham, N.T.; Holmes, P.V.; Stringham, J.M. Effects of Macular Xanthophyll Supplementation on Brain-Derived Neurotrophic Factor, pro-Inflammatory Cytokines, and Cognitive Performance. Physiol. Behav. 2019, 211, 112650. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Song, X.; Chen, G.-C.; Neelakantan, N.; van Dam, R.M.; Feng, L.; Yuan, J.-M.; Pan, A.; Koh, W.-P. Dietary Pattern in Midlife and Cognitive Impairment in Late Life: A Prospective Study in Chinese Adults. Am. J. Clin. Nutr. 2019, 110, 912–920. [Google Scholar] [CrossRef]
- Chen, X.; Maguire, B.; Brodaty, H.; O’Leary, F. Dietary Patterns and Cognitive Health in Older Adults: A Systematic Review. J. Alzheimers Dis. 2019, 67, 583–619. [Google Scholar] [CrossRef]
- Trichopoulou, A.; Kyrozis, A.; Rossi, M.; Katsoulis, M.; Trichopoulos, D.; La Vecchia, C.; Lagiou, P. Mediterranean Diet and Cognitive Decline over Time in an Elderly Mediterranean Population. Eur. J. Nutr. 2015, 54, 1311–1321. [Google Scholar] [CrossRef]
- Tanaka, T.; Talegawkar, S.A.; Jin, Y.; Colpo, M.; Ferrucci, L.; Bandinelli, S. Adherence to a Mediterranean Diet Protects from Cognitive Decline in the Invecchiare in Chianti Study of Aging. Nutrients 2018, 10, 2007. [Google Scholar] [CrossRef]
- Scarmeas, N.; Stern, Y.; Mayeux, R.; Manly, J.J.; Schupf, N.; Luchsinger, J.A. Mediterranean Diet and Mild Cognitive Impairment. Arch. Neurol. 2009, 66, 216–225. [Google Scholar] [CrossRef]
- Wu, L.; Sun, D. Adherence to Mediterranean Diet and Risk of Developing Cognitive Disorders: An Updated Systematic Review and Meta-Analysis of Prospective Cohort Studies. Sci. Rep. 2017, 7, 41317. [Google Scholar] [CrossRef]
- Giem, P.; Beeson, W.L.; Fraser, G.E. The Incidence of Dementia and Intake of Animal Products: Preliminary Findings from the Adventist Health Study. Neuroepidemiology 1993, 12, 28–36. [Google Scholar] [CrossRef]
- Péneau, S.; Galan, P.; Jeandel, C.; Ferry, M.; Andreeva, V.; Hercberg, S.; Kesse-Guyot, E.; SU.VI.MAX 2 Research Group. Fruit and Vegetable Intake and Cognitive Function in the SU.VI.MAX 2 Prospective Study. Am. J. Clin. Nutr. 2011, 94, 1295–1303. [Google Scholar] [CrossRef] [PubMed]
- Krikorian, R.; Nash, T.A.; Shidler, M.D.; Shukitt-Hale, B.; Joseph, J.A. Concord Grape Juice Supplementation Improves Memory Function in Older Adults with Mild Cognitive Impairment. Br. J. Nutr. 2010, 103, 730–734. [Google Scholar] [CrossRef]
- Lampe, J.W. Health Effects of Vegetables and Fruit: Assessing Mechanisms of Action in Human Experimental Studies. Am. J. Clin. Nutr. 1999, 70, 475S–490S. [Google Scholar] [CrossRef]
- Sala-Vila, A.; Valls-Pedret, C.; Rajaram, S.; Coll-Padrós, N.; Cofán, M.; Serra-Mir, M.; Pérez-Heras, A.M.; Roth, I.; Freitas-Simoes, T.M.; Doménech, M.; et al. Effect of a 2-Year Diet Intervention with Walnuts on Cognitive Decline. The Walnuts and Healthy Aging (WAHA) Study: A Randomized Controlled Trial. Am. J. Clin. Nutr. 2020, 111, 590–600. [Google Scholar] [CrossRef] [PubMed]
- Valls-Pedret, C.; Sala-Vila, A.; Serra-Mir, M.; Corella, D.; de la Torre, R.; Martínez-González, M.Á.; Martínez-Lapiscina, E.H.; Fitó, M.; Pérez-Heras, A.; Salas-Salvadó, J.; et al. Mediterranean Diet and Age-Related Cognitive Decline: A Randomized Clinical Trial. JAMA Intern. Med. 2015, 175, 1094–1103. [Google Scholar] [CrossRef] [PubMed]
- Rajaram, S.; Valls-Pedret, C.; Cofán, M.; Sabaté, J.; Serra-Mir, M.; Pérez-Heras, A.M.; Arechiga, A.; Casaroli-Marano, R.P.; Alforja, S.; Sala-Vila, A.; et al. The Walnuts and Healthy Aging Study (WAHA): Protocol for a Nutritional Intervention Trial with Walnuts on Brain Aging. Front. Aging Neurosci. 2017, 8. [Google Scholar] [CrossRef]
- Cardoso, B.R.; Apolinário, D.; da Silva Bandeira, V.; Busse, A.L.; Magaldi, R.M.; Jacob-Filho, W.; Cozzolino, S.M.F. Effects of Brazil Nut Consumption on Selenium Status and Cognitive Performance in Older Adults with Mild Cognitive Impairment: A Randomized Controlled Pilot Trial. Eur. J. Nutr. 2016, 55, 107–116. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, J.; Okereke, O.; Devore, E.; Rosner, B.; Breteler, M.; Grodstein, F. Long-Term Intake of Nuts in Relation to Cognitive Function in Older Women. J. Nutr. Health Aging 2014, 18, 496–502. [Google Scholar] [CrossRef] [PubMed]
- Arab, L.; Ang, A. A Cross Sectional Study of the Association between Walnut Consumption and Cognitive Function among Adult US Populations Represented in NHANES. J. Nutr. Health Aging 2015, 19, 284–290. [Google Scholar] [CrossRef]
- Bolling, B.W.; McKay, D.L.; Blumberg, J.B. The Phytochemical Composition and Antioxidant Actions of Tree Nuts. Asia Pac. J. Clin. Nutr. 2010, 19, 117–123. [Google Scholar]
- Chen, C.-Y.O.; Blumberg, J.B. Phytochemical Composition of Nuts. Asia Pac. J. Clin. Nutr. 2008, 17 (Suppl. S1), 329–332. [Google Scholar]
- Pribis, P.; Bailey, R.N.; Russell, A.A.; Kilsby, M.A.; Hernandez, M.; Craig, W.J.; Grajales, T.; Shavlik, D.J.; Sabatè, J. Effects of Walnut Consumption on Cognitive Performance in Young Adults. Br. J. Nutr. 2012, 107, 1393–1401. [Google Scholar] [CrossRef]
- Lee, J.; Torosyan, N.; Silverman, D.H. Examining the Impact of Grape Consumption on Brain Metabolism and Cognitive Function in Patients with Mild Decline in Cognition: A Double-Blinded Placebo Controlled Pilot Study. Exp. Gerontol. 2017, 87, 121–128. [Google Scholar] [CrossRef]
