Recent Advances on Nanoparticle Based Strategies for Improving Carotenoid Stability and Biological Activity
Abstract
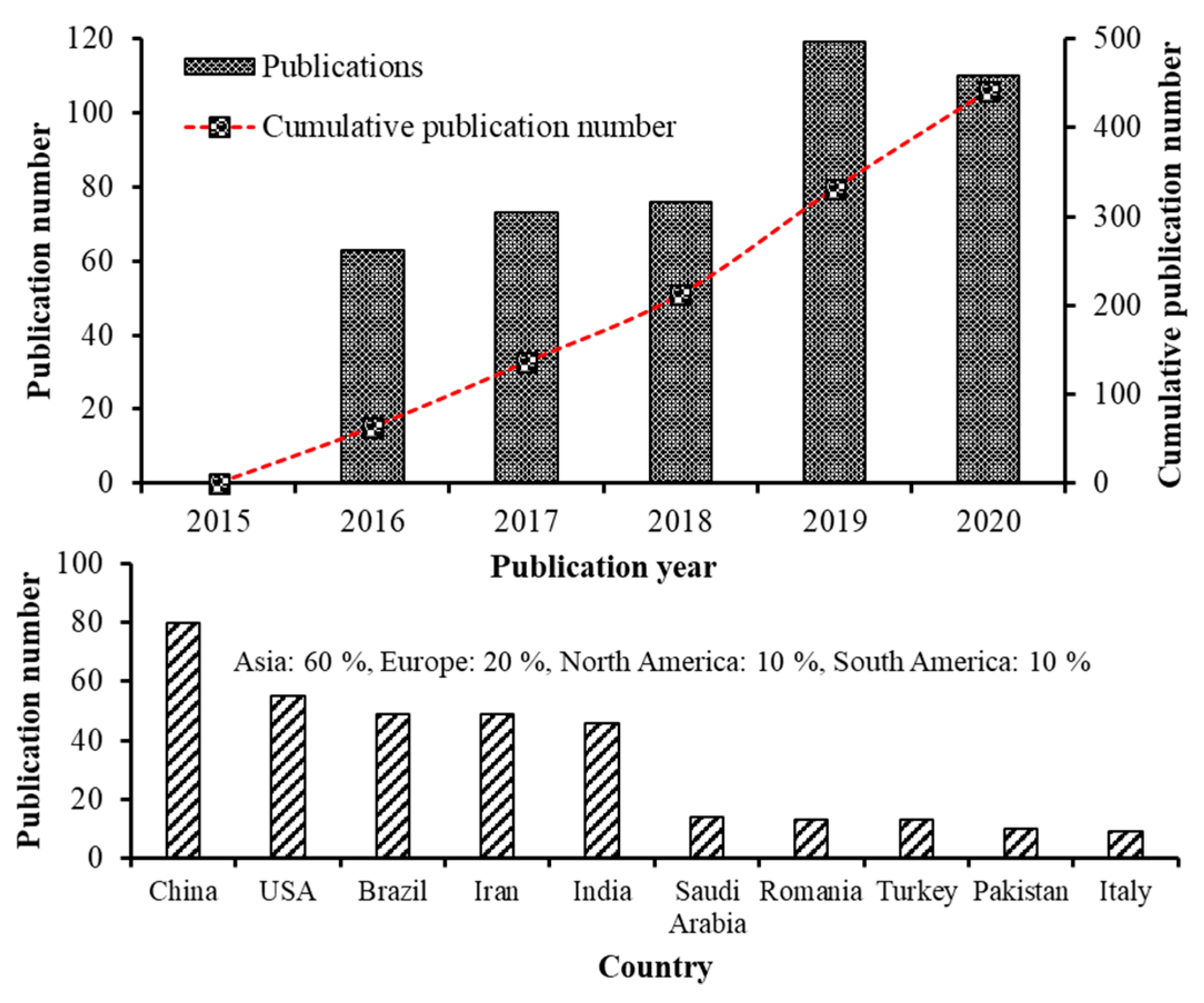
1. Introduction
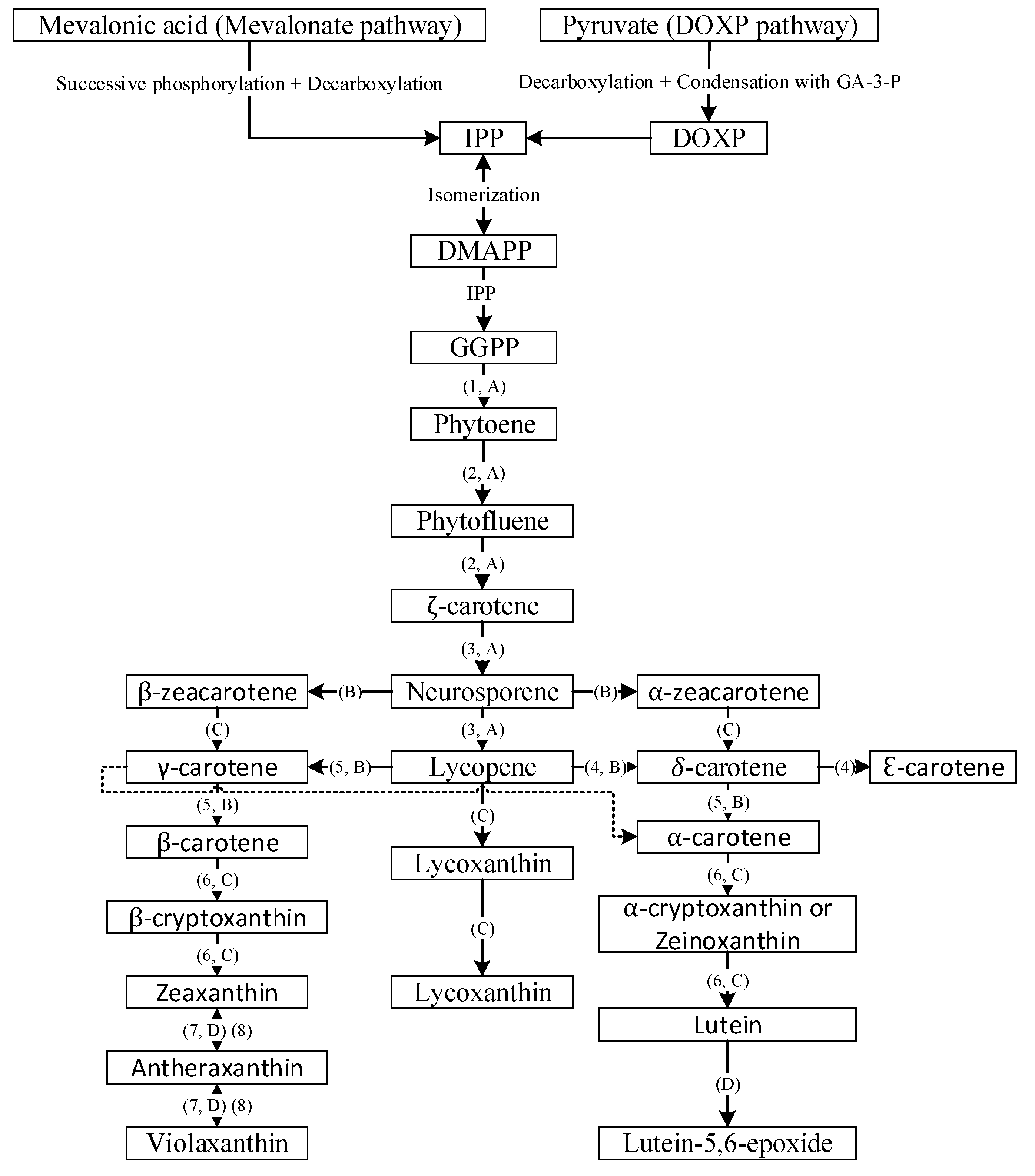
2. Carotenoid Biosynthesis and Stability-Overview
3. Conventional Microencapsulation vs. Nanoencapsulation
4. Preparation, Physicochemical Characterization, Stability Evaluation and Biological Activity
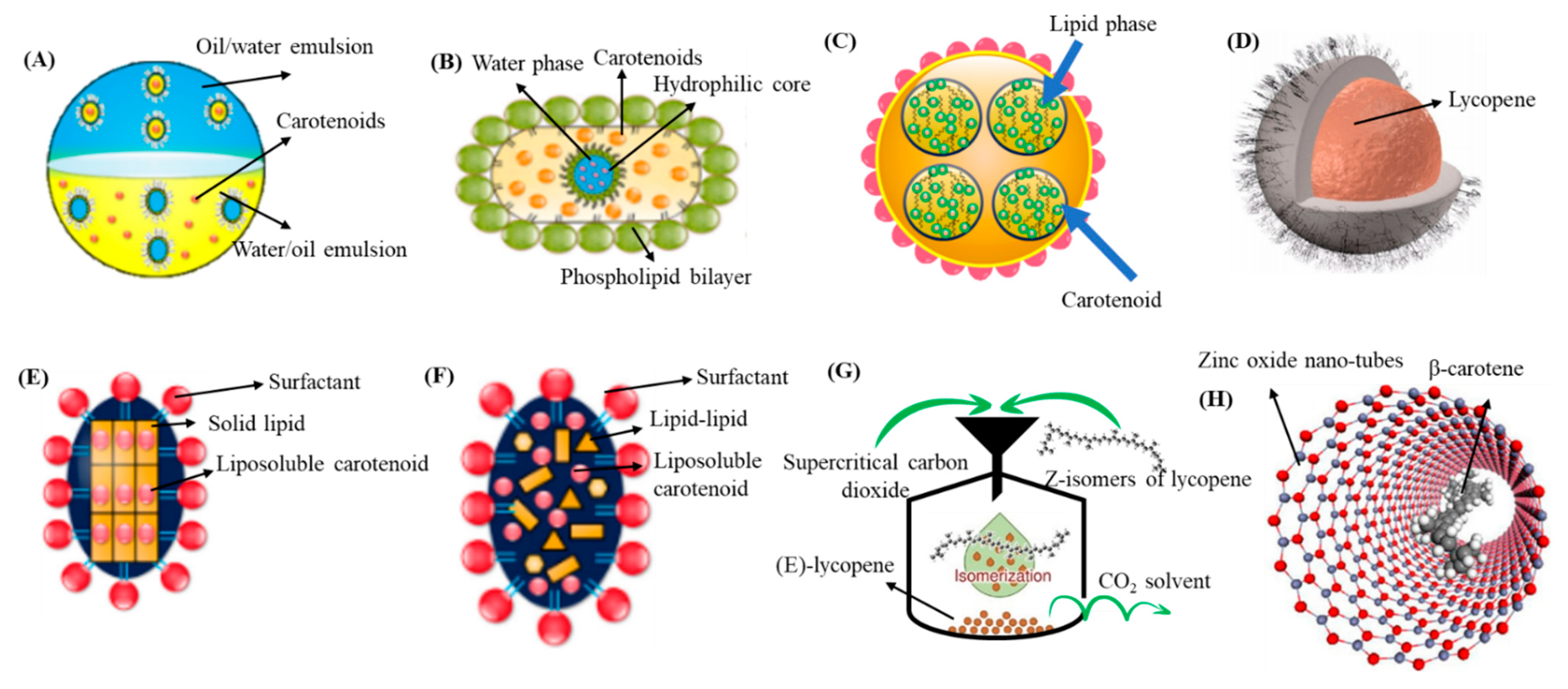
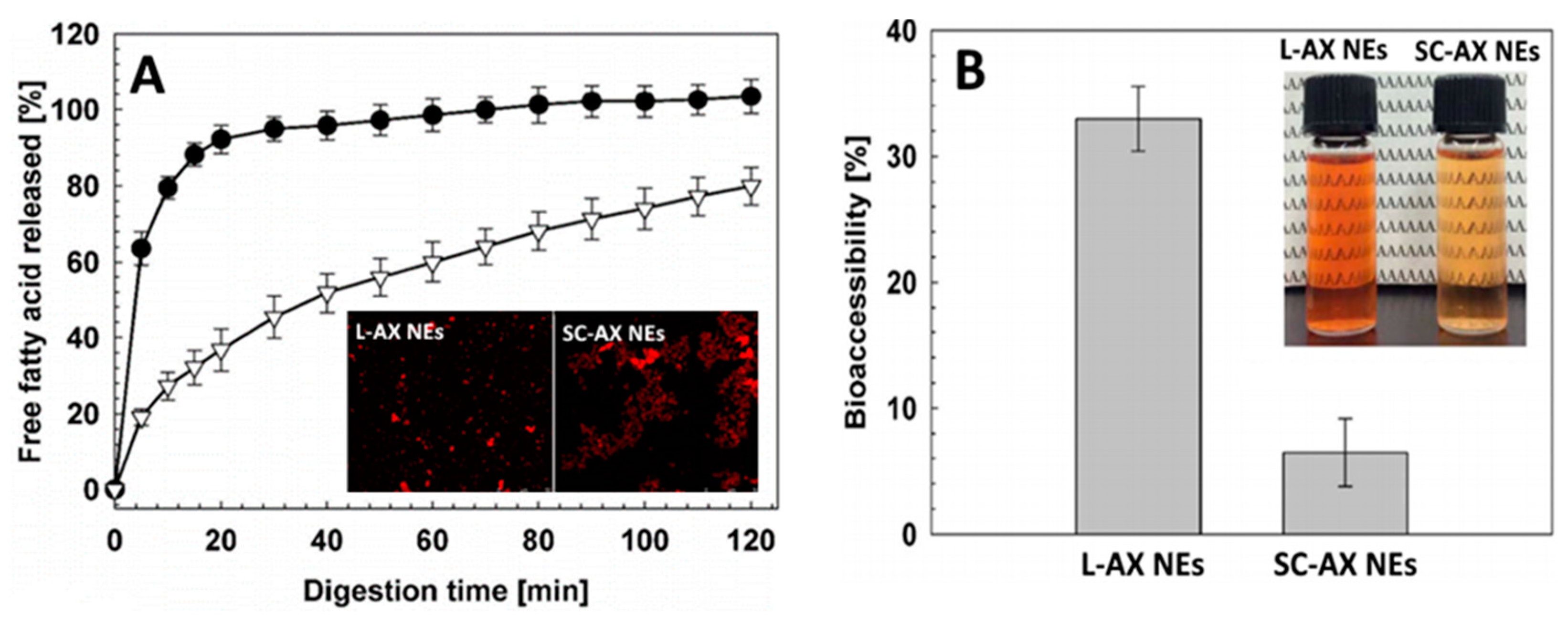
4.1. Nanoemulsion
4.2. Nanoliposomes
4.3. Polymeric/Biopolymeric Based Nanoparticles
4.3.1. Nanoparticles
4.3.2. Nanofiber, Nanocapsules and Micelles
4.4. Solid Lipid Nanoparticles (SLNPs) and Nanostructured Lipid Carriers (NLCs)
4.5. Supercritical Fluid-Based Nanoparticles
4.6. Metal/metal Oxide-Based Nanoparticles and Hybrid Nanocomposites
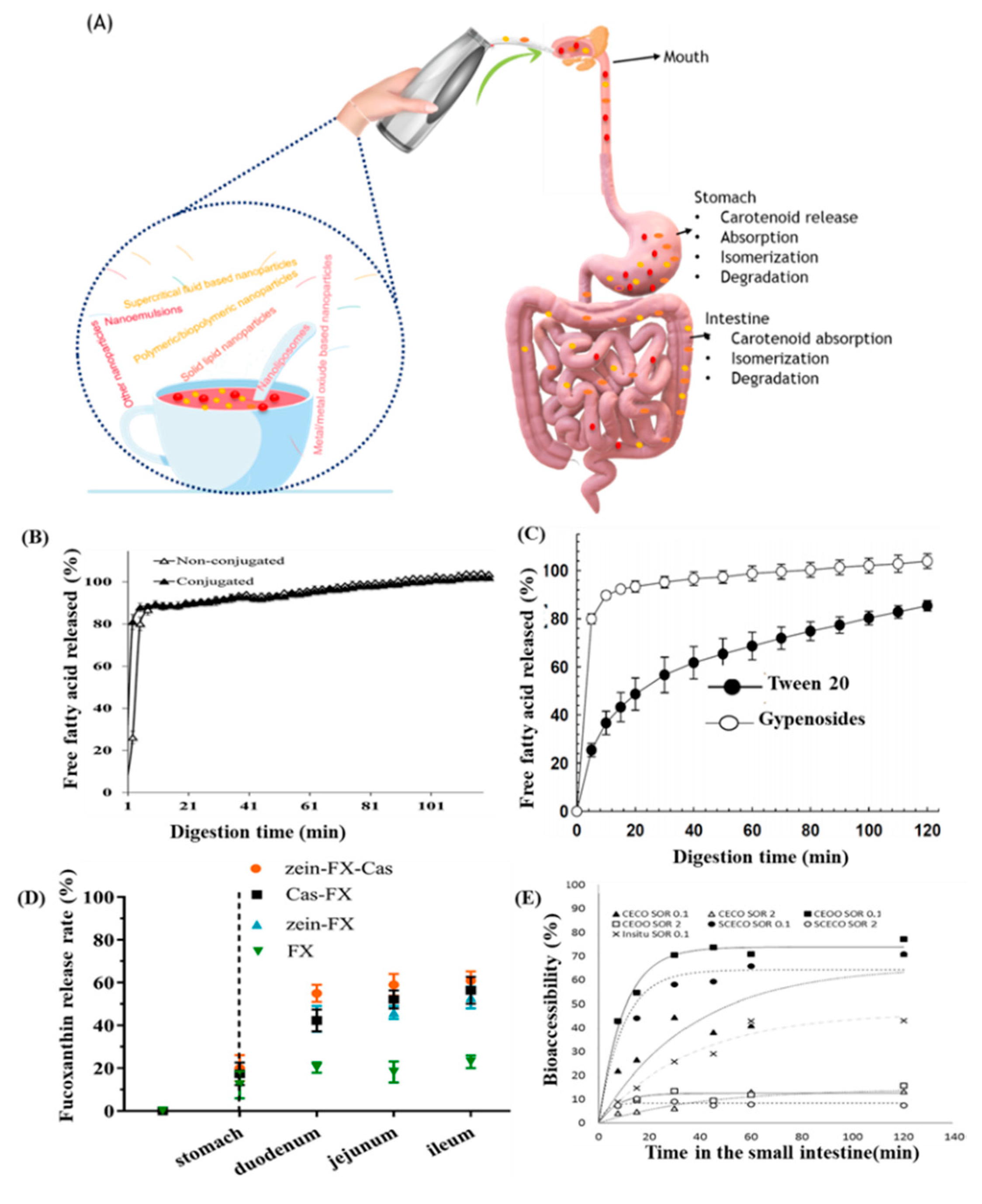
5. In Vitro Release, Gastrointestinal Absorption and Bioaccessibility/Bioavailability
6. Nanocarotenoids Application in Pharmaceutical, Nutraceutical and Food-An Overview
7. Conclusions and Future Prospective
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ABTS | 2,2′-Azino-bis (3-ethylbenzthiazoline-6-sulfonic acid) |
| BC-CR NEs | β-carotene enriched nanoemulsions prepared from Citrus reticulate |
| BC-MC/BO NEs | β-carotene enriched microbial carotenoids (Microbacterium sp.) and buriti oil nanoemulsions |
| CSZ-NPs | Caseinate-stabilized zein nanoparticles |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl |
| EE | Encapsulation efficiency |
| ES-LU-NFs | Electrospun lutein-loaded nanofibers |
| FX-CH-GL NGs | Fucoxanthin-loaded chitosan-glycolipid hybrid nanogels |
| IC50 | Half maximal inhibitory concentration |
| LGOSNPs | Lycopene-reduced graphene oxide-silver nanoparticles |
| LN-GNPs | Lycopene nanoemulsions carrying gold nanoparticles |
| NCs | Nanocapsules |
| NEs | Nanoemulsions |
| NFs | Nanofibers |
| NLs | Nanoliposomes |
| NLCs | Nanostructured lipid carriers |
| NPs | Nanoparticles |
| OEGCG-lycopene NPs | Oligomerized (-) epigallocatechin-3-O-gallate-lycopene nanoparticles |
| o/w | Oil-in-water |
| PCL | Poly-ε-caprolactone |
| PEG | Polyethylene glycol |
| PLGA | Poly(lactide-co-glycolide) |
| PLL | Poly-L-lysine |
| PVA | Polyvinyl alcohol |
| PVP/Z-AX NPs | Z-astaxanthin nanoparticles with polyvinylpyrrolidone |
| SEDS | Solution enhanced dispersion by supercritical fluids |
| SLNPs | Solid-lipid nanoparticles |
| w/o | Water-in-oil |
| ZP | Zeta potential |
| ZX-CE-LB NEs | Zeaxanthin-rich carotenoid extract from Lycium barbarum L. |
References
- Ellison, S.L. Carotenoids: Physiology. In Encyclopedia of Food and Health; Ellison, S.L. Academic Press: Oxford, UK, 2016; pp. 670–675. [Google Scholar]
- Soukoulis, C.; Bohn, T. A comprehensive overview on the micro- and nano-technological encapsulation advances for enhancing the chemical stability and bioavailability of carotenoids. Crit. Rev. Food Sci. Nutri. 2018, 58, 1–36. [Google Scholar] [CrossRef]
- Focsan, A.L.; Polyakov, N.E.; Kispert, L.D. Supramolecular carotenoid complexes of enhanced solubility and stability-the way of bioavailability improvement. Molecules 2019, 24, 3947. [Google Scholar] [CrossRef] [PubMed]
- Rostamabadi, H.; Falsafi, S.R.; Jafari, S.M. Nanoencapsulation of carotenoids within lipid-based nanocarriers. J. Cont. Rel. 2019, 298, 38–67. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, P.P.; Andrade, L.d.A.; Flôres, S.H.; Rios, A.D.O. Nanoencapsulation of carotenoids: A focus on different delivery systems and evaluation parameters. J. Food Sci. Technol. 2018, 55, 3851–3860. [Google Scholar] [CrossRef]
- Sun, X.; Cameron, R.G.; Manthey, J.A.; Hunter, W.B.; Bai, J. Microencapsulation of tangeretin in a citrus pectin mixture matrix. Foods 2020, 9, 1200. [Google Scholar] [CrossRef]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; Rodriguez-Torres, M.D.P.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef]
- Okonogi, S.; Riangjanapatee, P. Physicochemical characterization of lycopene-loaded nanostructured lipid carrier formulations for topical administration. Int. J. Pharm. 2015, 478, 726–735. [Google Scholar] [CrossRef] [PubMed]
- Rehman, A.; Tong, Q.; Jafari, S.M.; Assadpour, E.; Shehzad, Q.; Aadil, R.M.; Iqbal, M.W.; Rashed, M.M.A.; Mushtaq, B.S.; Ashraf, W. Carotenoid-loaded nanocarriers: A comprehensive review. Adv. Colloid Interface Sci. 2020, 275, 102048. [Google Scholar] [CrossRef]
- Usman, M.; Ho, Y.-S. A bibliometric study of the Fenton oxidation for soil and water remediation. J. Environ. Manag. 2020, 270, 110886. [Google Scholar] [CrossRef]
- Andreo-Martínez, P.; Oliva, J.; Giménez-Castillo, J.J.; Motas, M.; Quesada-Medina, J.; Cámara, M.Á. Science production of pesticide residues in honey research: A descriptive bibliometric study. Environ. Toxicol. Pharmacol. 2020, 79, 103413. [Google Scholar] [CrossRef]
- Zhang, C. Biosynthesis of carotenoids and apocarotenoids by microorganisms and their industrial potential. In Progress in Carotenoid Research; BoD—Books on Demand: Norderstedt, Germany, 2018; pp. 85–105. [Google Scholar]
- Inbaraj, B.S.; Chen, B.H. Carotenoids in tomato plants. In Tomatoes and Tomato Products-Nutritional, Medicinal and Therapeutic Properties; CRC Press: Boca Raton, FL, USA, 2008; pp. 133–164. [Google Scholar]
