Yogurt Fortification by the Addition of Microencapsulated Stripped Weakfish (Cynoscion guatucupa) Protein Hydrolysate
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Protein Hydrolysate
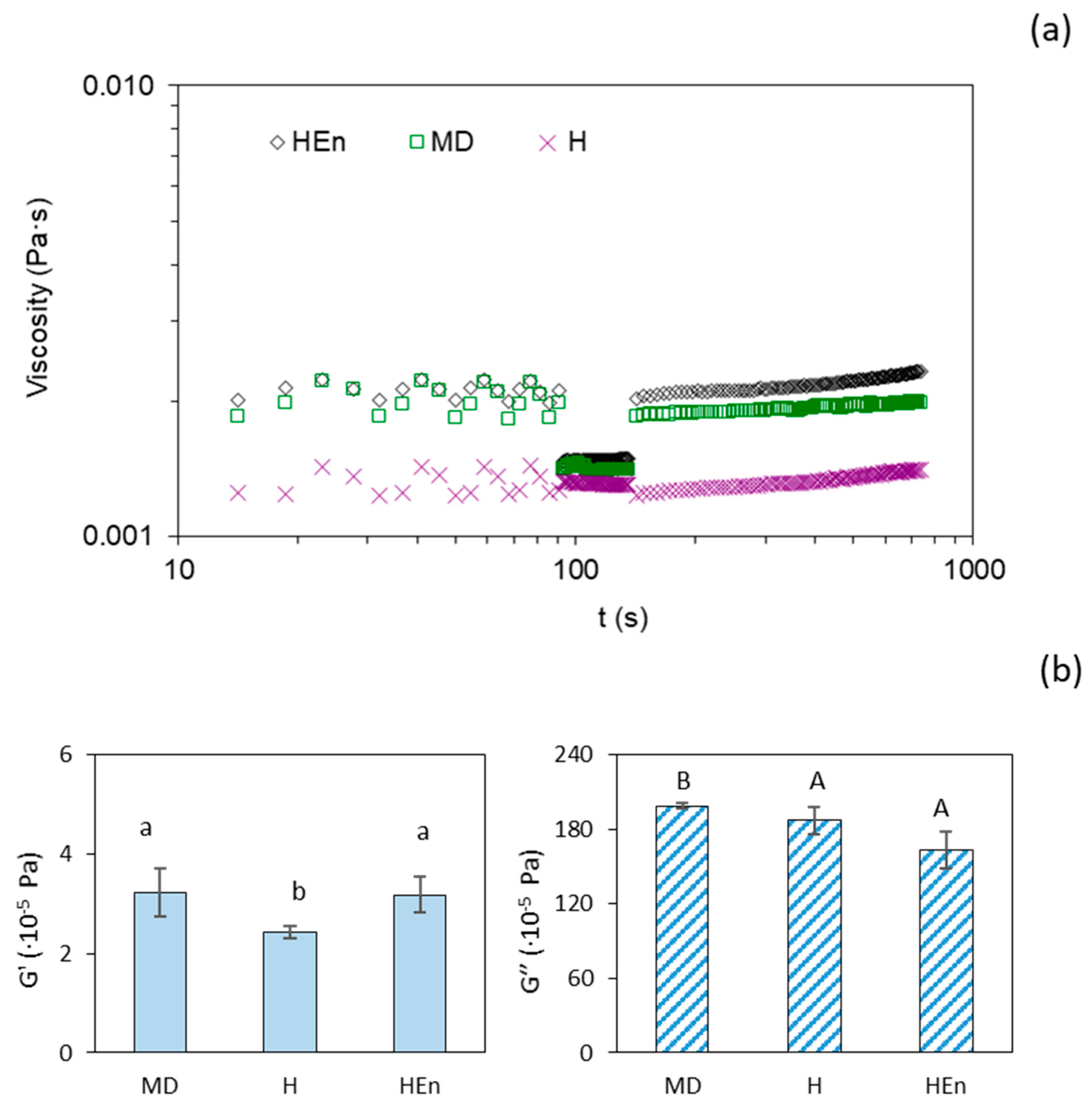
2.3. Microencapsulation
2.4. Yogurt Preparation
2.5. Yogurt Characterization
2.5.1. Proximal Analyses, Physicochemical Analysis and Color
2.5.2. Syneresis
2.5.3. Texture Analysis
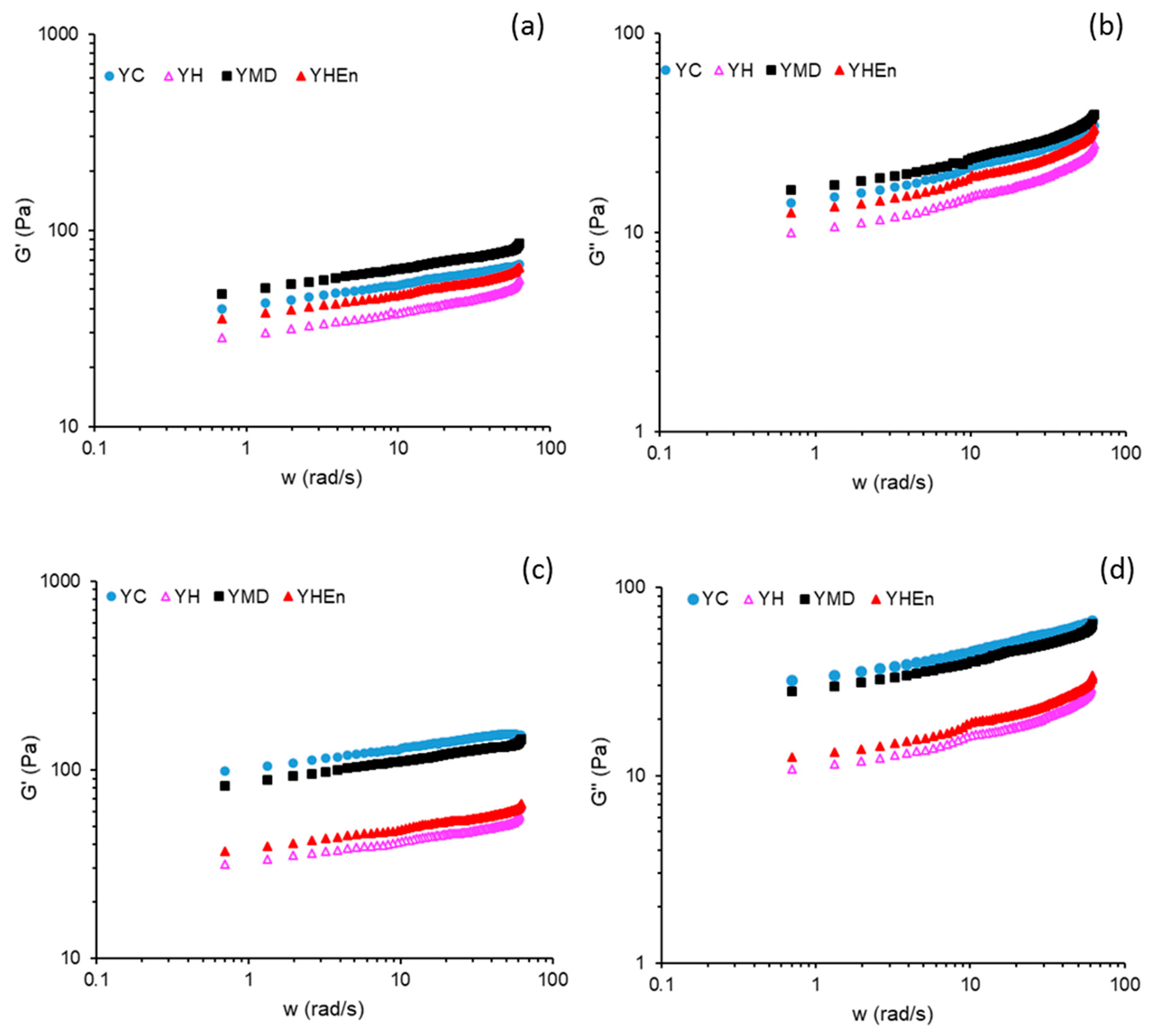
2.5.4. Rheological Analyses
2.6. Microbiological Analysis
2.7. Yogurt Bioactivity
2.7.1. Antioxidant Activity
ABTS Radical Scavenging Activity
Reducing Power
2.7.2. ACE Inhibitory Activity
2.8. Sensory Analysis
2.9. Statistical Analysis
3. Results and Discussion
3.1. Visual Appearance, Proximal Analyses and Physicochemical Properties of Yogurts
3.2. Textural and Viscoelastic Properties
3.3. Microbiological Analysis
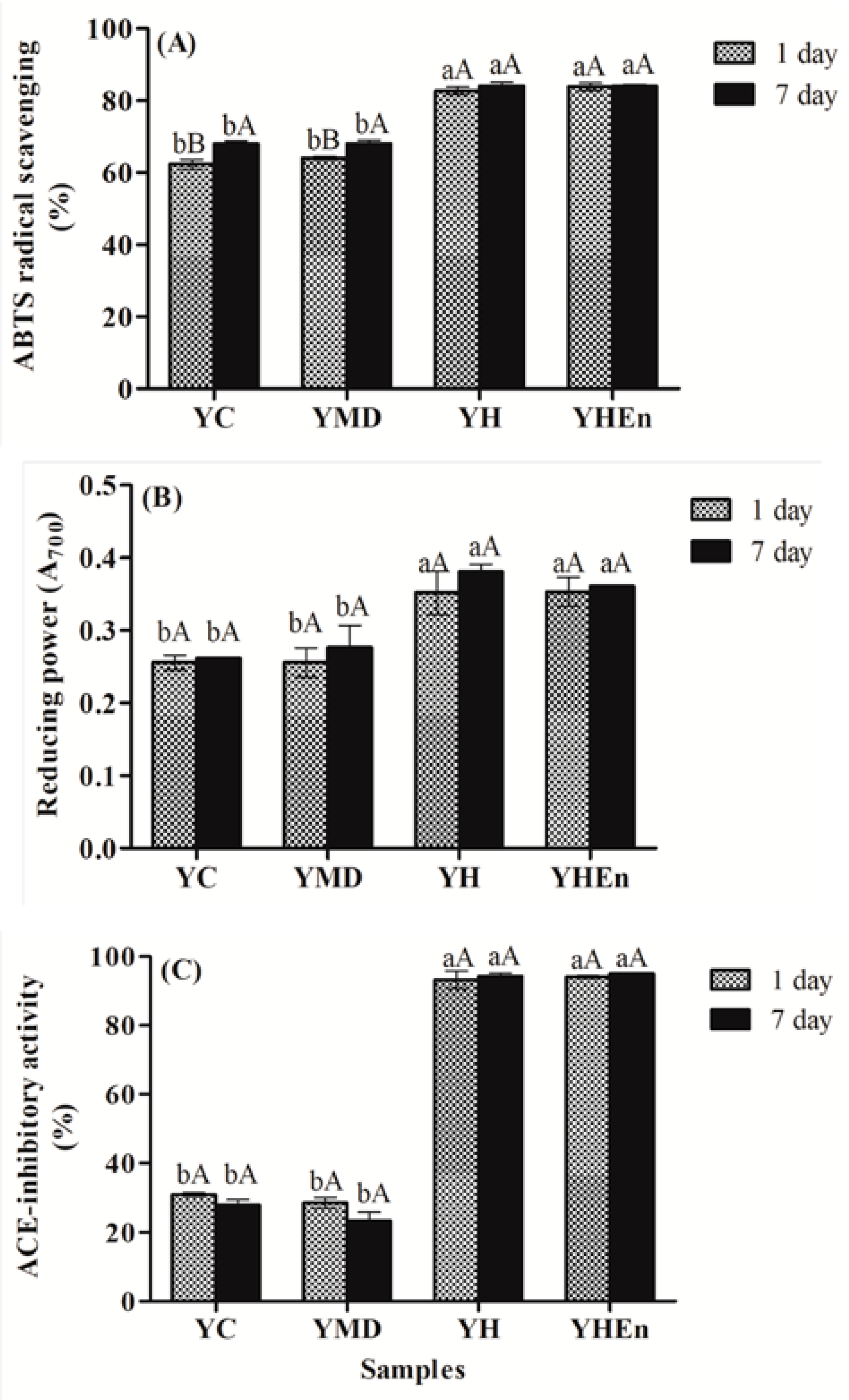
3.4. Bioactivity
3.4.1. Antioxidant Activity
3.4.2. ACE Inhibitory Activity
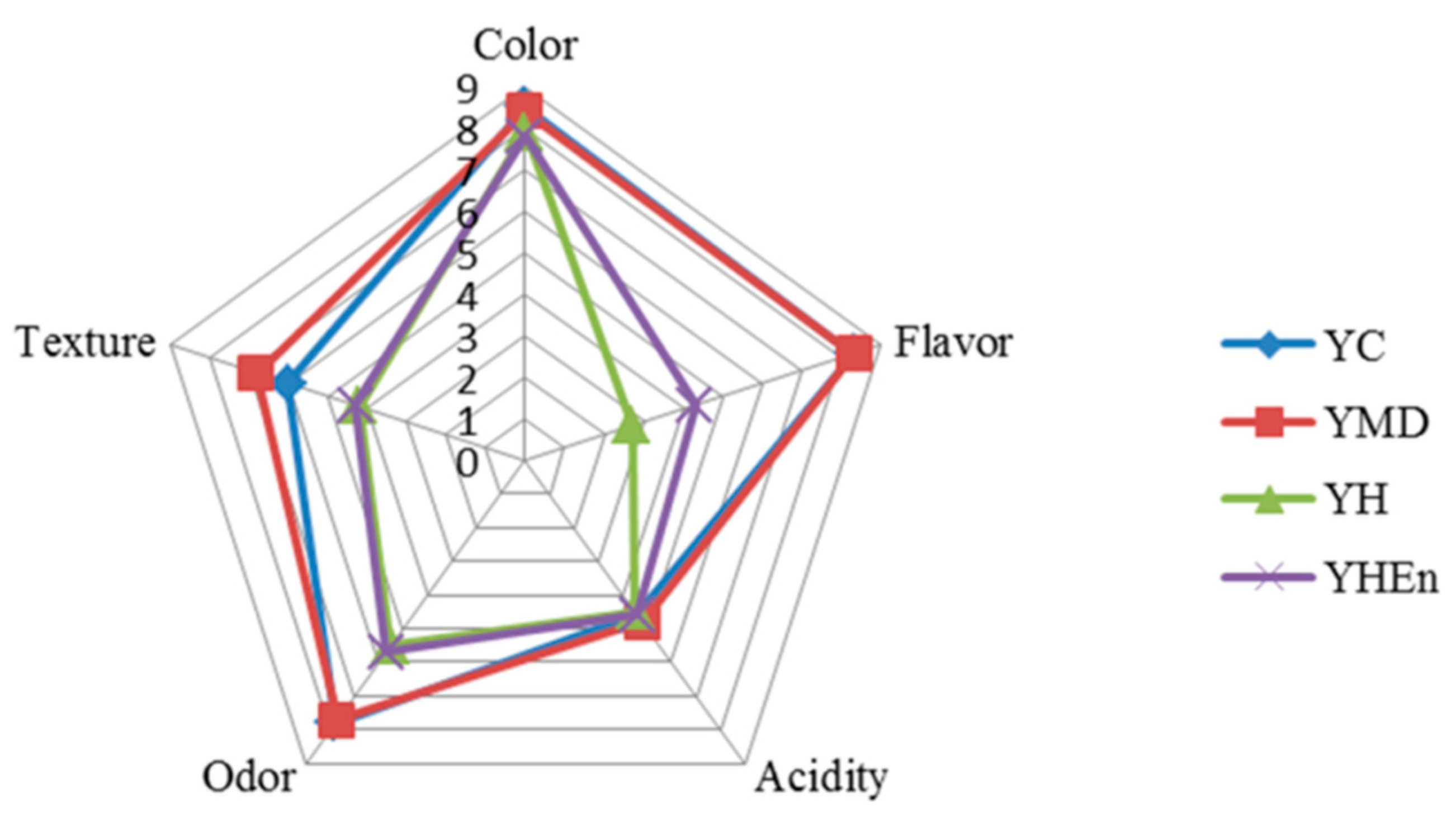
3.5. Sensory Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jaster, H.; Arend, G.D.; Rezzadori, K.; Chaves, V.C.; Reginatto, F.H.; Petrus, J.C.C. Enhancement of antioxidant activity and physicochemical properties of yogurt enriched with concentrated strawberry pulp obtained by block freeze concentration. Food Res. Int. 2018, 104, 119–125. [Google Scholar] [CrossRef]
- Ozturkoglu-Budak, S.; Akal, C.; Yetisemiyen, A. Effect of dried nut fortification on functional, physicochemical, textural, and microbiological properties of yogurt. J. Dairy Sci. 2016, 99, 8511–8523. [Google Scholar] [CrossRef] [Green Version]
- Gahruie, H.H.; Eskandari, M.H.; Mesbahi, G.; Hanifpour, M.A. Scientific and technical aspects of yogurt fortification: A review. Food Sci. Hum. Wellness 2015, 4, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Barkallah, M.; Dammak, M.; Louati, I.; Hentati, F.; Hadrich, B.; Mechichi, T.; Ayadi, M.A.; Fendri, M.; Attia, H.; Abdelkafi, S. Effect of Spirulina platensis fortification on physicochemical, textural, antioxidant and sensory properties of yogurt during fermentation and storage. LWT—Food Sci. Technol. 2017, 84, 323–330. [Google Scholar] [CrossRef]
