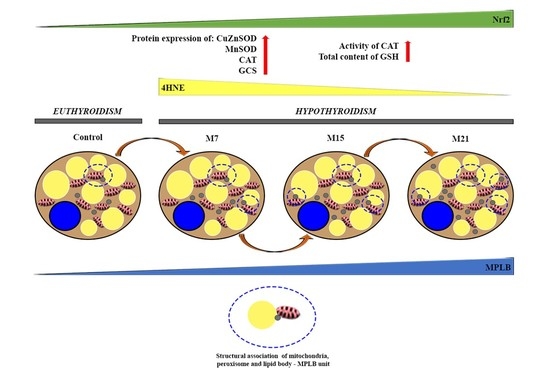
The Unity of Redox and Structural Remodeling of Brown Adipose Tissue in Hypothyroidism
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Experimental Design
2.2. Western Blotting
2.3. The activity of Antioxidant Defense Enzymes
2.4. Transmission Electron Microscopy & Immunogold Labeling
2.5. Immunofluorescence
2.6. Light Microscopy and Immunohistochemistry
2.7. Statistics
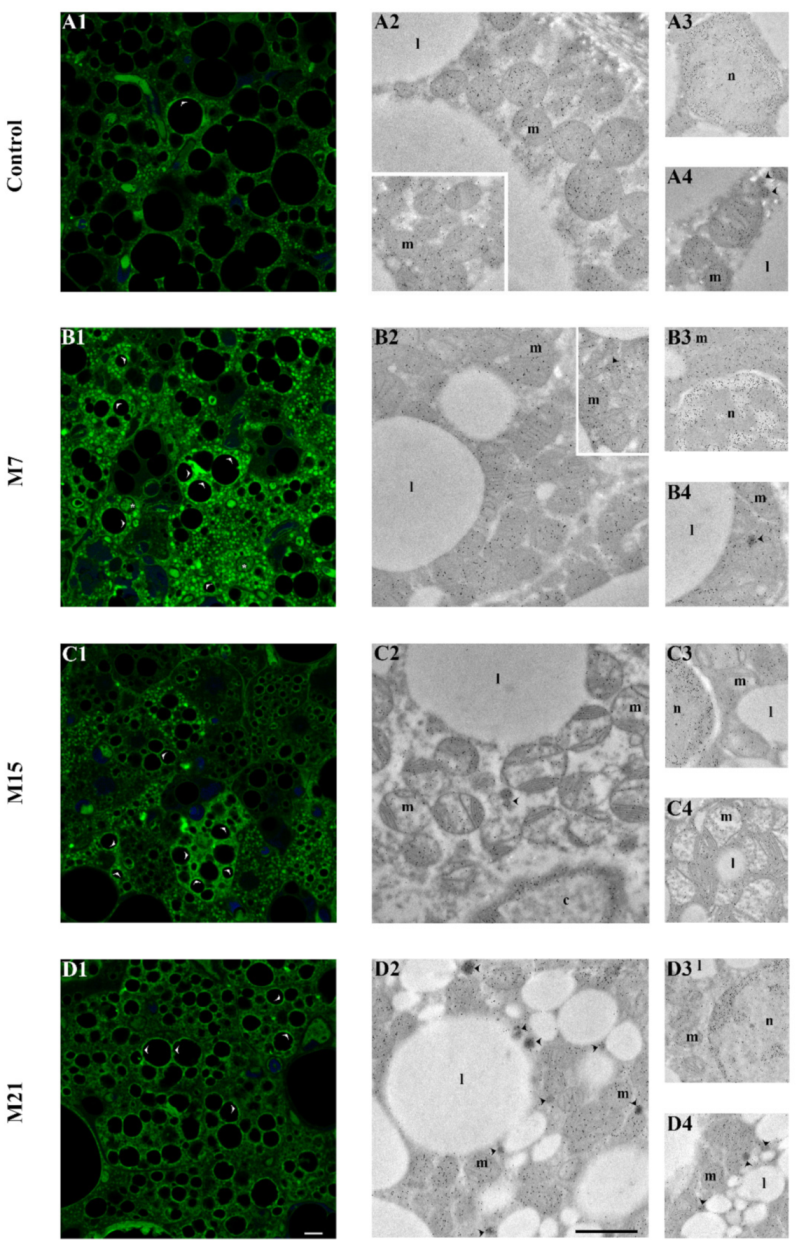
3. Results
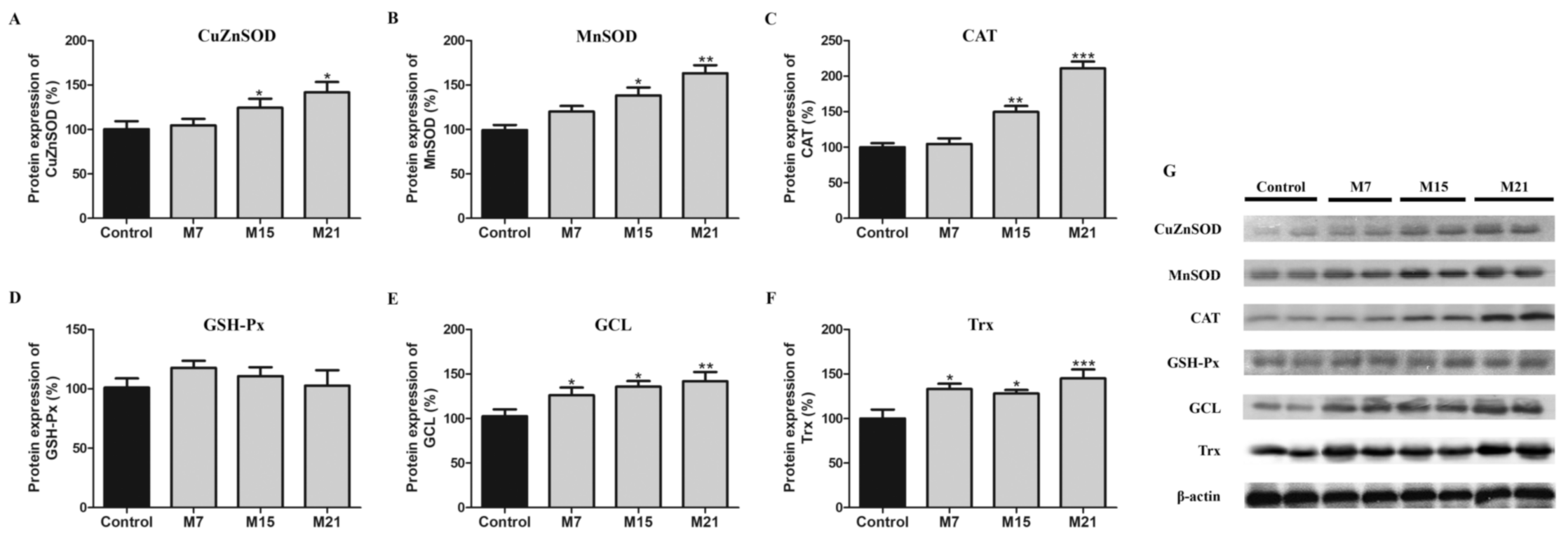
3.1. Hypothyroidism-Related Changes in Protein Expression of CuZnSOD, MnSOD, CAT, GSH-Px, GCL, and Trx
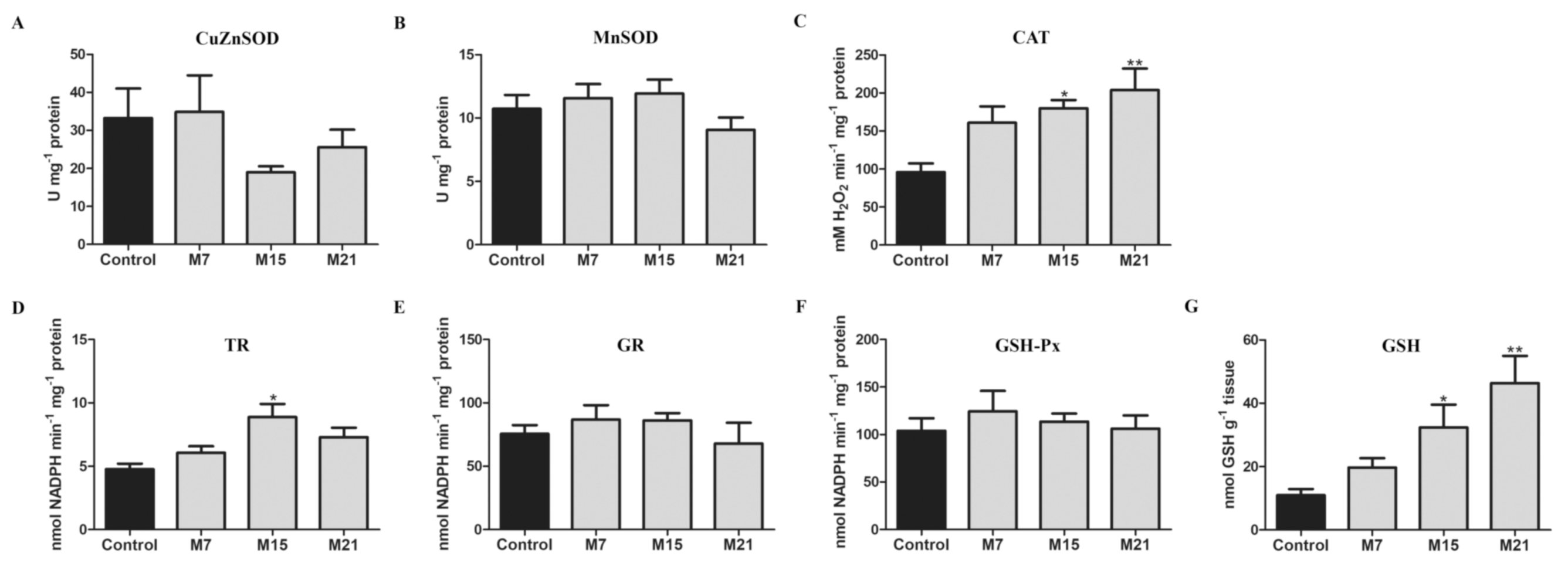
3.2. Hypothyroidism-Related Changes in Enzyme Activity of CuZnSOD, MnSOD, CAT, TR, GR, GSH-Px, and Content of GSH
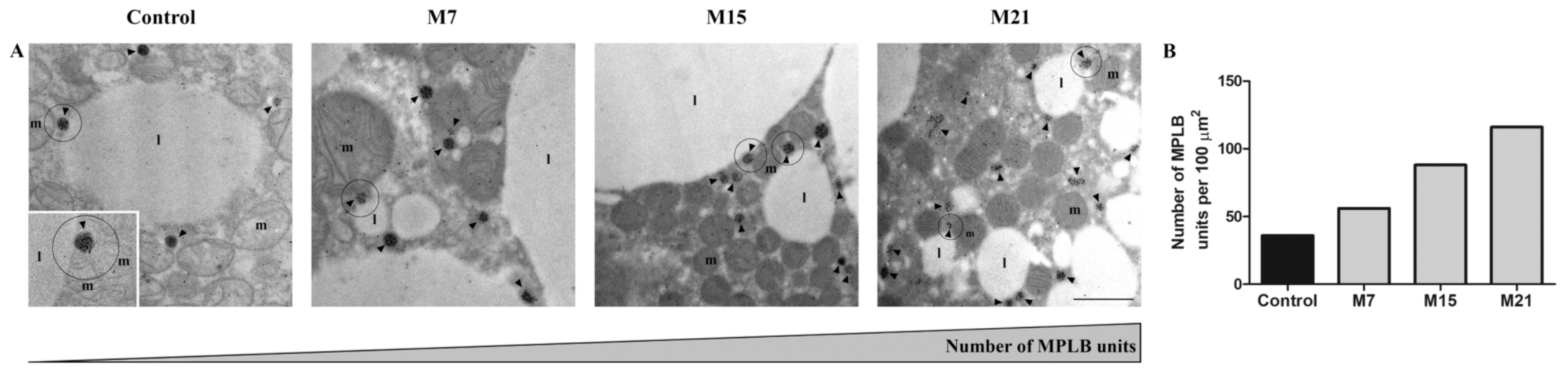
3.3. Changes in Subcellular Distribution of CuZnSOD, MnSOD, CAT, and GSH-Px during Hypothyroidism
3.4. Protein Expression of Nrf2 in BAT during Hypothyroidism
3.5. Tissue and Cell Distribution Patterns of 4-HNE-Modified Proteins during Hypothyroidism
4. Discussion
4.1. Hypothyroidism Induces Antioxidant Defense in BAT
4.2. Hypothyroidism Induces the Creation of Specific Organellar Units
4.3. Hypothyroidism-Induced Newly Established Localization of AD Enzymes
4.4. Transcriptional Control of Brown Adipose Tissue Redox Remodeling Involves Nrf2
4.5. Possible Mechanism of Redox-Structural Remodeling in Brown Adipose Tissue during Hypothyroidism through Secondary Messengers of Redox Signaling
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chiovato, L.; Magri, F.; Carlé, A. Hypothyroidism in Context: Where We’ve Been and Where We’re Going. Adv. Ther. 2019, 36, 47–58. [Google Scholar] [CrossRef]
- Brenta, G. Why Can Insulin Resistance Be a Natural Consequence of Thyroid Dysfunction? J. Thyroid Res. 2011, 2011, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Chung, G.E.; Kim, D.; Kim, W.; Yim, J.Y.; Park, M.J.; Kim, Y.J.; Yoon, J.H.; Lee, H.S. Non-alcoholic fatty liver disease across the spectrum of hypothyroidism. J. Hepatol. 2012, 57, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Sinha, R.A.; Singh, B.K.; Yen, P.M. Thyroid hormone regulation of hepatic lipid and carbohydrate metabolism. Trends Endocrinol. Metab. 2014, 25, 538–545. [Google Scholar] [CrossRef] [PubMed]
- Martinez, B.; Ortiz, R.M. Thyroid hormone regulation and insulin resistance: Insights from animals naturally adapted to fasting. Physiology 2017, 32, 141–151. [Google Scholar] [CrossRef]
- Halliwell, B. Biochemistry of oxidative stress. Biochem. Soc. Trans. 2007, 35, 1147–1150. [Google Scholar] [CrossRef]
- Roberts, C.K.; Sindhu, K.K. Oxidative stress and metabolic syndrome. Life Sci. 2009, 84, 705–712. [Google Scholar] [CrossRef]
- Xing, M. Oxidative stress: A new risk factor for thyroid cancer. Endocr. Relat. Cancer 2012, 19, 1–7. [Google Scholar] [CrossRef]
