Linking the Dynamic Changes in the In Vitro Antioxidant Activity of Carob Kibbles upon Roasting to the Chemical and Structural Changes Revealed by FTIR Spectroscopy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Standards
2.2. Plant Material and Roasting
2.3. Extraction Method
2.4. Determination of Total Polyphenolic Content
2.5. Determination of Proanthocyanidin Content
2.6. Determination of the Radical Scavenging Activity on DPPH
2.7. High Performance Liquid Chromatography (HPLC) Analysis
2.8. ATR-FTIR Spectroscopy
2.9. Statistical Analysis
3. Results and Discussion
3.1. Polyphenolic Content and Antioxidant Activity
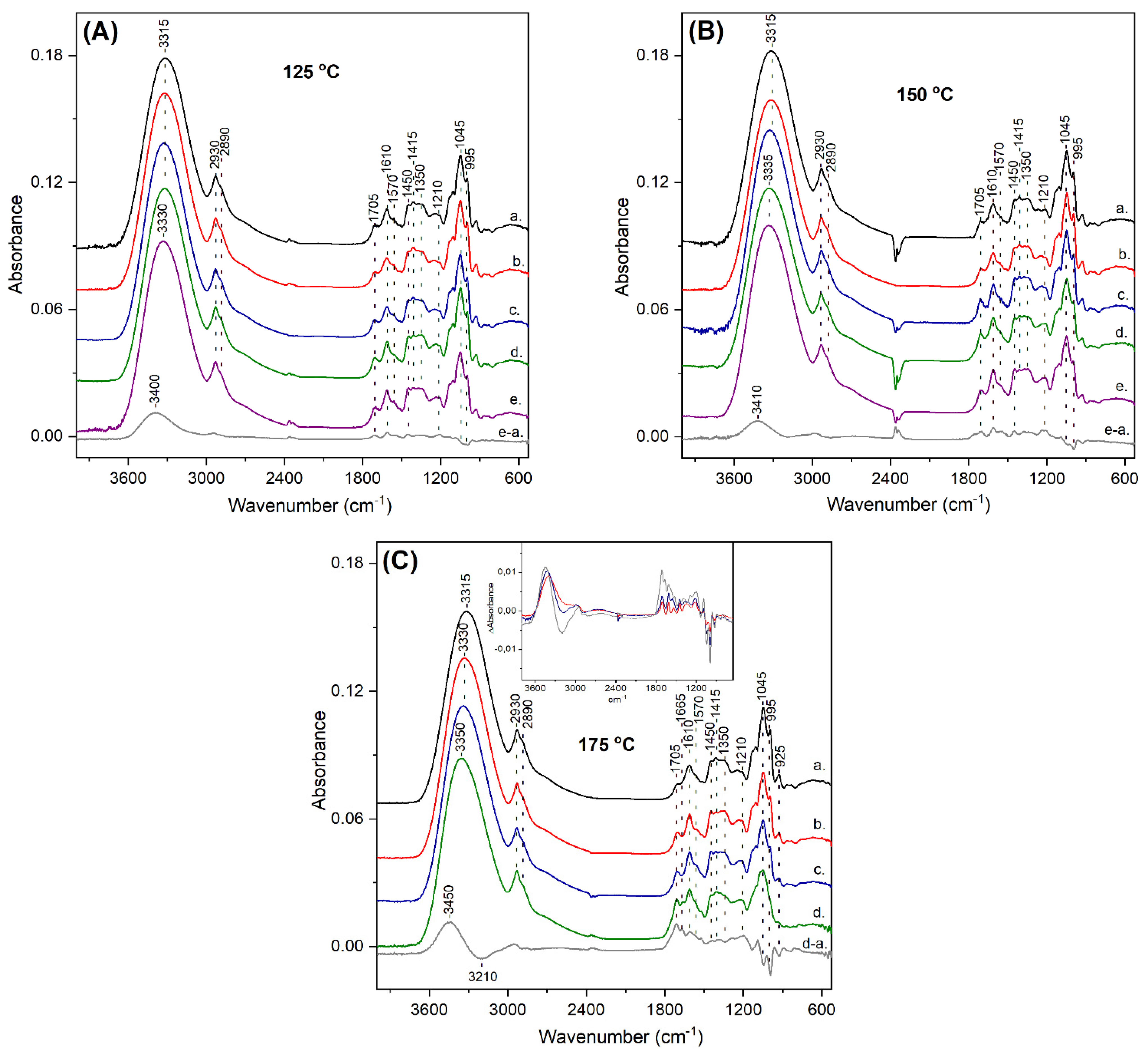
3.2. ATR-FTIR Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tous, J.; Romero, A.; Batlle, I. The Carob Tree: Botany, Horticulture, and Genetic Resources. In Horticultural Reviews; Janik, J., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2014; Volume 41, pp. 385–456. [Google Scholar]
- Loullis, A.; Pinakoulaki, E. Carob as cocoa substitute: A review on composition, health benefits and food applications. Eur. Food Res. Technol. 2018, 244, 959–977. [Google Scholar] [CrossRef]
- Papaefstathiou, E.; Agapiou, A.; Giannopoulos, S.; Kokkinofta, R. Nutritional characterization of carobs and traditional carob products. Food Sci. Nutr. 2018, 6, 2151–2161. [Google Scholar] [CrossRef]
- Barak, S.; Mudgil, D. Locust bean gum: Processing, properties and food applications-A review. Int. J. Biol. Macromol. 2014, 66, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Nasar-Abbas, S.M.; e-Huma, Z.; Vu, T.H.; Khan, M.K.; Esbenshade, H.; Jayasena, V. Carob Kibble: A Bioactive-Rich Food Ingredient. Compr. Rev. Food Sci. Food Saf. 2016, 15, 63–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodríguez-Solana, R.; Romano, A.; Moreno-Rojas, J.M. Carob Pulp: A Nutritional and Functional By-Product. Processes 2021, 9, 1146. [Google Scholar] [CrossRef]
