Characterization of Fruit Development, Antioxidant Capacity, and Potential Vasoprotective Action of Peumo (Cryptocarya alba), a Native Fruit of Chile
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Fruit Quality Assessments
2.3. Ethylene Production and CO2 Production
2.4. Determination of Antioxidant Capacity, Total Polyphenol Content, and Total Flavonoid Content during Fruit Development
2.4.1. The Ferric Reducing Antioxidant Power (FRAP) Assay
2.4.2. The Trolox Equivalent Antioxidant Capacity (TEAC) Assay
2.4.3. The 2,2-diphenyl-1-picrylhydrazyl (DPPH) Assay
2.4.4. The Oxygen Radical Absorbance Capacity (ORAC) Assay
2.4.5. Total Phenolic Content Determination
2.4.6. Total Flavonoid Content Determination
2.5. Bioactivity Assay of Ripe Peumo Extract
2.5.1. Functional Extract Preparation
2.5.2. Ultrahigh-Pressure Liquid Chromatography-Mass Spectrometry (U-HPLC/MS) Analysis
2.5.3. Cellular Antioxidant Activity
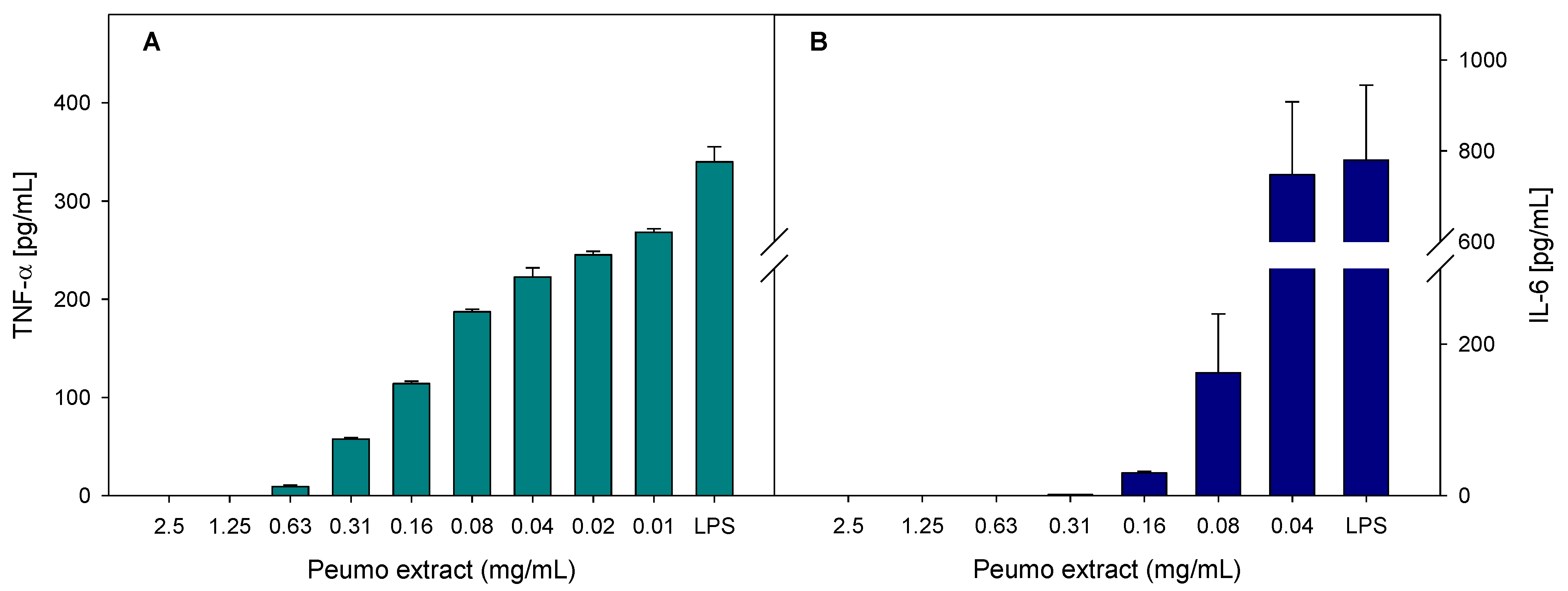
2.5.4. Anti-Inflammatory Activity
2.5.5. Animals
2.5.6. Rat Aortic Rings Preparation and Recording
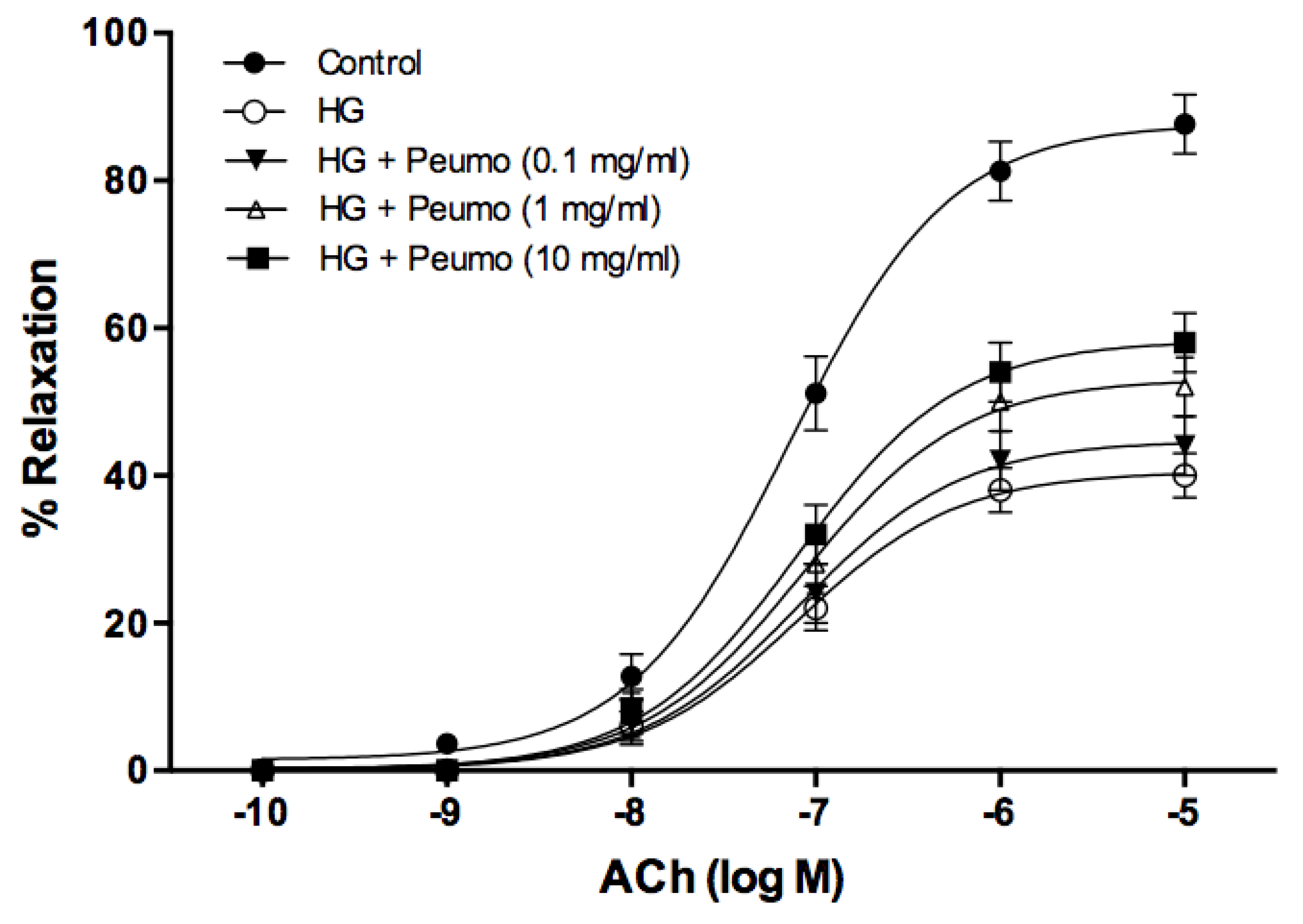
2.5.7. Evaluation of the Protective Effect of the Ripe Peumo Extracts
2.6. Statistical Analysis
3. Results
3.1. Characterization of Quality and Physiological Parameters during Fruit Development of the Peumo Fruit
3.2. Antioxidant Capacity, Total Polyphenol, and Flavonoid Content during Fruit Development of the Peumo Fruit
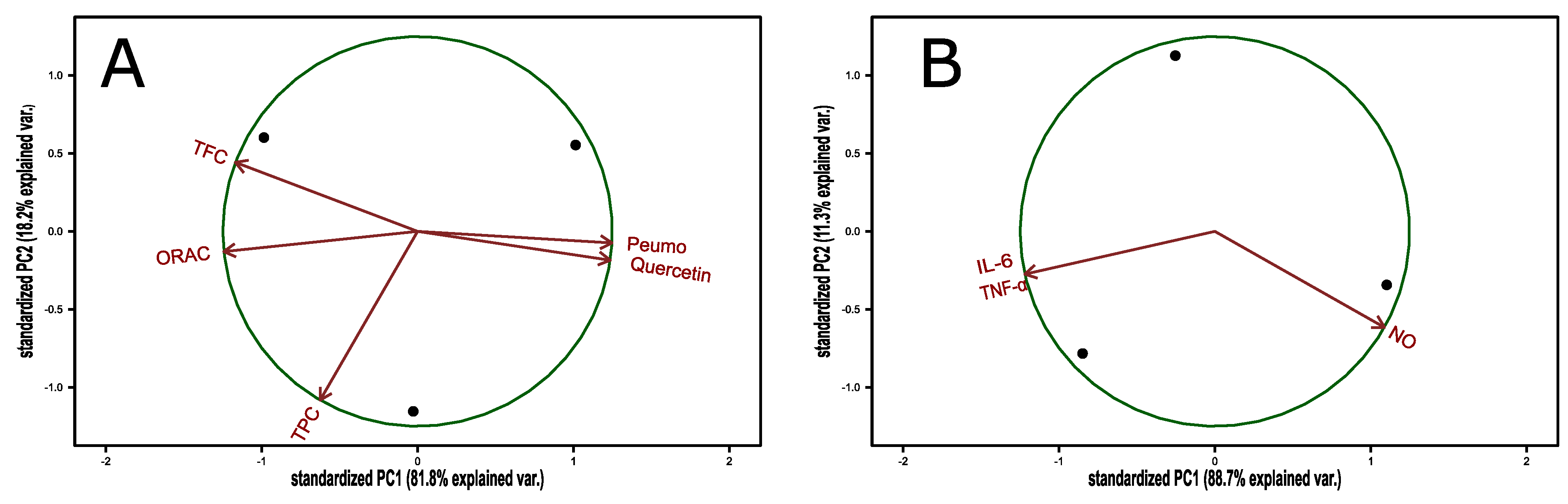
3.3. Composition of Peumo Fruit Extract
