Cyclooxygenase-2 Glycosylation Is Affected by Peroxynitrite in Endothelial Cells: Impact on Enzyme Activity and Degradation
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture and Treatment
2.2. Cytotoxicity Assay
2.3. Peroxynitrite Generation
2.4. RNA Isolation and Analysis
2.5. Western Blot Analysis
2.6. COX-2 Immunoprecipitation
2.7. Endoglycosidase H and PNGase F Digestion
2.8. Metabolic Labeling
2.9. Total Hexokinase Activity
2.10. Prostaglandins Measurement
2.11. Analysis of COX-2 Protein Degradation
2.12. Immunofluorescence Staining
2.13. Statistical Analysis
3. Results
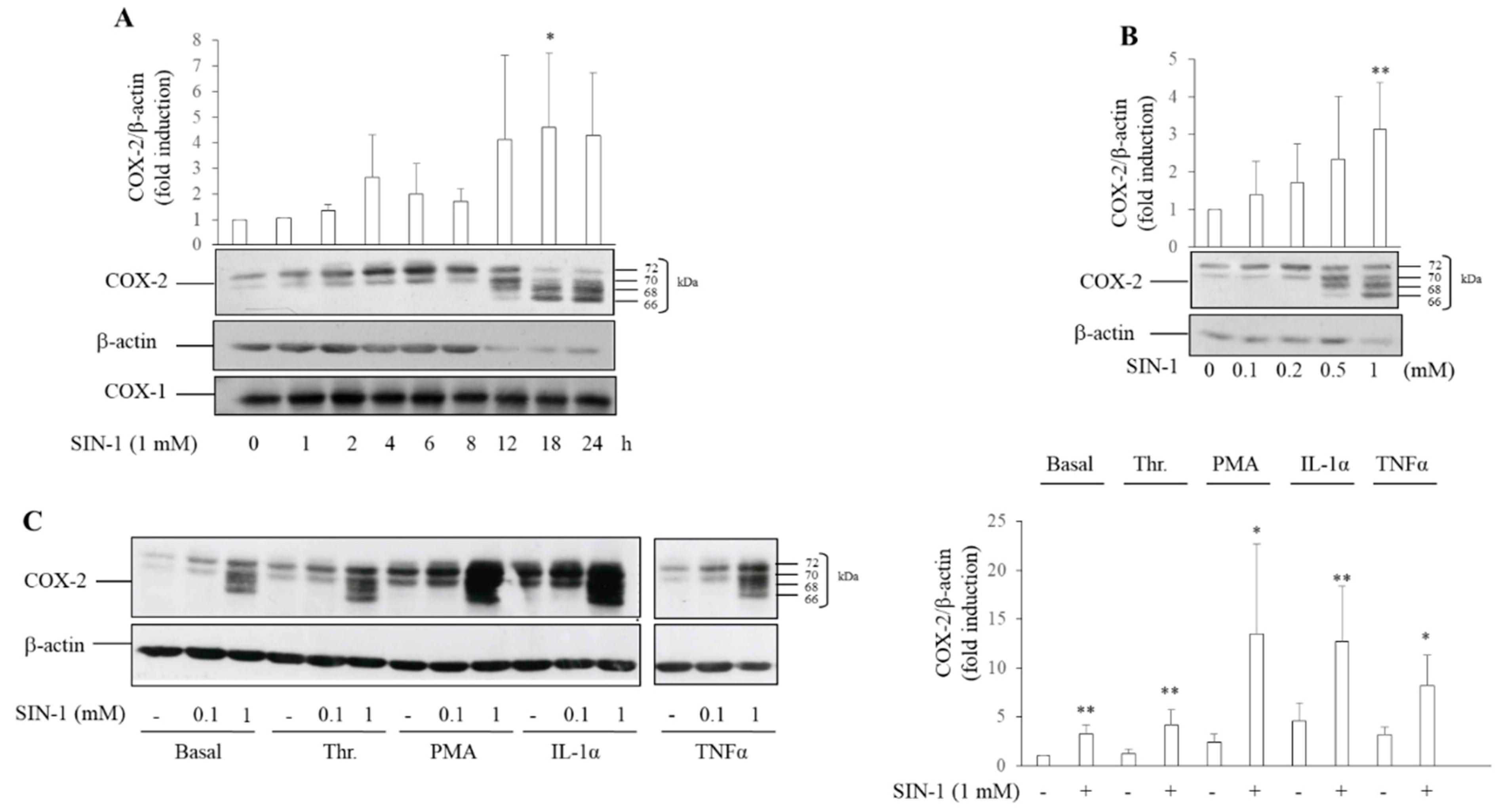
3.1. SIN-1 Induces COX-2 Expression in HUVEC
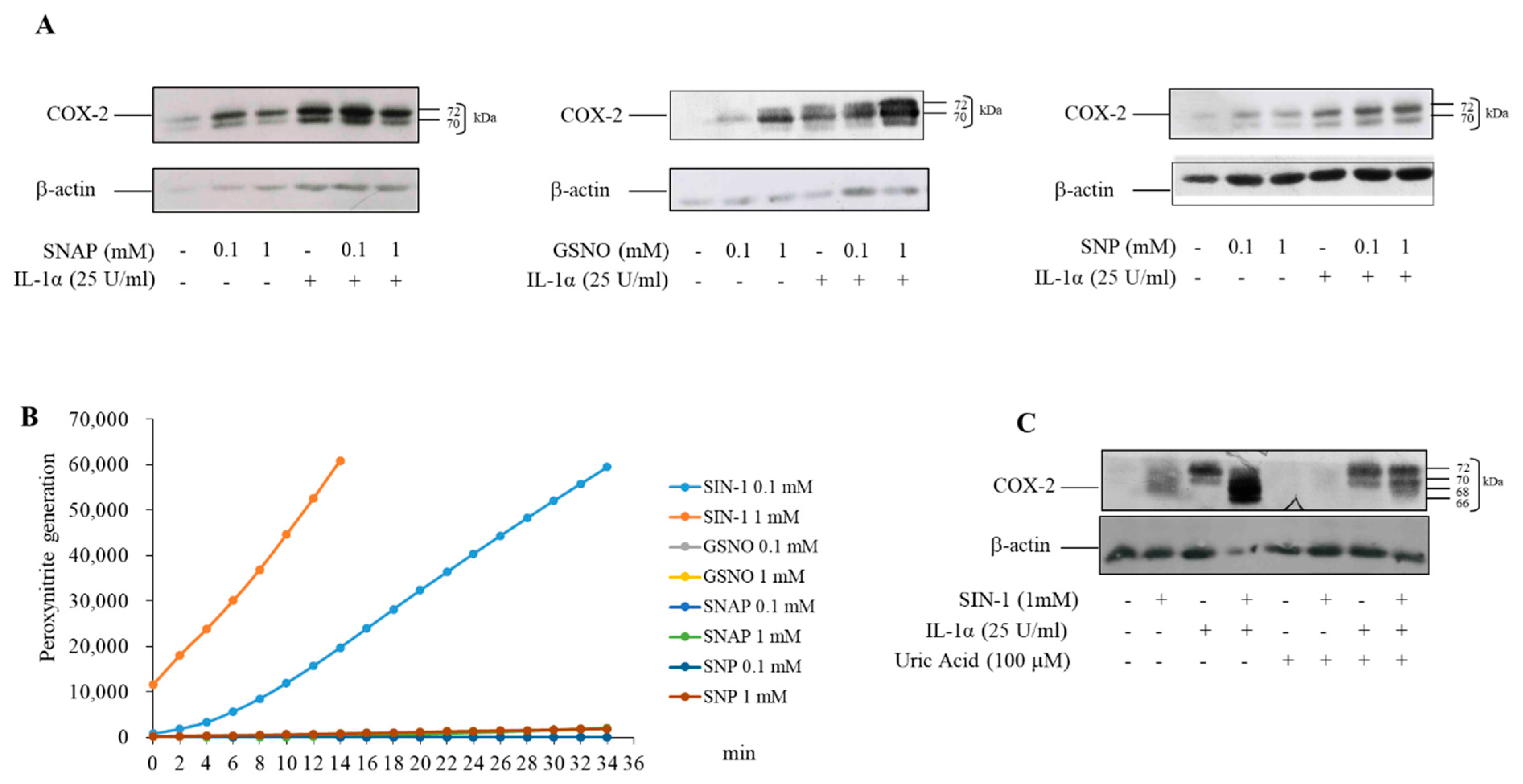
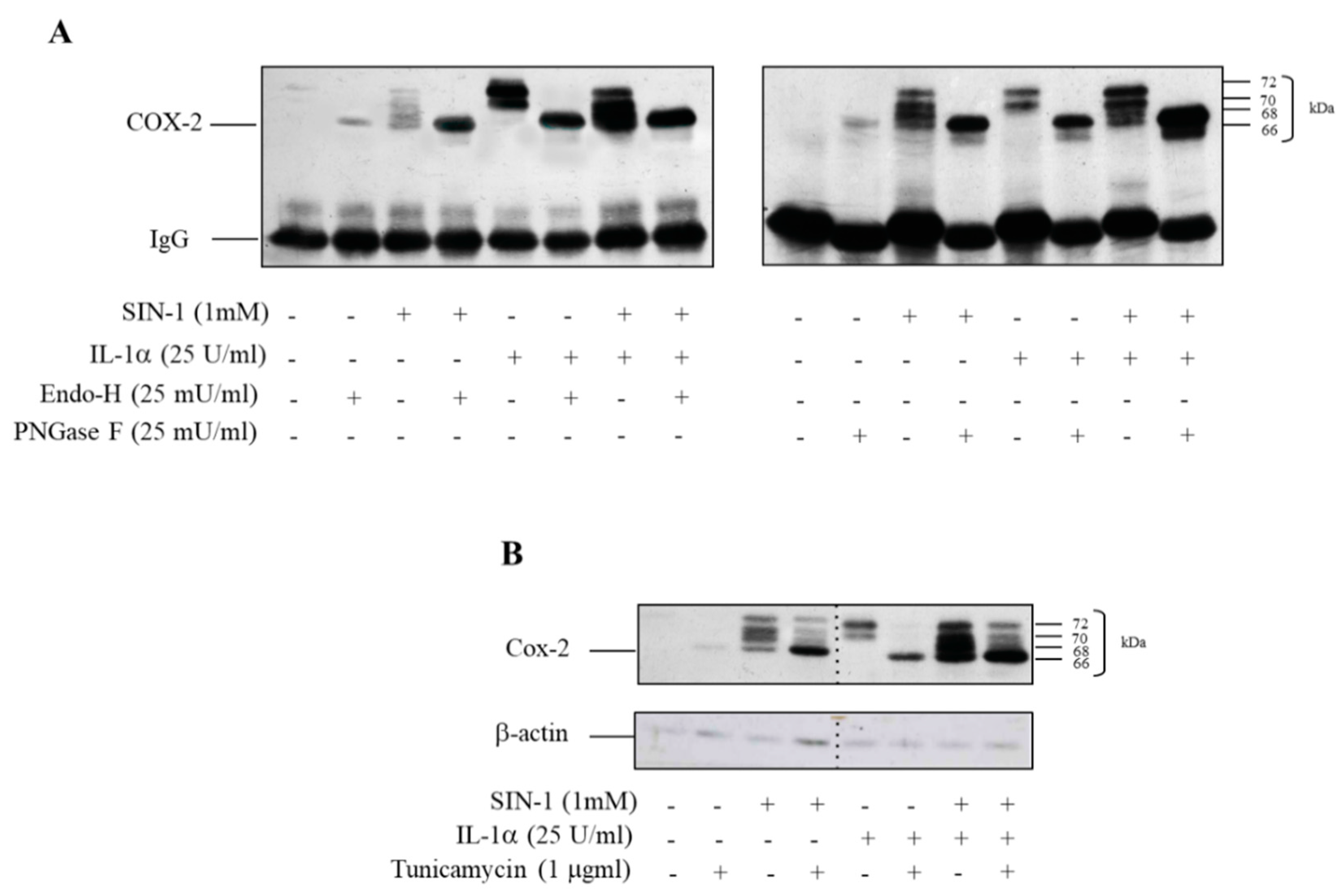
3.2. COX-2 Induced by SIN-1 Is Hypoglycosylated
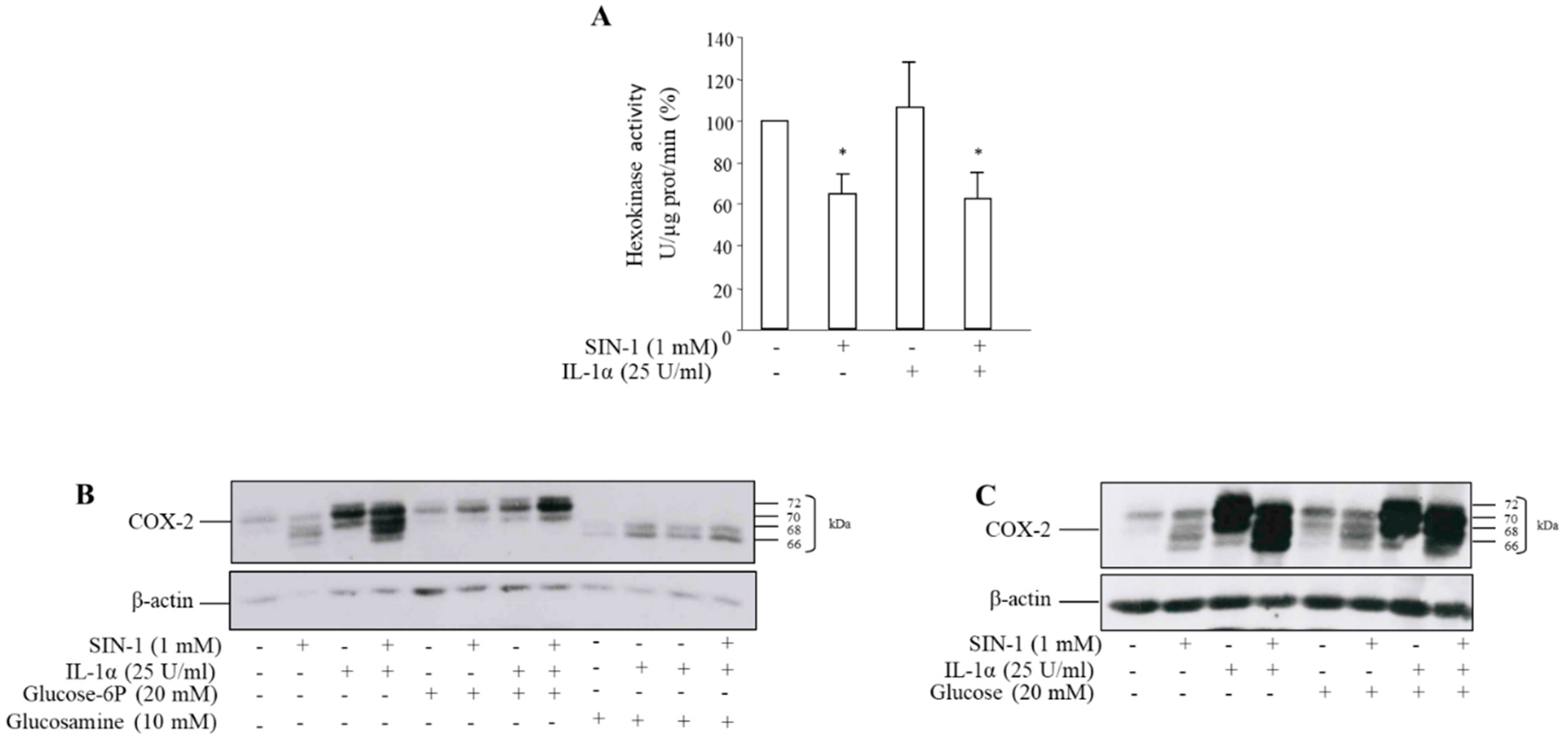
3.3. The Effect of SIN-1 on COX-2 Involves Hexokinase Activity
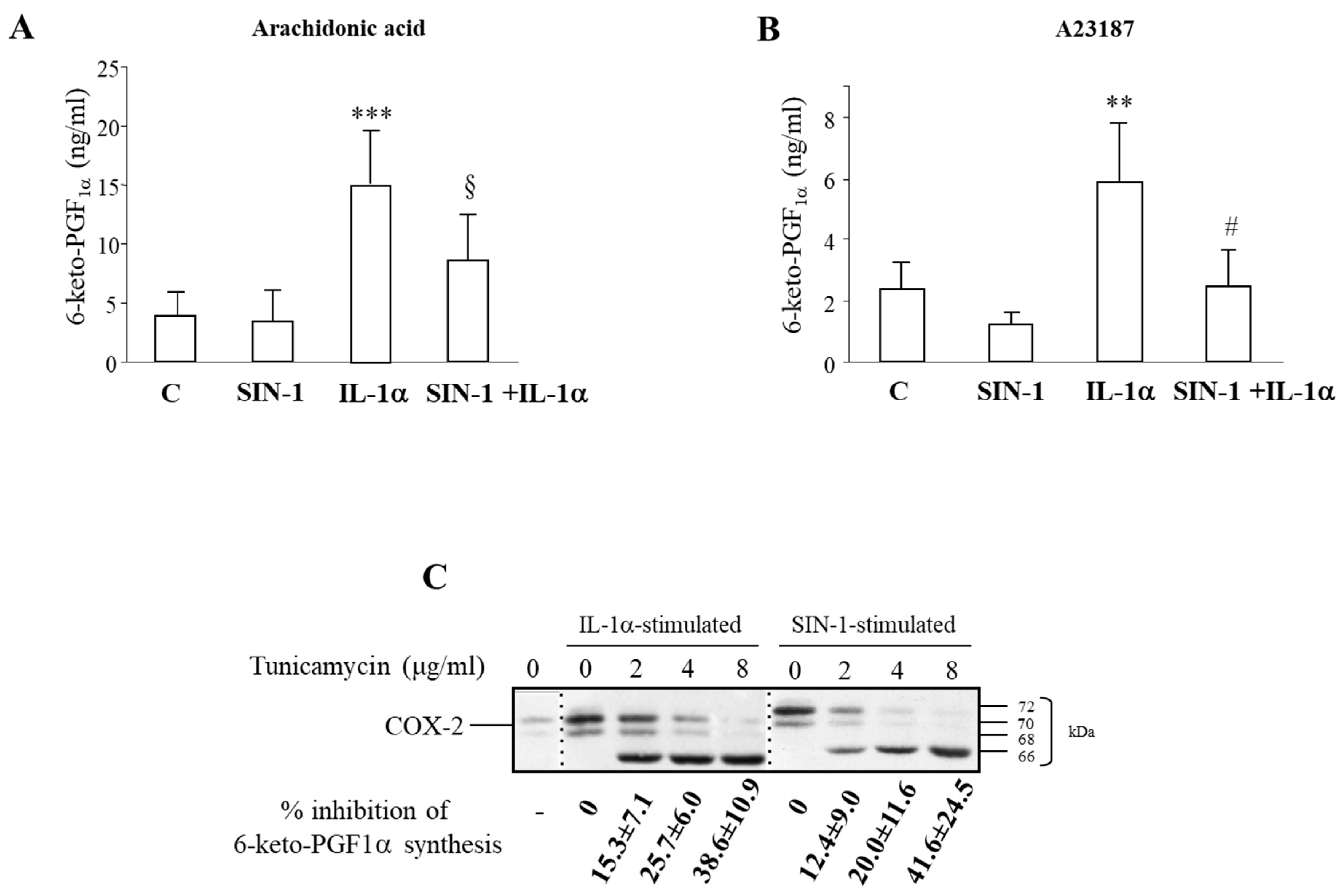
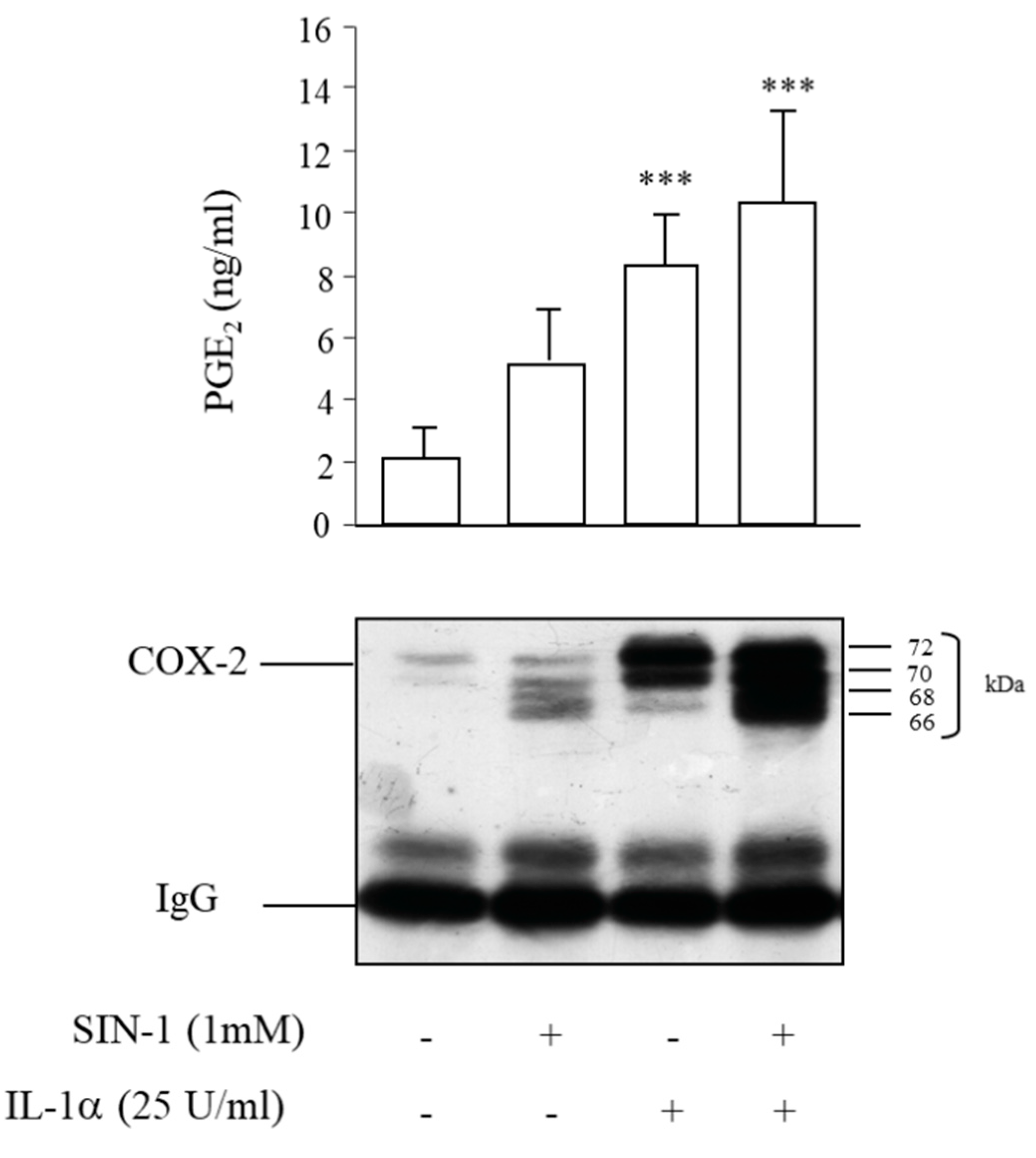
3.4. SIN-1 Impairs Prostaglandins Production in HUVEC
3.5. Partial Recovery of COX-2 Activity Impaired by SIN-1
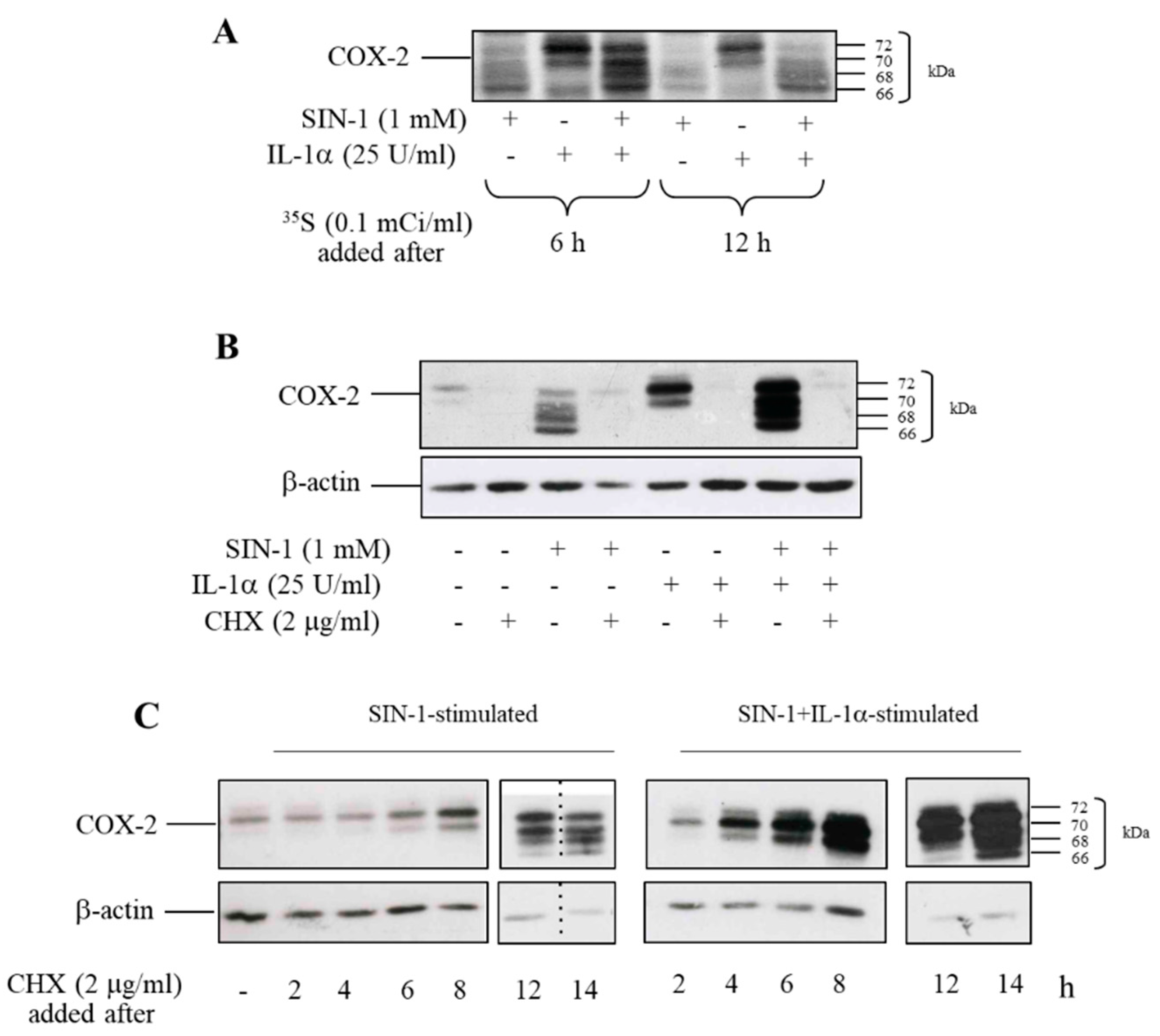
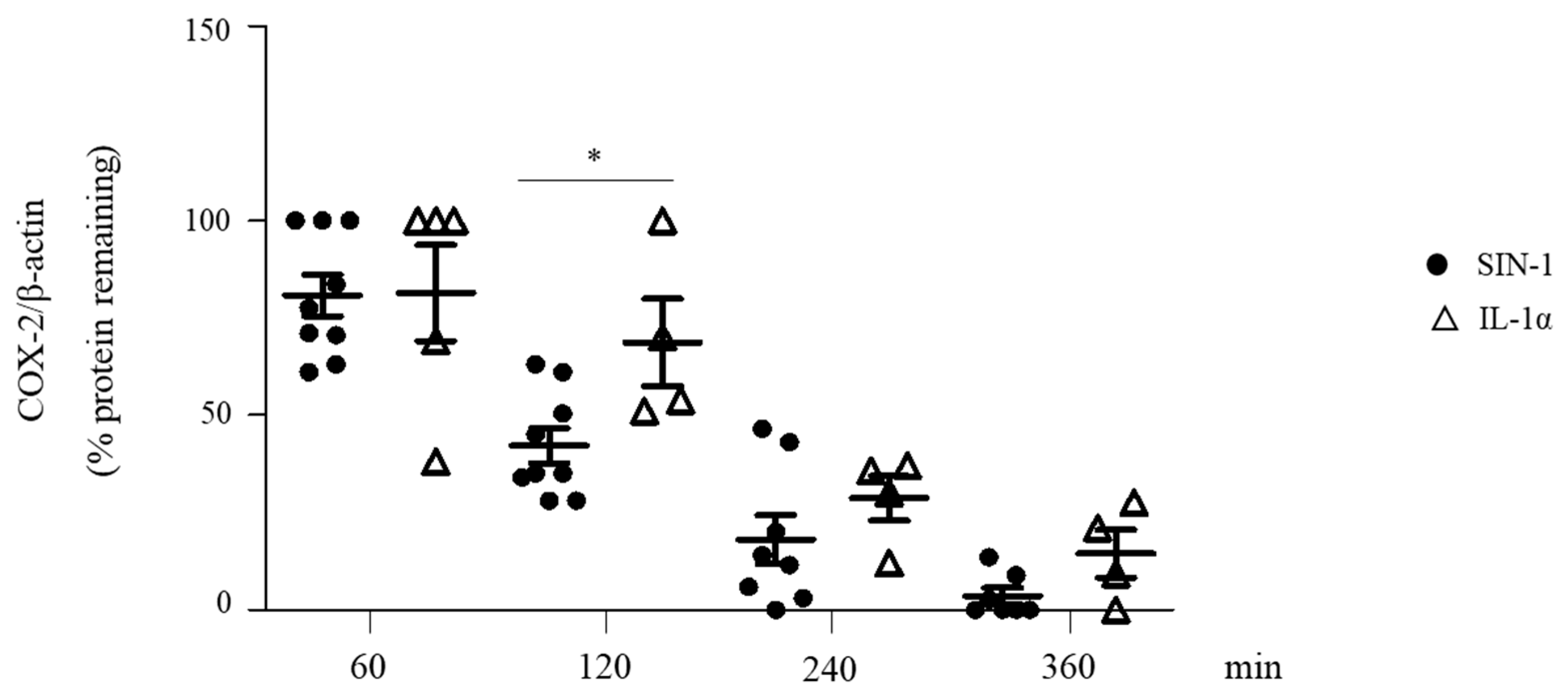
3.6. SIN-1 Accelerates the Turnover of Hypoglycosylated COX-2
3.7. Altered Intracellular Localization of Hypoglycosylated COX-2
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- O’Banion, M.K.; Sadowski, H.B.; Winn, V.; Young, D.A. A serum- and glucocorticoid-regulated 4-kilobase mRNA encodes a cyclooxygenase-related protein. J. Biol. Chem. 1991, 266, 23261–23267. [Google Scholar] [CrossRef]
- Smith, W.L.; DeWitt, D.L.; Garavito, R.M. Cyclooxygenases: Structural, cellular, and molecular biology. Annu. Rev. Biochem. 2000, 69, 145–182. [Google Scholar] [CrossRef]
- Otto, J.C.; Dewitt, D.L.; Smith, W.L. N-Glycosylation of Prostaglandin Endoperoxide Synthases-1 and Synthases-2 and Their Orientations in the Endoplasmic-Reticulum. J. Biol. Chem. 1993, 268, 18234–18242. [Google Scholar] [CrossRef]