- Brassesco, M.E.; Brandão, T.R.S.; Silva, C.L.M.; Pintado, M. Carob bean (Ceratonia siliqua L.): A new perspective for functional food. Trends Food Sci. Technol. 2021, 114, 310–322. [Google Scholar] [CrossRef]
- Farag, M.A.; El-Kersh, D.M.; Ehrlich, A.; Choucry, M.A.; El-Seedi, H.; Frolov, A.; Wessjohann, L.A. Variation in Ceratonia siliqua pod metabolome in context of its different geographical origin, ripening stage and roasting process. Food Chem. 2019, 283, 675–687. [Google Scholar] [CrossRef]
- Kyriacou, M.C.; Antoniou, C.; Rouphael, Y.; Graziani, G.; Kyratzis, A. Mapping the primary and secondary metabolomes of carob (Ceratonia siliqua L.) fruit and its postharvest antioxidant potential at critical stages of ripening. Antioxidants 2021, 10, 57. [Google Scholar] [CrossRef]
- Yousif, A.K.; Alghzawi, H.M. Processing and characterization of carob powder. Food Chem. 2000, 69, 283–287. [Google Scholar] [CrossRef]
- Şahin, H.; Topuz, A.; Pischetsrieder, M.; Özdemir, F. Effect of roasting process on phenolic, antioxidant and browning properties of carob powder. Eur. Food Res. Technol. 2009, 230, 155–161. [Google Scholar] [CrossRef]
- Vitali Čepo, D.; Mornar, A.; Nigović, B.; Kremer, D.; Radanović, D.; Vedrina Dragojević, I. Optimization of roasting conditions as an useful approach for increasing antioxidant activity of carob powder. LWT Food Sci. Technol. 2014, 58, 578–586. [Google Scholar] [CrossRef]
