Anti-Inflammatory Potential of Complex Extracts of Ligularia stenocephala Matsum. & Koidz. and Secale cereale L. Sprout in Chronic Gingivitis: In Vitro Investigation and Randomized Clinical Trial
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of TEES-10®
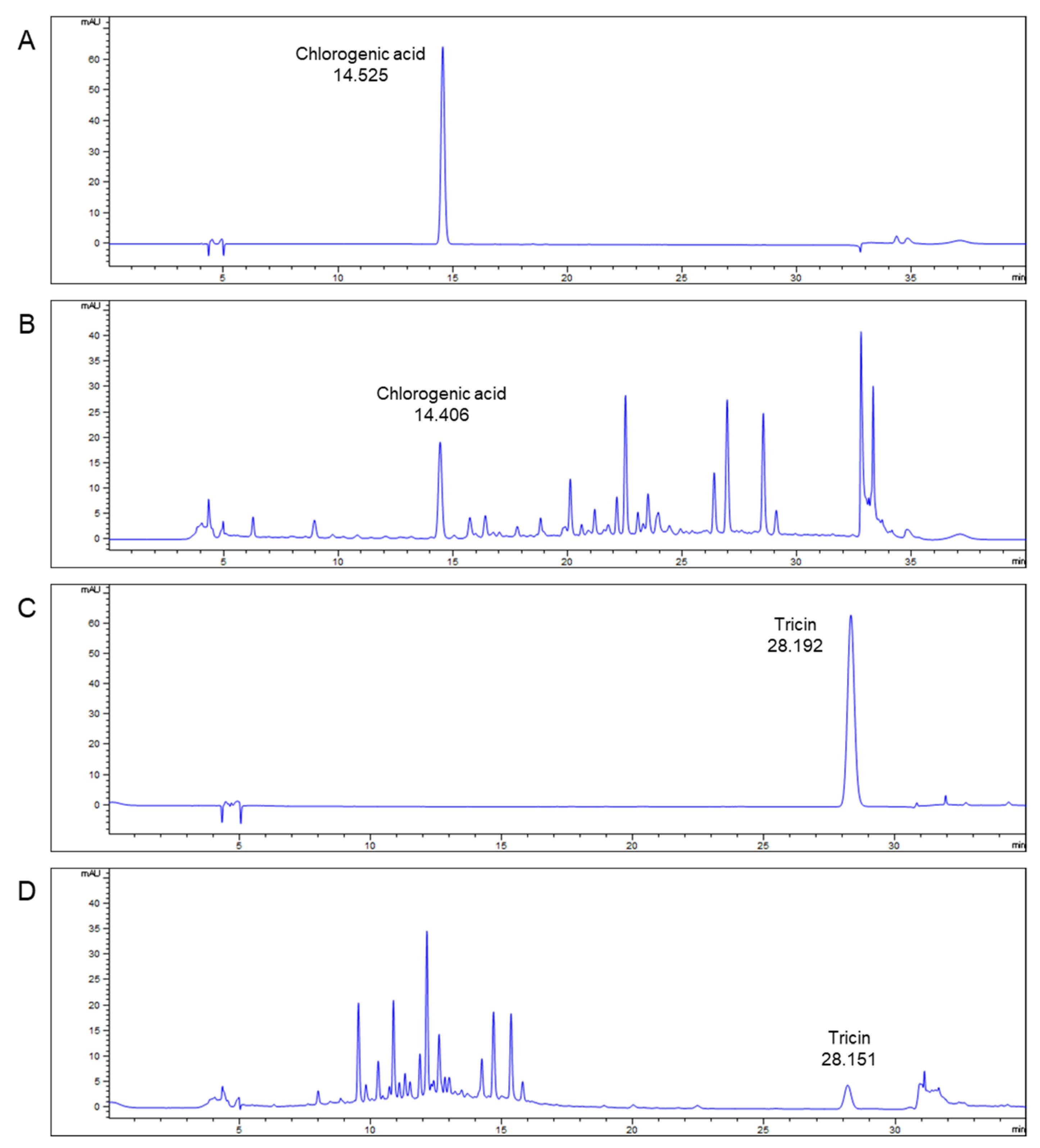
2.2. Qualitative Analysis of TEES-10®
2.3. In Vitro Investigation
2.3.1. Human PDL Cell Culture
2.3.2. Effect of TEES-10® on PDL Cell Viability and LPS Induced Inflammation
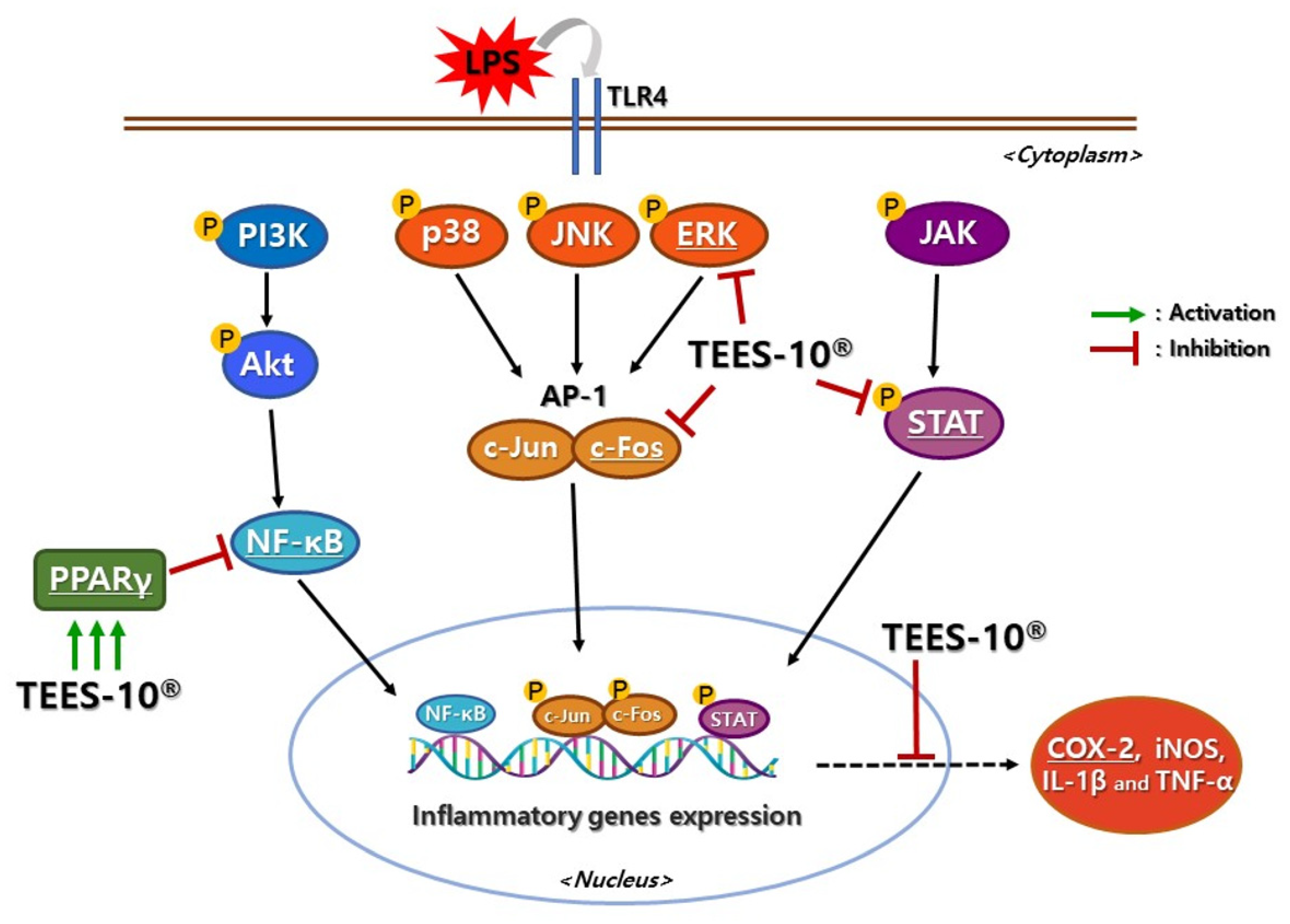
2.3.3. Effect of TEES-10® on the Expressions of Inflammatory Mediator
2.4. Double Blinded Randomized Clinical Trial
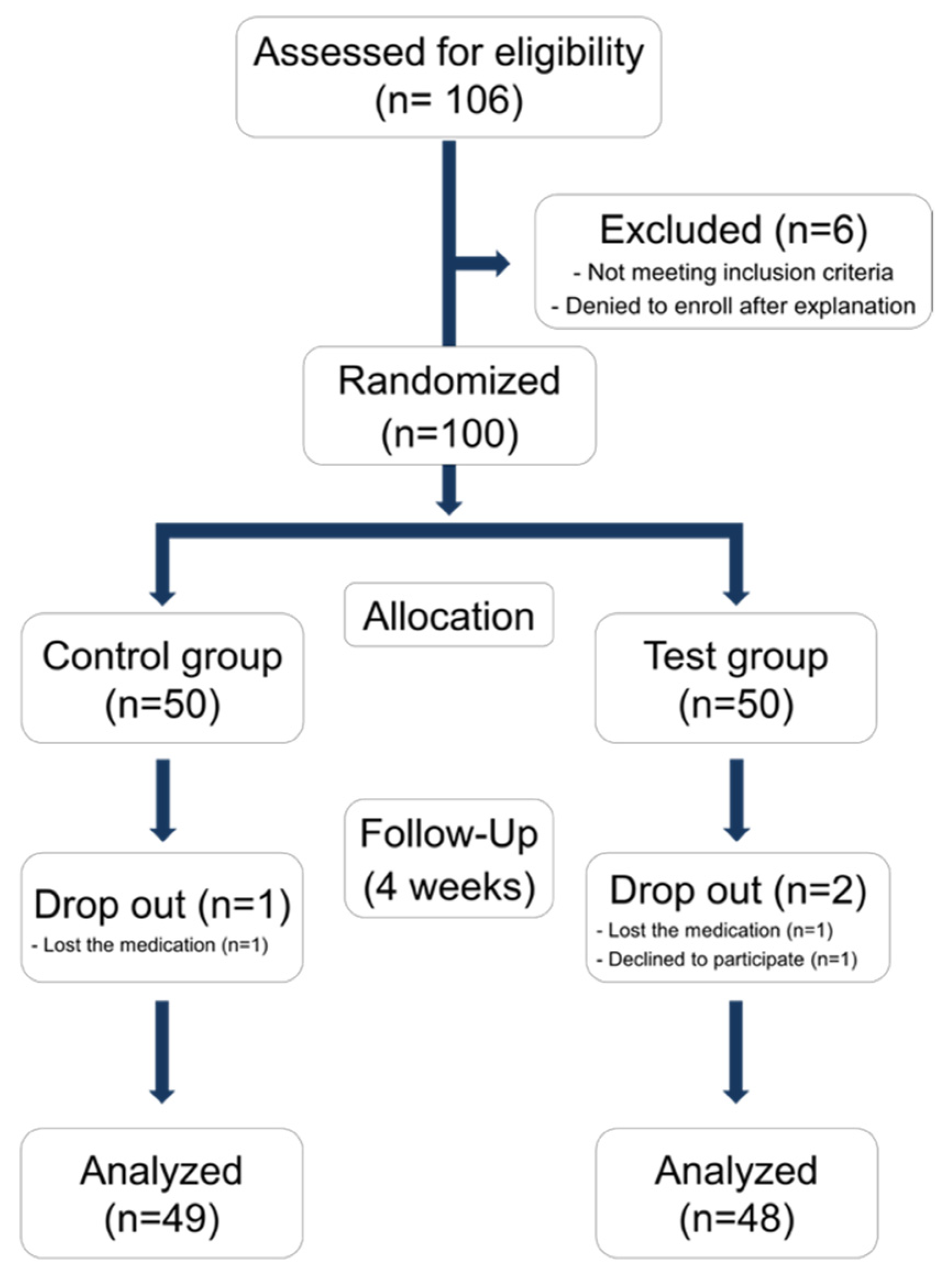
2.4.1. Study Design and Population
2.4.2. Participants
2.4.3. Sample Size Calculation
2.4.4. Clinical Evaluation
2.4.5. Salivary Biomarker Evaluation
2.5. Statistical Analysis
3. Results
3.1. Major Ingredient of TEES-10®—Qualitative Analysis of TEES-10®
3.2. In Vitro Investigation
3.2.1. Effect of TEES-10® on PDL Cell Viability
3.2.2. Effect of TEES-10® on LPS Induced Inflammation
3.2.3. Effect of TEES-10® on the Expression of Inflammatory Mediators in LPS-Treated PDL Cells
3.3. Randomized Controlled Study in Human
3.3.1. Demographic Analysis
3.3.2. Changes of Clinical Index
3.3.3. Changes in Number of Patients Diagnosed with Gingivitis
3.3.4. Changes of Salivary Biomarkers for Gingivitis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bartold, P.M.; Van Dyke, T.E. Host modulation: Controlling the inflammation to control the infection. Periodontol. 2000 2017, 75, 317–329. [Google Scholar] [CrossRef]
- Hajishengallis, G.; Chavakis, T.; Lambris, J.D. Current understanding of periodontal disease pathogenesis and targets for host-modulation therapy. Periodontol. 2000 2020, 84, 14–34. [Google Scholar] [CrossRef]