3.4. Bioactivity of Peumo Fruit Extracts
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
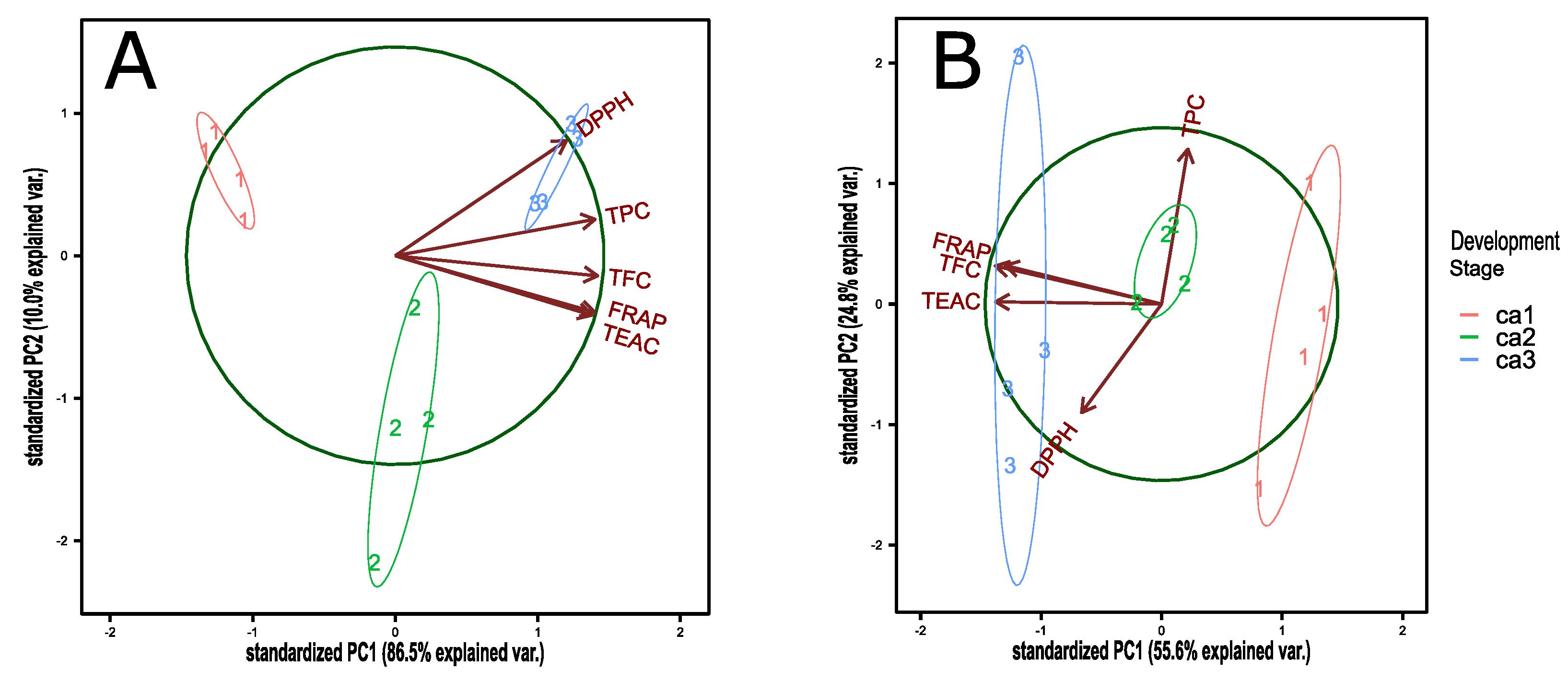
| TEAC | TPC | FRAP | DPPH | TFC | |
|---|---|---|---|---|---|
| TEAC | 0.836 *** | 0.979 *** | 0.632 * | 0.903 *** | |
| TPC | 0.836 *** | 0.847 *** | 0.848 *** | 0.918 *** | |
| FRAP | 0.979 *** | 0.847 *** | 0.647 * | 0.939 *** | |
| DPPH | 0.632 * | 0.848 *** | 0.647 * | 0.724 ** | |
| TFC | 0.903 *** | 0.918 *** | 0.939 *** | 0.724 ** | |
| Computed correlation used Pearson-method. | |||||
| TEAC | TPC | FRAP | DPPH | TFC | |
|---|---|---|---|---|---|
| TEAC | −0.227 | 0.732 ** | 0.258 | 0.929 *** | |
| TPC | −0.227 | 0.097 | −0.315 | 0.005 | |
| FRAP | 0.732 ** | 0.097 | 0.347 | 0.793 ** | |
| DPPH | 0.258 | −0.315 | 0.347 | 0.223 | |
| TFC | 0.929 *** | 0.005 | 0.793 ** | 0.223 | |
| Computed correlation used Pearson-method. | |||||
References
- Fuentes-Ramírez, A.; Pauchard, A.; Cavieres, L.A.; García, R.A. Survival and growth of Acacia dealbata vs. native trees across an invasion front in south-central Chile. For. Ecol. Manag. 2011, 261, 1003–1009. [Google Scholar] [CrossRef]
- Benedetti, S. Información Tecnológica de Productos Forestales No Madereros del Bosque Nativo en Chile; Monografía de peumo Cryptocarya alba (Mol) Looser; CONAF: Santiago, Chile, 2012; ISBN 978-956-318-066-4. Available online: https://investigacion.conaf.cl/archivos/repositorio_documento/2018/11/004_2011-DOCUMENTOS_MONOGRAFIA_PEUMO.pdf (accessed on 28 October 2021).