- Da Silva, S.C.; Fernandes, I.P.; Barros, L.; Fernandes, Â.; Alvez, M.J.; Calhelha, R.C.; Pereira, C.; Barreira, J.C.M.; Manrique, Y.; Colla, E.; et al. Spray-dried Spirulina platensis as an effective ingredient to improve yogurt formulations: Testing different encapsulating solutions. J. Funct. Foods 2019, 60, 103427. [Google Scholar] [CrossRef] [Green Version]
- Demirci, T.; Aktaş, K.; Sözeri, D.; Öztürk, H.İ.; Akın, N. Rice bran improve probiotic viability in yoghurt and provide added antioxidative benefits. J. Funct. Foods 2017, 36, 396–403. [Google Scholar] [CrossRef]
- Francisco, C.R.L.; Heleno, S.A.; Fernandes, I.P.M.; Barreira, J.C.M.; Calhelha, R.C.; Barros, L.; Gonçalves, O.H.; Ferreira, I.C.F.R.; Barreiro, M.F. Functionalization of yogurts with Agaricus bisporus extracts encapsulated in spray-dried maltodextrin crosslinked with citric acid. Food Chem. 2018, 245, 845–853. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shori, A.B.; Baba, A.S.; Chuah, P.F. The effects of fish collagen on the proteolysis of milk proteins, ACE inhibitory activity and sensory evaluation of plain- and Allium sativum-yogurt. J. Taiwan Inst. Chem. Eng. 2013, 44, 701–706. [Google Scholar] [CrossRef]
- Abdel-Hamid, M.; Romeih, E.; Huang, Z.; Enomoto, T.; Huang, L.; Li, L. Bioactive properties of probiotic set-yogurt supplemented with Siraitia grosvenorii fruit extract. Food Chem. 2020, 303, 125400. [Google Scholar] [CrossRef]
- Wulandani, B.R.D.; Marsono, Y.; Utami, T.; Rahayu, E.S. Potency of yogurt as angiotensin converting enzyme inhibitor with addition of Ficus glomerata Roxb fruit extract. Int. Food Res. J. 2018, 25, 1153–1158. [Google Scholar]
- Hemker, A.K.; Nguyen, L.T.; Karwe, M.; Salvi, D. Effects of pressure-assisted enzymatic hydrolysis on functional and bioactive properties of tilapia (Oreochromis niloticus) by-product protein hydrolysates. LWT—Food Sci. Technol. 2020, 122, 109003. [Google Scholar] [CrossRef]
- Neves, A.C.; Harnedy, P.A.; O’Keeffe, M.B.; FitzGerald, R.J. Bioactive peptides from Atlantic salmon (Salmo salar) with angiotensin converting enzyme and dipeptidyl peptidase IV inhibitory, and antioxidant activities. Food Chem. 2017, 218, 396–405. [Google Scholar] [CrossRef]
- Ambigaipalan, P.; Shahidi, F. Bioactive peptides from shrimp shell processing discards: Antioxidant and biological activities. J. Funct. Foods 2017, 34, 7–17. [Google Scholar] [CrossRef]