- Zhao, D.; Simon, J.E.; Wu, Q. A Critical Review on Grape Polyphenols for Neuroprotection: Strategies to Enhance Bioefficacy. Crit. Rev. Food Sci. Nutr. 2020, 60, 597–625. [Google Scholar] [CrossRef] [PubMed]
- Buendia, I.; Michalska, P.; Navarro, E.; Gameiro, I.; Egea, J.; León, R. Nrf2—ARE Pathway: An Emerging Target against Oxidative Stress and Neuroinflammation in Neurodegenerative Diseases. Pharmacol. Ther. 2016, 157, 84–104. [Google Scholar] [CrossRef]
- Bensalem, J.; Dudonné, S.; Etchamendy, N.; Pellay, H.; Amadieu, C.; Gaudout, D.; Dubreuil, S.; Paradis, M.-E.; Pomerleau, S.; Capuron, L.; et al. Polyphenols from Grape and Blueberry Improve Episodic Memory in Healthy Elderly with Lower Level of Memory Performance: A Bicentric Double-Blind, Randomized, Placebo-Controlled Clinical Study. J. Gerontol. A Biol. Sci. Med. Sci. 2019, 74, 996–1007. [Google Scholar] [CrossRef] [PubMed]
- Gopalan, A.; Reuben, S.C.; Ahmed, S.; Darvesh, A.S.; Hohmann, J.; Bishayee, A. The Health Benefits of Blackcurrants. Food Funct. 2012, 3, 795. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, A.; Salo, I.; Plaza, M.; Björck, I. Effects of a Mixed Berry Beverage on Cognitive Functions and Cardiometabolic Risk Markers; A Randomized Cross-over Study in Healthy Older Adults. PLoS ONE 2017, 12, e0188173. [Google Scholar] [CrossRef]
- Haskell-Ramsay, C.F.; Stuart, R.C.; Okello, E.J.; Watson, A.W. Cognitive and Mood Improvements Following Acute Supplementation with Purple Grape Juice in Healthy Young Adults. Eur. J. Nutr. 2017, 56, 2621–2631. [Google Scholar] [CrossRef] [PubMed]
- Krikorian, R.; Shidler, M.D.; Nash, T.A.; Kalt, W.; Vinqvist-Tymchuk, M.R.; Shukitt-Hale, B.; Joseph, J.A. Blueberry Supplementation Improves Memory in Older Adults. J. Agric. Food Chem. 2010, 58, 3996–4000. [Google Scholar] [CrossRef] [PubMed]
- Bowtell, J.L.; Aboo-Bakkar, Z.; Conway, M.E.; Adlam, A.-L.R.; Fulford, J. Enhanced Task-Related Brain Activation and Resting Perfusion in Healthy Older Adults after Chronic Blueberry Supplementation. Appl. Physiol. Nutr. Metab. 2017, 42, 773–779. [Google Scholar] [CrossRef]
- Traustadóttir, T.; Davies, S.S.; Stock, A.A.; Su, Y.; Heward, C.B.; Roberts, L.J.; Harman, S.M. Tart Cherry Juice Decreases Oxidative Stress in Healthy Older Men and Women. J. Nutr. 2009, 139, 1896–1900. [Google Scholar] [CrossRef]
- Chai, S.C.; Davis, K.; Zhang, Z.; Zha, L.; Kirschner, K.F. Effects of Tart Cherry Juice on Biomarkers of Inflammation and Oxidative Stress in Older Adults. Nutrients 2019, 11, 228. [Google Scholar] [CrossRef]
- Chai, S.C.; Jerusik, J.; Davis, K.; Wright, R.S.; Zhang, Z. Effect of Montmorency Tart Cherry Juice on Cognitive Performance in Older Adults: A Randomized Controlled Trial. Food Funct. 2019, 10, 4423–4431. [Google Scholar] [CrossRef]
- Siddarth, P.; Li, Z.; Miller, K.J.; Ercoli, L.M.; Merril, D.A.; Henning, S.M.; Heber, D.; Small, G.W. Randomized Placebo-Controlled Study of the Memory Effects of Pomegranate Juice in Middle-Aged and Older Adults. Am. J. Clin. Nutr. 2020, 111, 170–177. [Google Scholar] [CrossRef]
- Bookheimer, S.Y.; Renner, B.A.; Ekstrom, A.; Li, Z.; Henning, S.M.; Brown, J.A.; Jones, M.; Moody, T.; Small, G.W. Pomegranate Juice Augments Memory and FMRI Activity in Middle-Aged and Older Adults with Mild Memory Complaints. Evid. Based Complement. Alternat. Med. 2013, 2013, 946298. [Google Scholar] [CrossRef] [PubMed]
- Enogieru, A.B.; Haylett, W.; Hiss, D.C.; Bardien, S.; Ekpo, O.E. Rutin as a Potent Antioxidant: Implications for Neurodegenerative Disorders. Oxidative Med. Cell. Longev. 2018, 2018, 6241017. [Google Scholar] [CrossRef] [PubMed]
- Kean, R.J.; Lamport, D.J.; Dodd, G.F.; Freeman, J.E.; Williams, C.M.; Ellis, J.A.; Butler, L.T.; Spencer, J.P.E. Chronic Consumption of Flavanone-Rich Orange Juice Is Associated with Cognitive Benefits: An 8-Wk, Randomized, Double-Blind, Placebo-Controlled Trial in Healthy Older Adults. Am. J. Clin. Nutr. 2015, 101, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Bell, L.; Lamport, D.J.; Butler, L.T.; Williams, C.M. A Review of the Cognitive Effects Observed in Humans Following Acute Supplementation with Flavonoids, and Their Associated Mechanisms of Action. Nutrients 2015, 7, 10290–10306. [Google Scholar] [CrossRef]
- Magalingam, K.B.; Radhakrishnan, A.K.; Haleagrahara, N. Protective Mechanisms of Flavonoids in Parkinson’s Disease. Oxidative Med. Cell. Longev. 2015, 2015, 314560. [Google Scholar] [CrossRef]
- Nishimura, M.; Ohkawara, T.; Nakagawa, T.; Muro, T.; Sato, Y.; Satoh, H.; Kobori, M.; Nishihira, J. A Randomized, Double-Blind, Placebo-Controlled Study Evaluating the Effects of Quercetin-Rich Onions on Cognitive Function in Elderly Subjects. Funct. Foods Health Dis. 2017, 7, 353–374. [Google Scholar] [CrossRef]
- Marrelli, M.; Amodeo, V.; Statti, G.; Conforti, F. Biological Properties and Bioactive Components of Allium Cepa, L.: Focus on Potential Benefits in the Treatment of Obesity and Related Comorbidities. Molecules 2018, 24, 119. [Google Scholar] [CrossRef] [PubMed]
- Habtemariam, S. The Therapeutic Potential of Rosemary (Rosmarinus Officinalis) Diterpenes for Alzheimer’s Disease. Evid. Based Complement. Altern. Med. 2016, 2016, 2680409. [Google Scholar] [CrossRef] [PubMed]
- Sorond, F.A.; Hurwitz, S.; Salat, D.H.; Greve, D.N.; Fisher, N.D.L. Neurovascular Coupling, Cerebral White Matter Integrity, and Response to Cocoa in Older People. Neurology 2013, 81, 904–909. [Google Scholar] [CrossRef] [PubMed]