- Sevigny, M.B.; Li, C.F.; Alas, M.; Hughes-Fulford, M. Glycosylation regulates turnover of cyclooxygenase-2. FEBS Lett. 2006, 580, 6533–6536. [Google Scholar] [CrossRef][Green Version]
- Chandrasekharan, N.V.; Simmons, D.L. The cyclooxygenases. Genome Biol. 2004, 5, 241. [Google Scholar] [CrossRef]
- Yamamoto, K.; Arakawa, T.; Ueda, N.; Yamamoto, S. Transcriptional roles of nuclear factor kappa B and nuclear factor-interleukin-6 in the tumor necrosis factor alpha-dependent induction of cyclooxygenase-2 in MC3T3-E1 cells. J. Biol. Chem. 1995, 270, 31315–31320. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.L.; Chipman, J.G.; Robertson, D.L.; Erikson, R.L.; Simmons, D.L. Expression of a mitogen-responsive gene encoding prostaglandin synthase is regulated by mRNA splicing. Proc. Natl. Acad. Sci. USA 1991, 88, 2692–2696. [Google Scholar] [CrossRef] [PubMed]
- Lusis, A.J. Atherosclerosis. Nature 2000, 407, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Cipollone, F.; Cicolini, G.; Bucci, M. Cyclooxygenase and prostaglandin synthases in atherosclerosis: Recent insights and future perspectives. Pharmacol. Ther. 2008, 118, 161–180. [Google Scholar] [CrossRef]
- Tanabe, T.; Tohnai, N. Cyclooxygenase isozymes and their gene structures and expression. Prostaglandins Other Lipid Mediat. 2002, 68, 95–114. [Google Scholar] [CrossRef]
- Mitchell, J.A.; Kirkby, N.S. Eicosanoids, prostacyclin and cyclooxygenase in the cardiovascular system. Br. J. Pharmacol. 2019, 176, 1038–1050. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.Y.; Monslow, J.; Todd, L.; Lawson, J.; Pure, E.; FitzGerald, G.A. Cyclooxygenase-2 in endothelial and vascular smooth muscle cells restrains atherogenesis in hyperlipidemic mice. Circulation 2014, 129, 1761–1769. [Google Scholar] [CrossRef] [PubMed]
- Vila, L. Cyclooxygenase and 5-lipoxygenase pathways in the vessel wall: Role in atherosclerosis. Med. Res. Rev. 2004, 24, 399–424. [Google Scholar] [CrossRef]
- Reiss, A.B.; Edelman, S.D. Recent insights into the role of prostanoids in atherosclerotic vascular disease. Curr. Vasc. Pharmacol. 2006, 4, 395–408. [Google Scholar] [CrossRef] [PubMed]
