Methods to Determine Chain-Breaking Antioxidant Activity of Nanomaterials beyond DPPH•. A Review
Abstract
:1. Introduction
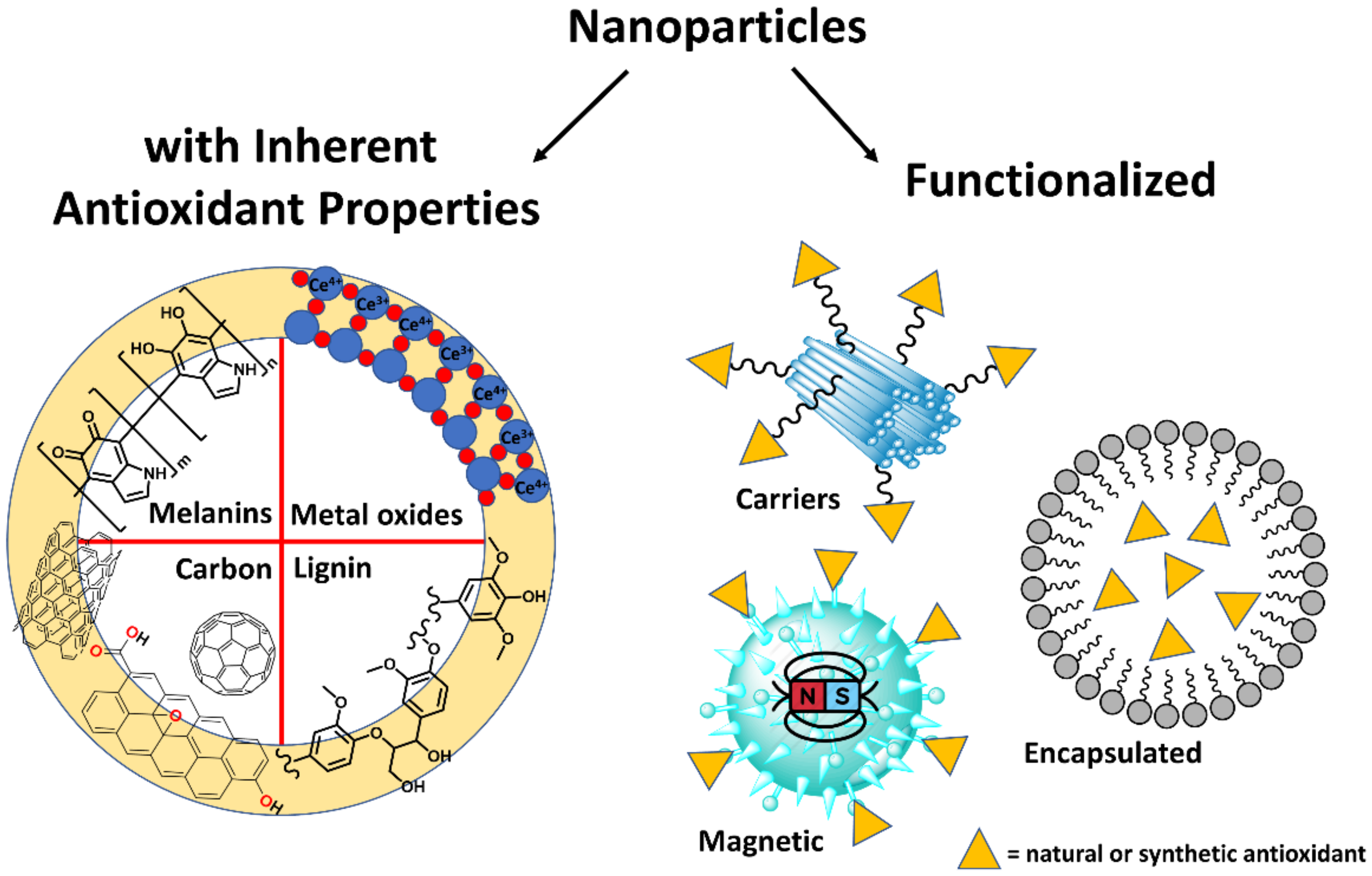
2. Recent Examples of Nanomaterials Used for Their Antioxidant Activity
2.1. Inherent Antioxidant Nanoparticles
2.2. Functionalized Nanoparticles
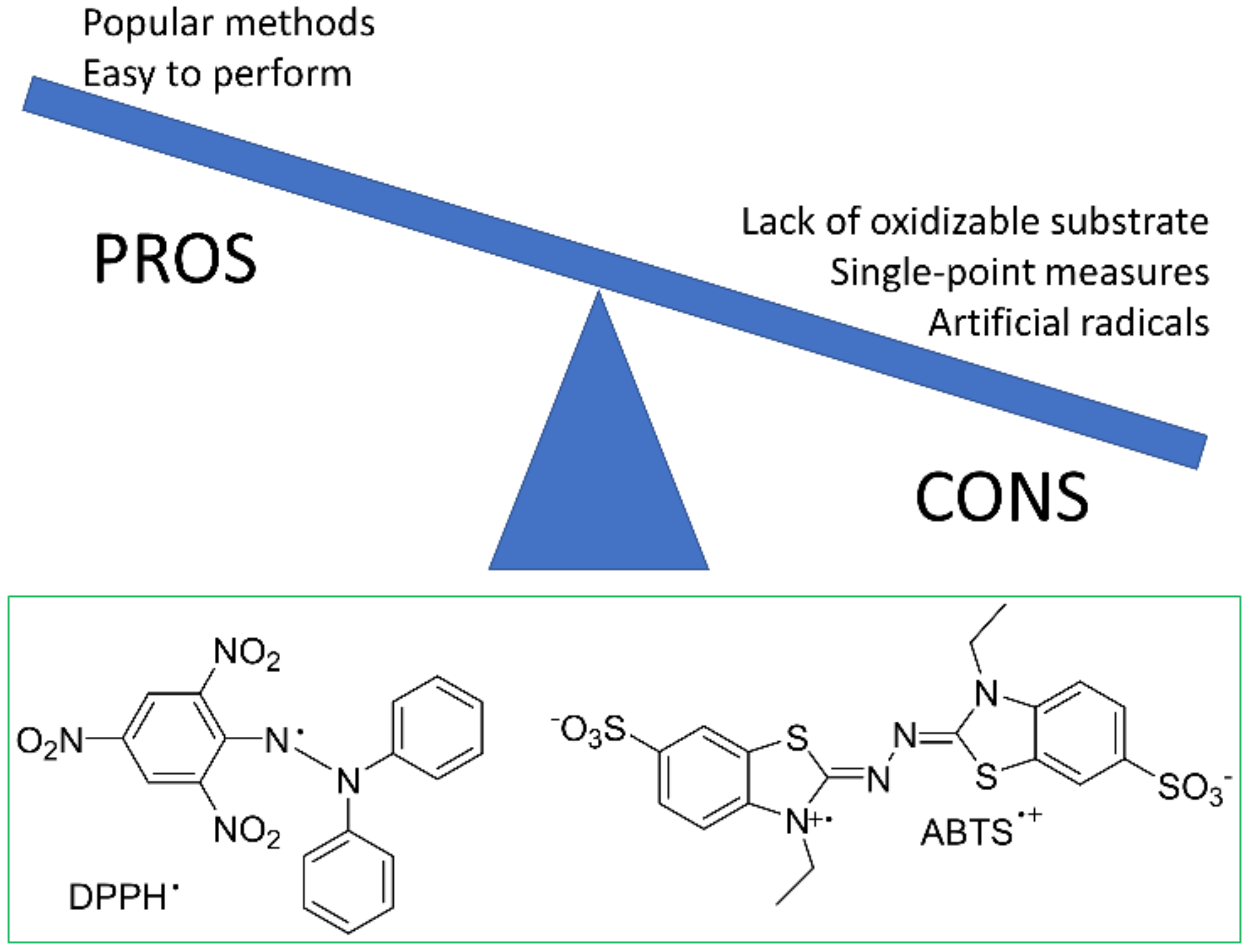
3. Why You Should Not Use Simplified Methods?