- Bullon, P.; Newman, H.N.; Battino, M. Obesity, diabetes mellitus, atherosclerosis and chronic periodontitis: A shared pathology via oxidative stress and mitochondrial dysfunction? Periodontol. 2000 2014, 64, 139–153. [Google Scholar] [CrossRef]
- Palipoch, S.; Koomhin, P. Oxidative Stress-Associated Pathology: A Review. Sains Malays. 2015, 44, 1441–1451. [Google Scholar] [CrossRef]
- Mancini, A.; Di Segni, C.; Raimondo, S.; Olivieri, G.; Silvestrini, A.; Meucci, E.; Currò, D. Thyroid Hormones, Oxidative Stress, and Inflammation. Mediat. Inflamm. 2016, 2016, 1–12. [Google Scholar] [CrossRef]
- Rani, V.; Deep, G.; Singh, R.K.; Palle, K.; Yadav, U.C.S. Oxidative stress and metabolic disorders: Pathogenesis and therapeutic strategies. Life Sci. 2016, 148, 183–193. [Google Scholar] [CrossRef]
- Halliwell, B. Cellular Stress and Protection Mechanisms. Biochem. Soc. Trans. 1996, 24, 1023–1027. [Google Scholar] [CrossRef]
- Sun, Y.; Oberley, L.W. Redox regulation of transcriptional activators. Free Radic. Biol. Med. 1996, 21, 335–348. [Google Scholar] [CrossRef]
- Lee, J.M.; Calkins, M.J.; Chan, K.; Kan, Y.W.; Johnson, J.A. Identification of the NF-E2-related factor-2-dependent genes conferring protection against oxidative stress in primary cortical astrocytes using oligonucleotide microarray analysis. J. Biol. Chem. 2003, 278, 12029–12038. [Google Scholar] [CrossRef]
- Suh, J.H.; Shenvi, S.V.; Dixon, B.M.; Liu, H.; Jaiswal, A.K.; Liu, R.M.; Hagen, T.M. Decline in transcriptional activity of Nrf2 causes age-related loss of glutathione synthesis, which is reversible with lipoic acid. Proc. Natl. Acad. Sci. USA 2004, 101, 3381–3386. [Google Scholar] [CrossRef]
- Ryoo, I.; Kwak, M.K. Regulatory crosstalk between the oxidative stress-related transcription factor Nfe2l2/Nrf2 and mitochondria. Toxicol. Appl. Pharmacol. 2018, 359, 24–33. [Google Scholar] [CrossRef]
- Schmidlin, C.J.; Dodson, M.B.; Madhavan, L.; Zhang, D.D. Redox regulation by NRF2 in aging and disease. Free Radic. Biol. Med. 2019, 134, 702–707. [Google Scholar] [CrossRef]
- Dalle-Donne, I.; Scaloni, A.; Giustarini, D.; Cavarra, E.; Tell, G.; Lungarella, G.; Colombo, R.; Rossi, R.; Milzani, A. Proteins as biomarkers of oxidattiv/e/nitrosative stress in diseases: The contribution of redox proteomics. Mass Spectrom. Rev. 2003, 24, 55–99. [Google Scholar] [CrossRef]
- Margaritelis, N.V.; Cobley, J.N.; Paschalis, V.; Veskoukis, A.S.; Theodorou, A.A.; Kyparos, A.; Nikolaidis, M.G. Going retro: Oxidative stress biomarkers in modern redox biology. Free Radic. Biol. Med. 2016, 98, 2–12. [Google Scholar] [CrossRef]
- Korac, B.; Kalezic, A.; Pekovic-Vaughan, V.; Korac, A. Redox changes in obesity, metabolic syndrome, and diabetes. Redox Biol. 2021, 101887. [Google Scholar] [CrossRef] [PubMed]
- Cannon, B.; Nedergaard, J. Brown adipose tissue: Function and physiological significance. Physiol. Rev. 2004, 84, 277–359. [Google Scholar] [CrossRef] [PubMed]