- Trombelli, L.; Farina, R.; Silva, C.O.; Tatakis, D.N. Plaque-induced gingivitis: Case definition and diagnostic considerations. J. Clin. Periodontol. 2018, 45, S44–S67. [Google Scholar] [CrossRef] [Green Version]
- Murakami, S.; Mealey, B.L.; Mariotti, A.; Chapple, I.L. Dental plaque–induced gingival conditions. J. Periodontol. 2018, 45, S17–S27. [Google Scholar] [CrossRef] [PubMed]
- Chapple, I.L.; Matthews, J.B. The role of reactive oxygen and antioxidant species in periodontal tissue destruction. Periodontol. 2000 2007, 43, 160–232. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Narula, S.C.; Sharma, R.K.; Tewari, S.; Sehgal, P.K. Vitamin E supplementation, superoxide dismutase status, and outcome of scaling and root planing in patients with chronic periodontitis: A randomized clinical trial. J. Periodontol. 2014, 85, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.C.; Kim, O.S.; Kim, O.J.; Kim, Y.J.; Chung, H.J. Antioxidant profile of whole saliva after scaling and root planing in periodontal disease. J. Periodontal. Implant. Sci. 2010, 40, 164–171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chapple, I.L. Potential mechanisms underpinning the nutritional modulation of periodontal inflammation. J. Am. Dent. Assoc. 2009, 140, 178–184. [Google Scholar] [CrossRef] [Green Version]
- Van der Velden, U.; Kuzmanova, D.; Chapple, I.L. Micronutritional approaches to periodontal therapy. J. Clin. Periodontol. 2011, 38 (Suppl. 11), 142–158. [Google Scholar] [CrossRef]
- Dawson, D.R., III; Branch-Mays, G.; Gonzalez, O.A.; Ebersole, J.L. Dietary modulation of the inflammatory cascade. Periodontol 2000 2014, 64, 161–197. [Google Scholar] [CrossRef]
- Isacco, C.G.; Ballini, A.; De Vito, D.; Nguyen, K.C.; Cantore, S.; Bottalico, L.; Quagliuolo, L.; Boccellino, M.; Di Domenico, M.; Santacroce, L.; et al. Rebalancing the Oral Microbiota as an Efficient Tool in Endocrine, Metabolic and Immune Disorders. Endocr. Metab. Immune Disord. Drug Targets 2021, 21, 777–784. [Google Scholar] [CrossRef]