- Rivero-Pino, F.; Espejo-Carpio, F.J.; Guadix, E.M. Bioactive fish hydrolysates resistance to food processing. LWT—Food Sci. Technol. 2020, 117, 108670. [Google Scholar] [CrossRef]
- Nimse, S.B.; Pal, D. Free radicals, natural antioxidants, and their reaction mechanisms. RSC Adv. 2015, 5, 27986–28006. [Google Scholar] [CrossRef] [Green Version]
- Ma, Y.S.; Zhao, H.J.; Zhao, X.H. Comparison of the Effects of the Alcalase-Hydrolysates of Caseinate, and of Fish and Bovine Gelatins on the Acidification and Textural Features of Set-Style Skimmed Yogurt-Type Products. Foods 2019, 8, 501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akbarbaglu, Z.; Jafari, S.M.; Sarabandi, K.; Mohammadi, M.; Heshmati, M.K.; Pezeshki, A. Influence of spray drying encapsulation on the retention of antioxidant properties and microstructure of flaxseed protein hydrolysates. Colloids Surf. B Biointerfaces 2019, 178, 421–429. [Google Scholar] [CrossRef] [PubMed]
- Murthy, L.N.; Phadke, G.G.; Mohan, C.O.; Chandra, M.V.; Annamalai, J.; Visnuvinayagam, S.; Ravishankar, C.N. Characterization of Spray-Dried Hydrolyzed Proteins from Pink Perch Meat Added with Maltodextrin and Gum Arabic. J. Aquat. Food Prod. Technol. 2017, 26, 913–928. [Google Scholar] [CrossRef]
- Mohan, A.; Rajendran, S.R.C.K.; He, Q.S.; Bazinet, L.; Udenigwe, C.C. Encapsulation of food protein hydrolysates and peptides: A review. RSC Adv. 2015, 5, 79270–79278. [Google Scholar] [CrossRef]
- Gómez-Mascaraque, L.G.; Miralles, B.; Recio, I.; López-Rubio, A. Microencapsulation of a whey protein hydrolysate within micro-hydrogels: Impact on gastrointestinal stability and potential for functional yoghurt development. J. Funct. Foods 2016, 26, 290–300. [Google Scholar] [CrossRef] [Green Version]
- Sarabandi, K.; Mahoonak, A.S.; Hamishekar, H.; Ghorbani, M.; Jafari, S.M. Microencapsulation of casein hydrolysates: Physicochemical, antioxidant and microstructure properties. J. Food Eng. 2018, 237, 86–95. [Google Scholar] [CrossRef]
- Zhu, F. Encapsulation and delivery of food ingredients using starch based systems. Food Chem. 2017, 229, 542–552. [Google Scholar] [CrossRef]
- Tan, S.; Zhong, C.; Langrish, T. Pre-gelation assisted spray drying of whey protein isolates (WPI) for microencapsulation and controlled release. LWT—Food Sci. Technol. 2020, 117, 108625. [Google Scholar] [CrossRef]