- Cheeke, P.R. Actual and Potential Applications of Yucca Schidigera and Quillaja Saponaria Saponins in Human and Animal Nutrition. In Saponins in Food, Feedstuffs and Medicinal Plants; Oleszek, W., Marston, A., Eds.; Proceedings of the Phythochemical Society of Europe book Series; Springer: Dordrecht, The Netherlands, 2000; Volume 45. [Google Scholar]
- Fuentes-Barros, G.; Castro-Saavedra, S.; Liberona, L.; Acevedo-Fuentes, W.; Tirapegui, C.; Mattar, C.; Cassels, B.K. Variation of the alkaloid content of Peumus boldus (boldo). Fitoterapia 2018, 127, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Vogel, H.; Razmilic, I.; San Martín, J.; Doll, U.; y González, B. Plantas Medicinales Chilenas. Experiencia de Domesticación y Cultivo de Boldo, Matico, Bailahuén, Canelo, Peumo y Maqui, 2nd ed.; Editorial de la Universidad de Talca: Talca, Chile, 2008; 194p. [Google Scholar]
- Avello-Lorca, M.; López Canales, C.; GaticaValenzuela, C.; Bustos Concha, E.; Chait, A.B.; Pastene Navarrete, C.E.; Bittner Berner, C.M. Antimicrobial effects of extracts from Chilean plants of Lauraceae and Atherospermataceae families. Rev. Cub. Plant. Med. 2012, 17, 73–83. [Google Scholar]
- Schmeda-Hirschmann, G.; Astudillo, L.; Bastida, J.; Codina, C.; de Arias, A.R.; Ferreira, M.E.; Inchaustti, A.; Yaluff, G. Cryptofolione derivatives from Cryptocarya alba fruits. J. Pharm. Pharmacol. 2010, 53, 563–567. [Google Scholar] [CrossRef]
- Domínguez, S.Y.; Martínez, E. Árboles de nuestros bosques. In Guía Didáctica; Ediciones Alfonso Martínez, S. L: Madrid, Spain, 1999. [Google Scholar]
- Simirgiotis, M.J. Antioxidant Capacity and HPLC-DAD-MS Profiling of Chilean Peumo (Cryptocarya alba) Fruits and Comparison with German Peumo (Crataegus monogyna) from Southern Chile. Molecules 2013, 18, 2061–2080. [Google Scholar] [CrossRef] [Green Version]
- Speisky, H.; López-Alarcón, C.; Gómez, M.; Fuentes, J.; Sandoval-Acuña, C. First Web-Based Database on Total Phenolics and Oxygen Radical Absorbance Capacity (ORAC) of Fruits Produced and Consumed within the South Andes Region of South America. J. Agric. Food Chem. 2012, 60, 8851–8859. [Google Scholar] [CrossRef]
- Ruiz, A.; Hermosí;n-Gutiérrez, I.; Mardones, C.; Vergara, C.; Herlitz, E.; Vega, M.; Dorau, C.; Winterhalter, P.; Von Baer, D. Polyphenols and Antioxidant Activity of Calafate (Berberis microphylla) Fruits and Other Native Berries from Southern Chile. J. Agric. Food Chem. 2010, 58, 6081–6089. [Google Scholar] [CrossRef]
- Fuentes, L.; Figueroa, C.R.; Valdenegro, M.; Vinet, R. Patagonian Berries: Healthy Potential and the Path to Becoming Functional Foods. Foods 2019, 8, 289. [Google Scholar] [CrossRef] [Green Version]
- Céspedes, C.; El-Hafidi, M.; Pavon, N.; Alarcon, J. Antioxidant and cardioprotective activities of phenolic extracts from fruits of Chilean blackberry Aristotelia chilenesis (Elaeocarpaceae). Maqui. Food Chem. 2008, 108, 820–829. [Google Scholar] [CrossRef]
- Miranda-Rottmann, S.; Aspillaga, A.A.; Pérez, D.D.; Vasquez, L.; Martinez, A.A.L.F.; Leighton, F. Juice and Phenolic Fractions of the Berry Aristotelia chilensis Inhibit LDL Oxidation in Vitro and Protect Human Endothelial Cells against Oxidative Stress. J. Agric. Food Chem. 2002, 50, 7542–7547. [Google Scholar] [CrossRef]
- Schreckinger, M.E.; Wang, J.; Yousef, G.; Lila, M.A.; de Mejia, E.G. Antioxidant Capacity and in Vitro Inhibition of Adipogenesis and Inflammation by Phenolic Extracts of Vaccinium floribundum and Aristotelia chilensis. J. Agric. Food Chem. 2010, 58, 8966–8976. [Google Scholar] [CrossRef]
- Paredes-López, O.; Cervantes-Ceja, M.L.; Vigna-Pérez, M.; Hernández-Pérez, T. Berries: Improving human health and healthy aging, and promoting quality life A review. Plant Foods Hum. Nutr. 2010, 65, 299–308. [Google Scholar] [CrossRef]
- AOAC (Association of Official Analytical Chemists). Official Methods of Analysis of AOAC International, 16th ed.; Association of Official Analytical Chemists Inc.: Washington, DC, USA, 1997. [Google Scholar]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van den Berg, R.; Haenen, G.R.; van den Berg, H.; Bast, A.A.L.T. Applicability of an improved Trolox equivalent antioxidant capacity (TEAC) assay for evaluation of antioxidant capacity measurements of mixtures. Food Chem. 1999, 66, 511–517. [Google Scholar] [CrossRef]