- Santilli, F.; D’Ardes, D.; Davi, G. Oxidative stress in chronic vascular disease: From prediction to prevention. Vasc. Pharmacol. 2015, 74, 23–37. [Google Scholar] [CrossRef]
- Perez-Torres, I.; Manzano-Pech, L.; Rubio-Ruiz, M.E.; Soto, M.E.; Guarner-Lans, V. Nitrosative Stress and Its Association with Cardiometabolic Disorders. Molecules 2020, 25, 2555. [Google Scholar] [CrossRef] [PubMed]
- Wattanapitayakul, S.K.; Weinstein, D.M.; Holycross, B.J.; Bauer, J.A. Endothelial dysfunction and peroxynitrite formation are early events in angiotensin-induced cardiovascular disorders. FASEB J. 2000, 14, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Szabo, C.; Ischiropoulos, H.; Radi, R. Peroxynitrite: Biochemistry, pathophysiology and development of therapeutics. Nat. Rev. Drug Discov. 2007, 6, 662–680. [Google Scholar] [CrossRef]
- Bachschmid, M.; Schildknecht, S.; Ullrich, V. Redox regulation of vascular prostanoid synthesis by the nitric oxide-superoxide system. Biochem. Biophys. Res. Commun. 2005, 338, 536–542. [Google Scholar] [CrossRef]
- Schildknecht, S.; Ullrich, V. Peroxynitrite as regulator of vascular prostanoid synthesis. Arch. Biochem. Biophys. 2009, 484, 183–189. [Google Scholar] [CrossRef]
- Eligini, S.; Barbieri, S.S.; Cavalca, V.; Camera, M.; Brambilla, M.; De Franceschi, M.; Tremoli, E.; Colli, S. Diversity and similarity in signaling events leading to rapid Cox-2 induction by tumor necrosis factor-alpha and phorbol ester in human endothelial cells. Cardiovasc. Res. 2005, 65, 683–693. [Google Scholar] [CrossRef] [PubMed]
- Repetto, G.; del Peso, A.; Zurita, J.L. Neutral red uptake assay for the estimation of cell viability/cytotoxicity. Nat. Protoc. 2008, 3, 1125–1131. [Google Scholar] [CrossRef]
- Eligini, S.; Habib, A.; Lebret, M.; Creminon, C.; Levy-Toledano, S.; Maclouf, J. Induction of cyclo-oxygenase-2 in human endothelial cells by SIN-1 in the absence of prostaglandin production. Br. J. Pharmacol. 2001, 133, 1163–1171. [Google Scholar] [CrossRef]