- Da Silva, M.M.; Paese, K.; Guterres, S.S.; Pohlmann, A.R.; Rutz, J.K.; Flores Cantillano, R.F.; Nora, L.; Rios, A.d.O. Thermal and ultraviolet–visible light stability kinetics of co-nanoencapsulated carotenoids. Food Bioprod. Process. 2017, 105, 86–94. [Google Scholar] [CrossRef]
- Mordi, R.C.; Ademosun, O.T.; Ajanaku, C.O.; Olanrewaju, I.O.; Walton, J.C. Free radical mediated oxidative degradation of carotenes and xanthophylls. Molecules 2020, 25, 1038. [Google Scholar] [CrossRef] [PubMed]
- Giménez, P.J.; Fernández-López, J.A.; Angosto, J.M.; Obón, J.M. Comparative thermal degradation patterns of natural yellow colorants used in foods. Plant Foods Hum. Nutr. 2015, 70, 380–387. [Google Scholar] [CrossRef]
- Do Amaral, P.H.R.; Andrade, P.L.; de Conto, L.C. Microencapsulation and its uses in food science and technology: A review. In Microencapsulation—Processes, Technologies and Industrial Applications; Salaün, F., Ed.; IntechOpen Limited: London, UK, 2019; pp. 1–18. [Google Scholar]
- Krajišnik, D.; Čalija, B.; Cekić, N. Polymeric microparticles and inorganic micro/nanoparticulate drug carriers: An overview and pharmaceutical application. In Microsized and Nanosized Carriers for Nonsteroidal Anti-Inflammatory Drugs; Čalija, B., Ed.; Academic Press: Boston, MA, USA, 2017; Chapter 15; pp. 31–67. [Google Scholar]
- Fu, F.; Hu, L. Temperature sensitive colour-changed composites. In Advanced High Strength Natural Fibre Composites in Construction; Fan, M., Fu, F., Eds.; Woodhead Publishing: Cambridge, UK, 2017; Chapter 15; pp. 405–423. [Google Scholar]
- Polyakov, N.E.; Kispert, L.D. Water soluble biocompatible vesicles based on polysaccharides and oligosaccharides inclusion complexes for carotenoid delivery. Carbohy. Poly. 2015, 128, 207–219. [Google Scholar] [CrossRef] [PubMed]
- García, J.M.; Giuffrida, D.; Dugo, P.; Mondello, L.; Osorio, C. Development and characterisation of carotenoid-rich microencapsulates from tropical fruit by-products and yellow tamarillo (Solanum betaceum Cav.). Powder Technol. 2018, 339, 702–709. [Google Scholar] [CrossRef]
- Ding, Z.; Tao, T.; Yin, X.; Prakash, S.; Wang, X.; Zhao, Y.; Han, J.; Wang, Z. Improved encapsulation efficiency and storage stability of spray dried microencapsulated lutein with carbohydrates combinations as encapsulating material. LWT 2020, 124, 109139. [Google Scholar] [CrossRef]
- Feng, Z.-Z.; Li, M.-Y.; Wang, Y.-T.; Zhu, M.-J. Astaxanthin from Phaffia rhodozyma: Microencapsulation with carboxymethyl cellulose sodium and microcrystalline cellulose and effects of microencapsulated astaxanthin on yogurt properties. LWT 2018, 96, 152–160. [Google Scholar] [CrossRef]
- Zhou, Q.; Yang, L.; Xu, J.; Qiao, X.; Li, Z.; Wang, Y.; Xue, C. Evaluation of the physicochemical stability and digestibility of microencapsulated esterified astaxanthins using in vitro and in vivo models. Food Chem. 2018, 260, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Tupuna, D.S.; Paese, K.; Guterres, S.S.; Jablonski, A.; Flôres, S.H.; Rios, A.d.O. Encapsulation efficiency and thermal stability of norbixin microencapsulated by spray-drying using different combinations of wall materials. Ind. Crops Prod. 2018, 111, 846–855. [Google Scholar] [CrossRef]
- Ursache, F.M.; Andronoiu, D.G.; Ghinea, I.O.; Barbu, V.; Ioniţă, E.; Cotârleţ, M.; Dumitraşcu, L.; Botez, E.; Râpeanu, G.; Stănciuc, N. Valorizations of carotenoids from sea buckthorn extract by microencapsulation and formulation of value-added food products. J. Food Eng. 2018, 219, 16–24. [Google Scholar] [CrossRef]
- Arenas-Jal, M.; Suñé-Negre, J.M.; García-Montoya, E. An overview of microencapsulation in the food industry: Opportunities, challenges, and innovations. Eur. Food Res. Technol. 2020, 246, 1371–1382. [Google Scholar] [CrossRef]
- Corrêa-Filho, L.C.; Moldão-Martins, M.; Alves, V.D. Advances in the application of microcapsules as carriers of functional compounds for food products. Appl. Sci. 2019, 9, 571. [Google Scholar] [CrossRef]
- Rashidinejad, A.; Jafari, S.M. Nanoencapsulation of bioactive food ingredients. In Handbook of Food Nanotechnology; Jafari, S.M., Ed.; Academic Press: Cambridge, MA, USA, 2020; Chapter 8; pp. 279–344. [Google Scholar]
- Yu, H.; Park, J.-Y.; Kwon, C.W.; Hong, S.-C.; Park, K.-M.; Chang, P.-S. An overview of nanotechnology in food science: Preparative methods, practical applications, and safety. J. Chem. 2018, 2018, 5427978. [Google Scholar] [CrossRef]
- Singh, T.; Shukla, S.; Kumar, P.; Wahla, V.; Bajpai, V.K.; Rather, I.A. Application of nanotechnology in food science: Perception and overview. Front. Microbiol. 2017, 8, 1501. [Google Scholar] [CrossRef]
- Baek, E.J.; Garcia, C.V.; Shin, G.H.; Kim, J.T. Improvement of thermal and UV-light stability of β-carotene-loaded nanoemulsions by water-soluble chitosan coating. Int. J. Biol. Macromol. 2020, 165, 1156–1163. [Google Scholar] [CrossRef]
- Barman, K.; Chowdhury, D.; Baruah, P.K. Development of β-carotene loaded nanoemulsion using the industrial waste of orange (Citrus reticulate) peel to improve in vitro bioaccessibility of carotenoids and use as natural food colorant. J. Food Process Preserv. 2020, 44, e14429. [Google Scholar] [CrossRef]