- Hong, J.Y.; Lee, J.S.; Choi, S.H.; Shin, H.S.; Park, J.C.; Shin, S.I.; Chung, J.H. A randomized, double-blind, placebo-controlled multicenter study for evaluating the effects of fixed-dose combinations of vitamin C, vitamin E, lysozyme, and carbazochrome on gingival inflammation in chronic periodontitis patients. BMC Oral Health 2019, 19, 40. [Google Scholar] [CrossRef] [PubMed]
- Debnath, T.; Kim, E.-K.; Deb Nath, N.C.; Lee, K.-G.J.F.; Immunology, A. Therapeutic effects of Ligularia stenocephala against inflammatory bowel disease by regulating antioxidant and inflammatory mediators. Food Agric. Immunol. 2017, 28, 1142–1154. [Google Scholar] [CrossRef] [Green Version]
- Andreasen, M.F.; Landbo, A.K.; Christensen, L.P.; Hansen, A.; Meyer, A.S. Antioxidant effects of phenolic rye (Secale cereale L.) extracts, monomeric hydroxycinnamates, and ferulic acid dehydrodimers on human low-density lipoproteins. J. Agric. Food Chem. 2001, 49, 4090–4096. [Google Scholar] [CrossRef] [PubMed]
- Preshaw, P.M. Host modulation therapy with anti-inflammatory agents. Periodontol 2000 2018, 76, 131–149. [Google Scholar] [CrossRef]
- Sulijaya, B.; Takahashi, N.; Yamazaki, K. Host modulation therapy using anti-inflammatory and antioxidant agents in periodontitis: A review to a clinical translation. Arch. Oral Biol. 2019, 105, 72–80. [Google Scholar] [CrossRef]
- Jeong, Y.Y.; Kim, M.S.; Lee, K.E.; Nam, O.H.; Jang, J.-H.; Choi, S.-C.; Lee, H.-S. Comparison of 2- and 3-Dimensional Cultured Periodontal Ligament Stem Cells; a Pilot Study. Appl. Sci. 2021, 11, 1083. [Google Scholar] [CrossRef]
- Liu, J.; Chen, S.; Ren, W.; Liu, J.; Yang, P.; Chen, Z.; Zhang, Q.; Yang, F. Lipopolysaccharide-induced suppression of periodontal ligament cell proliferation and apoptosis are strengthened under high glucose conditions. Arch. Oral Biol. 2017, 79, 70–76. [Google Scholar] [CrossRef]