- Valdenegro, M.; Fuentes, L.; Herrera, R.; Moya-Leon, M.A. Changes in antioxidant capacity during development and ripening of goldenberry (Physalis peruviana L.) fruit and in response to 1-methylcyclopropene treatment. Postharvest Biol. Technol. 2012, 67, 110–117. [Google Scholar] [CrossRef]
- Murcia, M.; Jiménez-Monreal, A.; García-Diz, L.; Carmona, M.; Maggi, L.; Martínez-Tomé, M. Antioxidant activity of minimally processed (in modified atmospheres), dehydrated and ready-to-eat vegetables. Food Chem. Toxicol. 2009, 47, 2103–2110. [Google Scholar] [CrossRef] [PubMed]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A., Jr. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Galati, E.M.; Mondello, M.R.; Giuffrida, D.; Dugo, G.; Miceli, N.; Pergolizzi, S.; Taviano, M.F. Chemical Characterization and Biological Effects of Sicilian Opuntia ficus indica (L.) Mill. Fruit Juice: Antioxidant and Antiulcerogenic Activity. J. Agric. Food Chem. 2003, 51, 4903–4908. [Google Scholar] [CrossRef]
- Chang, C.-C.; Yang, M.-H.; Wen, H.-M.; Chern, J.-C. Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J. Food Drug Anal. 2002, 10, 178–182. [Google Scholar]
- Fuentes, L.; Valdenegro, M.; Gómez, M.-G.; Ayala-Raso, A.; Quiroga, E.; Martinez, J.P.; Vinet, R.; Caballero, E.; Figueroa, C. Characterization of fruit development and potential health benefits of arrayan (Luma apiculata), a native berry of South America. Food Chem. 2016, 196, 1239–1247. [Google Scholar] [CrossRef]
- Viktorová, J.; Kumar, R.; Řehořová, K.; Hoang, L.; Ruml, T.; Figueroa, C.R.; Valdenegro, M.; Fuentes, L. Antimicrobial Activity of Extracts of Two Native Fruits of Chile: Arrayan (Luma apiculata) and Peumo (Cryptocarya alba). Antibiotics 2020, 9, 444. [Google Scholar] [CrossRef]
- Viktorova, J.; Stranska-Zachariasova, M.; Fenclova, M.; Vitek, L.; Hajslova, J.; Kren, V.; Ruml, T. Complex Evaluation of Antioxidant Capacity of Milk Thistle Dietary Supplements. Antioxidants 2019, 8, 317. [Google Scholar] [CrossRef] [Green Version]
- Dobiasová, S.; Řehořová, K.; Kučerová, D.; Biedermann, D.; Káňová, K.; Petrásková, L.; Koucká, K.; Václavíková, R.; Valentová, K.; Ruml, T.; et al. Multidrug Resistance Modulation Activity of Silybin Derivatives and Their Anti-Inflammatory Potential. Antioxidants 2020, 9, 455. [Google Scholar] [CrossRef]
- National Research Council (US) Committee for the Update of the Guide for the Care and Use of Laboratory Animals. In Guide for the Care and Use of Laboratory Animals, 8th ed.; National Academies Press: Washington, DC, USA, 2011.
- Vinet, R.; Brieva, C.; Pinardi, G.; Penna, M. Modulation of α-adrenergic-induced contractions by endothelium-derived relaxing factor in rat aorta. Gen. Pharmacol. Vasc. Syst. 1991, 22, 137–142. [Google Scholar] [CrossRef]
- Vinet, R.; Araos, P.; Gentina, J.C.; Knox, M.; Guzman, L. p-Coumaric acid reduces high glucose-mediated impairment of endothelium-dependent relaxation in rat aorta. BLACPMA 2014, 13, 232–237. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2010; ISBN 3-900051-07-0. [Google Scholar]
- Xu, J.; Zhao, Y.; Zhang, X.; Zhang, L.; Hou, Y.; Dong, W. Transcriptome Analysis and Ultrastructure Observation Reveal that Hawthorn Fruit Softening Is due to Cellulose/Hemicellulose Degradation. Front. Plant Sci. 2016, 7, 1524. [Google Scholar] [CrossRef] [Green Version]
- U.S. FDA and the Center for Food Safety and Applied Nutrition. Available online: http://www.foodscience.caes.uga.edu/extension/documents/fdaapproximatephoffoodslacf-phs.pdf (accessed on 28 October 2021).
- Maxie, E.C.; Catlin, P.B.; Hartman, H.T. Respiration and ripening of olive fruits. Proc. Am. Soc. Hortic. Sci. 1960, 75, 275–291. [Google Scholar]
- Kafkaletou, M.; Fasseas, C.; Tsantili, E. Increased firmness and modified cell wall composition by ethylene were reversed by the ethylene inhibitor 1-methylcyclopropene (1-MCP) in the non-climacteric olives harvested at dark green stage—Possible implementation of ethylene for olive quality. J. Plant Physiol. 2019, 238, 63–71. [Google Scholar] [CrossRef]