- Mansur, M.C.P.P.R.; Campos, C.; Vermelho, A.B.; Nobrega, J.; da Cunha Boldrini, L.; Balottin, L.; Lage, C.; Rosado, A.S.; Ricci-Júnior, E.; dos Santos, E.P. Photoprotective nanoemulsions containing microbial carotenoids and buriti oil: Efficacy and safety study. Arab. J. Chem. 2020, 13, 6741–6752. [Google Scholar] [CrossRef]
- Gasa-Falcon, A.; Arranz, E.; Odriozola-Serrano, I.; Martín-Belloso, O.; Giblin, L. Delivery of β-carotene to the in vitro intestinal barrier using nanoemulsions with lecithin or sodium caseinate as emulsifiers. LWT 2021, 135, 110059. [Google Scholar] [CrossRef]
- Borba, C.M.; Tavares, M.N.; Macedo, L.P.; Araújo, G.S.; Furlong, E.B.; Dora, C.L.; Burkert, J.F.M. Physical and chemical stability of β-carotene nanoemulsions during storage and thermal process. Food Res. Int. 2019, 121, 229–237. [Google Scholar] [CrossRef]
- Sotomayor-Gerding, D.; Oomah, B.D.; Acevedo, F.; Morales, E.; Bustamante, M.; Shene, C.; Rubilar, M. High carotenoid bioaccessibility through linseed oil nanoemulsions with enhanced physical and oxidative stability. Food Chem. 2016, 199, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Li, L.; Xiao, N.; Li, M.; Xie, X. Physical properties of oil-in-water nanoemulsions stabilized by OSA-modified starch for the encapsulation of lycopene. Colloids Surf. A Physicochem. Eng. Asp. 2018, 552, 59–66. [Google Scholar] [CrossRef]
- Zhao, C.; Wei, L.; Yin, B.; Liu, F.; Li, J.; Liu, X.; Wang, J.; Wang, Y. Encapsulation of lycopene within oil-in-water nanoemulsions using lactoferrin: Impact of carrier oils on physicochemical stability and bioaccessibility. Int. J. Biol. Macromol. 2020, 153, 912–920. [Google Scholar] [CrossRef]
- Pereira, M.C.; Hill, L.E.; Zambiazi, R.C.; Mertens-Talcott, S.; Talcott, S.; Gomes, C.L. Nanoencapsulation of hydrophobic phytochemicals using poly (dl-lactide-co-glycolide) (PLGA) for antioxidant and antimicrobial delivery applications: Guabiroba fruit (Campomanesia xanthocarpa O. Berg) study. LWT 2015, 63, 100–107. [Google Scholar] [CrossRef]
- Bezerra, P.Q.M.; Matos, M.F.R.d.; Ramos, I.G.; Magalhães-Guedes, K.T.; Druzian, J.I.; Costa, J.A.V.; Nunes, I.L. Innovative functional nanodispersion: Combination of carotenoid from Spirulina and yellow passion fruit albedo. Food Chem. 2019, 285, 397–405. [Google Scholar] [CrossRef] [PubMed]
- Yi, J.; Lam, T.I.; Yokoyama, W.; Cheng, L.W.; Zhong, F. Beta-carotene encapsulated in food protein nanoparticles reduces peroxyl radical oxidation in Caco-2 cells. Food Hydrocoll. 2015, 43, 31–40. [Google Scholar] [CrossRef]
- Rostamabadi, H.; Sadeghi Mahoonak, A.; Allafchian, A.; Ghorbani, M. Fabrication of β-carotene loaded glucuronoxylan-based nanostructures through electrohydrodynamic processing. Int. J. Biol. Macromol. 2019, 139, 773–784. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Yalcin, M.; Lin, Q.; Ardawi, M.-S.M.; Mousa, S.A. Self-assembly of green tea catechin derivatives in nanoparticles for oral lycopene delivery. J. Control. Release 2017, 248, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos, A.G.; Valim, M.O.; Amorim, A.G.N.; do Amaral, C.P.; de Almeida, M.P.; Borges, T.K.S.; Socodato, R.; Portugal, C.C.; Brand, G.D.; Mattos, J.S.C.; et al. Cytotoxic activity of poly-ε-caprolactone lipid-core nanocapsules loaded with lycopene-rich extract from red guava (Psidium guajava L.) on breast cancer cells. Food Res. Int. 2020, 136, 109548. [Google Scholar] [CrossRef]
- Dos Santos, P.P.; Paese, K.; Guterres, S.S.; Pohlmann, A.R.; Costa, T.H.; Jablonski, A.; Flôres, S.H.; Rios, A.D.O. Development of lycopene-loaded lipid-core nanocapsules: Physicochemical characterization and stability study. J. Nano Res. 2015, 17, 107. [Google Scholar] [CrossRef]
- Bolla, P.K.; Gote, V.; Singh, M.; Patel, M.; Clark, B.A.; Renukuntla, J. Lutein-loaded, biotin-decorated polymeric nanoparticles enhance lutein uptake in retinal cells. Pharmaceutics 2020, 12, 798. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Huo, P.; Ding, Z.; Kumar, P.; Liu, B. Preparation of lutein-loaded PVA/sodium alginate nanofibers and investigation of its release behavior. Pharmaceutics 2019, 11, 449. [Google Scholar] [CrossRef]
- Hafezi Ghahestani, Z.; Alebooye Langroodi, F.; Mokhtarzadeh, A.; Ramezani, M.; Hashemi, M. Evaluation of anti-cancer activity of PLGA nanoparticles containing crocetin. Artif. Cells Nanomed. Biotechnol. 2017, 45, 955–960. [Google Scholar] [CrossRef] [PubMed]
- Ravi, H.; Baskaran, V. Biodegradable chitosan-glycolipid hybrid nanogels: A novel approach to encapsulate fucoxanthin for improved stability and bioavailability. Food Hydrocoll. 2015, 43, 717–725. [Google Scholar] [CrossRef]
- Tan, C.; Feng, B.; Zhang, X.; Xia, W.; Xia, S. Biopolymer-coated liposomes by electrostatic adsorption of chitosan (chitosomes) as novel delivery systems for carotenoids. Food Hydrocoll. 2016, 52, 774–784. [Google Scholar] [CrossRef]
- Hassane Hamadou, A.; Huang, W.-C.; Xue, C.; Mao, X. Comparison of β-carotene loaded marine and egg phospholipids nanoliposomes. J. Food Eng. 2020, 283, 110055. [Google Scholar] [CrossRef]