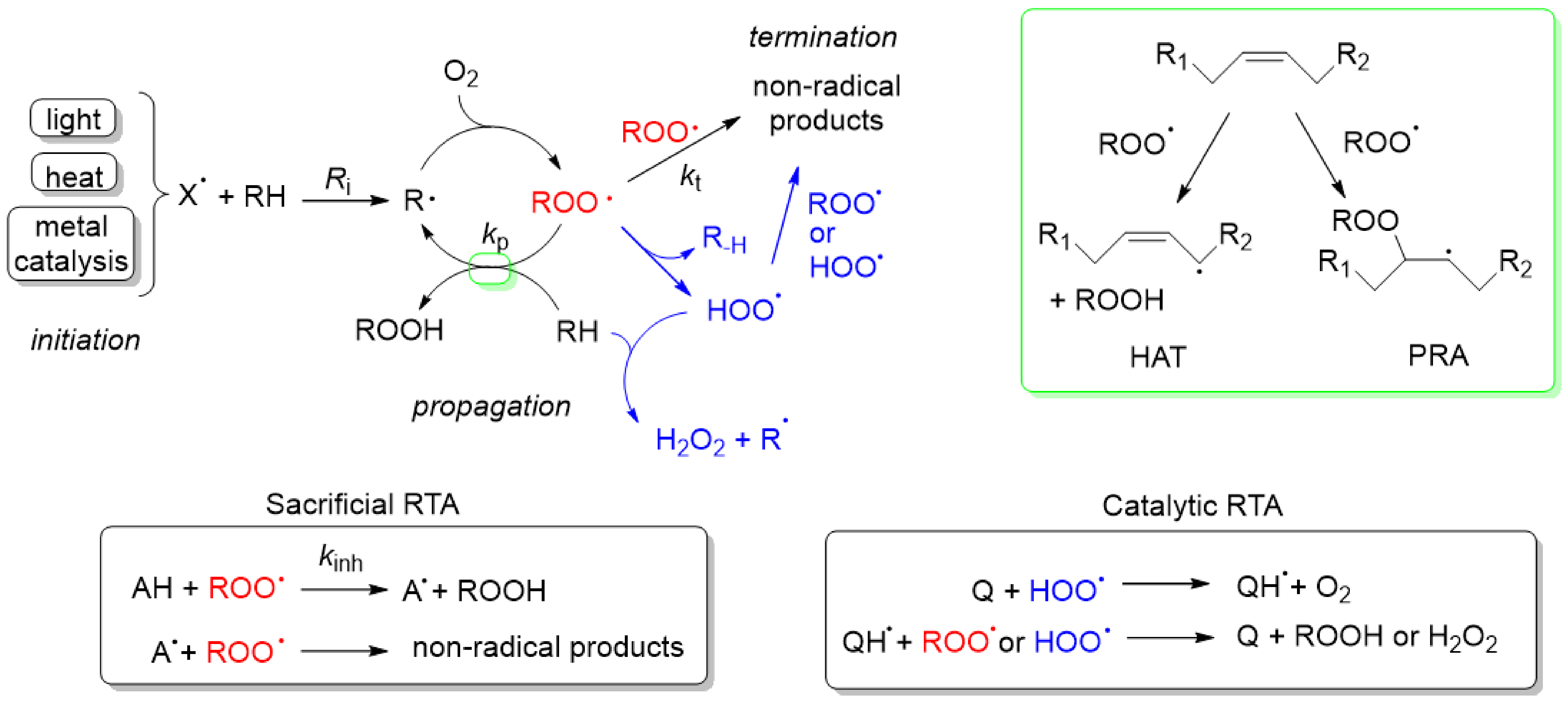
4. Autoxidation Mechanism. Why Focus on Peroxyl Radicals?
4.1. Autoxidation
4.2. Chain-Breaking or Radical-Trapping Antioxidants (RTA)
4.3. Why Focus on Peroxyl Radicals?
4.4. SOD-like Activity
5. How to Study Autoxidations Inhibited by Nanoantioxidants?
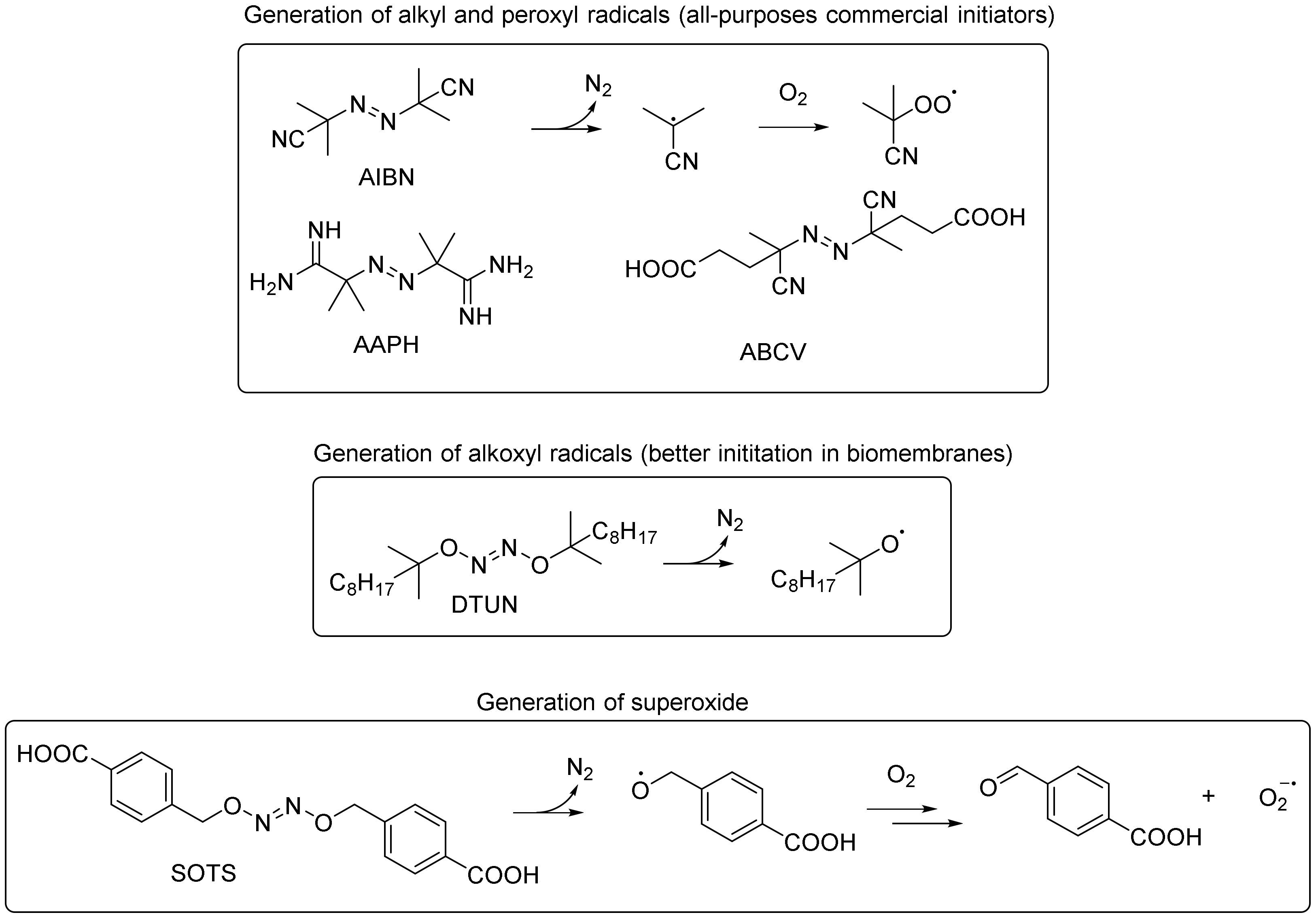
5.1. Initiation
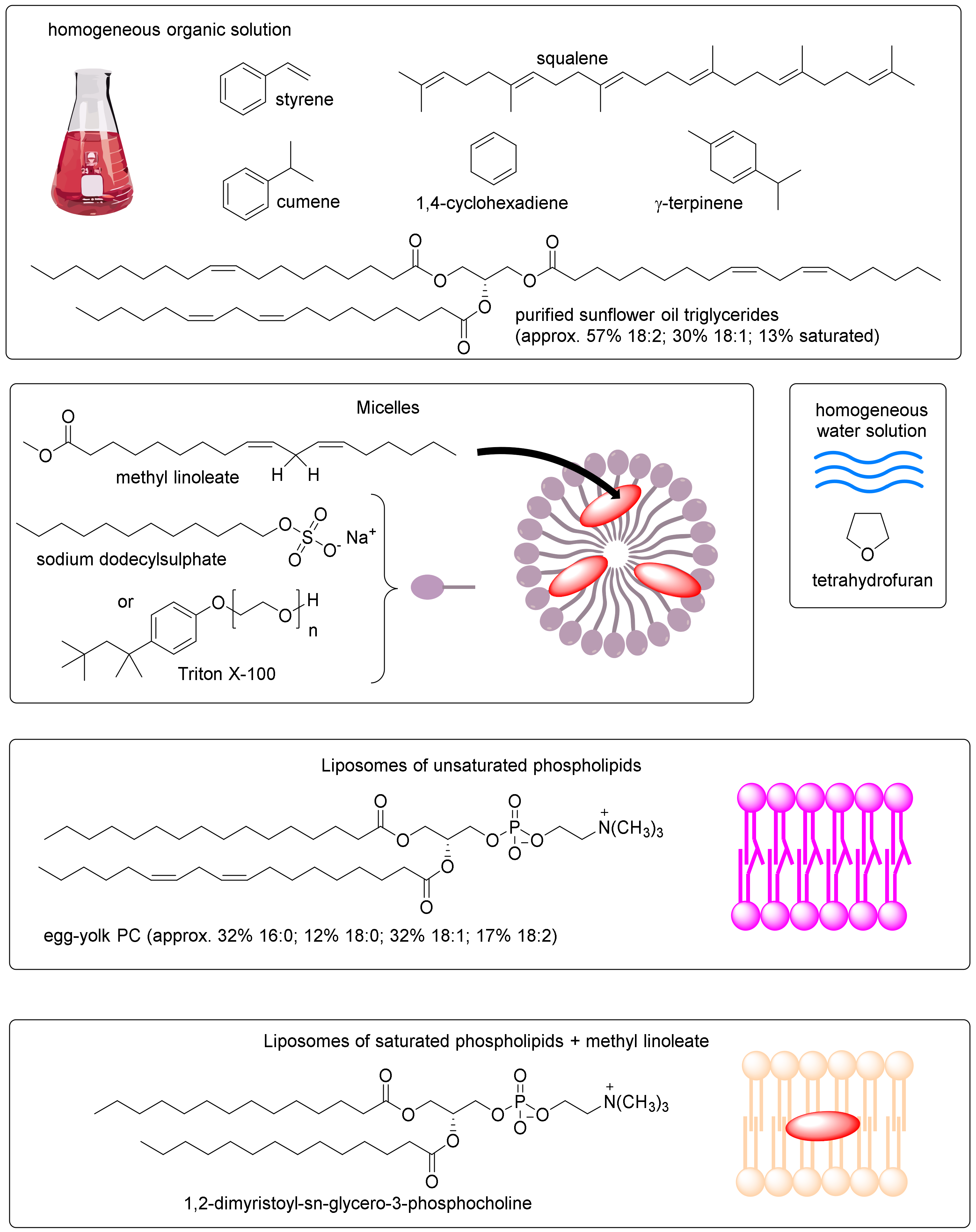
5.2. Oxidizable Substrates and Reaction Medium