- Eskelinen, M.H.; Ngandu, T.; Tuomilehto, J.; Soininen, H.; Kivipelto, M. Midlife Coffee and Tea Drinking and the Risk of Late-Life Dementia: A Population-Based CAIDE Study. J. Alzheimers Dis. 2009, 16, 85–91. [Google Scholar] [CrossRef]
- McCarty, M.F.; Assanga, S.B.I. Ferulic Acid May Target MyD88-Mediated pro-Inflammatory Signaling—Implications for the Health Protection Afforded by Whole Grains, Anthocyanins, and Coffee. Med. Hypotheses 2018, 118, 114–120. [Google Scholar] [CrossRef]
- Shah, S.P.; Duda, J.E. Dietary Modifications in Parkinson’s Disease: A Neuroprotective Intervention? Med. Hypotheses 2015, 85, 1002–1005. [Google Scholar] [CrossRef]
- Okello, E.J.; Mather, J. Comparative Kinetics of Acetyl- and Butyryl-Cholinesterase Inhibition by Green Tea Catechins|Relevance to the Symptomatic Treatment of Alzheimer’s Disease. Nutrients 2020, 12, 1090. [Google Scholar] [CrossRef]
- Mazzio, E.A.; Close, F.; Soliman, K.F.A. The Biochemical and Cellular Basis for Nutraceutical Strategies to Attenuate Neurodegeneration in Parkinson’s Disease. Int. J. Mol. Sci. 2011, 12, 506–569. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. PRISMA Group Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed]
- Kent, K.; Charlton, K.; Roodenrys, S.; Batterham, M.; Potter, J.; Traynor, V.; Gilbert, H.; Morgan, O.; Richards, R. Consumption of Anthocyanin-Rich Cherry Juice for 12 Weeks Improves Memory and Cognition in Older Adults with Mild-to-Moderate Dementia. Eur. J. Nutr. 2017, 56, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Remington, R.; Chan, A.; Lepore, A.; Kotlya, E.; Shea, T.B. Apple Juice Improved Behavioral but Not Cognitive Symptoms in Moderate-to-Late Stage Alzheimer’s Disease in an Open-Label Pilot Study. Am. J. Alzheimer’s Dis. Other Dement. 2010, 25, 367–371. [Google Scholar] [CrossRef]
- Miller, M.G.; Hamilton, D.A.; Joseph, J.A.; Shukitt-Hale, B. Dietary Blueberry Improves Cognition among Older Adults in a Randomized, Double-Blind, Placebo-Controlled Trial. Eur. J. Nutr. 2018, 57, 1169–1180. [Google Scholar] [CrossRef]
- Pengelly, A.; Snow, J.; Mills, S.Y.; Scholey, A.; Wesnes, K.; Butler, L.R. Short-Term Study on the Effects of Rosemary on Cognitive Function in an Elderly Population. J. Med. Food 2012, 15, 10–17. [Google Scholar] [CrossRef]
- Nooyens, A.C.J.; Bueno-de-Mesquita, H.B.; van Boxtel, M.P.J.; van Gelder, B.M.; Verhagen, H.; Verschuren, W.M.M. Fruit and Vegetable Intake and Cognitive Decline in Middle-Aged Men and Women: The Doetinchem Cohort Study. Br. J. Nutr. 2011, 106, 752–761. [Google Scholar] [CrossRef]
- Dai, Q.; Borenstein, A.R.; Wu, Y.; Jackson, J.C.; Larson, E.B. Fruit and Vegetable Juices and Alzheimer’s Disease: The Kame Project. Am. J. Med. 2006, 119, 751–759. [Google Scholar] [CrossRef]
- Godos, J.; Caraci, F.; Castellano, S.; Currenti, W.; Galvano, F.; Ferri, R.; Grosso, G. Association Between Dietary Flavonoids Intake and Cognitive Function in an Italian Cohort. Biomolecules 2020, 10, 1300. [Google Scholar] [CrossRef] [PubMed]
- Bondonno, C.P.; Downey, L.A.; Croft, K.D.; Scholey, A.; Stough, C.; Yang, X.; Considine, M.J.; Ward, N.C.; Puddey, I.B.; Swinny, E.; et al. The Acute Effect of Flavonoid-Rich Apples and Nitrate-Rich Spinach on Cognitive Performance and Mood in Healthy Men and Women. Food Funct. 2014, 5, 849–858. [Google Scholar] [CrossRef] [PubMed]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. “Mini-Mental State”. A Practical Method for Grading the Cognitive State of Patients for the Clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef]
- Crichton, G.E.; Elias, M.F.; Alkerwi, A. Chocolate Intake Is Associated with Better Cognitive Function: The Maine-Syracuse Longitudinal Study. Appetite 2016, 100, 126–132. [Google Scholar] [CrossRef]
- Haller, S.; Montandon, M.-L.; Rodriguez, C.; Herrmann, F.R.; Giannakopoulos, P. Impact of Coffee, Wine, and Chocolate Consumption on Cognitive Outcome and MRI Parameters in Old Age. Nutrients 2018, 10, 1391. [Google Scholar] [CrossRef]
- Ide, K.; Yamada, H.; Takuma, N.; Kawasaki, Y.; Harada, S.; Nakase, J.; Ukawa, Y.; Sagesaka, Y.M. Effects of Green Tea Consumption on Cognitive Dysfunction in an Elderly Population: A Randomized Placebo-Controlled Study. Nutr. J. 2016, 15, 49. [Google Scholar] [CrossRef]
- Ng, T.-P.; Feng, L.; Niti, M.; Kua, E.-H.; Yap, K.-B. Tea Consumption and Cognitive Impairment and Decline in Older Chinese Adults. Am. J. Clin. Nutr. 2008, 88, 224–231. [Google Scholar] [CrossRef]
- Kuriyama, S.; Hozawa, A.; Ohmori, K.; Shimazu, T.; Matsui, T.; Ebihara, S.; Awata, S.; Nagatomi, R.; Arai, H.; Tsuji, I. Green Tea Consumption and Cognitive Function: A Cross-Sectional Study from the Tsurugaya Project 1. Am. J. Clin. Nutr. 2006, 83, 355–361. [Google Scholar] [CrossRef]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An Overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef]
- Ebrahimi, A.; Schluesener, H. Natural Polyphenols against Neurodegenerative Disorders: Potentials and Pitfalls. Ageing Res. Rev. 2012, 11, 329–345. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Goel, N. Phenolic Acids: Natural Versatile Molecules with Promising Therapeutic Applications. Biotechnol. Rep. 2019, 24, e00370. [Google Scholar] [CrossRef]