- Pan, L.; Zhang, S.; Gu, K.; Zhang, N. Preparation of astaxanthin-loaded liposomes: Characterization, storage stability and antioxidant activity. CyTA J. Food 2018, 16, 607–618. [Google Scholar] [CrossRef]
- Pan, L.; Wang, H.; Gu, K. Nanoliposomes as vehicles for astaxanthin: Characterization, in vitro release evaluation and structure. Molecules 2018, 23, 2822. [Google Scholar] [CrossRef]
- Jiao, Y.; Li, D.; Liu, C.; Chang, Y.; Song, J.; Xiao, Y.J.R.A. Polypeptide–decorated nanoliposomes as novel delivery systems for lutein. RSC Adv. 2018, 8, 31372–31381. [Google Scholar] [CrossRef]
- Jain, A.; Sharma, G.; Thakur, K.; Raza, K.; Shivhare, U.S.; Ghoshal, G.; Katare, O.P. Beta-carotene-encapsulated solid lipid nanoparticles (BC-SLNs) as promising vehicle for cancer: An investigative assessment. AAPS Pharm. Sci. Tech. 2019, 20, 100. [Google Scholar] [CrossRef]
- Mehrad, B.; Ravanfar, R.; Licker, J.; Regenstein, J.M.; Abbaspourrad, A. Enhancing the physicochemical stability of β-carotene solid lipid nanoparticle (SLNP) using whey protein isolate. Food Res. Int. 2018, 105, 962–969. [Google Scholar] [CrossRef] [PubMed]
- Schjoerring-Thyssen, J.; Olsen, K.; Koehler, K.; Jouenne, E.; Rousseau, D.; Andersen, M.L. Morphology and structure of solid lipid nanoparticles loaded with high concentrations of β-carotene. J. Agric. Food Chem. 2019, 67, 12273–12282. [Google Scholar] [CrossRef]
- Nazemiyeh, E.; Eskandani, M.; Sheikhloie, H.; Nazemiyeh, H. Formulation and physicochemical characterization of lycopene-loaded solid lipid nanoparticles. Adv. Pharm. Bull. 2016, 6, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Neupane, Y.R.; Panda, B.P.; Kohli, K. Lipid Based nanoformulation of lycopene improves oral delivery: Formulation optimization, ex vivo assessment and its efficacy against breast cancer. J. Microencap. 2017, 34, 416–429. [Google Scholar] [CrossRef] [PubMed]
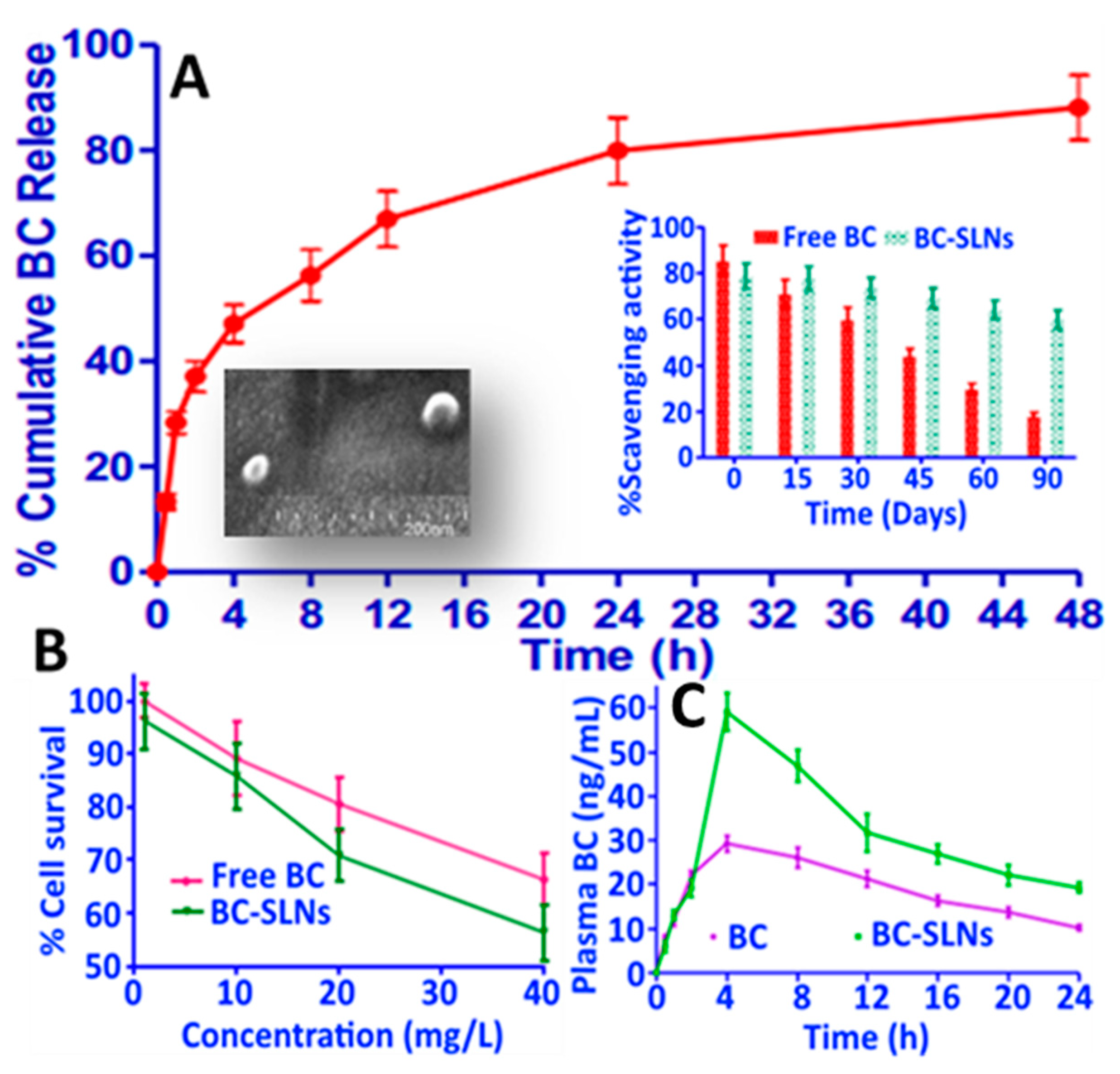
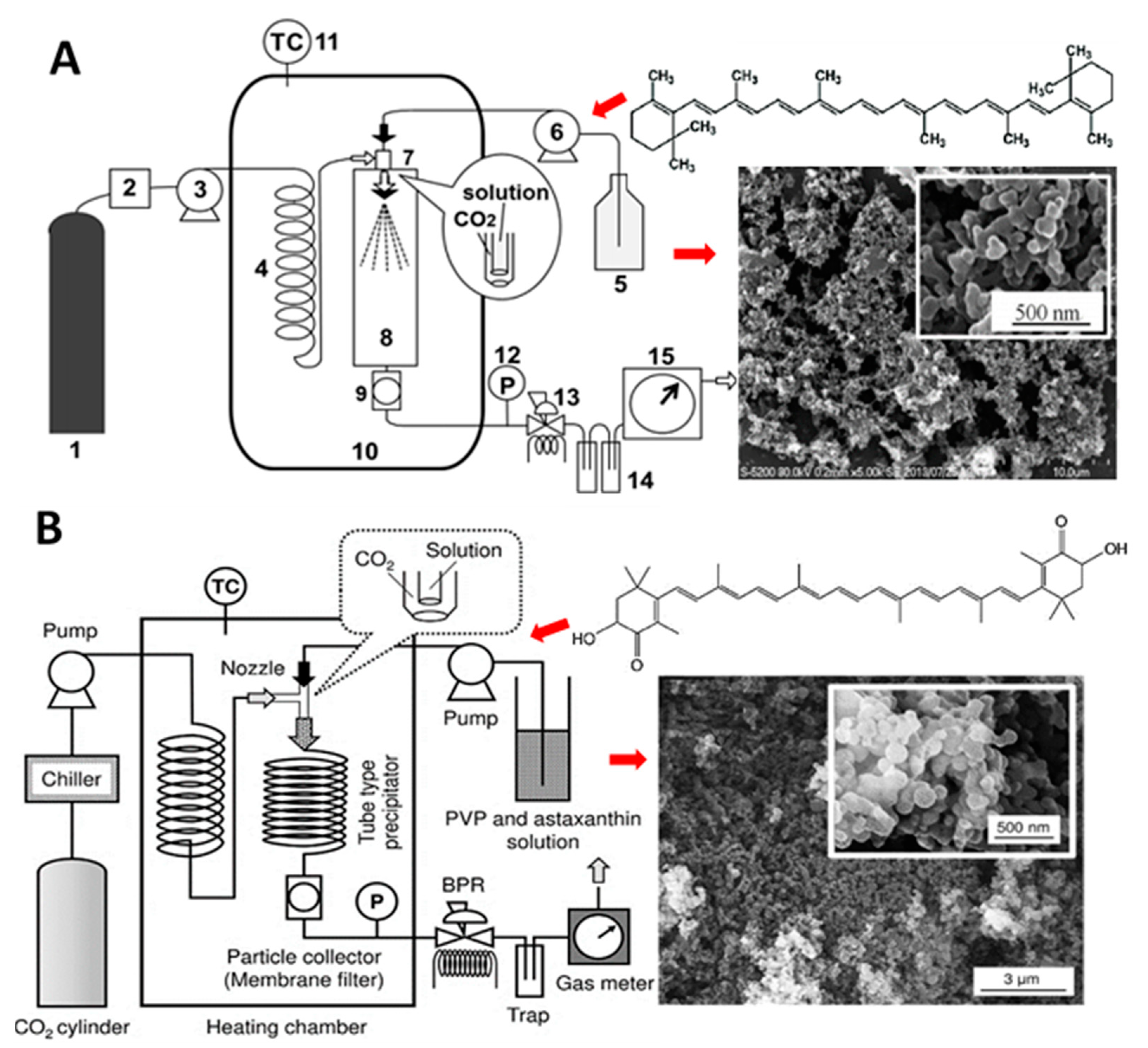
- Kaga, K.; Honda, M.; Adachi, T.; Honjo, M.; Kanda, H.; Goto, M. Nanoparticle formation of PVP/astaxanthin inclusion complex by solution-enhanced dispersion by supercritical fluids (SEDS): Effect of PVP and astaxanthin Z-isomer content. J. Supercrit. Fluids 2018, 136, 44–51. [Google Scholar] [CrossRef]
- Tirado, D.F.; Palazzo, I.; Scognamiglio, M.; Calvo, L.; Della Porta, G.; Reverchon, E. Astaxanthin encapsulation in ethyl cellulose carriers by continuous supercritical emulsions extraction: A study on particle size, encapsulation efficiency, release profile and antioxidant activity. J. Supercrit. Fluids 2019, 150, 128–136. [Google Scholar] [CrossRef]
- Patra, J.K.; Baek, K.-H. Novel green synthesis of gold nanoparticles using Citrullus lanatus rind and investigation of proteasome inhibitory activity, antibacterial, and antioxidant potential. Int. J. Nanomed. 2015, 10, 7253–7264. [Google Scholar]
- Huang, R.F.; Wei, Y.J.; Inbaraj, B.S.; Chen, B.H. Inhibition of colon cancer cell growth by nanoemulsion carrying gold nanoparticles and lycopene. Int. J. Nanomed. 2015, 10, 2823–2846. [Google Scholar]
- Shabestarian, H.; Homayouni-Tabrizi, M.; Soltani, M.; Namvar, F.; Azizi, S.; Mohamad, R.; Shabestarian, H. Green synthesis of gold nanoparticles using sumac aqueous extract and their antioxidant activity. J. Mat. Res. 2017, 20, 264–270. [Google Scholar] [CrossRef]
- Chakravarty, P.; Famili, A.; Nagapudi, K.; Al-Sayah, M.A. Using Supercritical fluid technology as a green alternative during the preparation of drug delivery systems. Pharmaceutics 2019, 11, 629. [Google Scholar] [CrossRef]
- Sengul, A.B.; Asmatulu, E. Toxicity of metal and metal oxide nanoparticles: A review. Environ. Chem. Let. 2020, 18, 1659–1683. [Google Scholar] [CrossRef]
- Seabra, A.B.; Durán, N. Nanotoxicology of metal oxide nanoparticles. Metals 2015, 5, 934–975. [Google Scholar] [CrossRef]
- Aswathanarayan, J.B.; Vittal, R.R. Nanoemulsions and their potential applications in food industry. Front. Sustain. Food Syst. 2019, 3, 1–21. [Google Scholar] [CrossRef]
- Agarwal, S.; Vivekanandan, S.; David, T.; Mitra, M.; Palanivelu, J.; Chidambaram, R. Nanoemulsions: Industrial production and food-grade applications. In Polymers for Agri-Food Applications; Gutiérrez, T.J., Ed.; Springer: New York, NY, USA, 2019; pp. 159–182. [Google Scholar]
- Chen, B.-H.; Stephen Inbaraj, B. Nanoemulsion and nanoliposome based strategies for improving anthocyanin stability and bioavailability. Nutrients 2019, 11, 1052. [Google Scholar] [CrossRef] [PubMed]
- Alliod, O.; Valour, J.-P.; Urbaniak, S.; Fessi, H.; Dupin, D.; Charcosset, C. Preparation of oil-in-water nanoemulsions at large-scale using premix membrane emulsification and Shirasu Porous Glass (SPG) membranes. Colloids Surf. A Physicochem. Eng. Asp. 2018, 557, 76–84. [Google Scholar] [CrossRef]
- Flores-Andrade, E.; Allende-Baltazar, Z.; Sandoval-González, P.E.; Jiménez-Fernández, M.; Beristain, C.I.; Pascual-Pineda, L.A. Carotenoid nanoemulsions stabilized by natural emulsifiers: Whey protein, gum arabic, and soy lecithin. J. Food Eng. 2021, 290, 110208. [Google Scholar] [CrossRef]
- Komaiko, J.; McClements, D.J. Low-energy formation of edible nanoemulsions by spontaneous emulsification: Factors influencing particle size. J. Food Eng. 2015, 146, 122–128. [Google Scholar] [CrossRef]