- Gunel, Z.; Tontul, İ.; Dincer, C.; Topuz, A.; Sahin-Nadeem, H. Influence of microwave, the combined microwave/hot air and only hot air roasting on the formation of heat-induced contaminants of carob powders. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2018, 35, 2332–2339. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Solana, R.; Salgado, J.M.; Pérez-Santín, E.; Romano, A. Effect of carob variety and roasting on the antioxidant capacity, and the phenolic and furanic contents of carob liquors. J. Sci. Food Agric. 2019, 99, 2697–2707. [Google Scholar] [CrossRef] [Green Version]
- Eldeeb, G.S.S.; Mosilhey, S.H. Roasting temperature impact on bioactive compounds and PAHs in Carob powder (Ceratonia siliqua L.). J. Food Sci. Technol. 2021. [Google Scholar] [CrossRef]
- Kumazawa, S.; Taniguchi, M.; Suzuki, Y.; Shimura, M.; Kwon, M.S.; Nakayama, T. Antioxidant activity of polyphenols in carob pods. J. Agric. Food Chem. 2002, 50, 373–377. [Google Scholar] [CrossRef]
- Papagiannopoulos, M.; Wollseifen, H.R.; Mellenthin, A.; Haber, B.; Galensa, R. Identification and quantification of polyphenols in carob fruits (Ceratonia siliqua L.) and derived products by HPLC-UV-ESI/MSn. J. Agric. Food Chem. 2004, 52, 3784–3791. [Google Scholar] [CrossRef] [PubMed]
- Cavdarova, M.; Makris, D.P. Extraction kinetics of phenolics from carob (Ceratonia siliqua L.) kibbles using environmentally benign solvents. Waste Biomass Valoriz. 2014, 5, 773–779. [Google Scholar] [CrossRef]
- Ydjedd, S.; Bouriche, S.; López-Nicolás, R.; Sánchez-Moya, T.; Frontela-Saseta, C.; Ros-Berruezo, G.; Rezgui, F.; Louaileche, H.; Kati, D.E. Effect of in vitro gastrointestinal digestion on encapsulated and nonencapsulated phenolic compounds of carob (Ceratonia siliqua L.) pulp extracts and their antioxidant capacity. J. Agric. Food Chem. 2017, 65, 827–835. [Google Scholar] [CrossRef]
- Goulas, V.; Georgiou, E. Utilization of carob fruit as sources of phenolic compounds with antioxidant potential: Extraction optimization and application in food models. Foods 2020, 9, 20. [Google Scholar] [CrossRef] [Green Version]