- Lima, K.O.; Alemán, A.; López-Caballero, M.E.; Gómez-Guillén, M.C.; Montero, M.P.; Prentice, C.; Huisa, A.J.T.; Monserrat, J.M. Characterization, stability, and in vivo effects in Caenorhabditis elegans of microencapsulated protein hydrolysates from stripped weakfish (Cynoscion guatucupa) industrial byproducts. Food Chem. 2021, 364, 130380. [Google Scholar] [CrossRef]
- Lima, K.O.; de Quadros, C.D.C.; da Rocha, M.; de Lacerda, J.T.J.G.; Juliano, M.A.; Dias, M.; Mendes, M.A.; Prentice, C. Bioactivity and bioaccessibility of protein hydrolyzates from industrial byproducts of Stripped weakfish (Cynoscion guatucupa). LWT—Food Sci. Technol. 2019, 111, 408–413. [Google Scholar] [CrossRef]
- Lee, W.J.; Lucey, J.A. Formation and Physical Properties of Yogurt. Asian Australas. J. Anim. Sci. 2010, 23, 1127–1136. [Google Scholar] [CrossRef]
- AOAC (Ed.) Official Methods of Analysis, 18th ed.; The Association of Official Analytical Chemists: Washington, DC, USA, 2005. [Google Scholar]
- Santillán-Urquiza, E.; Méndez-Rojas, M.Á.; Vélez-Ruiz, J.F. Fortification of yogurt with nano and micro sized calcium, iron and zinc, effect on the physicochemical and rheological properties. LWT—Food Sci. Technol. 2017, 80, 462–469. [Google Scholar] [CrossRef]
- Arancibia, M.Y.; López-Caballero, M.E.; Gómez-Guillén, M.C.; Montero, P. Release of volatile compounds and biodegradability of active soy protein lignin blend films with added citronella essential oil. Food Control 2014, 44, 7–15. [Google Scholar] [CrossRef] [Green Version]
- Zhang, T.; Jeong, C.H.; Cheng, W.N.; Bae, H.; Seo, H.G.; Petriello, M.C.; Han, S.G. Moringa extract enhances the fermentative, textural, and bioactive properties of yogurt. LWT—Food Sci. Technol. 2019, 101, 276–284. [Google Scholar] [CrossRef]
- Zheng, L.; Zhao, M.; Xiao, C.; Zhao, Q.; Su, G. Practical problems when using ABTS assay to assess the radical-scavenging activity of peptides: Importance of controlling reaction pH and time. Food Chem. 2016, 192, 288–294. [Google Scholar] [CrossRef] [PubMed]
- Canabady-Rochelle, L.L.S.; Harscoat-Schiavo, C.; Kessler, V.; Aymes, A.; Fournier, F.; Girardet, J.M. Determination of reducing power and metal chelating ability of antioxidant peptides: Revisited methods. Food Chem. 2015, 183, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Alemán, A.; Giménez, B.; Pérez-Santin, E.; Gómez-Guillén, M.C.; Montero, P. Contribution of Leu and Hyp residues to antioxidant and ACE-inhibitory activities of peptide sequences isolated from squid gelatin hydrolysate. Food Chem. 2011, 125, 334–341. [Google Scholar] [CrossRef] [Green Version]