- Kodama, T.; Honda, M.; Takemura, R.; Fukaya, T.; Uemori, C.; Kanda, H.; Goto, M. Effect of the Z-isomer content on nanoparticle production of lycopene using solution-enhanced dispersion by supercritical fluids (SEDS). J. Supercrit. Fluids 2018, 133, 291–296. [Google Scholar] [CrossRef]
- Monteiro, F.F.; Azevedo, D.L.; da Silva, E.C.; Ribeiro, L.A.; de Almeida Fonseca, A.L. Encapsulated β-carotene in ZnO nanotubes: Theoretical insight into the stabilization dynamics. Chem. Phys. Lett. 2015, 636, 62–66. [Google Scholar] [CrossRef]
- Khalid, N.; Shu, G.; Holland, B.J.; Kobayashi, I.; Nakajima, M.; Barrow, C.J. Formulation and characterization of O/W nanoemulsions encapsulating high concentration of astaxanthin. Food Res. Int. 2017, 102, 364–371. [Google Scholar] [CrossRef]
- Shu, G.; Khalid, N.; Chen, Z.; Neves, M.A.; Barrow, C.J.; Nakajima, M. Formulation and characterization of astaxanthin-enriched nanoemulsions stabilized using ginseng saponins as natural emulsifiers. Food Chem. 2018, 255, 67–74. [Google Scholar] [CrossRef]
- Murillo, A.G.; Aguilar, D.; Norris, G.H.; DiMarco, D.M.; Missimer, A.; Hu, S.; Smyth, J.A.; Gannon, S.; Blesso, C.N.; Luo, Y.; et al. Compared with powdered lutein, a lutein nanoemulsion increases plasma and liver lutein, protects against hepatic steatosis, and affects lipoprotein metabolism in guinea pigs. J. Nutri. 2016, 146, 1961–1969. [Google Scholar] [CrossRef] [PubMed]
- Hsu, H.J.; Huang, R.F.; Kao, T.H.; Inbaraj, B.S.; Chen, B.H. Preparation of carotenoid extracts and nanoemulsions from Lycium barbarum L. and their effects on growth of HT-29 colon cancer cells. Nanotechnology 2017, 28, 135103. [Google Scholar] [CrossRef] [PubMed]
- Ha, T.V.A.; Kim, S.; Choi, Y.; Kwak, H.-S.; Lee, S.J.; Wen, J.; Oey, I.; Ko, S. Antioxidant activity and bioaccessibility of size-different nanoemulsions for lycopene-enriched tomato extract. Food Chem. 2015, 178, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Escobar, M.P.; Pascual-Mathey, L.I.; Beristain, C.I.; Flores-Andrade, E.; Jiménez, M.; Pascual-Pineda, L.A. In vitro and In vivo antioxidant properties of paprika carotenoids nanoemulsions. LWT 2020, 118, 108694. [Google Scholar] [CrossRef]
- Chen, Z.; Shu, G.; Taarji, N.; Barrow, C.J.; Nakajima, M.; Khalid, N.; Neves, M.A. Gypenosides as natural emulsifiers for oil-in-water nanoemulsions loaded with astaxanthin: Insights of formulation, stability and release properties. Food Chem. 2018, 261, 322–328. [Google Scholar] [CrossRef]
- Steiner, B.M.; Shukla, V.; McClements, D.J.; Li, Y.O.; Sancho-Madriz, M.; Davidov-Pardo, G. Encapsulation of lutein in nanoemulsions stabilized by resveratrol and maillard conjugates. J. Food Sci. 2019, 84, 2421–2431. [Google Scholar] [CrossRef]
- Demirci, M.; Caglar, M.Y.; Cakir, B.; Gülseren, İ. 3–Encapsulation by nanoliposomes. In Nanoencapsulation Technologies for the Food and Nutraceutical Industries; Jafari, S.M., Ed.; Academic Press: Cambridge, MA, USA, 2017; pp. 74–113. [Google Scholar]
- De Freitas Zômpero, R.H.; López-Rubio, A.; de Pinho, S.C.; Lagaron, J.M.; de la Torre, L.G. Hybrid encapsulation structures based on β-carotene-loaded nanoliposomes within electrospun fibers. Colloids Surf. B Biointerfaces 2015, 134, 475–482. [Google Scholar] [CrossRef]
- Gulzar, S.; Benjakul, S. Characteristics and storage stability of nanoliposomes loaded with shrimp oil as affected by ultrasonication and microfluidization. Food Chem. 2020, 310, 125916. [Google Scholar] [CrossRef]
- Xia, S.; Tan, C.; Zhang, Y.; Abbas, S.; Feng, B.; Zhang, X.; Qin, F. Modulating effect of lipid bilayer–carotenoid interactions on the property of liposome encapsulation. Colloids Surf. B Biointerfaces 2015, 128, 172–180. [Google Scholar] [CrossRef]
- Sharma, M. Transdermal and intravenous nano drug delivery systems: Present and future. In Applications of Targeted Nano Drugs and Delivery Systems; Mohapatra, S.S., Ranjan, S., Dasgupta, N., Mishra, R.K., Thomas, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; Chapter 18; pp. 499–550. [Google Scholar]
- Jain, R.; Savla, H.; Naik, I.; Maniar, J.; Punjabi, K.; Vaidya, S.; Menon, M. Novel Nanotechnology based delivery systems for chemotherapy and prophylaxis of tuberculosis. In Handbook of Nanomaterials for Industrial Applications; Mustansar Hussain, C., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; Chapter 33; pp. 587–620. [Google Scholar]
- Rana, V.; Sharma, R. Recent advances in development of nano drug delivery. In Applications of Targeted Nano Drugs and Delivery Systems; Mohapatra, S.S., Ranjan, S., Dasgupta, N., Mishra, R.K., Thomas, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; Chapter 5; pp. 93–131. [Google Scholar]
- Detsi, A.; Kavetsou, E.; Kostopoulou, I.; Pitterou, I.; Pontillo, A.R.N.; Tzani, A.; Christodoulou, P.; Siliachli, A.; Zoumpoulakis, P. Nanosystems for the encapsulation of natural products: The case of chitosan biopolymer as a matrix. Pharmaceutics 2020, 12, 669. [Google Scholar] [CrossRef]
- Rahaiee, S.; Shojaosadati, S.A.; Hashemi, M.; Moini, S.; Razavi, S.H. Improvement of crocin stability by biodegradeble nanoparticles of chitosan-alginate. Int. J. Biol. Macromol. 2015, 79, 423–432. [Google Scholar] [CrossRef] [PubMed]