- Ghorbani, M.; Himms-Hagen, J. Appearance of brown adipocytes in white adipose tissue during CL 316,243-induced reversal of obesity and diabetes in Zucker fa/fa rats. Int. J. Obes. 1997, 21, 465–475. [Google Scholar] [CrossRef] [PubMed]
- Rothwell, N.J.; Stock, M.J. A role for brown adipose tissue in diet-induced thermogenesis. Obes. Res. 1997, 5, 650–656. [Google Scholar] [CrossRef]
- Nedergaard, J.; Bengtsson, T.; Cannon, B. Unexpected evidence for active brown adipose tissue in adult humans. Am. J. Physiol. Metab. 2007, 293, E444–E452. [Google Scholar] [CrossRef]
- Carey, A.L.; Kingwell, B.A. Brown adipose tissue in humans: Therapeutic potential to combat obesity. Pharmacol. Ther. 2013, 140, 26–33. [Google Scholar] [CrossRef]
- Saito, M. Human brown adipose tissue: Regulation and anti-obesity potential. Endocr. J. 2014, 61, 409–416. [Google Scholar] [CrossRef]
- Mory, G.; Ricquier, D.; Pesquiés, P.; Hémon, P. Effects of hypothyroidism on the brown adipose tissue of adult rats: Comparison with the effects of adaptation to cold. J. Endocrinol. 1981, 91, 515–524. [Google Scholar] [CrossRef]
- Rubio, A.; Raasmaja, A.; Silva, J.E. Thyroid hormone and norepinephrine signaling in brown adipose tissue. II: Differential effects of thyroid hormone on b3-adrenergic receptors in brown and white adipose tissue. Endocrinology 1995, 136, 3277–3284. [Google Scholar] [CrossRef]
- Silva, J.E. Thyroid Hormone Control of Thermogenesis and Energy Balance. Thyroid 1995, 5, 481–492. [Google Scholar] [CrossRef]
- Bianco, A.C.; McAninch, E.A. The role of thyroid hormone and brown adipose tissue in energy homoeostasis. Lancet Diabetes Endocrinol. 2013, 1, 250–258. [Google Scholar] [CrossRef]
- Hsieh, A.C.L.; Carlson, L.D. Role of the Thyroid in Metabolic Low Temperature. Am. J. Physiol. Leg. Content 1956, 188, 40–44. [Google Scholar] [CrossRef]
- Abelenda, M.; Puerta, M.L. Cold-induced thermogenesis in hypothyroid rats. Pflügers Arch. Eur. J. Physiol. 1990, 416, 663–666. [Google Scholar] [CrossRef]
- Zaninovich, A.A.; Raíces, M.; Rebagliati, I.; Ricci, C.; Hagmüller, K. Brown fat thermogenesis in cold-acclimated rats is not abolished by the suppression of thyroid function. Am. J. Physiol. Endocrinol. Metab. 2002, 283, 496–502. [Google Scholar] [CrossRef][Green Version]
- Laurberg, P.; Andersen, S.; Karmisholt, J. Cold Adaptation and Thyroid Hormone Metabolism. Horm 2005, 37, 545–549. [Google Scholar] [CrossRef]
- Petrović, V.; Korać, A.; Buzadžić, B.; Korać, B. The effects of L-arginine and L-NAME supplementation on redox-regulation and thermogenesis in interscapular brown adipose tissue. J. Exp. Biol. 2005, 208, 4263–4271. [Google Scholar] [CrossRef]
- Petrović, V.; Buzadžić, B.; Korać, A.; Vasilijević, A.; Janković, A.; Korać, B. Free radical equilibrium in interscapular brown adipose tissue: Relationship between metabolic profile and antioxidative defense. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2006, 142, 60–65. [Google Scholar] [CrossRef]