- Benedetti, S.; Prudencio, E.S.; Müller, C.M.O.; Verruck, S.; Mandarino, J.M.G.; Leite, R.S.; Petrus, J.C.C. Utilization of tofu whey concentrate by nano filtration process aimed at obtaining a functional fermented lactic beverage. J. Food Eng. 2016, 171, 222–229. [Google Scholar] [CrossRef]
- Córdova-Ramos, J.S.; Gonzales-Barron, U.; Cerrón-Mallqui, L.M. Physicochemical and sensory properties of yogurt as affected by the incorporation of jumbo squid (Dosidicus gigas) powder. LWT—Food Sci. Technol. 2018, 93, 506–510. [Google Scholar] [CrossRef] [Green Version]
- Sah, B.N.P.; Vasiljevic, T.; Mckechnie, S.; Donkor, O.N. Effect of refrigerated storage on probiotic viability and the production and stability of antimutagenic and antioxidant peptides in yogurt supplemented with pineapple peel. J. Dairy Sci. 2015, 98, 5905–5916. [Google Scholar] [CrossRef]
- Sah, B.N.P.; Vasiljevic, T.; McKechnie, S.; Donkor, O.N. Physicochemical, textural and rheological properties of probiotic yogurt fortified with fibre-rich pineapple peel powder during refrigerated storage. LWT—Food Sci. Technol. 2016, 65, 978–986. [Google Scholar] [CrossRef]
- Carmona, J.C.; Robert, P.; Vergara, C.; Sáenz, C. Microparticles of yellow-orange cactus pear pulp (Opuntia ficus-indica) with cladode mucilage and maltodextrin as a food coloring in yogurt. LWT—Food Sci. Technol. 2021, 138, 110672. [Google Scholar] [CrossRef]
- Öztürk, H.İ.; Aydın, S.; Sözeri, D.; Demirci, T.; Sert, D.; Akın, N. Fortification of set-type yoghurts with Elaeagnus angustifolia L. flours: Effects on physicochemical, textural, and microstructural characteristics. LWT—Food Sci. Technol. 2018, 90, 620–626. [Google Scholar] [CrossRef]
- Akalin, A.S.; Unal, G.; Dinkci, N.; Hayaloglu, A.A. Microstructural, textural, and sensory characteristics of probiotic yogurts fortified with sodium calcium caseinate or whey protein concentrate. J. Dairy Sci. 2012, 95, 3617–3628. [Google Scholar] [CrossRef]