- Hong, D.Y.; Lee, J.-S.; Lee, H.G. Chitosan/poly-γ-glutamic acid nanoparticles improve the solubility of lutein. Int. J. Biol. Macromol. 2016, 85, 9–15. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, S.; McClements, D.J.; Wang, D.; Xu, Y. Design of Astaxanthin-loaded core–shell nanoparticles consisting of chitosan oligosaccharides and poly(lactic-co-glycolic acid): Enhancement of water solubility, stability, and bioavailability. J. Agric. Food Chem. 2019, 67, 5113–5121. [Google Scholar] [CrossRef]
- Zielińska, A.; Carreiró, F.; Oliveira, A.M.; Neves, A.; Pires, B.; Venkatesh, D.N.; Durazzo, A.; Lucarini, M.; Eder, P.; Silva, A.M.; et al. Polymeric nanoparticles: Production, characterization, toxicology and ecotoxicology. Molecules 2020, 25, 3731. [Google Scholar] [CrossRef]
- Sardoiwala, M.N.; Kaundal, B.; Roy Choudhury, S. Development of engineered nanoparticles expediting diagnostic and therapeutic applications across blood–brain barrier. In Handbook of Nanomaterials for Industrial Applications; Mustansar Hussain, C., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; Chapter 37; pp. 696–709. [Google Scholar]
- Figueiredo-Junior, A.T.; dos Anjos, F.d.F.; Brito, F.d.C.d.M.; Viana, V.G.F.; Valença, S.S.; Lanzetti, M.; Finotelli, P.V. Bixin loaded on polymeric nanoparticles: Synthesis, characterization, and antioxidant applications in a biological system. Appl. Nanosci. 2021, 11, 63–78. [Google Scholar] [CrossRef]
- Da Silva, M.M.; Nora, L.; Cantillano, R.F.F.; Paese, K.; Guterres, S.S.; Pohlmann, A.R.; Costa, T.M.H.; Rios, A.D.O. The production, characterization, and the stability of carotenoids loaded in lipid-core nanocapsules. Food Bioprocess Technol. 2016, 9, 1148–1158. [Google Scholar] [CrossRef][Green Version]
- Jain, A.; Sharma, G.; Kushwah, V.; Ghoshal, G.; Jain, A.; Singh, B.; Shivhare, U.S.; Jain, S.; Katare, O.P. Beta carotene-loaded zein nanoparticles to improve the biopharmaceutical attributes and to abolish the toxicity of methotrexate: A preclinical study for breast cancer. Artif. Cells Nanomed. Biotechnol. 2018, 46, 402–412. [Google Scholar] [CrossRef] [PubMed]
- Lobato, K.B.D.S.; Paese, K.; Forgearini, J.C.; Guterres, S.S.; Jablonski, A.; Rios, A.D.O. Evaluation of stability of bixin in nanocapsules in model systems of photosensitization and heating. LWT 2015, 60, 8–14. [Google Scholar] [CrossRef]
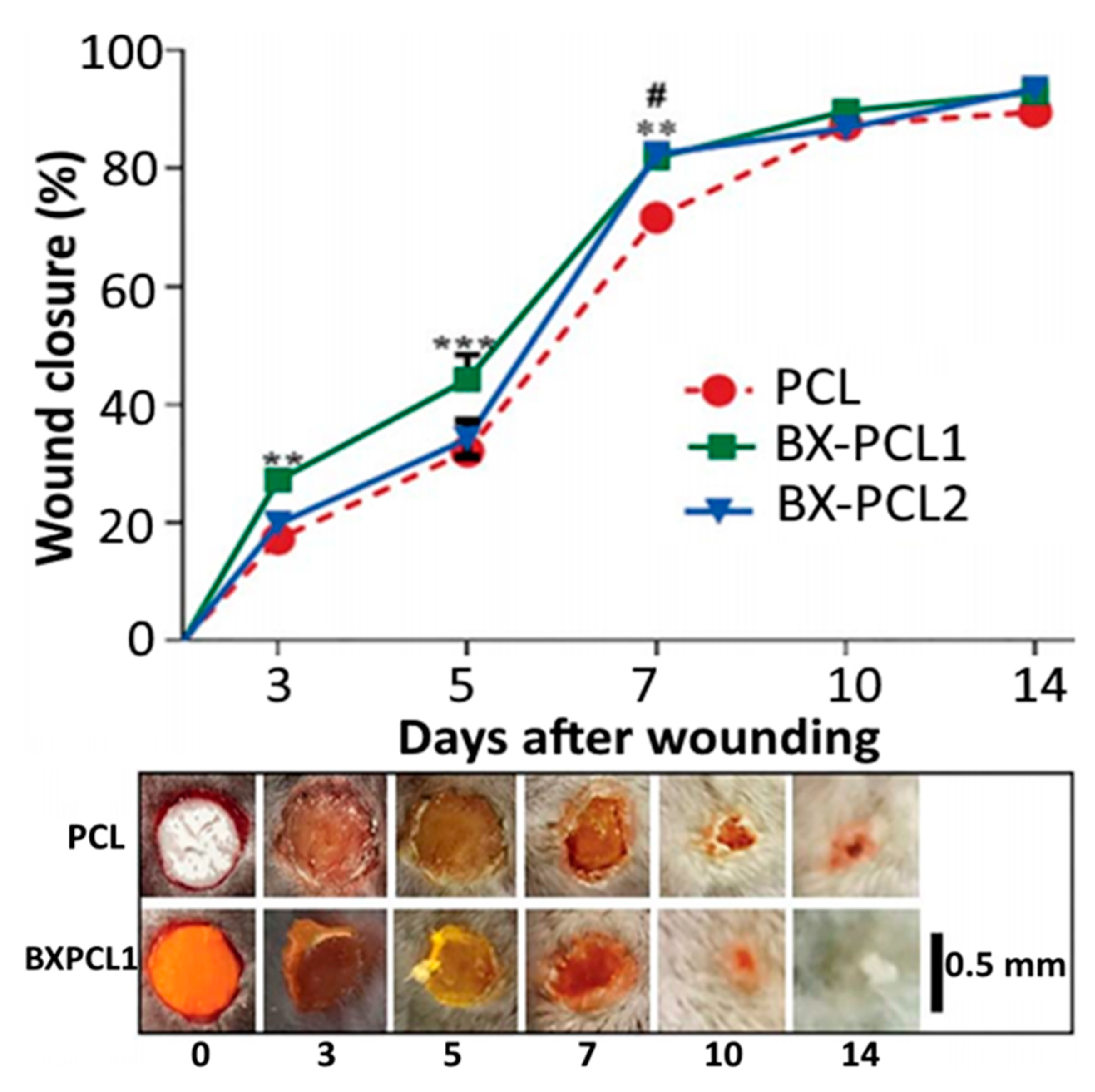
- Pinzón-García, A.D.; Cassini-Vieira, P.; Ribeiro, C.C.; de Matos Jensen, C.E.; Barcelos, L.S.; Cortes, M.E.; Sinisterra, R.D. Efficient cutaneous wound healing using bixin-loaded PCL nanofibers in diabetic mice. J. Biomed. Mat. Res. Part B App. Biomat. 2017, 105, 1938–1949. [Google Scholar] [CrossRef]
- Muhoza, B.; Zhang, Y.; Xia, S.; Cai, J.; Zhang, X.; Su, J. Improved stability and controlled release of lutein-loaded micelles based on glycosylated casein via Maillard reaction. J. Funct. Foods 2018, 45, 1–9. [Google Scholar] [CrossRef]
- Vieira, M.V.; Derner, R.B.; Lemos-Senna, E. Preparation and characterization of Haematococcus pluvialis carotenoid-loaded PLGA nanocapsules in a gel system with antioxidant properties for topical application. J. Drug Deliv. Sci. Technol. 2020, 61, 102099. [Google Scholar] [CrossRef]
- García-Pinel, B.; Porras-Alcalá, C.; Ortega-Rodríguez, A.; Sarabia, F.; Prados, J.; Melguizo, C.; López-Romero, J.M. Lipid-based nanoparticles: Application and recent advances in cancer treatment. Nanomaterials 2019, 9, 638. [Google Scholar] [CrossRef] [PubMed]
- Tekade, R.K.; Maheshwari, R.; Tekade, M.; Chougule, M.B. Solid lipid nanoparticles for targeting and delivery of drugs and genes. In Nanotechnology-Based Approaches for Targeting and Delivery of Drugs and Genes; Mishra, V., Kesharwani, P., Mohd Amin, M.C.I., Iyer, A., Eds.; Academic Press: Cambridge, MA, USA, 2017; Chapter 8; pp. 256–286. [Google Scholar]
- Ganesan, P.; Narayanasamy, D. Lipid nanoparticles: Different preparation techniques, characterization, hurdles, and strategies for the production of solid lipid nanoparticles and nanostructured lipid carriers for oral drug delivery. Sustain. Chem. Pharm. 2017, 6, 37–56. [Google Scholar] [CrossRef]
- Tamjidi, F.; Shahedi, M.; Varshosaz, J.; Nasirpour, A. Stability of astaxanthin-loaded nanostructured lipid carriers in beverage systems. J. Sci. Food Agric. 2018, 98, 511–518. [Google Scholar] [CrossRef]
- Zirak, M.B.; Pezeshki, A. Effect of surfactant concentration on the particle size, stability and potential zeta of beta carotene nano lipid carrier. Int. J. Curr. Microbiol. App. Sci 2015, 4, 924–932. [Google Scholar]
- Puglia, C.; Santonocito, D.; Musumeci, T.; Cardile, V.; Graziano, A.C.E.; Salerno, L.; Raciti, G.; Crascì, L.; Panico, A.M.; Puglisi, G. Nanotechnological approach to increase the antioxidant and cytotoxic efficacy of crocin and crocetin. Planta Med. 2019, 85, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Kammari, R.; Das, N.G.; Das, S.K. Nanoparticulate Systems for therapeutic and diagnostic applications. In Emerging Nanotechnologies for Diagnostics, Drug Delivery and Medical Devices; Mitra, A.K., Cholkar, K., Mandal, A., Eds.; Elsevier: Boston, MA, USA, 2017; Chapter 6; pp. 105–144. [Google Scholar]