- Scalbert, A.; Manach, C.; Morand, C.; Rémésy, C.; Jiménez, L. Dietary polyphenols and the prevention of diseases. Crit. Rev. Food Sci. Nutr. 2005, 45, 287–306. [Google Scholar] [CrossRef]
- Corsi, L.; Avallone, R.; Cosenza, F.; Farina, F.; Baraldi, C. Antiproliferative effects of Ceratonia siliqua L. on mouse hepatocellular carcinoma cell line. Fitoterapia 2002, 73, 674–684. [Google Scholar] [CrossRef]
- Custódio, L.; Fernandes, E.; Escapa, A.L.; Fajardo, A.; Aligué, R.; Alberício, F.; Neng, N.R.; Nogueira, J.M.F.; Romano, A. Antioxidant and cytotoxic activities of carob tree fruit pulps are strongly influenced by gender and cultivar. J. Agric. Food Chem. 2011, 59, 7005–7012. [Google Scholar] [CrossRef] [PubMed]
- Goulas, V.; Stylos, E.; Chatziathanasiadou, M.V.; Mavromoustakos, T.; Tzakos, A.G. Functional components of carob fruit: Linking the chemical and biological space. Int. J. Mol. Sci. 2016, 17, 875. [Google Scholar] [CrossRef]
- Ayache, S.B.; Saafi, E.B.; Emhemmed, F.; Flamini, G.; Achour, L.; Muller, C.D. Biological Activities of Aqueous Extracts from Carob. Molecules 2020, 25, 3120. [Google Scholar] [CrossRef]
- Gregoriou, G.; Neophytou, C.M.; Vasincu, A.; Gregoriou, Y.; Hadjipakkou, H.; Pinakoulaki, E.; Christodoulou, M.C.; Ioannou, G.D.; Stavrou, I.J.; Christou, A.; et al. Anti-cancer activity and phenolic content of extracts derived from cypriot carob (Ceratonia siliqua L.) pods using different solvents. Molecules 2021, 26, 5017. [Google Scholar] [CrossRef] [PubMed]