- Caleja, C.; Barros, L.; Antonio, A.L.; Carocho, M.; Oliveira, M.B.P.P.; Ferreira, I.C.F.R. Fortification of yogurts with different antioxidant preservatives: A comparative study between natural and synthetic additives. Food Chem. 2016, 210, 262–268. [Google Scholar] [CrossRef] [Green Version]
- Halim, N.R.A.; Yusof, H.M.; Sarbon, N.M. Functional and Bioactive Properties of Fish Protein Hydolysates and Peptides: A Comprehensive Review. Trends Food Sci. Technol. 2016, 51, 24–33. [Google Scholar] [CrossRef]
- Campo-Deaño, L.; Tovar, C.A. The effect of egg albumen on the viscoelasticity of crab sticks made from Alaska pollock and Pacific whiting surimi. Food Hydrocoll. 2009, 23, 1641–1646. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Y.; Liu, W. Dipole–dipole and H-bonding interactions signifi cantly enhance the multifaceted mechanical properties of thermoresponsive shape memory hydrogels. Adv. Funct. Mat. 2015, 25, 471–480. [Google Scholar] [CrossRef]
- Borderías, A.J.; Tovar, C.A.; Domínguez-Timón, F.; Díaz, M.T.; Pedrosa, M.M.; Moreno, H.M. Characterization of healthier mixed surimi gels obtained through partial substitution of myofibrillar proteins by isolate pea proteins. Food Hydrocoll. 2020, 107, 105976. [Google Scholar] [CrossRef]
- Piñeiro-Lago, L.; Franco, M.I.; Tovar, C.A. Changes in thermoviscoelastic and biochemical properties of Atroncau blancu and roxu Afuega’l Pitu cheese (PDO) during ripening. Food Res. Int. 2020, 137, 109693. [Google Scholar] [CrossRef]
- Moreno, H.M.; Bargiela, V.; Tovar, C.A.; Cando, D.; Borderias, A.J.; Herranz, B. High pressure applied to frozen flying fish (Parexocoetus brachyterus) surimi: Effect on physicochemical and rheological properties of gels. Food Hydrocoll. 2015, 48, 127–134. [Google Scholar] [CrossRef]
- Zhao, L.L.; Wang, X.L.; Liu, Z.P.; Sun, W.H.; Dai, Z.Y.; Ren, F.Z.; Mao, X.Y. Effect of α-lactalbumin hydrolysate-calcium complexes on the fermentation process and storage properties of yogurt. LWT—Food Sci. Technol. 2018, 88, 35–42. [Google Scholar] [CrossRef]
- Hervert, C.J.; Martin, N.H.; Boor, K.J.; Wiedmann, M. Survival and detection of coliforms, Enterobacteriaceae, and gram-negative bacteria in Greek yogurt. J. Dairy Sci. 2017, 100, 950–960. [Google Scholar] [CrossRef] [Green Version]
- Vedamuthu, E.R. Starter cultures for yogurt and fermented milks. In Manufacturing Yogurt and Fermented Milks; Chandan, R.C., Ed.; Blackwell Publishing Ltd.: Ames, IA, USA, 2006. [Google Scholar]