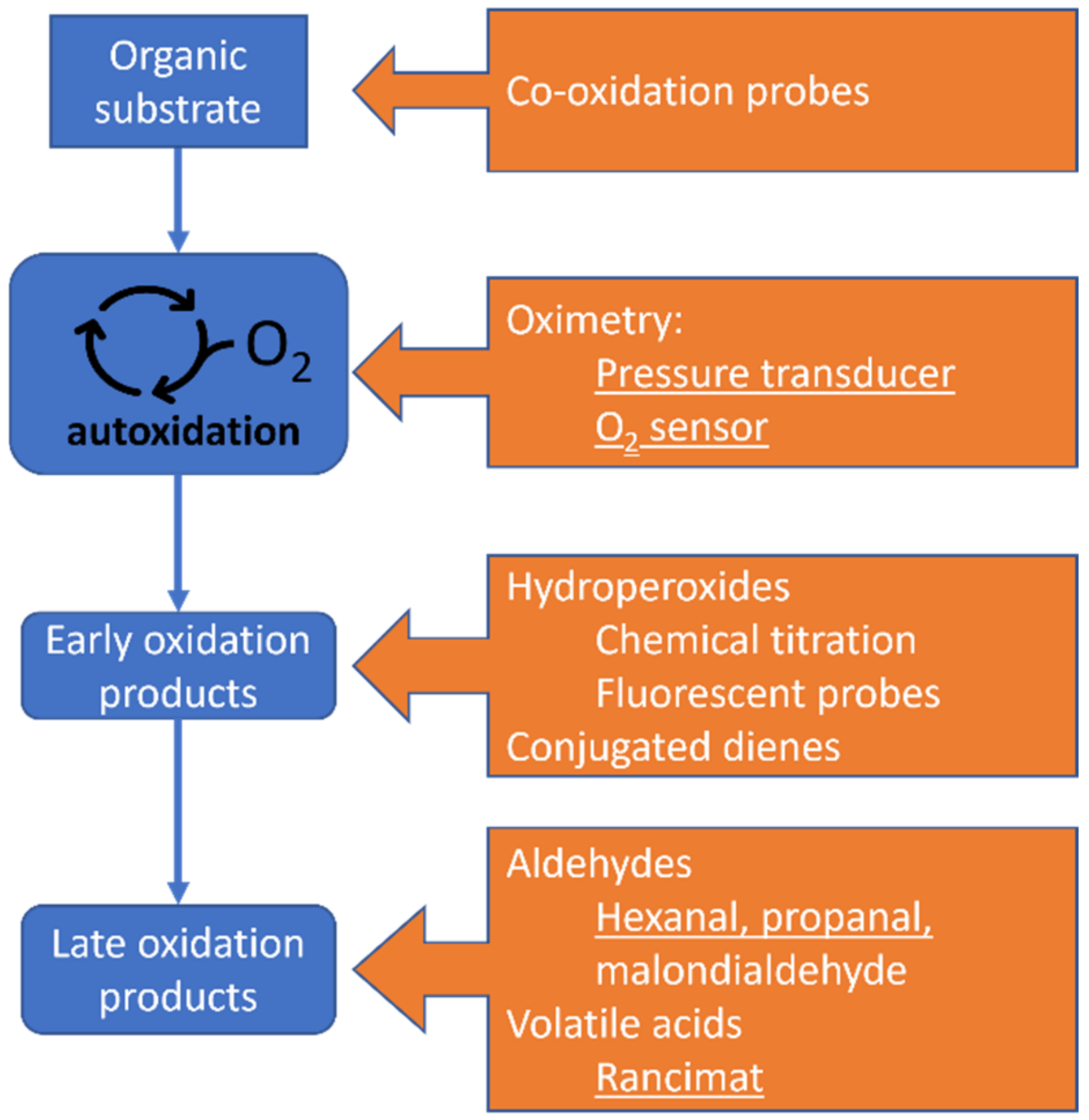
5.3. How to Study an Autoxidation?
5.3.1. Disappearance of Reactants
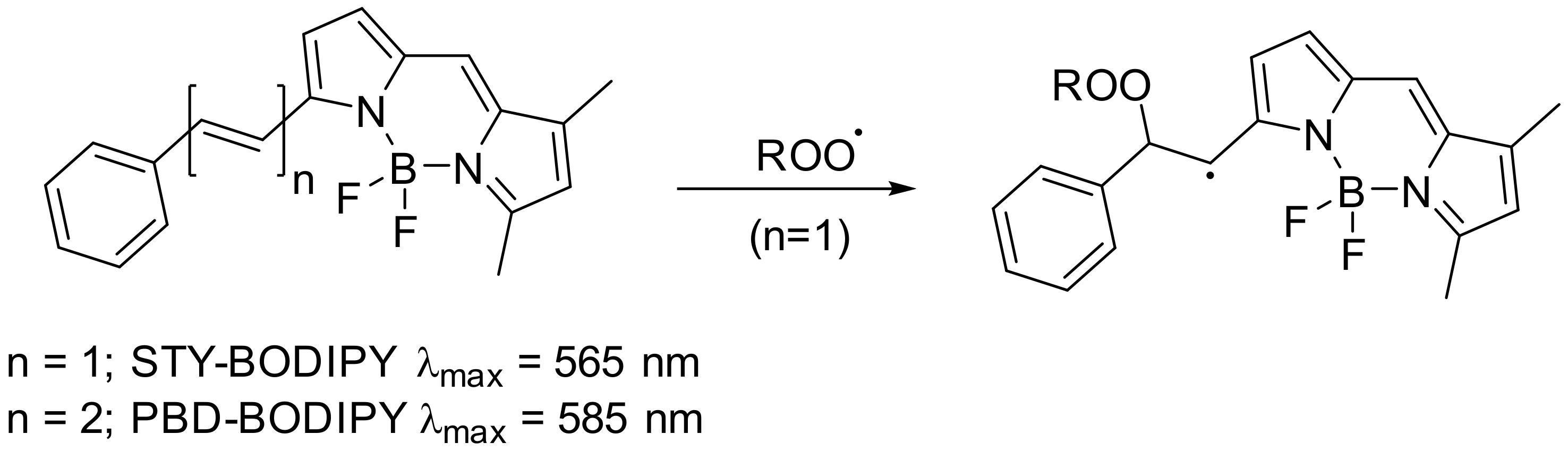
5.3.2. Formation of Early Products
5.3.3. Formation of Late Products
5.4. Advantages of Oximetry
5.5. Kinetic Analysis of O2 Consumption Plots
5.6. Catalytic Antioxidants
5.7. Studying Pro-Oxidant Activity
5.8. Limitations of Oximetry Methods
6. Conclusions
- (1)
- Is the nanomaterial able to stop the autoxidation of a relevant oxidizable substrate?
- (2)
- What is the kinh of the nanoantioxidant?
- (3)
- What is the duration of the inhibition period? Does it behave in a catalytic fashion?
- (4)
- Is the nanoantioxidant stable under the reaction conditions? Is it stable under air? Does it have a pro-oxidant effect the presence of alkyl and hydrogen peroxides?