- Habib, A.; Creminon, C.; Frobert, Y.; Grassi, J.; Pradelles, P.; Maclouf, J. Demonstration of an inducible cyclooxygenase in human endothelial cells using antibodies raised against the carboxyl-terminal region of the cyclooxygenase-2. J. Biol. Chem. 1993, 268, 23448–23454. [Google Scholar] [CrossRef]
- Trimble, R.B.; Maley, F. Optimizing Hydrolysis of N-Linked High-Mannose Oligosaccharides by “Endo-Beta-N-Acetylglucosaminidase-H”. Anal. Biochem. 1984, 141, 515–522. [Google Scholar] [CrossRef]
- Maley, F.; Trimble, R.B.; Tarentino, A.L.; Plummer, T.H. Characterization of Glycoproteins and Their Associated Oligosaccharides through the Use of Endoglycosidases. Anal. Biochem. 1989, 180, 195–204. [Google Scholar] [CrossRef]
- Rush, J.S. Role of Flippases in Protein Glycosylation in the Endoplasmic Reticulum. Lipid Insights 2015, 8, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Varki, A.; Gagneux, P. Biological Functions of Glycans. In Essentials of Glycobiology; Varki, A., Cummings, R.D., Esko, J.D., Stanley, P., Hart, G.W., Aebi, M., Darvill, A.G., Kinoshita, T., Packer, N.H., et al., Eds.; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 2015; pp. 77–88. [Google Scholar]
- Patel, R.N.; Attur, M.G.; Dave, M.N.; Patel, I.V.; Stuchin, S.A.; Abramson, S.B.; Amin, A.R. A novel mechanism of action of chemically modified tetracyclines: Inhibition of COX-2-mediated prostaglandin E-2 production. J. Immunol. 1999, 163, 3459–3467. [Google Scholar]
- Zhang, F.; Warskulat, U.; Wettstein, M.; Schreiber, R.; Henninger, H.P.; Decker, K.; Haussinger, D. Hyperosmolarity Stimulates Prostaglandin Synthesis and Cyclooxygenase-2 Expression in Activated Rat-Liver Macrophages. Biochem. J. 1995, 312, 135–143. [Google Scholar] [CrossRef]
- Jang, B.C.; Sung, S.H.; Park, J.G.; Park, J.W.; Bae, J.H.; Shin, D.H.; Park, G.Y.; Han, S.B.; Suh, S.I. Glucosamine hydrochloride specifically inhibits COX-2 by preventing COX-2 N-glycosylation and by increasing COX-2 protein turnover in a proteasome-dependent manner. J. Biol. Chem. 2007, 282, 27622–27632. [Google Scholar] [CrossRef] [PubMed]