- Petrović, V.; Buzadžić, B.; Korać, A.; Korać, B. Antioxidative defense and mitochondrial thermogenic response in brown adipose tissue. Genes Nutr. 2010, 5, 225–235. [Google Scholar] [CrossRef][Green Version]
- Lettieri-Barbato, D. Redox control of non-shivering thermogenesis. Mol. Metab. 2019, 25, 11–19. [Google Scholar] [CrossRef]
- Suter, E.R. The fine structure of brown adipose tissue. I. Cold-induced changes in the rat. J. Ultrasructure Res. 1969, 26, 216–241. [Google Scholar] [CrossRef]
- Korac, A.; Buzadzic, B.; Petrovic, V.; Vasilijevic, A.; Jankovic, A.; Micunovic, K.; Korac, B. The role of nitric oxide in remodeling of capillary network in rat interscapular brown adipose tissue after long-term cold acclimation. Histol. Histopathol. 2008, 23, 441–450. [Google Scholar] [CrossRef] [PubMed]
- Petrović, V.; Korać, A.; Buzadžić, B.; Vasilijević, A.; Janković, A.; Mićunović, K.; Korać, B. Nitric oxide regulates mitochondrial re-modelling in interscapular brown adipose tissue: Ultrastructural and morphometric-stereologic studies. J. Microsc. 2008, 232, 542–548. [Google Scholar] [CrossRef] [PubMed]
- Petrović, V.; Buzadžić, B.; Korać, A.; Vasilijević, A.; Janković, A.; Korać, B. NO modulates the molecular basis of rat interscapular brown adipose tissue thermogenesis. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2010, 152, 147–159. [Google Scholar] [CrossRef] [PubMed]
- Dicker, A.; Raasmaja, A.; Cannon, B.; Nedergaard, J. Increased α1-adrenoceptor density in brown adipose tissue indicates recruitment drive in hypothyroid rats. Am. J. Physiol. Endocrinol. Metab. 1992, 263. [Google Scholar] [CrossRef] [PubMed]
- Lapa, C.; Maya, Y.; Wagner, M.; Arias-Loza, P.; Werner, R.A.; Herrmann, K.; Higuchi, T. Activation of brown adipose tissue in hypothyroidism. Ann. Med. 2015, 47, 538–545. [Google Scholar] [CrossRef]
- Petrović, N.; Cvijić, G.; Davidović, V. The activity of antioxidant enzymes and the content of uncoupling protein-1 in the brown adipose tissue of hypothyroid rats: Comparison with effects of iopanoic acid. Physiol. Res. 2001, 50, 289–297. [Google Scholar]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the folin phenol reagent. Anal. Biochem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Misra, H.P.; Fridovich, I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J. Biol. Chem. 1972, 247, 3170–3175. [Google Scholar] [CrossRef]
- Luthman, M.; Holmgren, A. Rat Liver Thioredoxin and Thioredoxin Reductase: Purification and Characterization. Biochemistry 1982, 21, 6628–6633. [Google Scholar] [CrossRef]
- Glatzle, D.; Vuilleumier, J.P.; Weber, F.; Decker, K. Glutathione reductase test with whole blood, a convenient procedure for the assessment of the riboflavin status in humans. Experientia 1974, 30, 665–667. [Google Scholar] [CrossRef]
- Paglia, D.E.; Valentine, W.N. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J. Lab. Clin. Med. 1967, 70, 158–169. [Google Scholar]