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Helberg, J.; Pratt, D.A. Autoxidation vs. antioxidants—The fight for forever. Chem. Soc. Rev. 2021, 50, 7343–7358. [Google Scholar] [CrossRef]
- Valgimigli, L.; Pratt, D.A. Antioxidants in Chemistry and Biology. Encycl. Radic. Chem. Biol. Mater. 2012, 1623–1677. [Google Scholar] [CrossRef]
- Baschieri, A.; Amorati, R.; Benelli, T.; Mazzocchetti, L.; D’Angelo, E.; Valgimigli, L. Enhanced Antioxidant Activity under Biomimetic Settings of Ascorbic Acid Included in Halloysite Nanotubes. Antioxidants 2019, 8, 30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Medhe, S.; Bansal, P.; Srivastava, M.M. Enhanced antioxidant activity of gold nanoparticle embedded 3,6-dihydroxyflavone: A combinational study. Appl. Nanosci. 2014, 4, 153–161. [Google Scholar] [CrossRef] [Green Version]
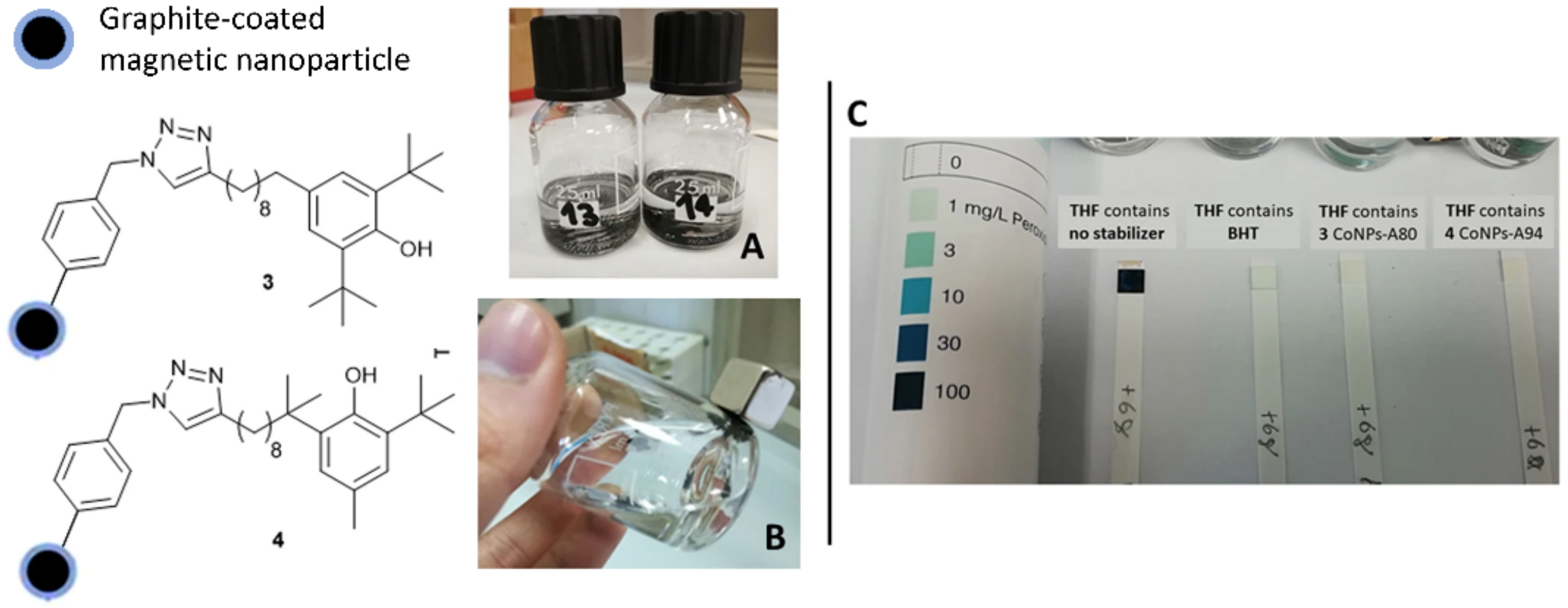
- Viglianisi, C.; Scarlini, A.; Tofani, L.; Menichetti, S.; Baschieri, A.; Amorati, R. Magnetic nanoantioxidants with improved radical-trapping stoichiometry as stabilizers for inhibition of peroxide formation in ethereal solvents. Sci. Rep. 2019, 9, 17219. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, C.A.; Ni, D.; Rosenkrans, Z.T.; Cai, W. Scavenging of reactive oxygen and nitrogen species with nanomaterials. Nano Res 2018, 11, 4955–4984. [Google Scholar] [CrossRef]
- Brindhadevi, K.; Samuel, M.S.; Verma, T.N.; Vasantharaj, S.; Sathiyavimal, S.; Saravanan, M.; Pugazhendhi, A.; Duc, P.A. Zinc oxide nanoparticles (ZnONPs)-induced antioxidants and photocatalytic degradation activity from hybrid grape pulp extract (HGPE). Biocatal. Agric. Biotechnol. 2020, 28, 101730. [Google Scholar] [CrossRef]
- Kokalari, I.; Gassino, R.; Giovannozzi, A.M.; Croin, L.; Gazzano, E.; Bergamaschi, E.; Rossi, A.M.; Perrone, G.; Riganti, C.; Ponti, J.; et al. Pro- and anti-oxidant properties of near-infrared (NIR) light responsive carbon nanoparticles. Free Radic. Biol. Med. 2019, 134, 165–176. [Google Scholar] [CrossRef]
- Mansour Lakourj, M.; Norouzian, R.-S.; Esfandyar, M.; Ghasemi mir, S. Conducting nanocomposites of polypyrrole-co-polyindole doped with carboxylated CNT: Synthesis approach and anticorrosion/antibacterial/antioxidation property. Mater. Sci. Eng. B 2020, 261, 114673. [Google Scholar] [CrossRef]
- Chenthamara, D.; Subramaniam, S.; Ramakrishnan, S.G.; Krishnaswamy, S.; Essa, M.M.; Lin, F.H.; Qoronfleh, M.W. Therapeutic efficacy of nanoparticles and routes of administration. Biomater Res. 2019, 23, 20. [Google Scholar] [CrossRef]
- Flieger, J.; Flieger, W.; Baj, J.; Maciejewski, R. Antioxidants: Classification, Natural Sources, Activity/Capacity Measurements, and Usefulness for the Synthesis of Nanoparticles. Materials 2021, 14, 4135. [Google Scholar] [CrossRef]
- Sharpe, E.; Andreescu, D.; Andreescu, S. Artificial nanoparticle antioxidants. In Oxidative Stress: Diagnostics, Prevention, and Therapy; ACS Publications: Washington, DC, USA, 2011; pp. 235–253. [Google Scholar] [CrossRef]
- Morry, J.; Ngamcherdtrakul, W.; Yantasee, W. Oxidative stress in cancer and fibrosis: Opportunity for therapeutic intervention with antioxidant compounds, enzymes, and nanoparticles. Redox. Biol. 2017, 11, 240–253. [Google Scholar] [CrossRef]
- Khalil, I.; Yehye, W.A.; Etxeberria, A.E.; Alhadi, A.A.; Dezfooli, S.M.; Julkapli, N.B.M.; Basirun, W.J.; Seyfoddin, A. Nanoantioxidants: Recent Trends in Antioxidant Delivery Applications. Antioxidants 2020, 9, 24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lvov, Y.; Wang, W.; Zhang, L.; Fakhrullin, R. Halloysite Clay Nanotubes for Loading and Sustained Release of Functional Compounds. Adv. Mater. 2016, 28, 1227–1250. [Google Scholar] [CrossRef] [PubMed]
- Gastaldi, L.; Ugazio, E.; Sapino, S.; Iliade, P.; Miletto, I.; Berlier, G. Mesoporous silica as a carrier for topical application: The Trolox case study. Phys. Chem. Chem. Phys. 2012, 14, 11318–11326. [Google Scholar] [CrossRef] [PubMed]
- Esch, F.; Fabris, S.; Zhou, L.; Montini, T.; Africh, C.; Fornasiero, P.; Comelli, G.; Rosei, R. Electron localization determines defect formation on ceria substrates. Science 2005, 309, 752–755. [Google Scholar] [CrossRef]
- Wu, H.; Li, F.; Wang, S.; Lu, J.; Li, J.; Du, Y.; Sun, X.; Chen, X.; Gao, J.; Ling, D. Ceria nanocrystals decorated mesoporous silica nanoparticle based ROS-scavenging tissue adhesive for highly efficient regenerative wound healing. Biomaterials 2018, 151, 66–77. [Google Scholar] [CrossRef]
- Tian, Z.; Li, X.; Ma, Y.; Chen, T.; Xu, D.; Wang, B.; Qu, Y.; Gao, Y. Quantitatively Intrinsic Biomimetic Catalytic Activity of Nanocerias as Radical Scavengers and Their Ability against H2O2 and Doxorubicin-Induced Oxidative Stress. ACS Appl. Mater. Interfaces 2017, 9, 23342–23352. [Google Scholar] [CrossRef]
- Zheng, Q.; Fang, Y.; Zeng, L.; Li, X.; Chen, H.; Song, H.; Huang, J.; Shi, S. Cytocompatible cerium oxide-mediated antioxidative stress in inhibiting ocular inflammation-associated corneal neovascularization. J. Mater. Chem. B 2019, 7, 6759–6769. [Google Scholar] [CrossRef]