- Darius, H.; Ahland, B.; Rucker, W.; Klaus, W.; Peskar, B.A.; Schror, K. The effects of molsidomine and its metabolite SIN-1 on coronary vessel tone, platelet aggregation, and eicosanoid formation in vitro--inhibition of 12-HPETE biosynthesis. J. Cardiovasc. Pharmacol. 1984, 6, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Nitz, R.E.; Fiedler, V.B. Molsidomine: Alternative approaches to treat myocardial ischemia. Pharmacotherapy 1987, 7, 28–37. [Google Scholar] [CrossRef]
- Davel, A.P.; Wenceslau, C.F.; Akamine, E.H.; Xavier, F.E.; Couto, G.K.; Oliveira, H.T.; Rossoni, L.V. Endothelial dysfunction in cardiovascular and endocrine-metabolic diseases: An update. Braz. J. Med. Biol. Res. 2011, 44, 920–932. [Google Scholar] [CrossRef] [PubMed]
- Kirkby, N.S.; Lundberg, M.H.; Wright, W.R.; Warner, T.D.; Paul-Clark, M.J.; Mitchell, J.A. COX-2 protects against atherosclerosis independently of local vascular prostacyclin: Identification of COX-2 associated pathways implicate Rgl1 and lymphocyte networks. PLoS ONE 2014, 9, e98165. [Google Scholar] [CrossRef]
- Iezzi, A.; Ferri, C.; Mezzetti, A.; Cipollone, F. COX-2: Friend or foe? Curr. Pharm. Des. 2007, 13, 1715–1721. [Google Scholar] [CrossRef]
- Ndengele, M.M.; Cuzzocrea, S.; Esposito, E.; Mazzon, E.; Di Paola, R.; Matuschak, G.M.; Salvemini, D. Cyclooxygenases 1 and 2 contribute to peroxynitrite-mediated inflammatory pain hypersensitivity. FASEB J. 2008, 22, 3154–3164. [Google Scholar] [CrossRef]
- Seo, J.Y.; Yu, J.H.; Lim, J.W.; Mukaida, N.; Kim, H. Nitric oxide-induced IL-8 expression is mediated by NF-kappaB and AP-1 in gastric epithelial AGS cells. J. Physiol. Pharmacol. 2009, 60 (Suppl. 7), 101–106. [Google Scholar]
- Yoshino, Y.; Yamamoto, S.; Kohsaka, S.; Oshiro, S.; Nakajima, K. Superoxide anion contributes to the induction of tumor necrosis factor alpha (TNFalpha) through activation of the MKK3/6-p38 MAPK cascade in rat microglia. Brain Res. 2011, 1422, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.M.; Song, J.D.; Chung, H.T.; Park, Y.C. Protein kinase CK2 mediates peroxynitrite-induced heme oxygenase-1 expression in articular chondrocytes. Int. J. Mol. Med. 2012, 29, 1039–1044. [Google Scholar] [CrossRef]