- Griffith, O.W. Determination of Glutathione and Glutathione Disulfide Using Glutathione Reductase and 2-vinylpyridine. Anal. Biochem. 1980, 106, 207–212. [Google Scholar] [CrossRef]
- Mano, T.; Sinohara, R.; Sawai, Y.; Oda, N.; Nishida, Y.; Mokuno, T.; Asano, K.; Ito, Y.; Kotake, M.; Hamada, M.; et al. Changes in lipid peroxidation and free radical scavengers in the brain of hyper- and hypothyroid aged rats. J. Endocrinol. 1995, 147, 361–365. [Google Scholar] [CrossRef]
- Yilmaz, S.; Ozan, S.; Benzer, F.; Canatan, H. Oxidative damage and antioxidant enzyme activities in experimental hypothyroidism. Cell Biochem. Funct. 2003, 21, 325–330. [Google Scholar] [CrossRef]
- Santi, A.; Duarte, M.M.M.F.; Moresco, R.N.; Menezes, C.; Bagatini, M.D.; Schetinger, M.R.C.; Loro, V.L. Association between thyroid hormones, lipids and oxidative stress biomarkers in overt hypothyroidism. Clin. Chem. Lab. Med. 2010, 48, 1635–1639. [Google Scholar] [CrossRef]
- Santi, A.; Duarte, M.M.M.F.; De Menezes, C.C.; Loro, V.L. Association of lipids with oxidative stress biomarkers in subclinical hypothyroidism. Int. J. Endocrinol. 2012, 2012. [Google Scholar] [CrossRef]
- Reddy, V.S.; Gouroju, S.; Suchitra, M.M.; Suresh, V.; Sachan, A.; Srinivasa Rao, P.V.L.N.; Bitla, A.R. Antioxidant defense in overt and subclinical hypothyroidism. Horm. Metab. Res. 2013, 45, 754–758. [Google Scholar] [CrossRef]
- Baskol, G.; Atmaca, H.; Tanriverdi, F.; Baskol, M.; Kocer, D.; Bayram, F. Oxidative stress and enzymatic antioxidant status in patients with hypothyroidism before and after treatment. Exp. Clin. Endocrinol. Diabetes 2007, 115, 522–526. [Google Scholar] [CrossRef]
- Dave, B.N.; Paradkar, N.M. Total superoxide dismutase, Cu/Zn superoxide dismutase and glutathione peroxidase in untreated hyperthyroidism and hypothyroidism. JK Sci. 2009, 11, 6–10. [Google Scholar]
- Pereira, B.; Fernando, L.; Costa, R.B.; Safi, D.A.; Bechara, E.J.; Curi, R. Hormonal regulation of superoxide dismutase, catalase, and glutathione peroxidase activities in rat macrophages. Biochem. Pharmacol. 1995, 50, 2093–2098. [Google Scholar] [CrossRef]
- Pereira, B.; Rosa, L.F.; Safi, D.A.; Bechara, E.J.; Curi, R. Control of superoxide dismutase, catalase and glutathione peroxidase activities in rat lymphoid organs by thyroid hormones. J. Endocrinol. 1994, 140, 73–77. [Google Scholar] [CrossRef]
- Cano-Europa, E.; Blas-Valdivia, V.; Lopez-Galindo, G.E.; Franco-Colin, M.; Pineda-Reynoso, M.; Hernandez-Garcia, A.; Ortiz-Butron, R. Methimazole-Induced Hypothyroidism Causes Alterationof The REDOX Environment, Oxidative Stress, Andhepatic Damage; Events Not Caused By Hypothyroidism Itself. Ann. Hepatol. 2010, 9, 80–88. [Google Scholar] [CrossRef]
- De Souza Cardoso, J.; Baldissarelli, J.; Reichert, K.P.; Teixeira, F.C.; Pereira Soares, M.S.; Chitolina Schetinger, M.R.; Morsch, V.M.; Farias Martins Filho, A.O.; Duarte Junior, H.R.; Ribeiro Coriolano, F.H.; et al. Neuroprotection elicited by resveratrol in a rat model of hypothyroidism: Possible involvement of cholinergic signaling and redox status. Mol. Cell. Endocrinol. 2021, 524, 1–8. [Google Scholar] [CrossRef]