- Han, S.I.; Lee, S.W.; Cho, M.G.; Yoo, J.M.; Oh, M.H.; Jeong, B.; Kim, D.; Park, O.K.; Kim, J.; Namkoong, E.; et al. Epitaxially Strained CeO2/Mn3O4 Nanocrystals as an Enhanced Antioxidant for Radioprotection. Adv. Mater. 2020, 32, e2001566. [Google Scholar] [CrossRef]
- Valgimigli, L.; Baschieri, A.; Amorati, R. Antioxidant activity of nanomaterials. J. Mater. Chem. B 2018, 6, 2036–2051. [Google Scholar] [CrossRef]
- Duan, H.; Wang, D.; Li, Y. Green chemistry for nanoparticle synthesis. Chem. Soc. Rev. 2015, 44, 5778–5792. [Google Scholar] [CrossRef]
- Gong, W.; Xiang, Z.; Ye, F.; Zhao, G. Composition and structure of an antioxidant acetic acid lignin isolated from shoot shell of bamboo (Dendrocalamus Latiforus). Ind. Crop. Prod. 2016, 91, 340–349. [Google Scholar] [CrossRef]
- Espinoza-Acosta, J.L.; Torres-Chávez, P.I.; Ramírez-Wong, B.; López-Saiz, C.M.; Montaño-Leyva, B. Antioxidant, antimicrobial, and antimutagenic properties of technical lignins and their applications. BioResources 2016, 11, 5452–5481. [Google Scholar] [CrossRef]
- Figueiredo, P.; Lintinen, K.; Hirvonen, J.T.; Kostiainen, M.A.; Santos, H.A. Properties and chemical modifications of lignin: Towards lignin-based nanomaterials for biomedical applications. Prog. Mater. Sci. 2018, 93, 233–269. [Google Scholar] [CrossRef]
- Piccinino, D.; Capecchi, E.; Tomaino, E.; Gabellone, S.; Gigli, V.; Avitabile, D.; Saladino, R. Nano-Structured Lignin as Green Antioxidant and UV Shielding Ingredient for Sunscreen Applications. Antioxidants 2021, 10, 274. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Fortunati, E.; Gao, D.; Balestra, G.M.; Giovanale, G.; He, X.; Torre, L.; Kenny, J.M.; Puglia, D. Valorization of Acid Isolated High Yield Lignin Nanoparticles as Innovative Antioxidant/Antimicrobial Organic Materials. ACS Sustain. Chem. Eng. 2018, 6, 3502–3514. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, M.; Yuan, Q.; Cheng, G. Controlled Preparation of Corncob Lignin Nanoparticles and their Size-Dependent Antioxidant Properties: Toward High Value Utilization of Lignin. ACS Sustain. Chem. Eng. 2019, 7, 17166–17174. [Google Scholar] [CrossRef]
- Trevisan, H.; Rezende, C.A. Pure, stable and highly antioxidant lignin nanoparticles from elephant grass. Ind. Crop. Prod. 2020, 145, 112105. [Google Scholar] [CrossRef]
- Liu, Y.; Ai, K.; Ji, X.; Askhatova, D.; Du, R.; Lu, L.; Shi, J. Comprehensive Insights into the Multi-Antioxidative Mechanisms of Melanin Nanoparticles and Their Application To Protect Brain from Injury in Ischemic Stroke. J. Am. Chem. Soc. 2017, 139, 856–862. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, T.; Ji, W.; Zhang, Y.; Wu, F.; Tang, Q.; Wei, H.; Mao, L.; Zhang, M. Zwitterionic Polydopamine Engineered Interface for In Vivo Sensing with High Biocompatibility. Angew. Chem. Int. Ed. 2020, 59, 23445–23449. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Gu, Z.; Zhu, F.; Li, Y. Structural and Functional Tailoring of Melanin-Like Polydopamine Radical Scavengers. CCS Chem. 2020, 2, 128–138. [Google Scholar] [CrossRef]
- Zhao, H.; Zeng, Z.; Liu, L.; Chen, J.; Zhou, H.; Huang, L.; Huang, J.; Xu, H.; Xu, Y.; Chen, Z.; et al. Polydopamine nanoparticles for the treatment of acute inflammation-induced injury. Nanoscale 2018, 10, 6981–6991. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Zhao, X.; Hu, T.; Han, Y.; Guo, B. Mussel-inspired, antibacterial, conductive, antioxidant, injectable composite hydrogel wound dressing to promote the regeneration of infected skin. J. Colloid Interface Sci. 2019, 556, 514–528. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Baschieri, A.; Mollica, F.; Valgimigli, L.; Cedrowski, J.; Litwinienko, G.; Amorati, R. Hydrogen Atom Transfer from HOO• to ortho-Quinones Explains the Antioxidant Activity of Polydopamine. Angew. Chem. Int. Ed. 2021, 60, 15220–15224. [Google Scholar] [CrossRef]
- Mavridi-Printezi, A.; Guernelli, M.; Menichetti, A.; Montalti, M. Bio-Applications of Multifunctional Melanin Nanoparticles: From Nanomedicine to Nanocosmetics. Nanomaterials 2020, 10, 2276. [Google Scholar] [CrossRef]
- Nakatsuka, N.; Hasani-Sadrabadi, M.M.; Cheung, K.M.; Young, T.D.; Bahlakeh, G.; Moshaverinia, A.; Weiss, P.S.; Andrews, A.M. Polyserotonin Nanoparticles as Multifunctional Materials for Biomedical Applications. ACS Nano 2018, 12, 4761–4774. [Google Scholar] [CrossRef]
- Zhou, X.; McCallum, N.C.; Hu, Z.; Cao, W.; Gnanasekaran, K.; Feng, Y.; Stoddart, J.F.; Wang, Z.; Gianneschi, N.C. Artificial Allomelanin Nanoparticles. ACS Nano 2019, 13, 10980–10990. [Google Scholar] [CrossRef]
- Liu, Q.; Zheng, J.; Guan, M.; Fang, X.; Wang, C.; Shu, C. Protective effect of C70-carboxyfullerene against oxidative-induced stress on postmitotic muscle cells. ACS Appl. Mater. Interfaces 2013, 5, 4328–4333. [Google Scholar] [CrossRef]