- Rico, D.; Martín-Diana, A.B.; Martínez-Villaluenga, C.; Aguirre, L.; Silván, J.M.; Dueñas, M.; De Luis, D.A.; Lasa, A. In vitro approach for evaluation of carob by-products as source bioactive ingredients with potential to attenuate metabolic syndrome (MetS). Heliyon 2019, 5, e01175. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.Y.; Qian, H.; Yao, W.R. Melanoidins produced by the Maillard reaction: Structure and biological activity. Food Chem. 2011, 128, 573–584. [Google Scholar] [CrossRef]
- Echavarría, A.P.; Pagán, J.; Ibarz, A. Melanoidins Formed by Maillard Reaction in Food and Their Biological Activity. Food Eng. Rev. 2012, 4, 203–223. [Google Scholar] [CrossRef]
- Coelho, C.; Ribeiro, M.; Cruz, A.C.S.; Domingues, M.R.M.; Coimbra, M.A.; Bunzel, M.; Nunes, F.M. Nature of phenolic compounds in coffee melanoidins. J. Agric. Food Chem. 2014, 62, 7843–7853. [Google Scholar] [CrossRef]
- Oracz, J.; Nebesny, E. Effect of roasting parameters on the physicochemical characteristics of high-molecular-weight Maillard reaction products isolated from cocoa beans of different Theobroma cacao L. groups. Eur. Food Res. Technol. 2019, 245, 111–128. [Google Scholar] [CrossRef]
- Oracz, J.; Zyzelewicz, D. In vitro antioxidant activity and ftir characterization of high-molecular weight melanoidin fractions from different types of cocoa beans. Antioxidants 2019, 8, 560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uysal, S.; Zengin, G.; Aktumsek, A.; Karatas, S. Chemical and biological approaches on nine fruit tree leaves collected from the Mediterranean region of Turkey. J. Funct. Foods 2016, 22, 518–532. [Google Scholar] [CrossRef]
- Quiroz-Reyes, C.N.; Fogliano, V. Design cocoa processing towards healthy cocoa products: The role of phenolics and melanoidins. J. Funct. Foods 2018, 45, 480–490. [Google Scholar] [CrossRef]