- Nedergaard, J.; Alexson, S.; Cannon, B. Cold adaptation in the rat: Increased brown fat peroxisomal β-oxidation relative to maximal mitochondrial oxidative capacity. Am. J. Physiol. Cell Physiol. 1980, 8. [Google Scholar] [CrossRef]
- Barja de Quiroga, G.; Lopez-Torres, M.; Perez-Campo, R.; Abelenda, M.; Paz Nava, M.; Puerta, M.L. Effect of cold acclimation on GSH, antioxidant enzymes and lipid peroxidation in brown adipose tissue. Biochem. J. 1991, 277, 289–292. [Google Scholar] [CrossRef]
- Fransen, M.; Nordgren, M.; Wang, B.; Apanasets, O. Role of peroxisomes in ROS/RNS-metabolism: Implications for human disease. Biochim. Biophys. Acta Mol. Basis Dis. 2012, 1822, 1363–1373. [Google Scholar] [CrossRef]
- Lismont, C.; Nordgren, M.; Van Veldhoven, P.P.; Fransen, M. Redox interplay between mitochondria and peroxisomes. Front. Cell Dev. Biol. 2015, 3. [Google Scholar] [CrossRef]
- Wanders, R.J.A. Metabolic functions of peroxisomes in health and disease. Biochimie 2014, 98, 36–44. [Google Scholar] [CrossRef]
- Park, H.; He, A.; Min, T.; Johnson, M.J.; Dean, M.J.; Pietka, T.A.; Chen, Y.; Zhang, X.; Hsu, F.-F.; Razani, B.; et al. Peroxisome-derived lipids regulate adipose thermogenesis by mediating cold-induced mitochondrial fission. J. Clin. Investig. 2018, 129, 694–711. [Google Scholar] [CrossRef]
- Schrader, M. Tubulo—Reticular Clusters of Peroxisomes in Living COS-7 Cells: Dynamic Behavior and Association with Lipid Droplets. J. Histochem. Cytochem. 2001, 49, 1421–1429. [Google Scholar] [CrossRef]
- Binns, D.; Januszewski, T.; Chen, Y.; Hill, J.; Markin, V.S.; Zhao, Y.; Gilpin, C.; Chapman, K.D.; Anderson, R.G.W.; Goodman, J.M. An intimate collaboration between peroxisomes and lipid bodies. J. Cell Biol. 2006, 173, 719–731. [Google Scholar] [CrossRef] [PubMed]
- Kunze, M.; Pracharoenwattana, I.; Smith, S.M.; Hartig, A. A central role for the peroxisomal membrane in glyoxylate cycle function. Biochim. Biophys. Acta 2006, 1763, 1441–1452. [Google Scholar] [CrossRef] [PubMed]
- Bartz, R.; Li, W.; Venables, B.; Zehmer, J.K.; Roth, M.R.; Welti, R.; Anderson, R.G.W.; Liu, P.; Chapman, K.D. Lipidomics reveals that adiposomes store ether lipids and mediate phospholipid traffic 1. J. Lipid Res. 2007, 48. [Google Scholar] [CrossRef]
- Lodhi, I.J.; Semenkovich, C.F. Peroxisomes: A nexus for lipid metabolism and cellular signaling. Cell Metab. 2014, 19, 380–392. [Google Scholar] [CrossRef] [PubMed]
- Olzmann, J.A.; Carvalho, P. Dynamics and functions of lipid droplets. Nat. Rev. Mol. Cell Biol. 2018, 20, 137–155. [Google Scholar] [CrossRef] [PubMed]
- Giacobino, J.P.; Moinat, M.; Muzzin, P.; Siegrist-Kaiser, C.A.; Seydoux, J.; Girardier, L. Peroxisomal oxidative capacity of brown adipose tissue depends on the thyroid status. Mol. Cell. Endocrinol. 1989, 61, 217–225. [Google Scholar] [CrossRef]