- Kim, D.H.; Jang, J.H.; Lee, B.N.; Chang, H.S.; Hwang, I.N.; Oh, W.M.; Kim, S.H.; Min, K.S.; Koh, J.T.; Hwang, Y.C. Anti-inflammatory and Mineralization Effects of ProRoot MTA and Endocem MTA in Studies of Human and Rat Dental Pulps In Vitro and In Vivo. J. Endod. 2018, 44, 1534–1541. [Google Scholar] [CrossRef]
- Kim, J.E.; Takanche, J.S.; Yun, B.S.; Yi, H.K. Anti-inflammatory character of Phelligridin D modulates periodontal regeneration in l ipopolysaccharide-induced human periodontal ligament cells. J. Periodontal Res. 2018, 53, 816–824. [Google Scholar] [CrossRef]
- Chapple, I.L.; Mealey, B.L.; Van Dyke, T.E.; Bartold, P.M.; Dommisch, H.; Eickholz, P.; Geisinger, M.L.; Genco, R.J.; Glogauer, M.; Goldstein, M. Periodontal health and gingival diseases and conditions on an intact and a reduced periodontium: Consensus report of workgroup 1 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Periodontol. 2018, 89, S74–S84. [Google Scholar] [CrossRef]
- Turesky, S.; Gilmore, N.D.; Glickman, I. Reduced plaque formation by the chloromethyl analogue of victamine C. J. Periodontol. 1970, 41, 41–43. [Google Scholar] [CrossRef] [Green Version]
- Lee, D.-K.; Kim, J.-K.; Kim, J.-S.; Park, K.-J.; Cha, D.-S.; Jeon, H. Inhibitory effect of Ligularia Stenocephala on the cancer metastasis. Nat. Prod. Sci. 2012, 18, 89–96. [Google Scholar]
- Yan, F.-l.; Wang, A.-x.; Jia, Z.-j. New phenol derivatives from Ligularia stenocephala. J. Chem. Res. 2004, 2004, 742–743. [Google Scholar] [CrossRef]
- Tungmunnithum, D.; Thongboonyou, A.; Pholboon, A.; Yangsabai, A. Flavonoids and Other Phenolic Compounds from Medicinal Plants for Pharmaceutical and Medical Aspects: An Overview. Medicines 2018, 5, 93. [Google Scholar] [CrossRef]
- Huyut, Z.; Beydemir, S.; Gulcin, I. Antioxidant and Antiradical Properties of Selected Flavonoids and Phenolic Compounds. Biochem. Res. Int. 2017, 2017, 7616791. [Google Scholar] [CrossRef] [PubMed]