- Acharya, K.R.; Sturrock, E.D.; Riordan, J.F.; Ehlers, M.R.W. ACE revisited: A new target for structure-based drug design. Nat. Rev. Drug Discov. 2003, 2, 891–902. [Google Scholar] [CrossRef]
- Hernández-Ledesma, B.; Del Mar Contreras, M.; Recio, I. Antihypertensive peptides: Production, bioavailability and incorporation into foods. Adv. Colloid Interface Sci. 2011, 165, 23–35. [Google Scholar] [CrossRef] [Green Version]
- Fitzgerald, R.J.; Murray, B.A.; Walsh, D.J. Hypotensive Peptides from Milk Proteins. J. Nutr. 2004, 134, 980–988. [Google Scholar] [CrossRef] [PubMed] [Green Version]
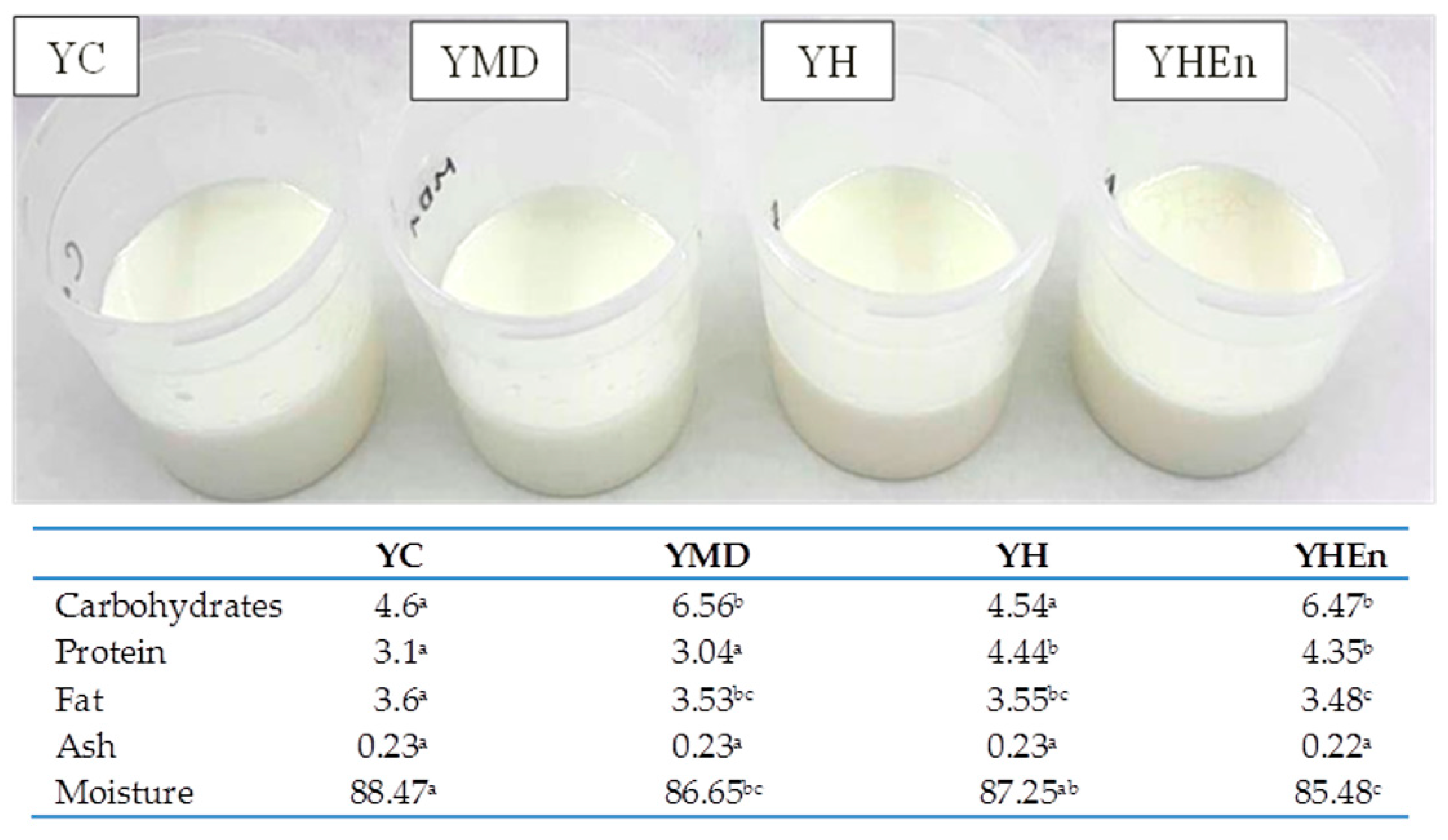
| Parameters | Time (Days) | Samples | |||
|---|---|---|---|---|---|
| YC | YMD | YH | YHEn | ||
| pH | 1 | 4.58 ± 0.01 bA | 4.61 ± 0.01 aA | 4.57 ± 0.01 bA | 4.53 ± 0.02 cA |
| 7 | 4.38 ± 0.01 bB | 4.43 ± 0.01 aB | 4.37 ± 0.01 bB | 4.35 ± 0.01 cB | |
| TA (% lactic acid) | 1 | 0.73 ± 0.01 bA | 0.73 ± 0.01 bB | 0.94 ± 0.02 aB | 0.95 ± 0.01 aB |
| 7 | 0.77 ± 0.04 bA | 0.75 ± 0.01 bA | 1.08 ± 0.01 aA | 1.09 ± 0.02 aA | |
| L* | 1 | 83.25 ± 0.12 aB | 82.64 ± 0.10 bB | 82.37 ± 0.07 bcB | 82.09 ± 0.19 cA |
| 7 | 83.59 ± 0.04 aA | 83.30 ± 0.15 bA | 82.80 ± 0.11 cA | 82.45 ± 0.03 dA | |
| a* | 1 | −1.53 ± 0.06 abA | −1.57 ± 0.03 bA | −1.42 ± 0.03 aA | −1.47 ± 0.04 abA |
| 7 | −1.49 ± 0.02 bcA | −1.54 ± 0.03 cA | −1.43 ± 0.03 abA | −1.42 ± 0.02 aB | |
| b* | 1 | 6.11 ± 0.08 bA | 6.21 ± 0.10 bA | 7.16 ± 0.04 aA | 7.22 ± 0.08 aA |
| 7 | 6.20 ± 0.06 bA | 6.20 ± 0.07 bA | 7.26 ± 0.10 aA | 7.26 ± 0.05 aA | |
| WI | 1 | 82.10 ± 0.12 aB | 81.50 ± 0.11 bA | 80.92 ± 0.06 cB | 80.63 ± 0.21 cA |
| 7 | 82.39 ± 0.06 aA | 82.12 ± 0.16 aA | 81.28 ± 0.14 bA | 80.96 ± 0.05 cA | |
| Syneresis (%) | 1 | 13.97 ± 0.63 aA | 11.0 ± 0.45 bA | 11.10 ± 0.43 bA | 9.25 ± 0.19 cA |
| 7 | 13.25 ± 1.12 aA | 9.89 ± 1.12 bcA | 10.53 ± 0.47 bB | 7.98 ± 0.79 cA | |
| Parameters | Time | Samples | |||
|---|---|---|---|---|---|
| (Days) | YC | YMD | YH | YHEn | |
| Firmness (N) | 1 | 1.11 ± 0.02 aB | 1.13 ± 0.02 aA | 0.99 ± 0.03 bA | 1.00 ± 0.02 bA |
| 7 | 1.34 ± 0.09 aA | 1.21 ± 0.10 abA | 0.94 ± 0.03 cA | 1.09 ± 0.08 bcA | |
| Cohesiveness | 1 | 0.69 ± 0.02 bA | 0.71 ± 0.01 bA | 0.77 ± 0.00 aB | 0.79 ± 0.03 aA |
| 7 | 0.67 ± 0.02 bA | 0.69 ± 0.04 bA | 1.29 ± 0.04 aA | 0.79 ± 0.11 bA | |