- Nerome, H.; Machmudah, S.; Fukuzato, R.; Higashiura, T.; Kanda, H.; Goto, M. Effect of solvent on nanoparticle production of β-carotene by a supercritical antisolvent process. Chem. Eng. Technol. 2016, 39, 1771–1777. [Google Scholar] [CrossRef]
- Sánchez-López, E.; Gomes, D.; Esteruelas, G.; Bonilla, L.; Lopez-Machado, A.L.; Galindo, R.; Cano, A.; Espina, M.; Ettcheto, M.; Camins, A.; et al. Metal-based nanoparticles as anti-microbial agents: An overview. Nanomaterials 2020, 10, 292. [Google Scholar] [CrossRef]
- Chavali, M.S.; Nikolova, M.P. Metal oxide nanoparticles and their applications in nanotechnology. SN Appl. Sci. 2019, 1, 607. [Google Scholar] [CrossRef]
- Sowani, H.; Mohite, P.; Damale, S.; Kulkarni, M.; Zinjarde, S. Carotenoid stabilized gold and silver nanoparticles derived from the Actinomycete Gordonia amicalis HS-11 as effective free radical scavengers. Enzym. Micro. Technol. 2016, 95, 164–173. [Google Scholar] [CrossRef]
- Zhang, X.-F.; Huang, F.-H.; Zhang, G.-L.; Bai, D.-P.; Massimo, D.F.; Huang, Y.-F.; Gurunathan, S. Novel biomolecule lycopene-reduced graphene oxide-silver nanoparticle enhances apoptotic potential of trichostatin A in human ovarian cancer cells (SKOV3). Int. J. Nanomed. 2017, 12, 7551–7575. [Google Scholar] [CrossRef] [PubMed]
- Hoshyar, R.; Khayati, G.R.; Poorgholami, M.; Kaykhaii, M. A novel green one-step synthesis of gold nanoparticles using crocin and their anti-cancer activities. J. Photochem. Photobiol. B Biol. 2016, 159, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Venil, C.K.; Malathi, M.; Velmurugan, P.; Renuka Devi, P. Green synthesis of silver nanoparticles using canthaxanthin from Dietzia maris AURCCBT01 and their cytotoxic properties against human keratinocyte cell line. J. Appl. Microbiol. 2020, 130, 1730–1744. [Google Scholar] [CrossRef] [PubMed]
- Dima, C.; Assadpour, E.; Dima, S.; Jafari, S.M. Bioavailability and bioaccessibility of food bioactive compounds; overview and assessment by in vitro methods. Compr. Rev. Food Sci. Food Saf. 2020, 19, 2862–2884. [Google Scholar] [CrossRef] [PubMed]
- Gumus, C.E.; Davidov-Pardo, G.; McClements, D.J. Lutein-enriched emulsion-based delivery systems: Impact of Maillard conjugation on physicochemical stability and gastrointestinal fate. Food Hydrocoll. 2016, 60, 38–49. [Google Scholar] [CrossRef]
- Li, H.; Xu, Y.; Sun, X.; Wang, S.; Wang, J.; Zhu, J.; Wang, D.; Zhao, L. Stability, bioactivity, and bioaccessibility of fucoxanthin in zein-caseinate composite nanoparticles fabricated at neutral pH by antisolvent precipitation. Food Hydrocoll. 2018, 84, 379–388. [Google Scholar] [CrossRef]
- Salvia-Trujillo, L.; Verkempinck, S.H.E.; Zhang, X.; Van Loey, A.M.; Grauwet, T.; Hendrickx, M.E. Comparative study on lipid digestion and carotenoid bioaccessibility of emulsions, nanoemulsions and vegetable-based in situ emulsions. Food Hydrocoll. 2019, 87, 119–128. [Google Scholar] [CrossRef]
- Xia, Z.; Han, Y.; Du, H.; McClements, D.J.; Tang, Z.; Xiao, H. Exploring the effects of carrier oil type on in vitro bioavailability of β-carotene: A cell culture study of carotenoid-enriched nanoemulsions. LWT 2020, 134, 110224. [Google Scholar] [CrossRef]
- Zanoni, F.; Vakarelova, M.; Zoccatelli, G. Development and characterization of astaxanthin-containing whey protein-based nanoparticles. Mar. Drugs 2019, 17, 627. [Google Scholar] [CrossRef]
- Fan, Y.; Gao, L.; Yi, J.; Zhang, Y.; Yokoyama, W. Development of β-carotene-loaded organogel-based nanoemulsion with improved in vitro and in vivo bioaccessibility. J. Agric. Food Chem. 2017, 65, 6188–6194. [Google Scholar] [CrossRef]
- Mok, I.-K.; Lee, J.K.; Kim, J.H.; Pan, C.-H.; Kim, S.M. Fucoxanthin bioavailability from fucoxanthin-fortified milk: In vivo and in vitro study. Food Chem. 2018, 258, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Stojiljkovic, N.; Ilic, S.; Jakovljevic, V.; Stojanovic, N.; Stojnev, S.; Kocic, H.; Stojanovic, M.; Kocic, G. The Encapsulation of lycopene in nanoliposomes enhances its protective potential in methotrexate-induced kidney injury model. Oxidative Med. Cell. Longev. 2018, 2018, 2627917. [Google Scholar] [CrossRef] [PubMed]
- Zare, M.; Norouzi Roshan, Z.; Assadpour, E.; Jafari, S.M. Improving the cancer prevention/treatment role of carotenoids through various nano-delivery systems. Crit. Rev. Food Sci. Nutr. 2021, 61, 522–534. [Google Scholar] [CrossRef] [PubMed]
- Ghaffari, M.; Dolatabadi, J.E.N. Nanotechnology for pharmaceuticals. In Industrial Applications of Nanomaterials; Thomas, S., Grohens, Y., Pottathara, Y.B., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; Chapter 17; pp. 475–502. [Google Scholar]
- Akhoond Zardini, A.; Mohebbi, M.; Farhoosh, R.; Bolurian, S. Production and characterization of nanostructured lipid carriers and solid lipid nanoparticles containing lycopene for food fortification. J. Food Sci. Technol. 2018, 55, 287–298. [Google Scholar] [CrossRef]
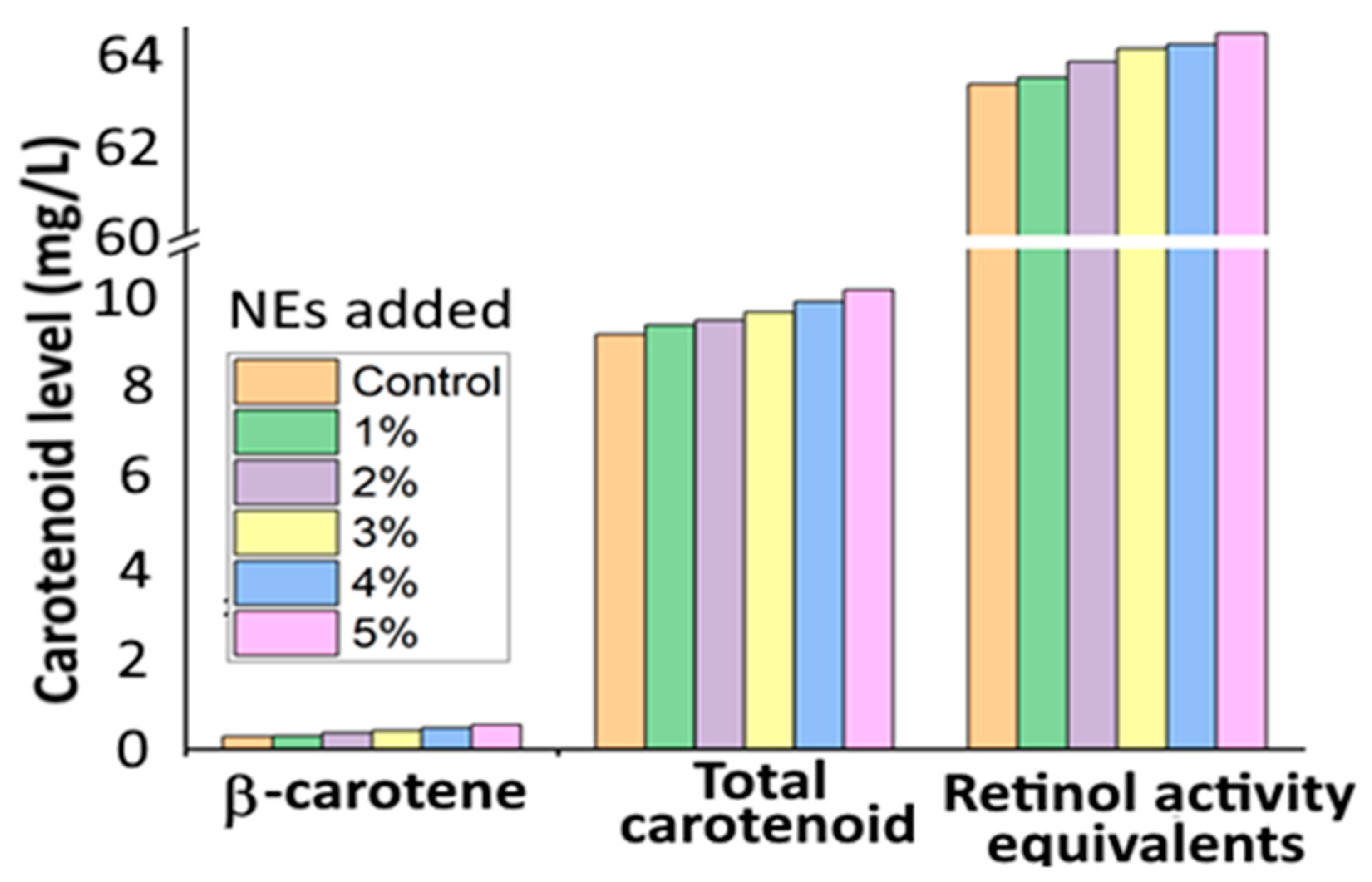
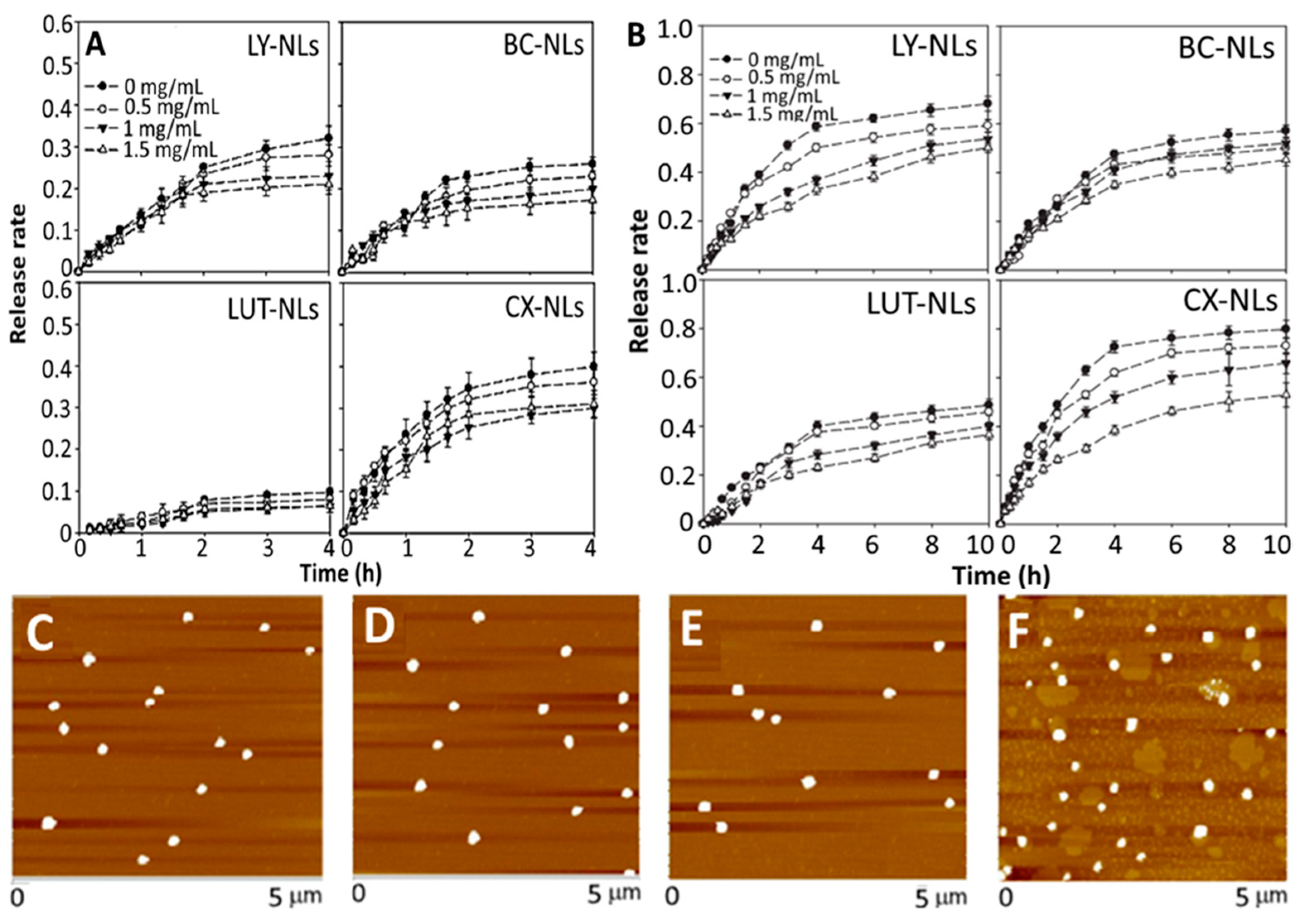
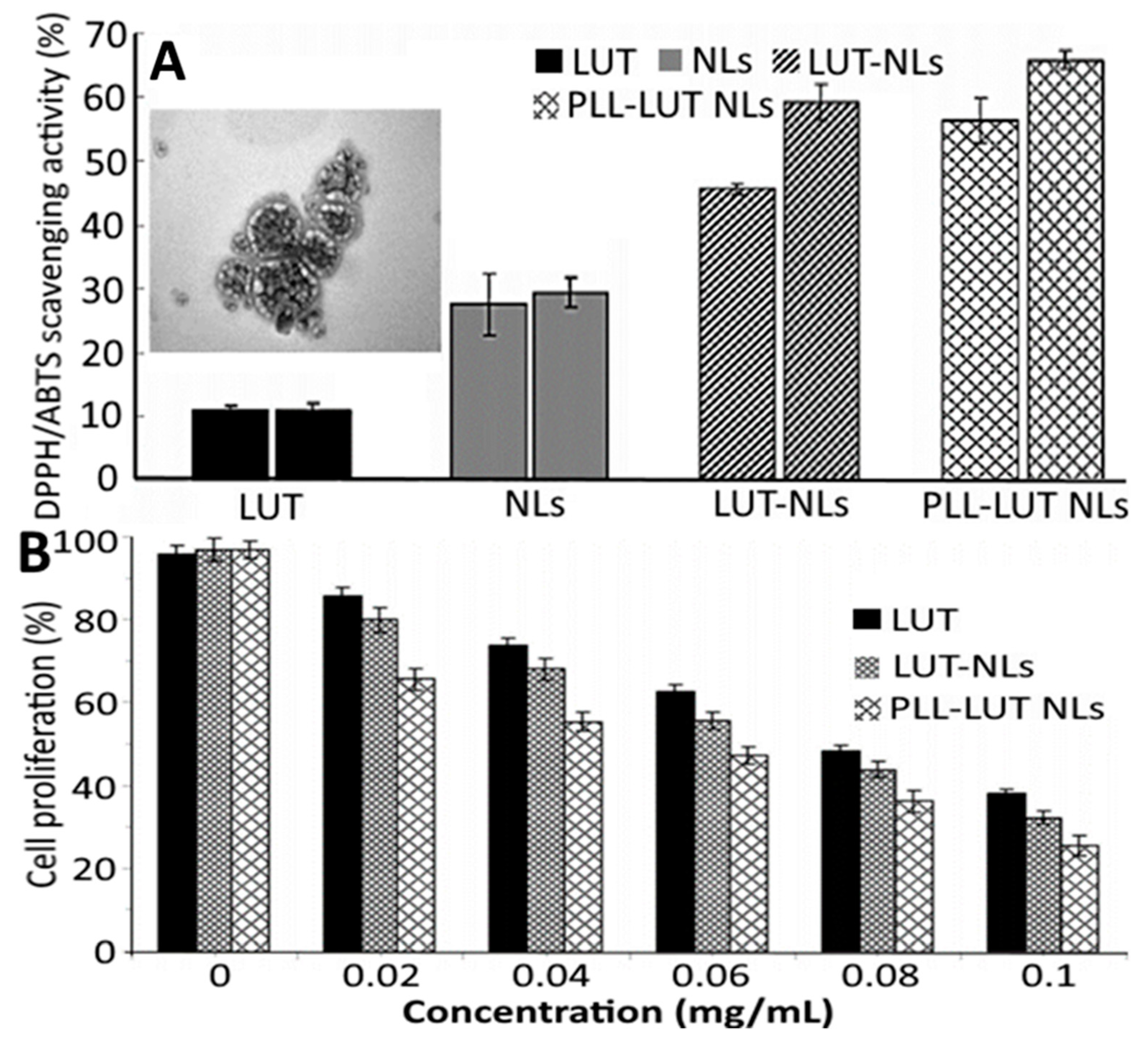
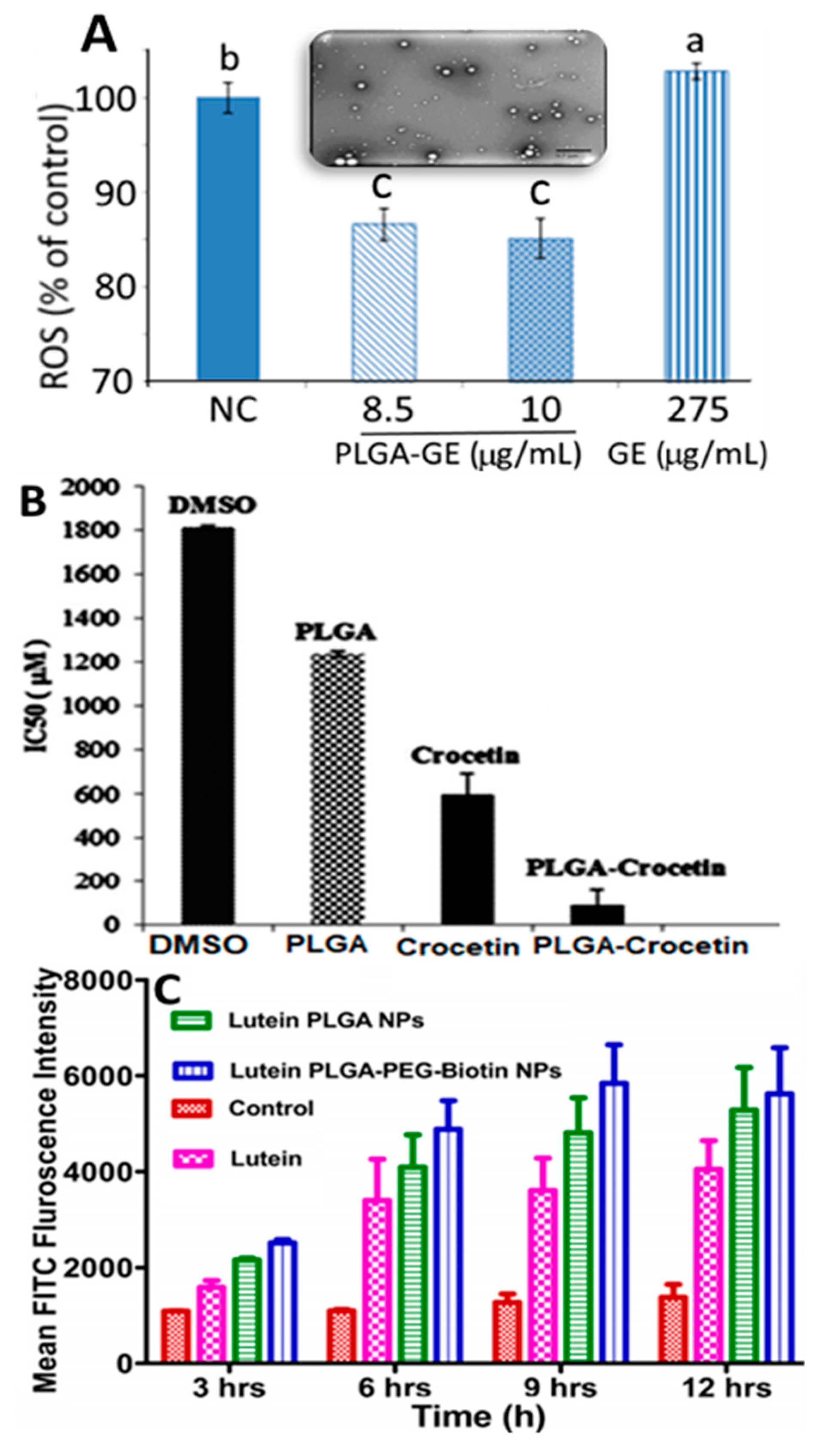


 and
and  represent bars corresponding to nitric oxide and 2,2-diphenylpicrylhydrazyl scavenging percentage values, respectively. Adapted with permission from Sowani, et al. [115].