- Sarikurkcu, C.; Arisoy, K.; Tepe, B.; Cakir, A.; Abali, G.; Mete, E. Studies on the antioxidant activity of essential oil and different solvent extracts of Vitex agnus castus L. fruits from Turkey. Food Chem. Toxicol. 2009, 47, 2479–2483. [Google Scholar] [CrossRef]
- Chen, Z.; Bertin, R.; Froldi, G. EC50 estimation of antioxidant activity in DPPH Å assay using several statistical programs. Food Chem. 2013, 138, 414–420. [Google Scholar] [CrossRef] [PubMed]
- Shimamura, T.; Sumikura, Y.; Yamazaki, T.; Tada, A.; Kashiwagi, T.; Ishikawa, H.; Matsui, T.; Sugimoto, N.; Akiyama, H.; Ukeda, H. Applicability of the DPPH assay for evaluating the antioxidant capacity of food additives-inter-laboratory evaluation study. Anal. Sci. 2014, 30, 717–721. [Google Scholar] [CrossRef] [Green Version]
- Bittová, M.; Krejzová, E.; Roblová, V. Monitoring of HPLC profiles of selected polyphenolic compounds in sea buckthorn (Hippophaë rhamnoides L.) plant parts during annual growth cycle and estimation of their antioxidant potential. Open Chem. 2014, 12, 1152–1161. [Google Scholar] [CrossRef]
- Coates, J. Interpretation of Infrared Spectra, A Practical Approach. In Encyclopedia of Analytical Chemistry; John Wiley & Sons, Ltd.: Chichester, UK, 2006; Volume 112, pp. 22–31. [Google Scholar]
- Grasel, F.D.S.; Ferrão, M.F.; Wolf, C.R. Development of methodology for identification the nature of the polyphenolic extracts by FTIR associated with multivariate analysis. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2016, 153, 94–101. [Google Scholar] [CrossRef]
- Mohsin, G.F.; Schmitt, F.J.; Kanzler, C.; Hoehl, A.; Hornemann, A. PCA-based identification and differentiation of FTIR data from model melanoidins with specific molecular compositions. Food Chem. 2019, 281, 106–113. [Google Scholar] [CrossRef]