- Solanki, I.; Parihar, P.; Parihar, M.S. Neurodegenerative Diseases: From Available Treatments to Prospective Herbal Therapy. Neurochem. Int. 2016, 95, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Lima, G.P.P.; Vianello, F.; Corrêa, C.R.; da Silva Campos, R.A.; Borguini, M.G. Polyphenols in Fruits and Vegetables and Its Effect on Human Health. Food Nutr. Sci. 2014, 5, 1065–1082. [Google Scholar] [CrossRef]
- Hornedo-Ortega, R.; Cerezo, A.B.; de Pablos, R.M.; Krisa, S.; Richard, T.; García-Parrilla, M.C.; Troncoso, A.M. Phenolic Compounds Characteristic of the Mediterranean Diet in Mitigating Microglia-Mediated Neuroinflammation. Front. Cell. Neurosci. 2018, 12, 373. [Google Scholar] [CrossRef] [PubMed]
- Airoldi, C.; La Ferla, B.; D’Orazio, G.; Ciaramelli, C.; Palmioli, A. Flavonoids in the Treatment of Alzheimer’s and Other Neurodegenerative Diseases. Curr. Med. Chem. 2018, 25, 3228–3246. [Google Scholar] [CrossRef]
- Arruda, H.S.; Neri-Numa, I.A.; Kido, L.A.; Maróstica Júnior, M.R.; Pastore, G.M. Recent Advances and Possibilities for the Use of Plant Phenolic Compounds to Manage Ageing-Related Diseases. J. Funct. Foods 2020, 75, 104203. [Google Scholar] [CrossRef]
- Bjørklund, G.; Dadar, M.; Chirumbolo, S.; Lysiuk, R. Flavonoids as Detoxifying and Pro-Survival Agents: What’s New? Food Chem. Toxicol. 2017, 110, 240–250. [Google Scholar] [CrossRef]
- Sarvestani, N.N.; Khodagholi, F.; Ansari, N.; Farimani, M.M. Involvement of P-CREB and Phase II Detoxifying Enzyme System in Neuroprotection Mediated by the Flavonoid Calycopterin Isolated from Dracocephalum Kotschyi. Phytomed. Int. J. Phytother. Phytopharmacol. 2013, 20, 939–946. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Pinilla, F.; Nguyen, T.T.J. Natural Mood Foods: The Actions of Polyphenols against Psychiatric and Cognitive Disorders. Nutr. Neurosci. 2012, 15, 127–133. [Google Scholar] [CrossRef]
- Rebas, E.; Rzajew, J.; Radzik, T.; Zylinska, L. Neuroprotective Polyphenols: A Modulatory Action on Neurotransmitter Pathways. Curr. Neuropharmacol. 2020, 18, 431–445. [Google Scholar] [CrossRef]
- Hanrahan, J.R.; Chebib, M.; Johnston, G.A.R. Flavonoid Modulation of GABA(A) Receptors. Br. J. Pharmacol. 2011, 163, 234–245. [Google Scholar] [CrossRef]
- Demir, Y.; Durmaz, L.; Taslimi, P.; Gulçin, İ. Antidiabetic Properties of Dietary Phenolic Compounds: Inhibition Effects on α-Amylase, Aldose Reductase, and α-Glycosidase. Biotechnol. Appl. Biochem. 2019, 66, 781–786. [Google Scholar] [CrossRef]
- Kim, Y.; Keogh, J.B.; Clifton, P.M. Polyphenols and Glycemic Control. Nutrients 2016, 8, 17. [Google Scholar] [CrossRef]
- Phan, H.T.T.; Samarat, K.; Takamura, Y.; Azo-Oussou, A.F.; Nakazono, Y.; Vestergaard, M.C. Polyphenols Modulate Alzheimer’s Amyloid Beta Aggregation in a Structure-Dependent Manner. Nutrients 2019, 11, 756. [Google Scholar] [CrossRef] [PubMed]
- Pezzuto, J.M.; Venkatasubramanian, V.; Hamad, M.; Morris, K.R. Unraveling the Relationship between Grapes and Health. J. Nutr. 2009, 139, 1783S–1787S. [Google Scholar] [CrossRef] [PubMed]
- Yuan, T.; Ma, H.; Liu, W.; Niesen, D.B.; Shah, N.; Crews, R.; Rose, K.N.; Vattem, D.A.; Seeram, N.P. Pomegranate’s Neuroprotective Effects against Alzheimer’s Disease Are Mediated by Urolithins, Its Ellagitannin-Gut Microbial Derived Metabolites. ACS Chem. Neurosci. 2016, 7, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Francis, S.T.; Head, K.; Morris, P.G.; Macdonald, I.A. The Effect of Flavanol-Rich Cocoa on the FMRI Response to a Cognitive Task in Healthy Young People. J. Cardiovasc. Pharmacol. 2006, 47 (Suppl. S2), S215–S220. [Google Scholar] [CrossRef] [PubMed]
- Mišík, M.; Hoelzl, C.; Wagner, K.-H.; Cavin, C.; Moser, B.; Kundi, M.; Simic, T.; Elbling, L.; Kager, N.; Ferk, F.; et al. Impact of Paper Filtered Coffee on Oxidative DNA-Damage: Results of a Clinical Trial. Mutat. Res. 2010, 692, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Siervo, M.; Lara, J.; Munro, A.; Tang, E.Y.H.; Rutjes, A.W.; Stephan, B. Dietary Interventions for Maintaining Cognitive Function in Cognitively Healthy People in Late Life. Cochrane Database Syst. Rev. 2015. [Google Scholar] [CrossRef]
- Vercambre, M.-N.; Berr, C.; Ritchie, K.; Kang, J.H. Caffeine and Cognitive Decline in Elderly Women at High Vascular Risk. J. Alzheimers Dis. 2013, 35, 413–421. [Google Scholar] [CrossRef] [PubMed]
- Medawar, E.; Huhn, S.; Villringer, A.; Veronica Witte, A. The Effects of Plant-Based Diets on the Body and the Brain: A Systematic Review. Transl. Psychiatry 2019, 9, 226. [Google Scholar] [CrossRef]
| Plant Food | Phytochemicals | Effects and Mechanisms | References |
|---|---|---|---|
| Nuts | Tocopherols, PUFA, especially α-linolenic acid (18:3 n–3, ALA, the plant-origin ω-3 fatty acid), carotenoids, tannins, naphthoquinones, phenolic acids (ellagic acid), phytosterols, polyphenols, melatonin, arginine, folates. | Antioxidant, anti-inflammatory. Improve antioxidant status, global cognition and perception, verbal fluency, delay onset of cognitive impairment in elderly subjects; improve inferential reasoning and cognitive functions in young adults. | [4,32,33,34,35,36,37,38,39,40] |
| Grapes | Stilbenes: trans-resveratrol, trans-resveratroloside, trans- pterostilbene, trans-picetannol, resveratrol. Phenolic-acids: caffeic acid, ferulic acid, p-coumaric acid, vanillic acid, gallic acid. Flavonoids: quercetin, kaempherol etc. | Antioxidant, anti-inflammatory. Protect against beta-amyloid peptide formation, increase the expression of Nrf2-related genes, modulate cerebral blood flow (CBF). Modulate glucose metabolism and inhibit MAO and GABA-ergic activities. Protect cerebral metabolism. Acute and chronic effect on cognition. | [30,41,42,43,44] |