- Sáez, F.A.; Aguayo, M.G.; Mendonça, R.T.; Fuentes, L.; Figueroa, C.R. Changes of cell wall-associated polysaccharides and sugars during development and ripening of arrayan (Luma apiculata) and lleuque (Prumnopitys andina) fruits. Acta Physiol. Plant 2022, 44, 9. [Google Scholar] [CrossRef]
- Zheng, Y.-Z.; Deng, G.; Liang, Q.; Chen, D.-F.; Guo, R.; Lai, R.-C. Antioxidant Activity of Quercetin and Its Glucosides from Propolis: A Theoretical Study. Sci. Rep. 2017, 7, 7543. [Google Scholar] [CrossRef] [Green Version]
- Dabeek, W.M.; Marra, M.V. Dietary Quercetin and Kaempferol: Bioavailability and Potential Cardiovascular-Related Bioactivity in Humans. Nutrients 2019, 11, 2288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, J. Dietary Flavonoid Aglycones and Their Glycosides: Which Show Better Biological Significance? Crit. Rev. Food Sci. Nutr. 2015, 57, 1874–1905. [Google Scholar] [CrossRef] [PubMed]
- Timmermann, B.N.; Valcic, S.; Liu, Y.-L.; Montenegro, G. Notes: Flavonols from Cryptocarya alba. Z. Nat. C 1995, 50, 898–899. [Google Scholar] [CrossRef]
- Kim, H.-Y.; Nam, S.-Y.; Hong, S.-W.; Kim, M.J.; Jeong, H.-J.; Kim, H.-M. Protective Effects of Rutin through Regulation of Vascular Endothelial Growth Factor in Allergic Rhinitis. Am. J. Rhinol. Allergy 2015, 29, e87–e94. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.-Y.; Huang, Y.-C.; Yang, M.-L.; Lee, C.-Y.; Chen, C.-J.; Yeh, C.-H.; Pan, P.-H.; Horng, C.-T.; Kuo, W.-H.; Kuan, Y.-H. Protective effect of rutin on LPS-induced acute lung injury via down-regulation of MIP-2 expression and MMP-9 activation through inhibition of Akt phosphorylation. Int. Immunopharmacol. 2014, 22, 409–413. [Google Scholar] [CrossRef]
- Wang, Y.P.; Wat, E.; Koon, C.M.; Wong, C.W.; Cheung, D.W.S.; Leung, P.C.; Zhao, Q.S.; Fung, K.P.; Lau, C.B.S. The beneficial potential of polyphenol-enriched fraction from Erigerontis Herba on metabolic syndrome. J. Ethnopharmacol. 2016, 187, 94–103. [Google Scholar] [CrossRef]
- Bujor, A.; Miron, A.; Luca, S.V.; Skalicka-Wozniak, K.; Silion, M.; Trifan, A.; Girard, C.; Demougeot, C.; Totoson, P. Vasorelaxant effects of Crataegus pentagyna: Links with arginase inhibition and phenolic profile. J. Ethnopharmacol. 2020, 252, 112559. [Google Scholar] [CrossRef]
- Peyrol, J.; Meyer, G.; Obert, P.; Dangles, O.; Pechère, L.; Amiot, M.-J.; Riva, C. Involvement of bilitranslocase and beta-glucuronidase in the vascular protection by hydroxytyrosol and its glucuronide metabolites in oxidative stress conditions. J. Nutr. Biochem. 2018, 51, 8–15. [Google Scholar] [CrossRef]
- Carullo, G.; Ahmed, A.; Fusi, F.; Sciubba, F.; Di Cocco, M.E.; Restuccia, D.; Spizzirri, U.G.; Saponara, S.; Aiello, F. Vasorelaxant Effects Induced by Red Wine and Pomace Extracts of Magliocco Dolce cv. Pharmaceutics 2020, 13, 87. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Hu, T.; Han, Y.; Huang, W.; Yuan, H.; Zhang, Y.-T.; Du, Y.; Jiang, Y.-W. Preventive Effects of Catechins on Cardiovascular Disease. Molecules 2016, 21, 1759. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mangels, D.R.; Mohler, E.R. Catechins as Potential Mediators of Cardiovascular Health. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 757–763. [Google Scholar] [CrossRef] [PubMed]
- Murray, M.; Walchuk, C.; Suh, M.; Jones, P.J. Green tea catechins and cardiovascular disease risk factors: Should a health claim be made by the United States Food and Drug Administration? Trends Food Sci. Technol. 2015, 41, 188–197. [Google Scholar] [CrossRef]
- Naveed, M.; Hejazi, V.; Abbas, M.; Kamboh, A.A.; Khan, G.J.; Shumzaid, M.; Ahmad, F.; Babazadeh, D.; FangFang, X.; Modarresi-Ghazani, F.; et al. Chlorogenic acid (CGA): A pharmacological review and call for further research. Biomed. Pharmacother. 2018, 97, 67–74. [Google Scholar] [CrossRef]
- Li, L.; Su, C.; Chen, X.; Wang, Q.; Jiao, W.; Luo, H.; Tang, J.; Wang, W.; Li, S.; Guo, S. Chlorogenic Acids in Cardiovascular Disease: A Review of Dietary Consumption, Pharmacology, and Pharmacokinetics. J. Agric. Food Chem. 2020, 68, 6464–6484. [Google Scholar] [CrossRef] [PubMed]