represent bars corresponding to nitric oxide and 2,2-diphenylpicrylhydrazyl scavenging percentage values, respectively. Adapted with permission from Sowani, et al. [115].
 and
and  represent bars corresponding to nitric oxide and 2,2-diphenylpicrylhydrazyl scavenging percentage values, respectively. Adapted with permission from Sowani, et al. [115].
represent bars corresponding to nitric oxide and 2,2-diphenylpicrylhydrazyl scavenging percentage values, respectively. Adapted with permission from Sowani, et al. [115].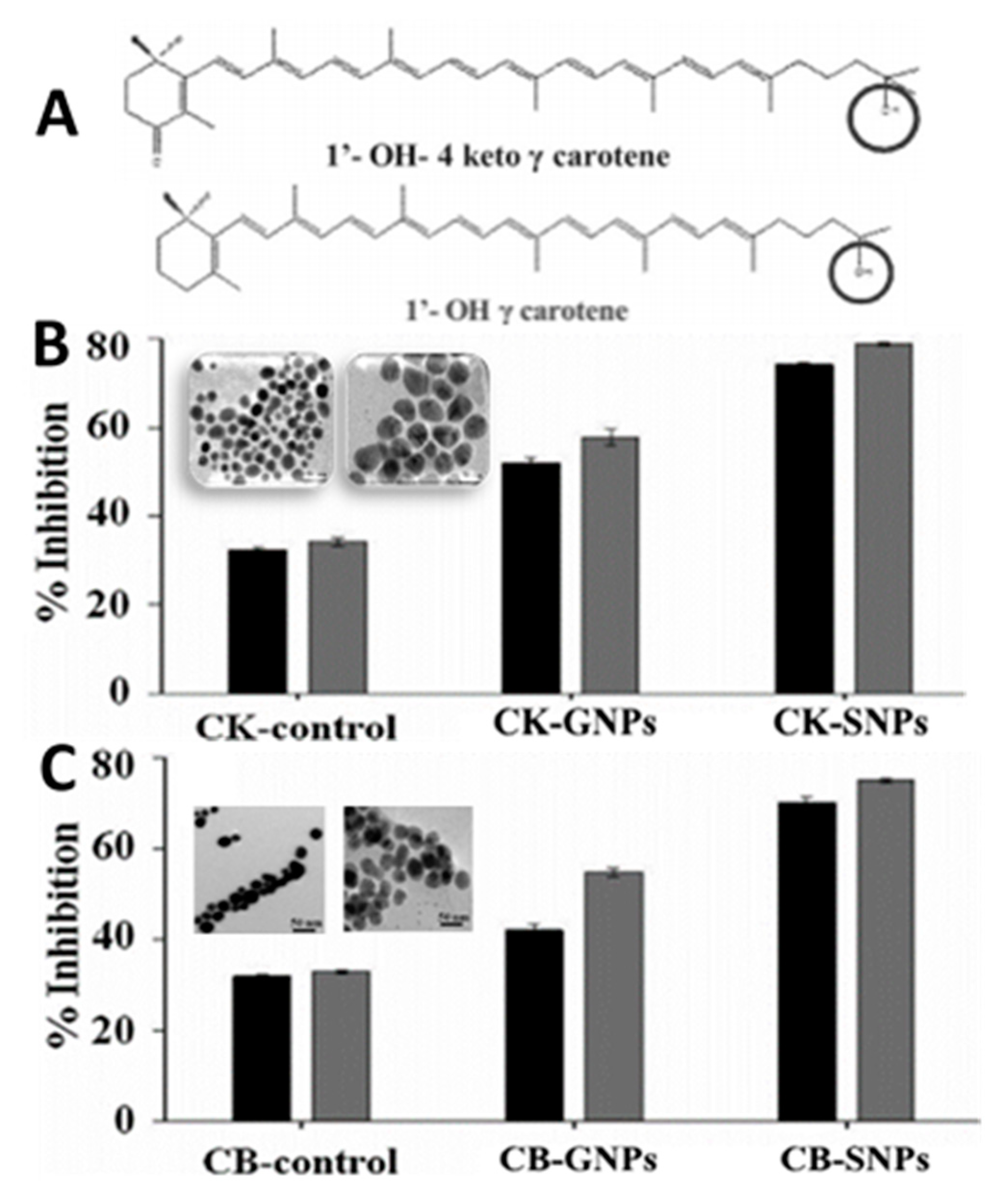

| Nanosystem | Carotenoids | Particle Size (nm) | EE (%) | Zeta Potential (mV) | Storage Stability (Days) | References |
|---|---|---|---|---|---|---|
| Nanoemulsions | β-carotene | 218 | NA | 40 | 21 at 37 °C | [32] |
| 143.7 | −38.2 | 30 at 25 °C | [33] | |||
| Microbial carotenoids | 142.1 | NA | 30 at 25 °C | [34] | ||
| Carotenoids | 290 to 350 | −53.4 to −58.8 | 21 at 25 °C | [35] | ||
| β-carotene | 198.4 to 315.6 | −29.9 to −38.5 | 90 at 4, 25, and 37 °C | [36] | ||
| Carotenoids | <200 | −30 to −45 | 35 at 25 °C | [37] | ||
| Lycopene | 145.1 to 161.9 | −19.7 to −20.7 | 1 at 25 °C | [38] | ||
| 200.1 to 287.1 | 61 to 89.1 | 20 to 45 | 42 at 4, 25, and 37 °C | [39] | ||
| Polymeric/biopolymeric NPs | Carotenoids | 153 | 83.7 | NA | NA | [40] |
| 84.4 | >96 | −41.3 to −43.6 | 60 at 41 °C | [41] | ||
| β-carotene | 77.8 to 371.8 | 98.7 to 99.1 | −37.8 to −29.9 | NA | [42] | |
| β-carotene | 70.4 | 97.4 | NA | NA | [43] | |
| Lycopene | 152 | 89 | 58.3 | NA | [44] | |
| ~ 200 | >95 | −36 | 210 at 5 °C | [45] | ||
| 193 | NA | −11.5 | 14 at 25 °C | [46] | ||
| Lutein | <250 | 74.5 | −27.2 | NA | [47] | |
| Lutein | 240 to 340 | ~91.9 | NA | NA | [48] | |
| Crocetin | 288 to 584 | 59.6 to 97.2 | NA | NA | [49] | |
| Fucoxanthin | 200 to 500 | 47 to 90 | 30 to 50 | 6 at 37 °C | [50] | |
| Nanoliposomes/liposomes | Carotenoids | 70 to100 | 75 | −5.3 | NA | [51] |
| β-carotene | 162.8 to 365.8 | ~98 | 64.5 to 42.6 | 70 at 4 °C | [52] | |
| Astaxanthin | 80.6 | 97.6 | 31.8 | 15 at 4 and 25 °C | [53] | |
| 60 to 80 | 97.4 | NA | NA | [54] | ||
| Lutein | 264.8 to 367.1 | 91.8 to 92.9 | −34.3 to −27.9 | NA | [55] | |
| SLNPs and NLCs | β-carotene SLNPs | 200 to 400 | 53.4 to 68.3 | −6.1 to −9.3 | 90 at 5, 25, and 40 °C | [56] |
| <220 | NA | 20 to 30 | 10 at 25 °C | [57] | ||
| 120 | NA | −30 | 56 at 25 °C | [58] | ||
| Lycopene SLNPs | 125 to 166 | 86.6 to 98.4 | NA | 60 at 4 °C | [59] | |
| Lycopene NLCs | 157 to 166 | > 99 | −74.2 to −74.6 | 120 at 4, 30, and 40 °C | [8] | |
| 121.9 | 84.50 | −29 | 90 at 25 °C | [60] | ||
| Supercritical fluid-based NPs | Astaxanthin | 150 to 175 | NA | NA | NA | [61] |
| 266 | 84 | NA | NA | [62] | ||
| Metal/metal oxide-based NPs and hybrid nanocomposites | Carotenoids | 20 to 140 | NA | NA | NA | [63] |
| Lycopene | 3 to 5 | −48.5 | 90 at 4 and 25 °C | [64] | ||
| 20.8 | −25.3 | NA | [65] |
| Nanosystem | Advantages and Disadvantages | References |
|---|---|---|
| Nanoemulsions | Advantages
| [2,5] |
| Polymeric/biopolymeric NPs | Advantages
| [3,5] |
| Nanoliposomes/liposomes | Advantages
| [5,9] |
| SLNPs | Advantages
| [2,5,9] |
| NLCs | Advantages
| [2,5] |
| Supercritical fluid-based NPs | Advantages
| [2,9,66] |
| Metal/metal oxide-based NPs and hybrid nanocomposites | Advantages
| [67,68] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sridhar, K.; Inbaraj, B.S.; Chen, B.-H. Recent Advances on Nanoparticle Based Strategies for Improving Carotenoid Stability and Biological Activity. Antioxidants 2021, 10, 713. https://doi.org/10.3390/antiox10050713
Sridhar K, Inbaraj BS, Chen B-H. Recent Advances on Nanoparticle Based Strategies for Improving Carotenoid Stability and Biological Activity. Antioxidants. 2021; 10(5):713. https://doi.org/10.3390/antiox10050713
Chicago/Turabian StyleSridhar, Kandi, Baskaran Stephen Inbaraj, and Bing-Huei Chen. 2021. "Recent Advances on Nanoparticle Based Strategies for Improving Carotenoid Stability and Biological Activity" Antioxidants 10, no. 5: 713. https://doi.org/10.3390/antiox10050713
APA StyleSridhar, K., Inbaraj, B. S., & Chen, B.-H. (2021). Recent Advances on Nanoparticle Based Strategies for Improving Carotenoid Stability and Biological Activity. Antioxidants, 10(5), 713. https://doi.org/10.3390/antiox10050713