| Berries | Quercetin, myricetin, ellagic acid, stilbenoids, kaempferol, vitamin C, proanthocyanidins, ellagitannins and phenolic acids. | Antioxidant, anti-inflammatory (multiple pathways), increase enzymatic antioxidant defenses (GSH). Antimicrobial, improve glucoregulation, interaction with microbiota, CBF. Increase digit vigilance reaction time, executive and memory functions. Increase neurogenesis. | [4,45,46,47,48,49] |
| Cherries | Proanthocyanins, anthocyanins (cyanidin-3-glucosylrutinoside, cyanidin-3-rutinoside, cyanindin-3-glucoside, and their agylcone, cyanidin), flavonols, melatonin. | Antioxidant, anti-inflammatory. Reduce the I/R-induced F2-isoprostane response and basal urinary excretion of oxidized nucleic acids (8-hydroxy-29-deoxyguanosine, 8-hydroxyguanosine or 8-OHdG). | [50,51,52] |
| Pomegranate | Ellagitannin, anthocyanins, flavan 3-ols, flavonols, catechins. | Antioxidant, anti-inflammatory. Pomegranate juice inhibited inflammation and amyloidogenesis in IL-1β-stimulated SK-N-SH cells. | [53,54] |
| Oranges | Flavanones (hesperidin), flavonols (rutin and quercetin). | Anti-inflammatory, anticancer, antithrombotic, cytoprotective and vasoprotective, inhibit Aβ25–35 fibril formation and aggregation in vitro, prevent mitochondrial damage, reduce OxS marker and proinflammatory cytokine (IL-1 β, TNF-α) generation, enhance antioxidant enzymes, improve CBF, and cognitive function. | [55,56,57] |
| Onion | Flavonoids (quercetin), phytosterols, saponins and sulphur-containing compounds, like N-acetylcysteine (NAC), S-methyl-L-cysteine, and S-propyl-L-cysteine sulfoxide. | Antioxidant, anti-inflammatory, anti-viral. Up-regulation of SIRT-1 and gluco-regulation (quercetin). Helps protect against cognitive decline. | [58,59] |
| Rosemary | Carnosic acid and carnosol (diterpens) are the two major antioxidants. | Antioxidant, anti-inflammatory. The two diterpens can induce Nrf2 and phase II detoxifying enzymes. Neuroprotective effects in vitro on human brain cells. AChE and BChE inhibition, DA neuron protection; metalloproteinase induction (carnosic acid). | [43,59,60,61] |
| Chocolate | Large amounts of the flavan-3-ol epicatechin, catechin, oligomeric procyanidins. | Antioxidant, anti-inflammatory. Stimulation of BDNF synthesis (neurogenesis, neuroplasticity), improvement of neurovascular function, CBF, BOLD. | [4,47,62] |
| Coffee | Rich source of caffein, and phenolic acids, especially chlorogenic acids, ferulic acid, hydroxycinnamic acids, 4-caffeoylquinic acid and 5-caffeoylquinic acid. | Antioxidant, anti-inflammatory, phase II detoxifying enzymes. Improves IR (antidiabetic). Acute and chronic effects on cognition, with J shaped curve on dementia for chronic consumption. | [4,63,64] |
| Tea | Major source of catechins (epicatechins and procyanidins) especially EGCG, theaflavin, caffeine. | Antioxidant, anti-inflammatory. EGCG inhibits AChE and BuChE, modulates the accumulation of amyloid fibrils and α-synuclein in vitro. Modulation of pro-apoptotic genes. Calming effect. Lipoxygenase inhibitor. | [57,58,65,66,67] |
| Authors, Year | Food | Study Design | Country | Subject Numbers and Characteristics | Sex | Age (Years) | Results | Efficacy |
|---|---|---|---|---|---|---|---|---|
| Arab & Ang, 2015 [37] | Nuts | Cross-sectional study of 2 groups of subjects | USA | 12,693, US civilian population (excluded who had a stroke or a neurological disorder), aged 20–59 years (5356) and >60 (7337). | 7070 F | 37.4, 70.1 mean | Significant, positive associations between walnut consumption and cognitive functions among all adults, regardless of age, gender or ethnicity. | SE |
| Bondonno et al., 2014 [76] | Flavonoid-rich apples and spinach | Crossover RCT | Australia | 30 healthy volunteers; 4 intervents in random order: (1) control: low flavonoid apple control (C) and low nitrate C; (2) apple: high flavonoid apple active (A) and low nitrate C; (3) spinach: low flavonoid apple C and nitrate-rich spinach A; (4) apple + spinach: high flavonoid apple A and nitrate-rich spinach A. | 24 F | 47.3 mean | No significant effect was observed on cognitive function. | NE |
| Bookheimer et al., 2013 [54] | Pomegranate juice | Placebo RCT | USA | 28 non-demented, elderly subjects; 15 pomegranate juice, 13 placebo flavor-matched drink. | 21 F | 62.5 mean | Pomegranate group showed a significant improvement in memory scores (recall measure and long-term retrieval) and increased task-related brain activation in healthy elderly subjects. | SE |
| Bowtell et al., 2017 [49] | Blueberry concentrate juice | Double-blind placebo RCT | UK | 26 healthy elderly subjects, 12 blueberry concentrated juice, 14 isoenergetic placebo. | 13 F | 68.2 mean | Non significant improvement in working memory (2-back test) in the blueberry versus the placebo group. | NSE in cognitive subsets |
| Cardoso et al., 2016 [35] | Brazil nuts | Placebo RCT | Brazil | 31 subjects >60 years with MCI: 16 brazil nuts, 15 control group. | 22 F | 77.7 mean | Changes in the total score not significantly different between groups. Statistically significant improvement only in verbal fluency and constructional praxis compared with control group. | NSE total score/SE in cognitive subsets |