- Nemeth, J.F.; Hochgesang, G.P., Jr.; Marnett, L.J.; Caprioli, R.M. Characterization of the glycosylation sites in cyclooxygenase-2 using mass spectrometry. Biochemistry Us 2001, 40, 3109–3116. [Google Scholar] [CrossRef]
- Adeva-Andany, M.M.; Perez-Felpete, N.; Fernandez-Fernandez, C.; Donapetry-Garcia, C.; Pazos-Garcia, C. Liver glucose metabolism in humans. Biosci. Rep. 2016, 36, e00416. [Google Scholar] [CrossRef] [PubMed]
- Heneberg, P. Redox Regulation of Hexokinases. Antioxid. Redox Signal. 2019, 30, 415–442. [Google Scholar] [CrossRef] [PubMed]
- Rinis, N.; Golden, J.E.; Marceau, C.D.; Carette, J.E.; Van Zandt, M.C.; Gilmore, R.; Contessa, J.N. Editing N-Glycan Site Occupancy with Small-Molecule Oligosaccharyltransferase Inhibitors. Cell Chem. Biol. 2018, 25, 1231–1241. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, P.; Youhnovski, N.; Daiber, A.; Balan, A.; Arsic, M.; Bachschmid, M.; Przybylski, M.; Ullrich, V. Specific nitration at tyrosine 430 revealed by high resolution mass spectrometry as basis for redox regulation of bovine prostacyclin synthase. J. Biol. Chem. 2003, 278, 12813–12819. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, Y.; Uno, E.; Sakuma, S. Effects of reactive oxygen and nitrogen species on cyclooxygenase-1 and -2 activities. Prostaglandins Leukot. Essent. Fatty Acids 2004, 71, 335–340. [Google Scholar] [CrossRef]
- Kim, S.F. The role of nitric oxide in prostaglandin biology; update. Nitric Oxide 2011, 25, 255–264. [Google Scholar] [CrossRef]
- Mbonye, U.R.; Song, I. Posttranscriptional and posttranslational determinants of cyclooxygenase expression. BMB Rep. 2009, 42, 552–560. [Google Scholar] [CrossRef]
- Mbonye, U.R.; Yuan, C.; Harris, C.E.; Sidhu, R.S.; Song, I.; Arakawa, T.; Smith, W.L. Two distinct pathways for cyclooxygenase-2 protein degradation. J. Biol. Chem. 2008, 283, 8611–8623. [Google Scholar] [CrossRef]
- Yuan, C.; Smith, W.L. A cyclooxygenase-2-dependent prostaglandin E2 biosynthetic system in the Golgi apparatus. J. Biol. Chem. 2015, 290, 5606–5620. [Google Scholar] [CrossRef]