- Kaur, P.; Sandhu, K.S.; Bangar, S.P.; Purewal, S.S.; Kaur, M.; Ilyas, R.A.; Asyraf, M.R.M.; Razman, M.R. Unraveling the Bioactive Profile, Antioxidant and DNA Damage Protection Potential of Rye (Secale cereale) Flour. Antioxidants 2021, 10, 1214. [Google Scholar] [CrossRef] [PubMed]
- Prakash, S.; Kumar, M.; Kumari, N.; Thakur, M.; Rathour, S.; Pundir, A.; Sharma, A.K.; Bangar, S.P.; Dhumal, S.; Singh, S. Plant-Based Antioxidant Extracts and Compounds in the Management of Oral Cancer. Antioxidants 2021, 10, 1358. [Google Scholar] [CrossRef] [PubMed]
- Williamson, G.; Barron, D.; Shimoi, K.; Terao, J. In vitro biological properties of flavonoid conjugates found in vivo. Free Radic. Res. 2005, 39, 457–469. [Google Scholar] [CrossRef]
- Williams, R.J.; Spencer, J.P.; Rice-Evans, C. Flavonoids: Antioxidants or signalling molecules? Free Radic. Biol. Med. 2004, 36, 838–849. [Google Scholar] [CrossRef]
- Sanz, I.; Alonso, B.; Carasol, M.; Herrera, D.; Sanz, M. Nonsurgical treatment of periodontitis. J. Evid. Based Dent. Pract. 2012, 12, 76–86. [Google Scholar] [CrossRef]
- Bunte, K.; Hensel, A.; Beikler, T. Polyphenols in the prevention and treatment of periodontal disease: A systematic review of in vivo, ex vivo and in vitro studies. Fitoterapia 2019, 132, 30–39. [Google Scholar] [CrossRef]
- Bapat, S.; Nagarajappa, R.; Ramesh, G.; Bapat, K. Effect of propolis mouth rinse on oral microorganisms—A randomized controlled trial. Clin. Oral Investig. 2021, 1–8. [Google Scholar] [CrossRef]
- Chava, V.K.; Vedula, B.D. Thermo-reversible green tea catechin gel for local application in chronic periodontitis: A 4-week clinical trial. J. Periodontol. 2013, 84, 1290–1296. [Google Scholar] [CrossRef]
- Park, J.-Y.; Ko, K.-A.; Lee, J.-Y.; Oh, J.-W.; Lim, H.-C.; Lee, D.-W.; Choi, S.-H.; Cha, J.-K. Clinical and Immunological Efficacy of Mangosteen and Propolis Extracted Complex in Patients with Gingivitis: A Multi-Centered Randomized Controlled Clinical Trial. Nutrients 2021, 13, 2604. [Google Scholar] [CrossRef]