| G0′ (Pa) | 1 | 40.71 ± 0.02 | 48.24 ± 0.06 | 27.62 ± 0.08 | 35.08 ± 0.07 |
| 7 | 101.94 ± 0.09 | 85.00 ± 0.06 | 31.14 ± 0.11 | 36.98 ± 0.07 | |
| n′ | 1 | 0.116 ± 0.001 | 0.122 ± 0.001 | 0.144 ± 0.003 | 0.131 ± 0.002 |
| 7 | 0.105 ± 0.001 | 0.117 ± 0.004 | 0.129 ± 0.004 | 0.121 ± 0.002 | |
| R2 (Equation (2)) | 1 | 0.998 | 0.988 | 0.958 | 0.972 |
| 7 | 0.986 | 0.993 | 0.922 | 0.975 | |
| G0″ (Pa) | 1 | 13.11 ± 0.06 | 14.71 ± 0.09 | 8.81 ± 0.08 | 11.24 ± 0.08 |
| 7 | 30.72 ± 0.06 | 23.33 ± 0.08 | 9.39 ± 0.10 | 11.05 ± 0.09 | |
| n″ | 1 | 0.214 ± 0.003 | 0.208 ± 0.005 | 0.236 ± 0.006 | 0.226 ± 0.005 |
| 7 | 0.177 ± 0.002 | 0.191 ± 0.003 | 0.239 ± 0.008 | 0.232 ± 0.006 | |
| R2 (Equation (3)) | 1 | 0.977 | 0.947 | 0.937 | 0.946 |
| 7 | 0.988 | 0.976 | 0.913 | 0.946 | |
| tanδ | 1 | 0.355 ± 0.007 aA | 0.343 ± 0.008 aA | 0.356 ± 0.007 aA | 0.358 ± 0.011 aA |
| 7 | 0.333 ± 0.012 aB | 0.338 ± 0.005 aA | 0.343 ± 0.004 aB | 0.345 ± 0.011 aA | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lima, K.O.; da Rocha, M.; Alemán, A.; López-Caballero, M.E.; Tovar, C.A.; Gómez-Guillén, M.C.; Montero, P.; Prentice, C. Yogurt Fortification by the Addition of Microencapsulated Stripped Weakfish (Cynoscion guatucupa) Protein Hydrolysate. Antioxidants 2021, 10, 1567. https://doi.org/10.3390/antiox10101567
Lima KO, da Rocha M, Alemán A, López-Caballero ME, Tovar CA, Gómez-Guillén MC, Montero P, Prentice C. Yogurt Fortification by the Addition of Microencapsulated Stripped Weakfish (Cynoscion guatucupa) Protein Hydrolysate. Antioxidants. 2021; 10(10):1567. https://doi.org/10.3390/antiox10101567
Chicago/Turabian StyleLima, Karina Oliveira, Meritaine da Rocha, Ailén Alemán, María Elvira López-Caballero, Clara A. Tovar, María Carmen Gómez-Guillén, Pilar Montero, and Carlos Prentice. 2021. "Yogurt Fortification by the Addition of Microencapsulated Stripped Weakfish (Cynoscion guatucupa) Protein Hydrolysate" Antioxidants 10, no. 10: 1567. https://doi.org/10.3390/antiox10101567
APA StyleLima, K. O., da Rocha, M., Alemán, A., López-Caballero, M. E., Tovar, C. A., Gómez-Guillén, M. C., Montero, P., & Prentice, C. (2021). Yogurt Fortification by the Addition of Microencapsulated Stripped Weakfish (Cynoscion guatucupa) Protein Hydrolysate. Antioxidants, 10(10), 1567. https://doi.org/10.3390/antiox10101567