- Qiao, Y.; Zhang, P.; Wang, C.; Ma, L.; Su, M. Reducing X-Ray Induced Oxidative Damages in Fibroblasts with Graphene Oxide. Nanomaterials 2014, 4, 522–534. [Google Scholar] [CrossRef] [Green Version]
- Shieh, Y.-T.; Wang, W.-W. Radical scavenging efficiencies of modified and microwave-treated multiwalled carbon nanotubes. Carbon 2014, 79, 354–362. [Google Scholar] [CrossRef]
- Huang, Y.; Liu, C.; Pu, F.; Liu, Z.; Ren, J.; Qu, X. A GO-Se nanocomposite as an antioxidant nanozyme for cytoprotection. Chem Commun 2017, 53, 3082–3085. [Google Scholar] [CrossRef]
- Cheng, X.; Ni, X.; Wu, R.; Chong, Y.; Gao, X.; Ge, C.; Yin, J.J. Evaluation of the structure-activity relationship of carbon nanomaterials as antioxidants. Nanomedicine 2018, 13, 733–747. [Google Scholar] [CrossRef]
- Qiu, Y.; Wang, Z.; Owens, A.C.; Kulaots, I.; Chen, Y.; Kane, A.B.; Hurt, R.H. Antioxidant chemistry of graphene-based materials and its role in oxidation protection technology. Nanoscale 2014, 6, 11744–11755. [Google Scholar] [CrossRef] [Green Version]
- Luo, M.; Boudier, A.; Clarot, I.; Maincent, P.; Schneider, R.; Leroy, P. Gold Nanoparticles Grafted by Reduced Glutathione With Thiol Function Preservation. Colloid Interface Sci. Commun. 2016, 14, 8–12. [Google Scholar] [CrossRef]
- Saravani, R.; Sargazi, S.; Saravani, R.; Rabbani, M.; Rahdar, A.; Taboada, P. Newly crocin-coated magnetite nanoparticles induce apoptosis and decrease VEGF expression in breast carcinoma cells. J. Drug Deliv. Sci. Technol. 2020, 60, 101987. [Google Scholar] [CrossRef]
- Deligiannakis, Y.; Sotiriou, G.A.; Pratsinis, S.E. Antioxidant and antiradical SiO2 nanoparticles covalently functionalized with gallic acid. ACS Appl. Mater. Interfaces 2012, 4, 6609–6617. [Google Scholar] [CrossRef] [PubMed]
- Massaro, M.; Amorati, R.; Cavallaro, G.; Guernelli, S.; Lazzara, G.; Milioto, S.; Noto, R.; Poma, P.; Riela, S. Direct chemical grafted curcumin on halloysite nanotubes as dual-responsive prodrug for pharmacological applications. Colloids Surf. B Biointerfaces 2016, 140, 505–513. [Google Scholar] [CrossRef] [PubMed]
- Arriagada, F.; Gunther, G.; Morales, J. Nanoantioxidant-Based Silica Particles as Flavonoid Carrier for Drug Delivery Applications. Pharmaceutics 2020, 12, 302. [Google Scholar] [CrossRef] [Green Version]
- Arriagada, F.; Gunther, G.; Nos, J.; Nonell, S.; Olea-Azar, C.; Morales, J. Antioxidant Nanomaterial Based on Core-Shell Silica Nanospheres with Surface-Bound Caffeic Acid: A Promising Vehicle for Oxidation-Sensitive Drugs. Nanomaterials 2019, 9, 214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Massaro, M.; Riela, S.; Guernelli, S.; Parisi, F.; Lazzara, G.; Baschieri, A.; Valgimigli, L.; Amorati, R. A synergic nanoantioxidant based on covalently modified halloysite-trolox nanotubes with intra-lumen loaded quercetin. J. Mater. Chem. B 2016, 4, 2229–2241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, T.; Xiao, P.; Zhang, J.; Jia, R.; Nawaz, H.; Chen, Z.; Zhang, J. Multifunctional Cellulose Ester Containing Hindered Phenol Groups with Free-Radical-Scavenging and UV-Resistant Activities. ACS Appl. Mater. Interfaces 2019, 11, 4302–4310. [Google Scholar] [CrossRef] [PubMed]
- Katana, B.; Rouster, P.; Varga, G.; Muráth, S.; Glinel, K.; Jonas, A.M.; Szilagyi, I. Self-Assembly of Protamine Biomacromolecule on Halloysite Nanotubes for Immobilization of Superoxide Dismutase Enzyme. ACS Applied Bio Mater. 2020, 3, 522–530. [Google Scholar] [CrossRef]
- Le, D.; Dilger, M.; Pertici, V.; Diabate, S.; Gigmes, D.; Weiss, C.; Delaittre, G. Ultra-Fast Synthesis of Multivalent Radical Nanoparticles by Ring-Opening Metathesis Polymerization-Induced Self-Assembly. Angew. Chem. Int. Ed. 2019, 58, 4725–4731. [Google Scholar] [CrossRef]
- Genovese, D.; Baschieri, A.; Vona, D.; Baboi, R.E.; Mollica, F.; Prodi, L.; Amorati, R.; Zaccheroni, N. Nitroxides as Building Blocks for Nanoantioxidants. ACS Appl. Mater. Interfaces 2021, 13, 31996–32004. [Google Scholar] [CrossRef]
- Soule, B.P.; Hyodo, F.; Matsumoto, K.; Simone, N.L.; Cook, J.A.; Krishna, M.C.; Mitchell, J.B. The chemistry and biology of nitroxide compounds. Free Radic. Biol. Med. 2007, 42, 1632–1650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shah, S.T.; Yehye, W.A.; Saad, O.; Simarani, K.; Chowdhury, Z.; Alhadi, A.A.; Al-Ani, L.A. Surface Functionalization of Iron Oxide Nanoparticles with Gallic Acid as Potential Antioxidant and Antimicrobial Agents. Nanomaterials 2017, 7, 306. [Google Scholar] [CrossRef]
- Shah, S.T.; Yehye, W.A.; Chowdhury, Z.Z.; Simarani, K. Magnetically directed antioxidant and antimicrobial agent: Synthesis and surface functionalization of magnetite with quercetin. PeerJ 2019, 7, e7651. [Google Scholar] [CrossRef] [Green Version]
- Viglianisi, C.; Di Pilla, V.; Menichetti, S.; Rotello, V.M.; Candiani, G.; Malloggi, C.; Amorati, R. Linking an a-tocopherol derivative to Cobalt(0) nanomagnets: Magnetically responsive antioxidants with superior radical trapping activity and reduced cytotoxicity. Chem.-Eur. J. 2014, 20, 6857–6860. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, W.; Compton, R.G. Investigation of single-drug-encapsulating liposomes using the nano-impact method. Angew. Chem. Int. Ed. 2014, 53, 13928–13930. [Google Scholar] [CrossRef]