| Chai et al., 2019 [52] | Montmorency tart cherry juice | Placebo RCT | USA | 37 adults aged 65–80 years with normal cognitive function enrolled, 34 completed: 17 tart cherry juice, 17 control juice. | 20 F | 65–80 | Significantly higher memory scores and learning task performances in the tart cherry compared to the control group. Significant improvement of sustained attention and spatial working memory in the within-group in tart cherry juice compared with corresponding baseline values. | SE |
| Crichton et al., 2016 [78] | Chocolate | Cross-sectional study | Australia | 968 non demented community dwelling; frequency intake of chocolate assessment (never/rarely or at least once/wk). | 558 F | 23–98, 61.9 mean | More frequent chocolate consumption was significantly associated with better performance in all tests, with the exception of working memory. | SE all domains except working memory |
| Dai et al., 2006 [74] | Fruit and vegetable juice | Prospective study (8 years: 4 follow-up waves, each 2 years apart) | USA | 1589 non demented aged >65 years. | 864 F | >65, 71.8 mean | The risk for probable AD was significantly reduced among people who drank fruit and vegetable juices 3 or more times per wk, compared with those who drank these juices less than once per wk. | SE |
| Eskelinen et al., 2009 [63] | Green tea and coffee | Prospective study (follow-up: 21 years) | Finland | 1409 elderly subjects randomly selected from the survivors of a population-based cohort previously surveyed at midlife visit. | 874 F | 71.3 mean | Moderate coffee consumption at midlife may decrease the risk of dementia/AD later in life. No association between tea consumption and risk of dementia (small sample) | SE for coffee/ NSE for tea |
| Godos et al., 2020 [75] | Flavonoid | Prospective study (follow-up: 24 mo) | Italy | 883 subjects divided by quartiles of total polyphenol intake: Q1 (n = 184), Q2 (n = 237), Q3 (n = 253) and Q4 (n = 209). | 382 F | 64.9 mean | Significant inverse association between higher dietary intake of total flavonoids and impaired cognitive status. Among individual subclasses of flavonoids, flavan-3-ols, catechins, anthocyanins and flavonols, and among individual polyphenols only quercetin, were associated with cognitive health. | SE |
| Haller et al., 2018 [79] | Coffee, wine and chocolate | Prospective study (follow-up: 3 years) | Switzerland | 145 community-based elderly individuals with preserved cognition aged 69–86 years. | 81 F | 69–86, 73.8 mean | Moderate consumption of caffeinate (coffee and chocolate) was related significantly to better cognitive outcome. In contrast, increased consumption of wine was related to an unfavorable cognitive evolution. | SE caffeinate; NE wine |
| Haskell-Ramsay et al., 2017 [47] | Purple grape juice | Double-blind, counterbalanced-crossover placebo RCT | UK | 20 healthy young adults. | 13 F | 18–35 | Purple grape juice significantly improved reaction time on a composite attention measure compared to placebo. | SE |
| Ide et al., 2016 [80] | Green tea | Placebo RCT | Japan | 27 elderly nursing home residents with cognitive dysfunction, 17 assigned to green tea and 16 to placebo. | 29 F | 84.8 mean | Changes in cognitive scores after 1 year of green tea consumption were not significantly different compared with that of the placebo group. | NE |
| Kean et al., 2015 [56] | Orange juice | Double-blind, crossover, placebo RCT | UK | 37 healthy elderly subjects aged 60–81: high flavanone 100% juice and low flavanone control juice. | 24 F | 60–81, 66.7 mean | Global cognitive function was significantly better after 8 wk of consumption of high flavanone (HF) orange juice relative to 8 wk of consumption of the control low-flavanone (LF) juice; better performance in sustained attention and episodic memory when the HF drink rather LF drink was consumed during the first arm. | SE |
| Kent et al., 2017 [69] | Cherry juice | Placebo RCT | Australia | 49 elderly subjects with mild-to-moderate AD recruited, 42 completed: 21 cherry juice, 21 (controls) apple juice. | not specified | 79.7 mean | Significant improvements in verbal fluency, short-term memory and long-term memory in the cherry juice group. No significant improvements from baseline in the control group. | SE |
| Krikorian et al., 2010 [30] | Concord grape juice | Double-blind, placebo RCT | USA | 12 elderly subjects with acquired early memory decline (not dementia); 5 Concord grape juice 100%, 7 placebo juice. | 4 F | 78.2 mean | Significant improvement in measures of verbal learning and non-significant enhancement of verbal and spatial recall. | SE in cognitive subsets |
| Krikorian et al., 2010 [48] | Wild blueberry juice | Double-blind, placebo RCT | USA | 9 elderly subjects with early memory changes in wild blueberry juice; 7 control group and beverage as in Krikorian study [30]. | 4 F | 76.2 mean | Statistically significant improvement of memory function (paired associate learning and word list recall). | SE |
| Kuriyama et al., 2006 [82] | Green, black or oolong tea | Cross-sectional study | Japan | 1003 elderly subjects >70 (excluding subjects with missing data on body weight, height, blood glucose concentrations, blood pressure, or depressive symptoms) | 531 F | 74 mean | Regular green tea consumers were less likely to develop cognitive impairment. On the other hand, black or oolong tea consumption was not correlated with significantly lower risk of developing a cognitive impairment | SE green tea/NSE other teas |