- Miao, M.; Xiang, L. Pharmacological action and potential targets of chlorogenic acid. Adv. Pharmacol. 2020, 87, 71–88. [Google Scholar] [CrossRef]
- Rodríguez, K.; Ah-Hen, K.; Vega-Galvez, A.; López, J.; Quispe-Fuentes, I.; Lemus-Mondaca, R.; Galvez-Ranilla, L. Changes in bioactive compounds and antioxidant activity during convective drying of murta (Ugni molinae T.) berries. Int. J. Food Sci. Technol. 2013, 49, 990–1000. [Google Scholar] [CrossRef]
- Puente-Díaz, L.; Ah-Hen, K.; Vega-Gálvez, A.; Lemus-Mondaca, R.; Di Scala, K. Combined infrared-convective drying of murta (Ugni molinae Turcz.) berries: Kinetic modeling and quality assessment. Dry Technol. 2013, 31, 329–338. [Google Scholar] [CrossRef]
- Castellon, X.; Bogdanova, V. Chronic Inflammatory Diseases and Endothelial Dysfunction. Aging Dis. 2016, 7, 81–89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, D.; Hu, M.-J.; Wang, Y.-Q.; Cui, Y.-L. Antioxidant Activities of Quercetin and Its Complexes for Medicinal Application. Molecules 2019, 24, 1123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lesjak, M.; Beara, I.; Simin, N.; Pintać, D.; Majkić, T.; Bekvalac, K.; Orčić, D.; Mimica-Dukić, N. Antioxidant and anti-inflammatory activities of quercetin and its derivatives. J. Funct. Foods 2018, 40, 68–75. [Google Scholar] [CrossRef]
- Zeng, Y.; Song, J.; Zhang, M.; Wang, H.; Zhang, Y.; Suo, H. Comparison of In Vitro and In Vivo Antioxidant Activities of Six Flavonoids with Similar Structures. Antioxidants 2020, 9, 732. [Google Scholar] [CrossRef]
- Rauf, A.; Imran, M.; Abu-Izneid, T.; Iahtisham-Ul-Haq; Patel, S.; Pan, X.; Naz, S.; Silva, A.S.; Saeed, F.; Suleria, H.A.R. Proanthocyanidins: A comprehensive review. Biomed. Pharmacother. 2019, 116, 108999. [Google Scholar] [CrossRef] [PubMed]
- Aziz, N.; Kim, M.-Y.; Cho, J.Y. Anti-inflammatory effects of luteolin: A review of in vitro, in vivo, and in silico studies. J. Ethnopharmacol. 2018, 225, 342–358. [Google Scholar] [CrossRef]
- Nakanishi, T.; Mukai, K.; Yumoto, H.; Hirao, K.; Hosokawa, Y.; Matsuo, T. Anti-inflammatory effect of catechin on cultured human dental pulp cells affected by bacteria-derived factors. Eur. J. Oral Sci. 2010, 118, 145–150. [Google Scholar] [CrossRef]
- Davignon, J.; Ganz, P. Role of Endothelial Dysfunction in Atherosclerosis. Circulation 2004, 109 (Suppl. S23), III-27–III-32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knox, M.; Vinet, R.; Fuentes, L.; Morales, B.; Martínez, J.L. A Review of Endothelium-Dependent and -Independent Vasodilation Induced by Phytochemicals in Isolated Rat Aorta. Animals 2019, 9, 623. [Google Scholar] [CrossRef] [Green Version]
- Chan, E.C.H.; Pannangpetch, P.; Woodman, O. Relaxation to Flavones and Flavonols in Rat Isolated Thoracic Aorta: Mechanism of Action and Structure-Activity Relationships. J. Cardiovasc. Pharmacol. 2000, 35, 326–333. [Google Scholar] [CrossRef] [PubMed]
- Jofré, I.; Pezoa, C.; Cuevas, M.; Scheuermann, E.; Freires, I.A.; Rosalen, P.L.; de Alencar, S.M.; Romero, F. Antioxidant and Vasodilator Activity of Ugni molinae Turcz. (Murtilla) and Its Modulatory Mechanism in Hypotensive Response. Oxidative Med. Cell. Longev. 2016, 2016, 6513416. [Google Scholar] [CrossRef] [Green Version]
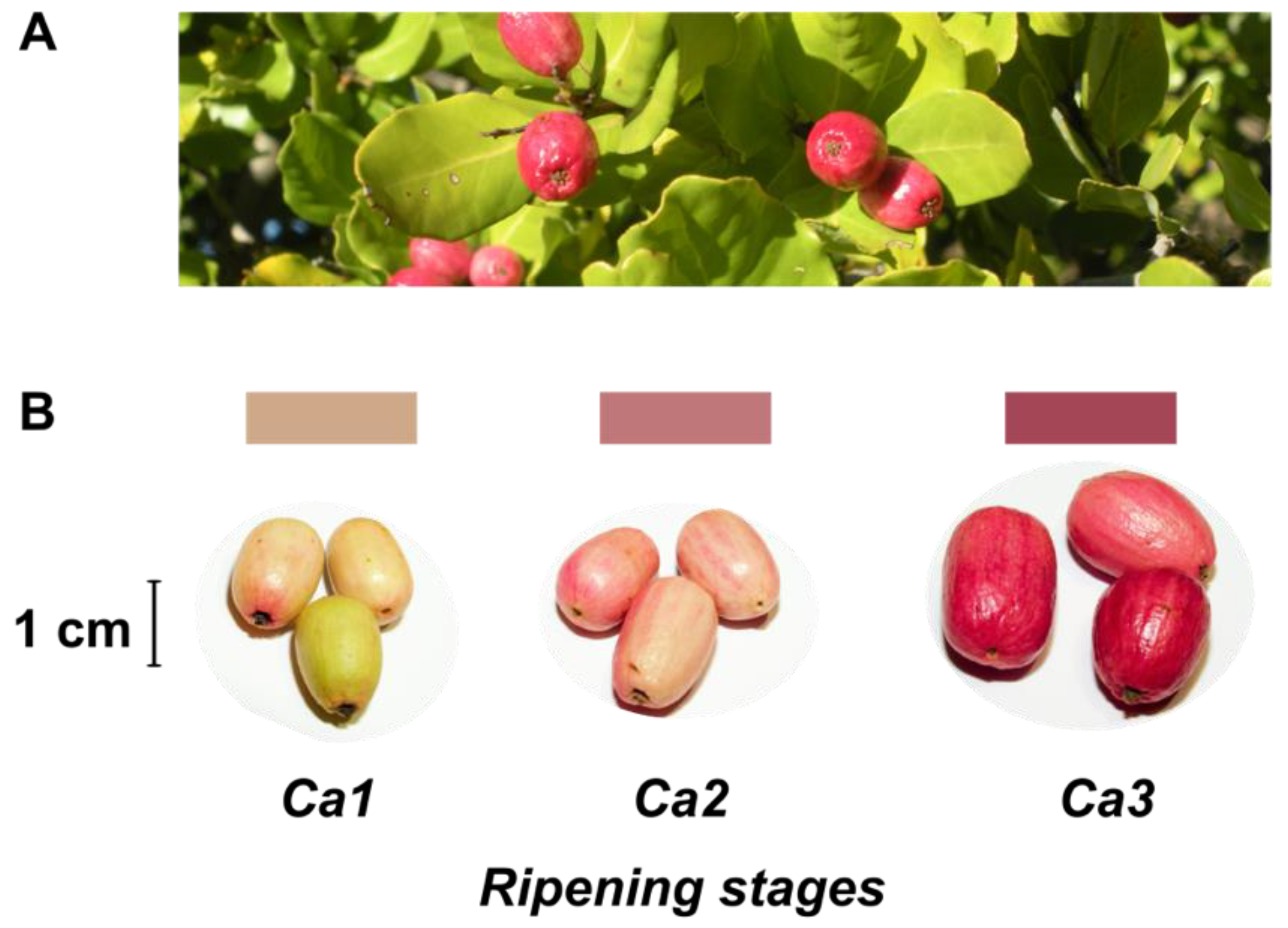
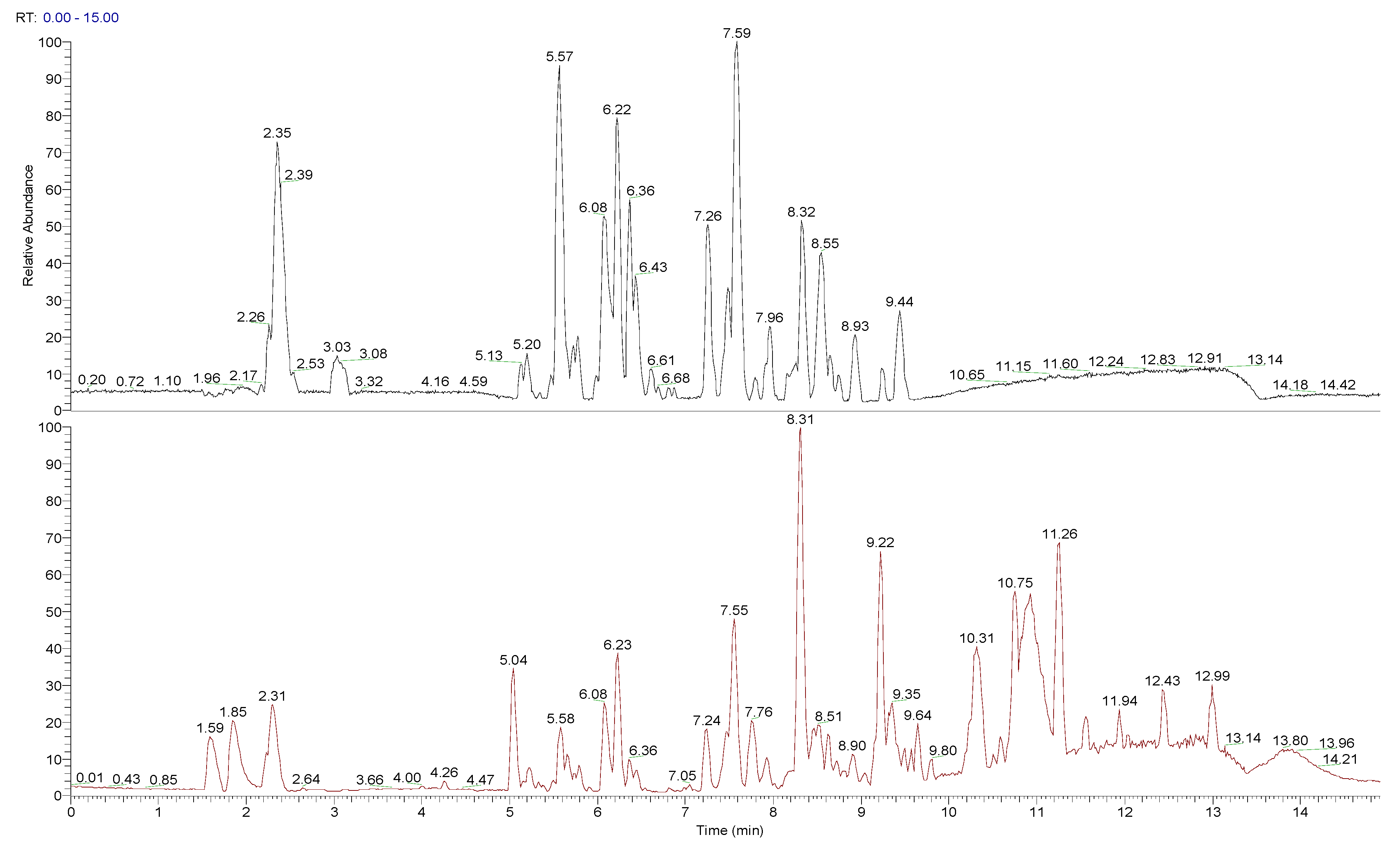
| Developmental Stages | |||||||
|---|---|---|---|---|---|---|---|
| Parameter | Harvest Season 2017 | Harvest Season 2018 | |||||
| Ca1 | Ca2 | Ca3 | Ca1 | Ca2 | Ca3 | ||
| Ethylene production | nd* | nd* | nd* | nd* | nd* | nd* | |
| Oxygen consumption (mg Kg−1 h−1) | 6.73 ± 0.00 a | 5.85 ± 0.04 b | 1.88 ± 0.04 c | 6.98 ± 0.01 a | 5.99 ± 0.02 b | 2,07 ± 0.02 c | |
| CO2 production (mg Kg−1 h−1) | 8.53 ± 0.00 a | 7.49 ± 0.04 b | 3.29 ± 0.04 c | 6.86 ± 0.04 a | 6.43 ± 0.02 b | 5.30 ± 0.51 c | |
| Firmness (N) | 7.54 ± 0.20 a | 5.99 ± 0.23 b | 4.97 ± 0.15 c | 7.93 ± 0.76 a | 6.04 ± 1.14 b | 5.03 ± 0.65 c | |
| pH | 5.66 ± 0.00 c | 5.75 ± 0.00 b | 5.90 ± 0.01 a | 6.12 ± 0.05 a | 6.26 ± 0.02 a | 6.26 ± 0.03 a | |