- Ueno, N.; Murakami, M.; Tanioka, T.; Fujimori, K.; Tanabe, T.; Urade, Y.; Kudo, I. Coupling between cyclooxygenase, terminal prostanoid synthase, and phospholipase A2. J. Biol. Chem. 2001, 276, 34918–34927. [Google Scholar] [CrossRef]
- Muller-Decker, K.; Scholz, K.; Neufang, G.; Marks, F.; Furstenberger, G. Localization of prostaglandin-H synthase-1 and -2 in mouse skin: Implications for cutaneous function. Exp. Cell Res. 1998, 242, 84–91. [Google Scholar] [CrossRef]
- Korbecki, J.; Baranowska-Bosiacka, I.; Gutowska, I.; Chlubek, D. The effect of reactive oxygen species on the synthesis of prostanoids from arachidonic acid. J. Physiol. Pharmacol. 2013, 64, 409–421. [Google Scholar] [PubMed]
- Cooke, C.L.; Davidge, S.T. Peroxynitrite increases iNOS through NF-kappaB and decreases prostacyclin synthase in endothelial cells. Am. J. Physiol. Cell Physiol. 2002, 282, C395–C402. [Google Scholar] [CrossRef]
- Ji, Y.S.; Xu, Q.; Schmedtje, J.F., Jr. Hypoxia induces high-mobility-group protein I(Y) and transcription of the cyclooxygenase-2 gene in human vascular endothelium. Circ. Res. 1998, 83, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Virag, L.; Szabo, E.; Gergely, P.; Szabo, C. Peroxynitrite-induced cytotoxicity: Mechanism and opportunities for intervention. Toxicol. Lett. 2003, 140, 113–124. [Google Scholar] [CrossRef]
- Pacher, P.; Beckman, J.S.; Liaudet, L. Nitric oxide and peroxynitrite in health and disease. Physiol. Rev. 2007, 87, 315–424. [Google Scholar] [CrossRef]
- Hemanth Kumar, K.; Tamatam, A.; Pal, A.; Khanum, F. Neuroprotective effects of Cyperus rotundus on SIN-1 induced nitric oxide generation and protein nitration: Ameliorative effect against apoptosis mediated neuronal cell damage. Neurotoxicology 2013, 34, 150–159. [Google Scholar] [CrossRef] [PubMed]
- Yang, E.S.; Park, J.W. Antioxidant enzyme inhibitors enhance peroxynitrite-induced cell death in U937 cells. Mol. Cell Biochem. 2007, 301, 61–68. [Google Scholar] [CrossRef]