| Lee et al., 2017 [41] | Grape freeze-dried powder | Double-blind, placebo RCT | USA | 10 elderly subjects with MCI randomized to consume an active grape formulation (n = not specified) or a matched placebo formulation (n = not specified). | 5 F | 66–82, 72.2 mean | Supplementation with grapes did not change neuropsychological battery measures but showed a protective effect on brain metabolism. | NE/NSE in subgroup |
| Miller et al., 2018 [71] | Tifblue blueberry lyophilized | Double-blind, placebo RCT | USA | 37 elderly subjects with cognitive decline as the ages: 18 blueberry, 19 control beverage. | 24 F | 60–75 | Subjects in the blueberry group showed, relatively to controls, significantly reduction in reaction times, fewer repetition errors in long term memory test and reduced switch cost on a task-switching test across study visits. | SE |
| Ng et al., 2008 [81] | Tea and coffee | Cross-sectional study plus prospective study (follow-up 1–2 years, median 16 mo) | China | Cross sectional on 2194 and prospective study on 1438 healthy adults >55 years. | 1323 F | 65.6 mean | More frequent tea consumption, of any kind, was associated with lower risk of cognitive impairment and cognitive decline. No association btw coffee and cognitive impairment | SE for teas; NE for coffee |
| Nilsson et al., 2017 [46] | Mixture of berries beverage | Crossover RCT | Sweden | 40 healthy 50–70 years old volunteers, 20 berry beverage and 20 control 5 wks, 5 wks interval then reverse 5 wks. | 30 F | 50–70 | Subjects performed significantly better in the working memory test after the berry beverage compared to after the control beverage. No significant effects on the other test variables were observed. | SE only 1 domain |
| Nishimura et al., 2017 [59] | Onion powder | Double-blind, placebo RCT | Japan | 50 healthy or with MCI subjects, randomized: 25 quercetin-rich onion powder and 25 a placebo onion powder without detectable quercetin. | 25 F | 65–84 | No differences in MMSE and cognitive impairment rating scale scores between the two groups. Only in younger subjects the MMSE scores were significantly higher in the active test food group than in the placebo food group at wk 24. | NE/SE in subgroup |
| Nooyens et al., 2011 [73] | Fruit and vegetable | Prospective study (follow-up: 5 years) | The Netherlands | A general population sample of 2613 subjects (excluding those who reported having experienced a stroke); habitual amount and frequency of fruit and vegetable intake studied in association with baseline and change in cognitive function. | 1325 F | 43–70 | Total intakes of fruits, legumes and juices were not associated with baseline or change in cognitive function. High intakes of some subgroups of fruits and vegetables (such as nuts, cabbage and root vegetables) were associated with significantly better cognitive function at baseline and/or smaller decline in cognitive domains. | SE subgroups of fruits and vegetables |
| O’Brien et al., 2014 [36] | Nuts | Prospective study (follow-up: 6 years) | USA | 15,467 women >70 years without a story of stroke. | 15,467 F | 74.3 mean | Higher total nut intake over the long term was associated with significantly better cognitive global performance at older ages. | SE |
| Pengelly et al., 2012 [72] | Rosemary | Double-blind, repeated-measures, crossover, placebo RCT | USA | 28 healthy and non-smoking elderly subjects aged 65–90 years cognitively tested 1, 2.5, 4, and 6 h following a placebo and four different doses of dried rosemary. | 20 F | 65–90, 75 mean | Significant improvement in measures of “speed of memory” compared with placebo with the lowest dose (750 mg) of rosemary, worsening with highest dose (6000 mg). | SE with lowest dose |
| Pribis et al., 2012 [40] | Walnuts | Double-blind, crossover, placebo RCT | USA | 47 college students: 23 walnut-placebo group, 24 placebo-walnut group. | not specified | 18–25, 20.6 mean | No significant increases were detected for non-verbal reasoning or memory on the walnut supplemented. Significant, moderate effect size increase in inferential reasoning. | NE/SE in cognitive subsets |
| Remington et al., 2010 [70] | Apple juice | Open-label pilot study | USA | 21 nursing home residents with moderate-to-late-stage AD, no control group. | not specified | 72–93 | No change in cognitive performance; significant improvement reported by caregivers in behavioral and psychological symptoms associated with dementia. | NE |
| Sala-Vila et al., 2020 [32] | Walnuts | Dual center, single blind, parallel-group RCT | USA/Spain | 657 cognitively healthy elders, 336 walnut diet and 321 control diet. | 439 F | 63–79 | Walnut supplementation for 2 years had no effect on cognition in healthy elders; analyses by site suggest that walnuts might delay cognitive decline in subgroups at higher risk. | NE/NSE in subgroup |
| Siddarth et al., 2020 [53] | Pomegranate juice | Double-blind, 2-group parallel RCT | USA | 200 subjects with normal aging or MCI: 98 pomegranate juice, 102 placebo drink. | 174 F | 50–75 | Daily consumption of pomegranate juice has shown to stabilize the ability to learn visual information over a 12-mo period. | NSE |