- Farhoosh, R.; Johnny, S.; Asnaashari, M.; Molaahmadibahraseman, N.; Sharif, A. Structure-antioxidant activity relationships of o-hydroxyl, o-methoxy, and alkyl ester derivatives of p-hydroxybenzoic acid. Food Chem. 2016, 194, 128–134. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, H.; Troise, A.D.; Fogliano, V. Melanoidins from Coffee, Cocoa, and Bread Are Able to Scavenge α-Dicarbonyl Compounds under Simulated Physiological Conditions. J. Agric. Food Chem. 2019, 67, 10921–10929. [Google Scholar] [CrossRef] [PubMed] [Green Version]
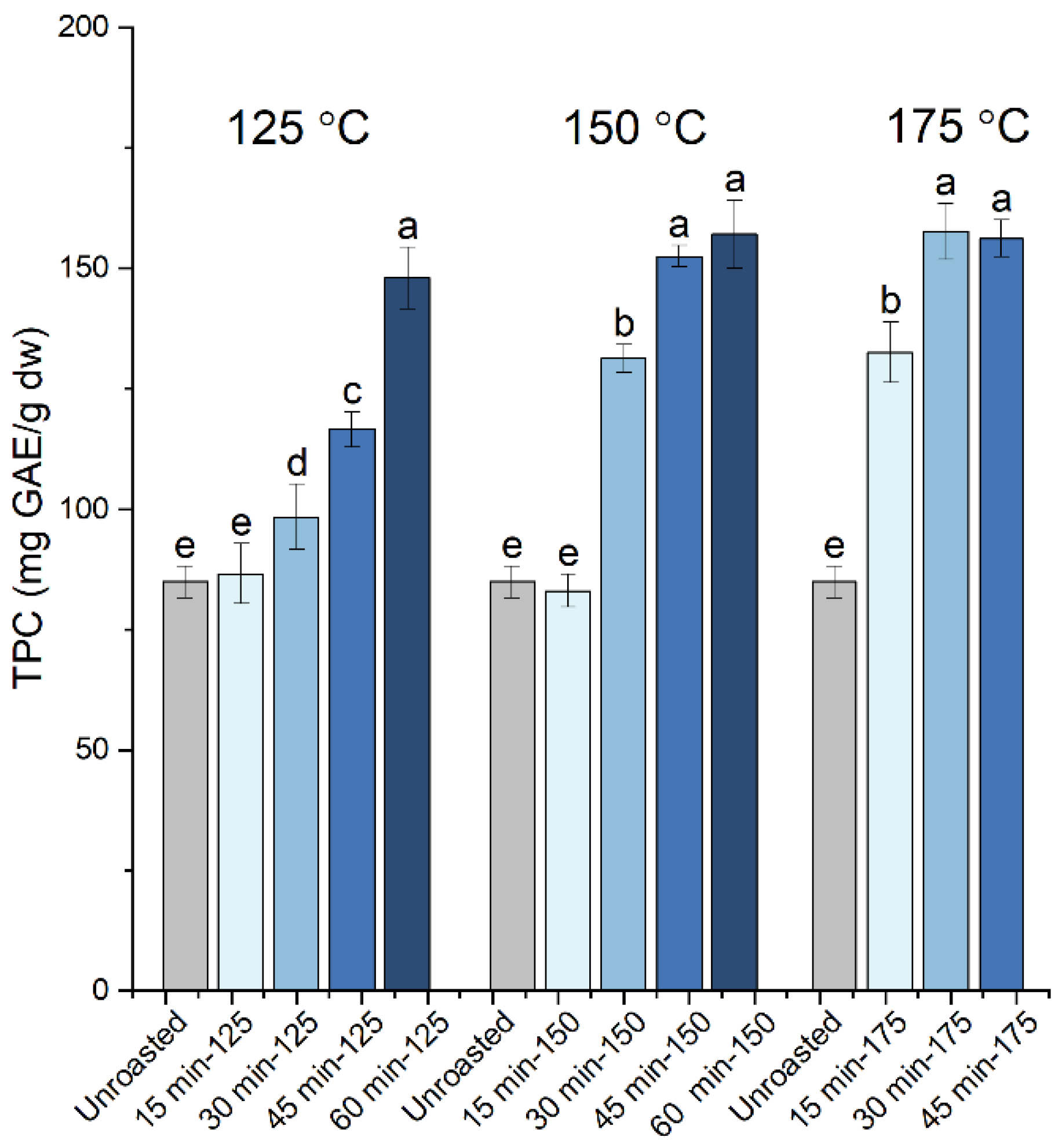
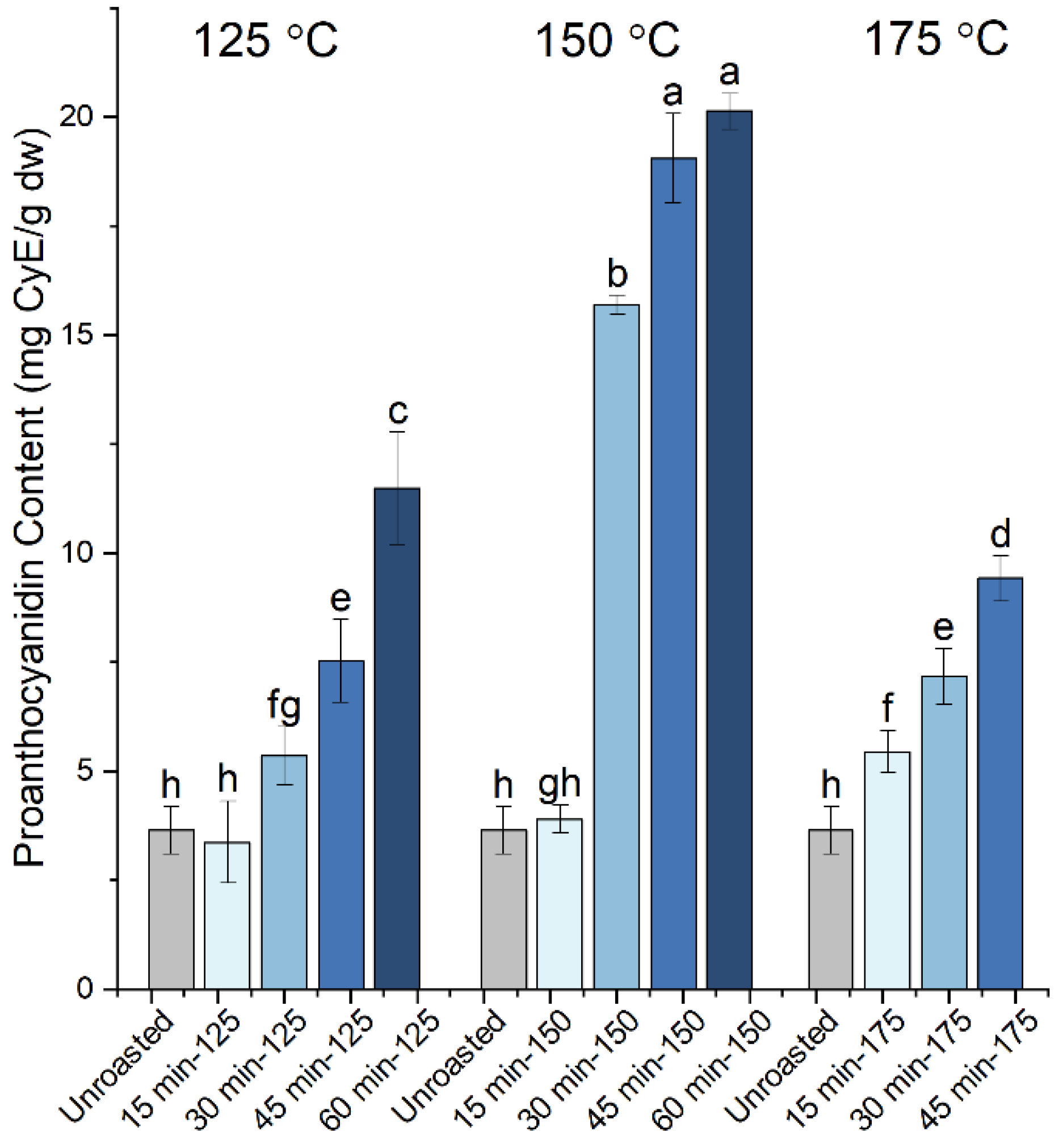
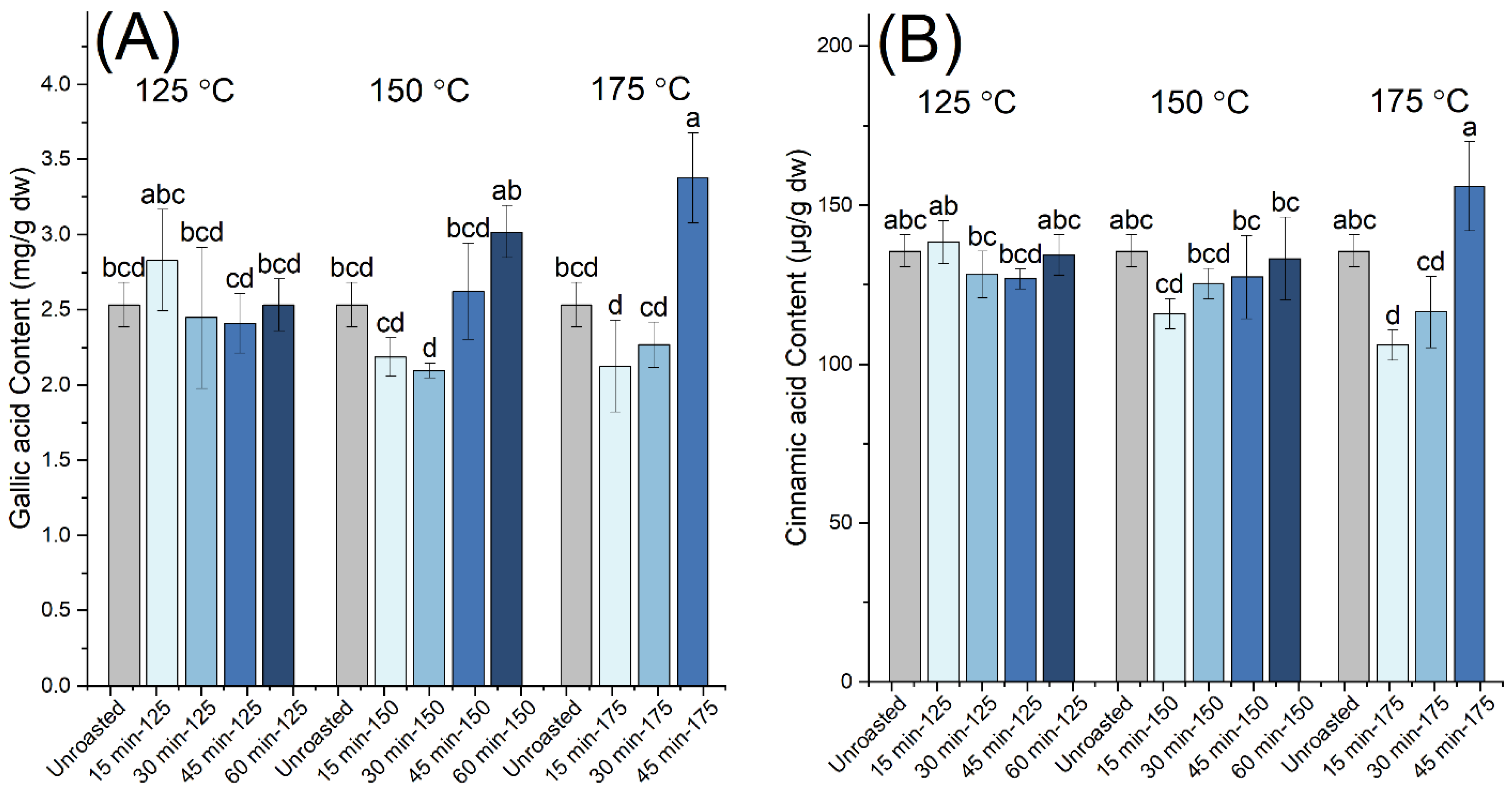
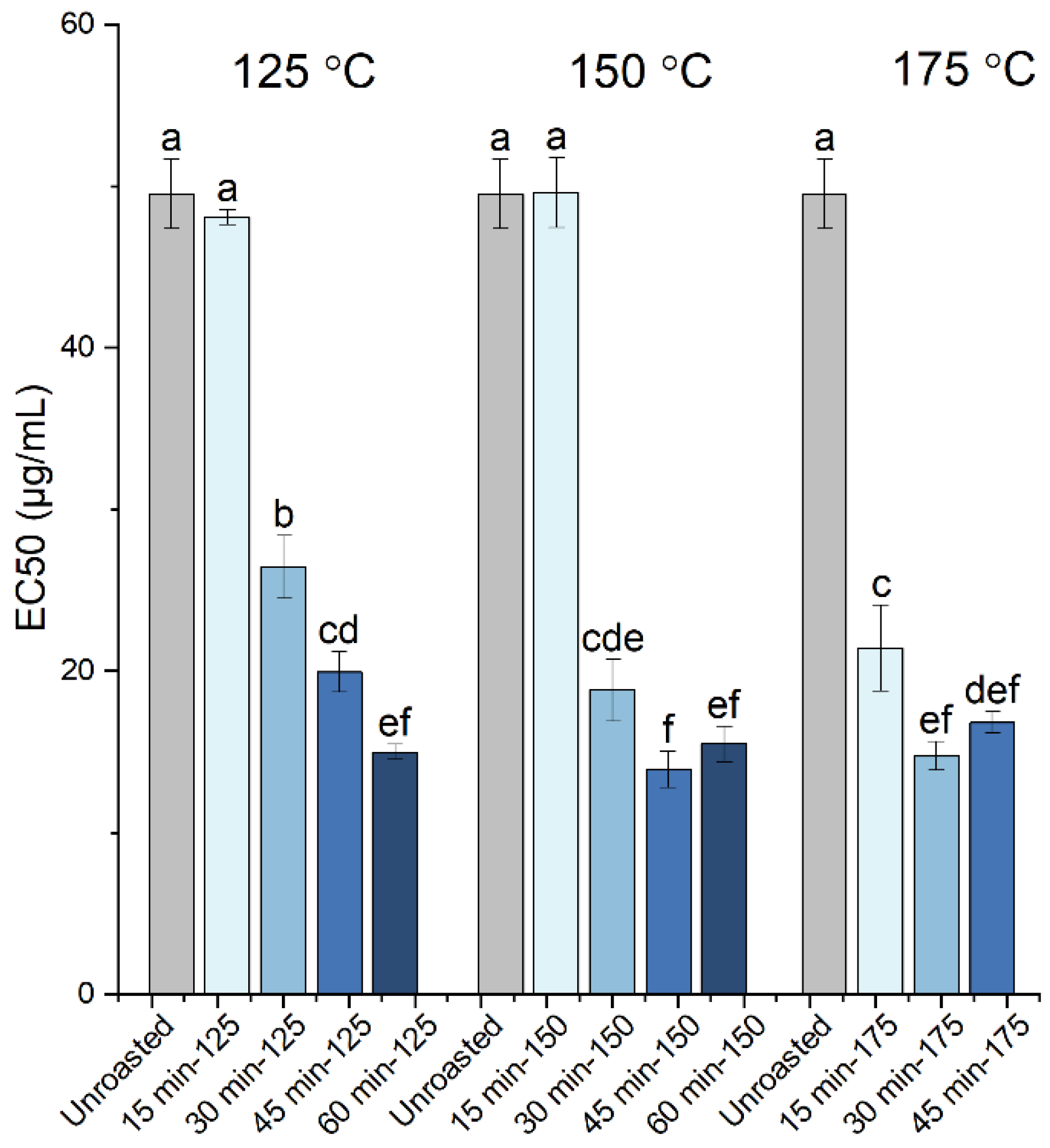
| Roasting Conditions | TPC (mg GAE/g dw) | Proanthocyanidins (mg CyE/g dw) | Gallic Acid (mg/g dw) | Cinnamic Acid (μg/g dw) | EC50 (μg/mL) | TEAC | |
|---|---|---|---|---|---|---|---|
| Unroasted | 84.9 ± 3.3 e | 3.64 ± 0.55 h | 2.53 ± 0.15 bcd | 135.5 ± 5.0 abc | 49.5 ± 2.2 a | 0.23 ± 0.01 | |
| 125 °C | 15 min | 86.6 ± 6.1 e | 3.37 ± 0.93 h | 2.83 ± 0.34 abc | 138.3 ± 6.8 ab | 48.1 ± 0.5 a | 0.24 ± 0.00 |
| 30 min | 98.4 ± 6.8 d | 5.36 ± 0.67 fg | 2.45 ± 0.47 bcd | 128.2 ± 7.3 bc | 26.5 ± 1.9 b | 0.43 ± 0.03 | |
| 45 min | 116.5 ± 3.6 c | 7.53 ± 0.95 e | 2.41 ± 0.20 cd | 126.8 ± 3.1 bcd | 20.0 ± 1.2 cd | 0.56 ± 0.03 | |
| 60 min | 148.0 ± 6.5 a | 11.49 ± 1.29 c | 2.53 ± 0.17 bcd | 134.3 ± 6.4 abc | 15.0 ± 0.5 ef | 0.77 ± 0.03 | |
| 150 °C | 15 min | 83.1 ± 3.5 e | 3.90 ± 0.32 gh | 2.19 ± 0.13 cd | 115.8 ± 4.7 cd | 49.6 ± 2.2 a | 0.23 ± 0.01 |
| 30 min | 131.3 ± 2.9 b | 15.69 ± 0.21 b | 2.10 ± 0.05 d | 125.4 ± 4.8 bcd | 18.8 ± 1.9 cde | 0.55 ± 0.03 | |
| 45 min | 152.5 ± 2.3 a | 19.05 ± 1.02 a | 2.62 ± 0.32 bcd | 127.3 ± 13.1 bc | 13.9 ± 1.1 f | 0.79 ± 0.03 | |
| 60 min | 157.1 ± 7.2 a | 20.12 ± 0.42 a | 3.02 ± 0.18 ab | 133.1 ± 13.0 bc | 15.5 ± 1.1 ef | 0.72 ± 0.03 | |