- Hong, I.; Pae, H.C.; Song, Y.W.; Cha, J.K.; Lee, J.S.; Paik, J.W.; Choi, S.H. Oral Fluid Biomarkers for Diagnosing Gingivitis in Human: A Cross-Sectional Study. J. Clin. Med. 2020, 9, 1720. [Google Scholar] [CrossRef]
- Listgarten, M. Periodontal probing: What does it mean? J. Clin. Periodontol. 1980, 7, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Prakash, S.; Kumari, N.; Pundir, A.; Punia, S.; Saurabh, V.; Choudhary, P.; Changan, S.; Dhumal, S.; Pradhan, P.C.; et al. Beneficial Role of Antioxidant Secondary Metabolites from Medicinal Plants in Maintaining Oral Health. Antioxidants 2021, 10, 1061. [Google Scholar] [CrossRef] [PubMed]
- Cassidy, A.; Minihane, A.M. The role of metabolism (and the microbiome) in defining the clinical efficacy of dietary flavonoids. Am. J. Clin. Nutr. 2017, 105, 10–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Placebo Group | Test Group | p-Value | |
|---|---|---|---|
| Age (years) Male/Female | 32.04 ± 1.80 22/27 | 32.98 ± 1.97 22/26 | p > 0.05 |
| p > 0.05 | |||
| Height (cm) | 167.95 ± 1.28 | 166.70 ± 1.20 | p > 0.05 |
| Weight (kg) | 65.39 ± 2.19 | 63.72 ± 2.10 | p > 0.05 |
| Placebo Group | Test Group | |||
|---|---|---|---|---|
| At Baseline | At 4 Weeks | At Baseline | At 4 Weeks | |
| GI | 0.88 ± 0.06 | 0.88 ± 0.06 | 0.88 ± 0.06 | 0.74 ± 0.07 (a) (d) |
| PI | 0.89 ± 0.11 | 1.01 ± 0.12 | 0.96 ± 0.11 | 1.05 ± 0.13 |
| Sum of BOP | 8.51 ± 0.74 | 8.57 ± 0.69 | 8.63 ± 0.77 | 6.44 ± 0.69 (b) (d) |
| PD (mm) | 3.00 ± 0.03 | 3.10 ± 0.04 (c) | 3.11 ± 0.04 | 3.05 ± 0.04 (b) (c) |
| At Baseline | At 4 Weeks | McNemar | ||||
|---|---|---|---|---|---|---|
| Placebo | Test | Placebo | Test | Placebo | Test | |
| Gingivitis | 40 | 42 | 42 | 30 | p > 0.05 | p < 0.01 |
| Healthy | 9 | 6 | 7 | 18 | ||