| Valls-Pedret et al., 2015 [33] | MD+ olive oil/nuts | Parallel-group RCT | Spain | 334 subjects at high cardiovascular risk but without cardiovascular disease. | 233 F | 55–80 | A Mediterranean diet (MD) supplemented with olive oil or nuts is associated with significant improvement in cognitive function. | SE |
| Classes | Phenolic Compounds | Sources | ||
|---|---|---|---|---|
| FLAVONOIDS | Anthocyanins | Aurantidin, cyanidin, luteolinidin, rosinidin, petunidin, malvidin, peonidin, etc. | Fruit, vegetables, nuts, medicinal plants, especially cranberries, black currants, red grapes, raspberries, strawberries, blueberries and blackberries. | |
| Isoflavanoids | Isoflavons | Genistenin, daizenin, glycitein. | Legumes, medicinal plants, especially soy, but also lupin, fava beans, kudzu. | |
| Isoflavans | Equol. | |||
| Flavanols, flavan-3-ols or catechins | Catechins, epicatechins, epigallocatechin (EGC), epicatechin gallate (ECG), epigallocatechin gallate (EGCG). | Fruit, medicinal plants and others, especially tea, chocolate. | ||
| Flavanones | Eriodictyol, narigenin, hesperitin, naringenin, abyssinones, hesperidin. | Fruit, medicinal plants and others, especially citrus fruits (orange, lemon) and grapes. | ||
| Flavonols | Kaempferol, myricetin, fisetin, rutin, quercetin. | Fruit, medicinal plants and others, especially onions, kale, lettuce, tomatoes, apples, grapes and berries. | ||
| Flavones | Apigenin, tangeretin, baicalein, rhoifolin. | Fruit, vegetables, medicinal plants, especially celery, parsley, red peppers, chamomile, mint, ginkgo biloba, peels of citrus fruits. | ||
| Chalcones | Fruit, vegetables, nuts, medicinal plants, especially tomatoes, pears, strawberries, bearberries and certain wheat products. | |||
| NON FLAVONOIDS | Phenolic acids | Hydroxybenzoic acids | Benzoic acid, gallic acid, salicylic acid, vanillic acid, ellagic acid. | Fruits, vegetables, medicinal plants, grains and others, especially coffee, tea, berries, pomegranate. |
| Hydroxycinnamic acids | Caffeic acid, chlorogenic acid, ferulic acid, sinapic acid, cumaric acid, gallic acid, rosmarinic acid. | |||
| Curcuminoids | Curcumin. | Tumeric | ||
| Stilbenes | Resveratrol. | Fruit, nuts, medicinal plants and others, especially grapes and red wine. | ||
| Hypothesized Effect | Overview |
|---|---|
| Direct oxidants scavenge | Scavenge superoxide and hydroxyl radicals, peroxyl radicals, NO, carbon-center free radicals, singlet oxygen and lipid free radicals, and peroxynitrite [84,86]. |
| Modulation of cellular signaling pathways | Neuroprotection via the activation of pro-survival pathways (ERK1/2, PI3K/Akt, PKC), and inhibition of pro-apoptotic pathways (JNK, p. 38) [84,86,90]. |
| Mitigation of mitochondrial dysfunction | Modulating mitochondrial dynamics, function, and biogenesis [19,58,90]. |
| Induction of antioxidant and phase II detoxification enzyme expression | GSH, CAT, SOD, CYP 450, glutathione S-transferase, NAD(P)H-quinone oxidoreductase, and UDP-glucuronosyl transferase (via Nrf2) [64,84,86,90,91,92]. This effect could be mediated by induction of cell’s adaptative response caused by polyphenol’s pro-oxidant activities [10,11,19]. |
| Modulation of synaptic plasticity (SP) | Modulation of synaptic morphology, neuroreceptors, kinase activity, release of BDNF, CREB [42,47,93]. |
| Anxiolytic effect | Via modulation of cortisol and GABA receptors and suppression of the activity of MAO [47,93,94,95]. |
| Increased CBF | Improving vasodilatation, angiogenesis and functional hyperemia/neurovascular coupling (NVC) [42,62]. |
| Metal chelation | Decreasing metal accumulation in neurons [84,90,91]. |
| Anti-inflammatory activities | Attenuating the expression of several pro-inflammatory pathways (OX-2, iNOS, NF-kB, IL-6, IL-1β, IL-1α, TNF-α, p38 etc.), inhibiting microglial and astrocytic overactivation [86,90]. |
| Anti-protein formation and aggregation | Modulating the formation and/or aggregation of α-synuclein protein, amyloid-β plaques, neurofibrillary tangles [86,90], and TAU proteins [86]. |
| Glucose homeostasis/hypoglycemic effect | Improving insulin-sensitivity, and peripheral glucose uptake; stimulating insulin secretion, delaying glucose absorption, protecting pancreatic β-cells, suppressing glucose release from the liver, inhibiting aldose reductase and AGEs generation [91,96,97]. |
| Protection against amyloid toxicity | Decreasing the accumulation of amyloid fibrils and α-synuclein as shown in vitro [41,89,98]. |
| Catecholamines increase (dopamine and ACh) | Stimulating the tyrosine hydroxylase expression/activity, inhibiting AChE and MAO expression/activity, activating dopamine receptors [66,86,90,94]. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baroni, L.; Sarni, A.R.; Zuliani, C. Plant Foods Rich in Antioxidants and Human Cognition: A Systematic Review. Antioxidants 2021, 10, 714. https://doi.org/10.3390/antiox10050714
Baroni L, Sarni AR, Zuliani C. Plant Foods Rich in Antioxidants and Human Cognition: A Systematic Review. Antioxidants. 2021; 10(5):714. https://doi.org/10.3390/antiox10050714
Chicago/Turabian StyleBaroni, Luciana, Anna Rita Sarni, and Cristina Zuliani. 2021. "Plant Foods Rich in Antioxidants and Human Cognition: A Systematic Review" Antioxidants 10, no. 5: 714. https://doi.org/10.3390/antiox10050714
APA StyleBaroni, L., Sarni, A. R., & Zuliani, C. (2021). Plant Foods Rich in Antioxidants and Human Cognition: A Systematic Review. Antioxidants, 10(5), 714. https://doi.org/10.3390/antiox10050714