| 175 °C | 15 min | 132.5 ± 6.2 b | 5.44 ± 0.49 f | 2.13 ± 0.31 d | 106.0 ± 4.8 d | 21.4 ± 2.7 c | 0.47 ± 0.04 |
| 30 min | 157.7 ± 5.9 a | 7.17 ± 0.65 e | 2.27 ± 0.15 cd | 116.4 ± 11.3 cd | 14.7 ± 0.9 ef | 0.75 ± 0.03 | |
| 45 min | 156.2 ± 3.9 a | 9.42 ± 0.51 d | 3.38 ± 0.30 a | 155.9 ± 14.0 a | 16.8 ± 0.7 def | 0.66 ± 0.02 | |
| TPC | PC | GA | CIA | EC50 | |
|---|---|---|---|---|---|
| TPC | 1 | 0.71042 | 0.31998 | 0.09808 | −0.91444 |
| PC | 1 | 0.21168 | 0.1464 | −0.67768 | |
| GA | 1 | 0.84864 | −0.12447 | ||
| CIA | 1 | 0.00249 | |||
| EC50 | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grigoriou, A.M.; Pinakoulaki, E. Linking the Dynamic Changes in the In Vitro Antioxidant Activity of Carob Kibbles upon Roasting to the Chemical and Structural Changes Revealed by FTIR Spectroscopy. Antioxidants 2021, 10, 2025. https://doi.org/10.3390/antiox10122025
Grigoriou AM, Pinakoulaki E. Linking the Dynamic Changes in the In Vitro Antioxidant Activity of Carob Kibbles upon Roasting to the Chemical and Structural Changes Revealed by FTIR Spectroscopy. Antioxidants. 2021; 10(12):2025. https://doi.org/10.3390/antiox10122025
Chicago/Turabian StyleGrigoriou, Anna Marina, and Eftychia Pinakoulaki. 2021. "Linking the Dynamic Changes in the In Vitro Antioxidant Activity of Carob Kibbles upon Roasting to the Chemical and Structural Changes Revealed by FTIR Spectroscopy" Antioxidants 10, no. 12: 2025. https://doi.org/10.3390/antiox10122025
APA StyleGrigoriou, A. M., & Pinakoulaki, E. (2021). Linking the Dynamic Changes in the In Vitro Antioxidant Activity of Carob Kibbles upon Roasting to the Chemical and Structural Changes Revealed by FTIR Spectroscopy. Antioxidants, 10(12), 2025. https://doi.org/10.3390/antiox10122025