- Girnun, G.D.; Domann, F.E.; Moore, S.A.; Robbins, M.E.C. Identification of a functional peroxisome proliferator-activated receptor response element in the rat catalase promoter. Mol. Endocrinol. 2002, 16, 2793–2801. [Google Scholar] [CrossRef]
- Milani, P.; Gagliardi, S.; Cova, E.; Cereda, C. SOD1 transcriptional and posttranscriptional regulation and its potential implications in ALS. Neurol. Res. Int. 2011, 2011, 1–9. [Google Scholar] [CrossRef]
- Kim, Y.S.; Vallur, P.G.; Phaëton, R.; Mythreye, K.; Hempel, N. Insights into the dichotomous regulation of SOD2 in cancer. Antioxidants 2017, 6, 86. [Google Scholar] [CrossRef]
- Tonelli, C.; Chio, I.I.C.; Tuveson, D.A. Transcriptional Regulation by Nrf2. Antioxid. Redox Signal. 2018, 29, 1727–1745. [Google Scholar] [CrossRef]
- Rani, N.; Arya, D.S. Chrysin rescues rat myocardium from ischemia-reperfusion injury via PPAR-γ/Nrf2 activation. Eur. J. Pharmacol. 2020, 883, 1–31. [Google Scholar] [CrossRef]
- Piantadosi, C.A.; Carraway, M.S.; Babiker, A.; Suliman, H.B. Heme oxygenase-1 regulates cardiac mitochondrial biogenesis via nrf2-mediated transcriptional control of nuclear respiratory factor-1. Circ. Res. 2008, 103, 1232–1240. [Google Scholar] [CrossRef]
- Shen, W.; Liu, K.; Tian, C.; Yang, L.; Li, X.; Ren, J.; Packer, L.; Cotman, C.W.; Liu, J. R-α-Lipoic acid and acetyl-L-carnitine complementarily promote mitochondrial biogenesis in murine 3T3-L1 adipocytes. Diabetologia 2008, 51, 165–174. [Google Scholar] [CrossRef]
- Holmström, K.M.; Baird, L.; Zhang, Y.; Hargreaves, I.; Chalasani, A.; Land, J.M.; Stanyer, L.; Yamamoto, M.; Dinkova-Kostova, A.T.; Abramov, A.Y. Nrf2 impacts cellular bioenergetics by controlling substrate availability for mitochondrial respiration. Biol. Open 2013, 2, 761–770. [Google Scholar] [CrossRef]
- Ichimura, Y.; Waguri, S.; Sou, Y.-s.; Kageyama, S.; Hasegawa, J.; Ishimura, R.; Saito, T.; Yang, Y.; Kouno, T.; Fukutomi, T.; et al. Phosphorylation of p62 Activates the Keap1-Nrf2 Pathway during Selective Autophagy. Mol. Cell 2013, 51, 618–631. [Google Scholar] [CrossRef]
- Kovac, S.; Angelova, P.R.; Holmström, K.M.; Zhang, Y.; Dinkova-Kostova, A.T.; Abramov, A.Y. Nrf2 regulates ROS production by mitochondria and NADPH oxidase. Biochim. Biophys. Acta Gen. Subj. 2014, 1850, 794–801. [Google Scholar] [CrossRef]
- Ludtmann, M.H.R.; Angelova, P.R.; Zhang, Y.; Abramov, A.Y.; Dinkova-Kostova, A.T. Nrf2 affects the efficiency of mitochondrial fatty acid oxidation. Biochem. J. 2014, 457, 415–424. [Google Scholar] [CrossRef]
- Holmström, K.M.; Kostov, R.V.; Dinkova-Kostova, A.T. The multifaceted role of Nrf2 in mitochondrial function. Curr. Opin. Toxicol. 2016, 2, 80–91. [Google Scholar] [CrossRef]
- Merry, T.L.; Ristow, M. Nuclear factor erythroid-derived 2-like 2 (NFE2L2, Nrf2) mediates exercise-induced mitochondrial biogenesis and the anti-oxidant response in mice. J. Physiol. 2016, 594, 5195–5207. [Google Scholar] [CrossRef]