| TA (%) | nd* | nd* | nd* | nd* | nd* | nd* | |
| SSC (°Brix) | 24.72 ± 2.18 b | 32.50 ± 1.78 a | 38.06 ± 4.16 a | 30.00 ± 2.50 a | 25.83 ± 1.44 a | 29.17 ± 1.44 a | |
| Length (cm) | 1.25 ± 0,02 b | 1.34 ± 0.02 b | 1.59 ± 0.05 a | 1.76 ± 0.10 b | 2.08 ± 0.04 a | 2.10 ± 0.04 a | |
| Diameter (cm) | 0.91 ± 0.02 c | 0.97 ± 0.01 b | 1.08 ± 0.02 a | 1.21 ± 0.04 a | 1.25 ± 0.02 a | 1.22 ± 0.01 a | |
| L/D | 1.38 ± 0.02 b | 1.38 ± 0.02 b | 1.47 ± 0.03 a | 1.45 ± 0.05 b | 1.66 ± 0.02 a | 1.73 ± 0.05 a | |
| FW (g) | 0.58 ± 0.03 c | 0.79 ± 0.02 b | 1.19 ± 0.05 a | 1.75 ± 0.17 a | 2.08 ± 0.08 a | 1.99 ± 0.05 a | |
| DW (g) | 0.32 ± 0.02 c | 0.46 ± 0.01 b | 0.69 ± 0.03 a | 1.07 ± 0.11 a | 1.26 ± 0.06 a | 1.13 ± 0.03 a | |
| Humiditywet basis | 43.10 ± 0.43 a | 41.77 ± 0.54 b | 40.34 ± 0.47 b | 42.29 ± 0.46 a | 41.35 ± 1.20 a | 40.70 ± 0.63 b | |
| Humiditydry basis | 75.76 ± 0.29 a | 71.74 ± 0.05 b | 67.61 ± 0.07 b | 73.27 ± 0.16 a | 70.49 ± 0.55 a | 66.86 ± 0.12 b | |
| Water activity | 0.81 ± 0.01 a | 0.78 ± 0.02 a | 0.77 ± 0.04 a | 0.78 ± 0.02 a | 0.76 ± 0.02 a | 0.78 ± 0.05 a | |
| Color (CIElab*) | L* | 69.67 ± 0.88 a | 67.13 ± 1.08 a | 68.21 ± 1.20 a | 71.83 ± 0.82 a | 58.17 ± 1.03 b | 43.14 ± 1.10 c |
| a* | 10.66 ±1.28 b | 14.19 ± 1.81 a | 13.43 ± 1.89 a | 10.64 ± 0.78 c | 29.44 ± 1.04 b | 41.14 ± 0.76 a | |
| b* | 19.36 ± 0.63 a | 20.05 ± 1.38 a | 17.80 ± 1.31 b | 18.57 ± 0.48 a | 11.70 ± 0.59 b | 11.10 ± 0.28 b | |
| C | 22.10 ± 1.43 a | 24.56 ± 2.28 a | 22.31 ± 2.30 a | 21.07 ± 2.47 c | 31.87 ± 0.98 b | 42.61 ± 0.77 a | |
| h° | 61.16 ± 0.56 a | 54.71 ± 1.03 b | 52.97 ± 1.17 b | 60.27 ± 2.30 a | 21.92 ± 1.49 b | 15.12 ± 0.33 c | |
| Development Stage | TPC [mgGAE/gFW] | TFC [mgQE/gFW] | FRAP [μmol FeSO4/gFW] | TEAC [mmol TE/gFW] | DPPH [IC50 μg/mL] | ORAC [mmol TE/gFW] | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Season 2017 | ||||||||||||
| Ca1 | 11.19 ± 0.7.4 | b | 7.34 ± 0.27 | c | 28.62 ± 0.95 | b | 4.34 ± 0.34 | c | 6.84 ± 0.17 | b | n.a. | |
| Ca2 | 13.70 ± 1.03 | b | 8.52 ± 0.33 | b | 35.96 ± 0.36 | a | 7.23 ± 0.32 | b | 6.89 ± 0.54 | b | n.a. | |
| Ca3 | 17.87 ± 1.57 | a | 9.21 ± 0.28 | a | 38.34 ± 0.33 | a | 8.09 ± 0.22 | a | 8.72 ± 0.14 | a | n.a. | |
| Season 2018 | ||||||||||||
| Ca1 | 12.85 ± 1.16 | c | 6.98 ± 0.21 | c | 29.49 ± 2.36 | b | 5.02 ± 0.39 | b | 7.69 ± 0.90 | b | 0.208 ± 0.010 | a |
| Ca2 | 15.15 ± 0.71 | a | 8.46 ± 0.39 | b | 35.94 ± 1.23 | a | 7.12 ± 0.18 | a | 7.09 ± 0.19 | a | 0.199 ± 0.002 | a |
| Ca3 | 17.61 ± 0.60 | a | 9.44 ± 0.18 | a | 37.08 ± 0.75 | a | 7.91 ± 0.30 | a | 8.35 ± 0.53 | a | 0.188 ± 0.002 | a |
| Blueberry | 2.75 ± 0.2 | 2.12 ± 0.44 | 4.95 ± 0.28 | 1.25 ± 0.30 | 11.36 ± 0.96 | 0.032 ± 0.000 | ||||||
| RT (min) | [M + X]+ (m/z) | [M − X]− (m/z) | [M] (m/z) | Fragments | MF | Tentative Compound |
|---|---|---|---|---|---|---|
| 2.35 | 343.1236 [M + H]+ | 341.1112 [M − H]− 683.2303 [2M − H]− | 342 | −89.0227, 101.0226, 119.0330, 143.0329, 161.0433, 179.0537 | C12H22O11 | Sucrose |
| 5.04 | 316.2121 [M + H]+ | 315 | 102.0916, 123.1169, 184.1695, 255.1590 | C19H25NO3 | Cryprochine Isocryprochine | |
| 5.13 | 867.2132 [M + H]+ | 865.2036 [M − H]− | 866 | −287.0587 −407.0808 −451.1076 −577.1406 −695.1471 713.1580 −739.1739 −847.0959 | C45H38O18 | Procyanidin C1 |
| 5.20 | 579.1500 [M + H]+ 1155.2760 [2M + H]+ | 577.1387 [M − H]− 1153.2665 [2M − H]− | 578 | −289.0744 −407.0809 −425.0918 −451.1076 | C30H26O12 | Procyanidin B1 Procyanidin B2 |
| 5.57 | 355.1025 [M + H]+ 377.0845 [M + Na]+ | 353.0900 [M − H]− 707.1879 [2M − H]− | 354 | 135.0458 179.0362 | C16H18O9 | 4-Caffeoylquinic acid |
| 579.1500 [M + H]+ | 577.1386 [M − H]− | 578 | C30H26O12 | Procyanidin B1 Procyanidin B2 | ||
| 867.2131 [M + H]+ | 865.2020 [M − H]− | 866 | C45H38O18 | Procyanidin C1 | ||
| 6.08 | 355.1027 [M + H]+ 377.0846 [M + Na]+ 731.1894 [2M + Na]+ | 353.0903 [M − H]− 707.1885 [2M − H]− | 354 | 191.0575 | C16H18O9 | Chlorogenic acid |
| 6.22 | 291.0863 [M + H]+ 313.0680 [M + Na]+ | 289.0737 [M − H]− 353.0900 579.1549 [2M − H]− | 290 | −179.0357, 205.0516 245.0811 | C15H14O6 | Catechin, Epicatechin |