| Chi square | p > 0.05 | p < 0.01 | ||||
| Placebo Group (n = 49) | Test Group (n = 48) | |||
|---|---|---|---|---|
| At Baseline | At 4 Weeks | At Baseline | At 4 Weeks | |
| MMP-8 (ng/mL) | 43.82 ± 11.29 | 37.85 ± 5.54 | 72.11 ± 14.78 | 24.41 ± 4.06 (a) (b) |
| MMP-9 (ng/mL) | 136.58 ± 27.88 | 194.21 ± 50.54 | 249.93 ± 86.36 | 142.97 ± 38.87 (a) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hong, I.; Park, J.-Y.; Noh, Y.-H.; Jeon, S.-H.; Paik, J.-W.; Lee, J.-S.; Choi, S.-H.; Cha, J.-K. Anti-Inflammatory Potential of Complex Extracts of Ligularia stenocephala Matsum. & Koidz. and Secale cereale L. Sprout in Chronic Gingivitis: In Vitro Investigation and Randomized Clinical Trial. Antioxidants 2021, 10, 1586. https://doi.org/10.3390/antiox10101586
Hong I, Park J-Y, Noh Y-H, Jeon S-H, Paik J-W, Lee J-S, Choi S-H, Cha J-K. Anti-Inflammatory Potential of Complex Extracts of Ligularia stenocephala Matsum. & Koidz. and Secale cereale L. Sprout in Chronic Gingivitis: In Vitro Investigation and Randomized Clinical Trial. Antioxidants. 2021; 10(10):1586. https://doi.org/10.3390/antiox10101586
Chicago/Turabian StyleHong, Inpyo, Jin-Young Park, Yoo-Hun Noh, Su-Hee Jeon, Jeong-Won Paik, Jung-Seok Lee, Seong-Ho Choi, and Jae-Kook Cha. 2021. "Anti-Inflammatory Potential of Complex Extracts of Ligularia stenocephala Matsum. & Koidz. and Secale cereale L. Sprout in Chronic Gingivitis: In Vitro Investigation and Randomized Clinical Trial" Antioxidants 10, no. 10: 1586. https://doi.org/10.3390/antiox10101586
APA StyleHong, I., Park, J.-Y., Noh, Y.-H., Jeon, S.-H., Paik, J.-W., Lee, J.-S., Choi, S.-H., & Cha, J.-K. (2021). Anti-Inflammatory Potential of Complex Extracts of Ligularia stenocephala Matsum. & Koidz. and Secale cereale L. Sprout in Chronic Gingivitis: In Vitro Investigation and Randomized Clinical Trial. Antioxidants, 10(10), 1586. https://doi.org/10.3390/antiox10101586