- Lo, S.C.; Hannink, M. PGAM5 tethers a ternary complex containing Keap1 and Nrf2 to mitochondria. Exp. Cell Res. 2008, 314, 1789–1803. [Google Scholar] [CrossRef]
- Strom, J.; Xu, B.; Tian, X.; Chen, Q.M. Nrf2 protects mitochondrial decay by oxidative stress. FASEB J. 2016, 30, 66–80. [Google Scholar] [CrossRef] [PubMed]
- Tsushima, M.; Liu, J.; Hirao, W.; Yamazaki, H.; Tomita, H.; Itoh, K. Emerging evidence for crosstalk between Nrf2 and mitochondria in physiological homeostasis and in heart disease. Arch. Pharm. Res. 2019, 43, 286–296. [Google Scholar] [CrossRef] [PubMed]
- Ayala, A.; Muñoz, M.F.; Argüelles, S. Lipid peroxidation: Production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid. Med. Cell. Longev. 2014, 1–31. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Sano, M.; Shinmura, K.; Tamaki, K.; Katsumata, Y.; Matsuhashi, T.; Morizane, S.; Ito, H.; Hishiki, T.; Endo, J.; et al. 4-Hydroxy-2-nonenal protects against cardiac ischemia-reperfusion injury via the Nrf2-dependent pathway. J. Mol. Cell. Cardiol. 2010, 49, 576–586. [Google Scholar] [CrossRef]
- Huang, Y.; Li, W.; Kong, A.N.T. Anti-oxidative stress regulator NF-E2-related factor 2 mediates the adaptive induction of antioxidant and detoxifying enzymes by lipid peroxidation metabolite 4-hydroxynonenal. Cell Biosci. 2012, 2, 40. [Google Scholar] [CrossRef]
- Chapple, S.J.; Cheng, X.; Mann, G.E. Effects of 4-hydroxynonenal on vascular endothelial and smooth muscle cell redox signaling and function in health and disease. Redox Biol. 2013, 1, 319–331. [Google Scholar] [CrossRef]
- López-Bernardo, E.; Anedda, A.; Sánchez-Pérez, P.; Acosta-Iborra, B.; Cadenas, S. 4-Hydroxynonenal induces Nrf2-mediated UCP3 upregulation in mouse cardiomyocytes. Free Radic. Biol. Med. 2015, 88, 427–438. [Google Scholar] [CrossRef]
- Castro, J.P.; Jung, T.; Grune, T.; Siems, W. 4-Hydroxynonenal (HNE) modified proteins in metabolic diseases. Free Radic. Biol. Med. 2017, 111, 309–315. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aleksic, M.; Kalezic, A.; Saso, L.; Jankovic, A.; Korac, B.; Korac, A. The Unity of Redox and Structural Remodeling of Brown Adipose Tissue in Hypothyroidism. Antioxidants 2021, 10, 591. https://doi.org/10.3390/antiox10040591
Aleksic M, Kalezic A, Saso L, Jankovic A, Korac B, Korac A. The Unity of Redox and Structural Remodeling of Brown Adipose Tissue in Hypothyroidism. Antioxidants. 2021; 10(4):591. https://doi.org/10.3390/antiox10040591
Chicago/Turabian StyleAleksic, Marija, Andjelika Kalezic, Luciano Saso, Aleksandra Jankovic, Bato Korac, and Aleksandra Korac. 2021. "The Unity of Redox and Structural Remodeling of Brown Adipose Tissue in Hypothyroidism" Antioxidants 10, no. 4: 591. https://doi.org/10.3390/antiox10040591
APA StyleAleksic, M., Kalezic, A., Saso, L., Jankovic, A., Korac, B., & Korac, A. (2021). The Unity of Redox and Structural Remodeling of Brown Adipose Tissue in Hypothyroidism. Antioxidants, 10(4), 591. https://doi.org/10.3390/antiox10040591