- Mattart, L.; Calay, D.; Simon, D.; Roebroek, L.; Caesens-Koenig, L.; Van Steenbrugge, M.; Tevel, V.; Michiels, C.; Arnould, T.; Boudjeltia, K.Z.; et al. The peroxynitrite donor 3-morpholinosydnonimine activates Nrf2 and the UPR leading to a cytoprotective response in endothelial cells. Cell Signal. 2012, 24, 199–213. [Google Scholar] [CrossRef]
- Harris, R.E. Cyclooxygenase-2 (cox-2) blockade in the chemoprevention of cancers of the colon, breast, prostate, and lung. Inflammopharmacology 2009, 17, 55–67. [Google Scholar] [CrossRef]
- Rigas, A.; Rigas, B.; Glassman, M.; Yen, Y.Y.; Lan, S.J.; Petridou, E.; Hsieh, C.C.; Trichopoulos, D. Breast-feeding and maternal smoking in the etiology of Crohn’s disease and ulcerative colitis in childhood. Ann. Epidemiol. 1993, 3, 387–392. [Google Scholar] [CrossRef]
- Alexanian, A.; Sorokin, A. Cyclooxygenase 2: Protein-protein interactions and posttranslational modifications. Physiol. Genom. 2017, 49, 667–681. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eligini, S.; Colli, S.; Habib, A.; Aldini, G.; Altomare, A.; Banfi, C. Cyclooxygenase-2 Glycosylation Is Affected by Peroxynitrite in Endothelial Cells: Impact on Enzyme Activity and Degradation. Antioxidants 2021, 10, 496. https://doi.org/10.3390/antiox10030496
Eligini S, Colli S, Habib A, Aldini G, Altomare A, Banfi C. Cyclooxygenase-2 Glycosylation Is Affected by Peroxynitrite in Endothelial Cells: Impact on Enzyme Activity and Degradation. Antioxidants. 2021; 10(3):496. https://doi.org/10.3390/antiox10030496
Chicago/Turabian StyleEligini, Sonia, Susanna Colli, Aida Habib, Giancarlo Aldini, Alessandra Altomare, and Cristina Banfi. 2021. "Cyclooxygenase-2 Glycosylation Is Affected by Peroxynitrite in Endothelial Cells: Impact on Enzyme Activity and Degradation" Antioxidants 10, no. 3: 496. https://doi.org/10.3390/antiox10030496
APA StyleEligini, S., Colli, S., Habib, A., Aldini, G., Altomare, A., & Banfi, C. (2021). Cyclooxygenase-2 Glycosylation Is Affected by Peroxynitrite in Endothelial Cells: Impact on Enzyme Activity and Degradation. Antioxidants, 10(3), 496. https://doi.org/10.3390/antiox10030496