| 6.35 | 470.1659 [M + H]+ 492.1486 [M + Na]+ | 468.1540 [M − H]− 937.3156 [2M − H]− | 469 | 292.1217, 424.1651 | Unidentified | |
| 6.43 | 355.1024 [M + H]+ 731.1821 [2M + Na]+ | 353.0901 [M − H]− 707.1879 [2M − H]− | 354 | −191.0568 | C16H18O9 | Analogue of chlorogenic acid 4-Caffeoylquinic acid |
| 7.26 | 465.1031 [M + H]+ 487.0849 [M + Na]+ | 463.0912 [M − H]− | 464 | 301.0380 178.9999 151.0046 | C21H20O11 | Isoquercitirin Hyperoside |
| 7.48 | 435.0926 [M + H]+ 457.0743 [M + Na]+ | 433.0801 [M − H]− | 434 | −301.0381 | C20H18O11 | Reynoutrin Quercetin 3-O-α-D-arabinopyranoside Quercetin 3-O-xyloside |
| 551.1037 [M + H]+ 573.0854 [M + Na]+ | 549.0919 | 550 | (−)-Rubrichalcolactone | |||
| 505.1018 [M − H]− | 504 | (6-(5,7-dihydroxy-2-(4-hydroxy-3-methoxyphenyl)-4-oxo-4H-chromen-8-yl)-3,4,5-trihydroxytetrahydro-2H-pyran-2-yl)methyl acetate | ||||
| 7.59 | 449.1081 [M + H]+ | 447.0963 [M − H]− | 448 | −301.0378 | C21H20O11 | Quercetin 3-O-α-D-rhamnopyranoside |
| 7.76 | 353.1361 [M + H]+ 376.2120 [M + Na]+ | 351.1412 [M − H]− 375.1473 [M + Na−H]− | 352 | 335.1254 | C22H40O3 | cryptorigidifoliol A |
| 7.96 | 463.1238 [M + H]+ | 461.1119 [M − H]− | 462 | C22H22O11 | Isorhamnetin-3-O-rhamnoside Luteolin 7-O-glucuronide | |
| 8.30 | 360.2155 [M + H]+ | 359.1525 | 358 | 313.1465, 327.1466, 341.1624 | C20H24O6 | (+)-Lariciresinol 4-O-Methylcedrusin |
| 8.93 | 263.1641 [M + H]+ 285.1458 [M + Na]+ | 262 | 165.0546 | C16H22O3 | 1′R*,3′S*,4′R*,5′S*,6S-6-[(4′-ethyl-9′-oxabicycle[3.3.1]non-6′-en-3′-yl)methyl]- 5,6- dihydro-2H-pyran-2-one | |
| 9.21 | 376.2599 [M + H]+ 398.2415 [M + Na]+ | 374.2470 [M − H]− | 375 | 209.1284 275.1754 293.1861 302.1864 | Unidentified | |
| 9.44 | 315.1620 | −118.0428 −163.0409 −271.1726 | C16H12O7 | Sexangularetin | ||
| 10.31 | 305.1747 [M + H]+ 327.1568 [M + Na]+ | 304 | Unidentified | |||
| 10.75 | 247.1694 [M + H]+ 269.1513 [M + Na]+ | 246 | 173.1328 229.1592 | C15H18O3 | Ethyl 5-hydroxy-7-phenyl-2,6-heptadienoate | |
| 11.26 | 249.1850 [M + H]+ 271.1668 [M + Na]+ | 248 | 133.1016 231.1750 | C16H24O2 | (4R,6S)-10-Phenyl-1-decene-4,6-diol | |
| Antioxidant capacity of peumo extract | ||||||
| ORAC (mmol/g DW | 0.637 ± 0.061 | |||||
| TPC (mgGAE/gDW) | 23.81 ± 3.06 | |||||
| TFC (mgQE/gDW) | 18.84 ± 3.33 | |||||
| Sample | IC50 [mg/L] |
|---|---|
| Peumo | 14.8 ± 1.5 |
| Quercetin | 6.3 ± 0.6 |
| Sample\Activity | IC50 [mg/L] | ||
|---|---|---|---|
| NO | TNF-α | IL-6 | |
| Peumo | 13.2 ± 0.5 b | 129.5 ± 3.5 b | 40.0 ± 4.0 b |
| Quercetin | 4.4 ± 0.6 a | 8.7 ± 0.7 a | 5.0 ± 0.5 a |
| Indomethacin | 19.4 ± 0.3 c | >40 | >40 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valdenegro, M.; Bernales, M.; Knox, M.; Vinet, R.; Caballero, E.; Ayala-Raso, A.; Kučerová, D.; Kumar, R.; Viktorová, J.; Ruml, T.; et al. Characterization of Fruit Development, Antioxidant Capacity, and Potential Vasoprotective Action of Peumo (Cryptocarya alba), a Native Fruit of Chile. Antioxidants 2021, 10, 1997. https://doi.org/10.3390/antiox10121997
Valdenegro M, Bernales M, Knox M, Vinet R, Caballero E, Ayala-Raso A, Kučerová D, Kumar R, Viktorová J, Ruml T, et al. Characterization of Fruit Development, Antioxidant Capacity, and Potential Vasoprotective Action of Peumo (Cryptocarya alba), a Native Fruit of Chile. Antioxidants. 2021; 10(12):1997. https://doi.org/10.3390/antiox10121997
Chicago/Turabian StyleValdenegro, Mónika, Maricarmen Bernales, Marcela Knox, Raúl Vinet, Eduardo Caballero, Aníbal Ayala-Raso, Denisa Kučerová, Rohitesh Kumar, Jitka Viktorová, Tomáš Ruml, and et al. 2021. "Characterization of Fruit Development, Antioxidant Capacity, and Potential Vasoprotective Action of Peumo (Cryptocarya alba), a Native Fruit of Chile" Antioxidants 10, no. 12: 1997. https://doi.org/10.3390/antiox10121997
APA StyleValdenegro, M., Bernales, M., Knox, M., Vinet, R., Caballero, E., Ayala-Raso, A., Kučerová, D., Kumar, R., Viktorová, J., Ruml, T., Figueroa, C. R., & Fuentes, L. (2021). Characterization of Fruit Development, Antioxidant Capacity, and Potential Vasoprotective Action of Peumo (Cryptocarya alba), a Native Fruit of Chile. Antioxidants, 10(12), 1997. https://doi.org/10.3390/antiox10121997