- Yang, J.H.; Lee, S.Y.; Han, Y.S.; Park, K.C.; Choy, J.H. Efficient transdermal penetration and improved stability of L-ascorbic acid encapsulated in an inorganic nanocapsule. Bull. Korean Chem. Soc. 2003, 24, 499–503. [Google Scholar]
- Fu, Y.; Zhao, D.; Yao, P.; Wang, W.; Zhang, L.; Lvov, Y. Highly aging-resistant elastomers doped with antioxidant-loaded clay nanotubes. ACS Appl. Mater. Interfaces 2015, 7, 8156–8165. [Google Scholar] [CrossRef] [PubMed]
- Massaro, M.; Piana, S.; Colletti, C.G.; Noto, R.; Riela, S.; Baiamonte, C.; Giordano, C.; Pizzolanti, G.; Cavallaro, G.; Milioto, S.; et al. Multicavity halloysite-amphiphilic cyclodextrin hybrids for co-delivery of natural drugs into thyroid cancer cells. J. Mater. Chem. B 2015, 3, 4074–4081. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vergaro, V.; Lvov, Y.M.; Leporatti, S. Halloysite clay nanotubes for resveratrol delivery to cancer cells. Macromol. Biosci. 2012, 12, 1265–1271. [Google Scholar] [CrossRef]
- Foti, M.C. Use and Abuse of the DPPH• Radical. J. Agric. Food Chem. 2015, 63, 8765–8776. [Google Scholar] [CrossRef]
- Shah, R.; Farmer, L.A.; Zilka, O.; Van Kessel, A.T.M.; Pratt, D.A. Beyond DPPH: Use of Fluorescence-Enabled Inhibited Autoxidation to Predict Oxidative Cell Death Rescue. Cell Chem Biol 2019, 26, 1594–1607. [Google Scholar] [CrossRef]
- Amorati, R.; Menichetti, S.; Viglianisi, C.; Foti, M.C. Proton-electron transfer pathways in the reactions of peroxyl and DPPH radicals with hydrogen-bonded phenols. Chem. Commun. 2012, 48, 11904–11906. [Google Scholar] [CrossRef]
- Pichla, M.; Bartosz, G.; Pienkowska, N.; Sadowska-Bartosz, I. Possible artefacts of antioxidant assays performed in the presence of nitroxides and nitroxide-containing nanoparticles. Anal. Biochem. 2020, 597, 113698. [Google Scholar] [CrossRef]
- Amorati, R.; Valgimigli, L. Advantages and limitations of common testing methods for antioxidants. Free Radic. Res. 2015, 49, 633–649. [Google Scholar] [CrossRef]
- Kumar, A.; Prasad, A.; Sedlarova, M.; Ksas, B.; Havaux, M.; Pospisil, P. Interplay between antioxidants in response to photooxidative stress in Arabidopsis. Free Radic. Biol. Med. 2020, 160, 894–907. [Google Scholar] [CrossRef]
- Frankel, E.N. Chemistry of extra virgin olive oil: Adulteration, oxidative stability, and antioxidants. J. Agric. Food Chem. 2010, 58, 5991–6006. [Google Scholar] [CrossRef] [PubMed]
- Denisov, E.T.; Khudyakov, I.V. Mechanisms of action and reactivities of the free radicals of inhibitors. Chem. Rev. 2002, 87, 1313–1357. [Google Scholar] [CrossRef]
- Jiang, X.; Stockwell, B.R.; Conrad, M. Ferroptosis: Mechanisms, biology and role in disease. Nat. Rev. Mol. Cell Biol. 2021, 22, 266–282. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Li, Y.; Hu, Z.; Yue, X.; Proetto, M.T.; Jones, Y.; Gianneschi, N.C. Mimicking Melanosomes: Polydopamine Nanoparticles as Artificial Microparasols. ACS Cent. Sci. 2017, 3, 564–569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, Z.; Lou, R.; Pan, W.; Li, N.; Tang, B. Nanoenzymes in disease diagnosis and therapy. Chem. Commun. 2020, 56, 15513–15524. [Google Scholar] [CrossRef]
- Feng, W.; Han, X.; Hu, H.; Chang, M.; Ding, L.; Xiang, H.; Chen, Y.; Li, Y. 2D vanadium carbide MXenzyme to alleviate ROS-mediated inflammatory and neurodegenerative diseases. Nat. Commun. 2021, 12, 2203. [Google Scholar] [CrossRef] [PubMed]
- Harrison, K.A.; Haidasz, E.A.; Griesser, M.; Pratt, D.A. Inhibition of hydrocarbon autoxidation by nitroxide-catalyzed cross-dismutation of hydroperoxyl and alkylperoxyl radicals. Chem. Sci. 2018, 9, 6068–6079. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Foti, M.C.; Ingold, K.U. Mechanism of inhibition of lipid peroxidation by gamma-terpinene, an unusual and potentially useful hydrocarbon antioxidant. J. Agric. Food Chem. 2003, 51, 2758–2765. [Google Scholar] [CrossRef] [Green Version]
- Niki, E. Role of vitamin E as a lipid-soluble peroxyl radical scavenger: In vitro and in vivo evidence. Free Radic. Biol. Med. 2014, 66, 3–12. [Google Scholar] [CrossRef]
- Cedrowski, J.; Litwinienko, G.; Baschieri, A.; Amorati, R. Hydroperoxyl Radicals (HOO• ): Vitamin E Regeneration and H-Bond Effects on the Hydrogen Atom Transfer. Chem.-Eur. J. 2016, 22, 16441–16445. [Google Scholar] [CrossRef]
- Baschieri, A.; Valgimigli, L.; Gabbanini, S.; DiLabio, G.A.; Romero-Montalvo, E.; Amorati, R. Extremely Fast Hydrogen Atom Transfer between Nitroxides and HOO• Radicals and Implication for Catalytic Coantioxidant Systems. J. Am. Chem. Soc. 2018, 140, 10354–10362. [Google Scholar] [CrossRef] [PubMed]
- Denisov, E.T. Cyclic mechanisms of chain termination in the oxidation of organic compounds. Russ. Chem. Rev. 1996, 65, 505–520. [Google Scholar] [CrossRef]
- Valgimigli, L.; Amorati, R.; Petrucci, S.; Pedulli, G.F.; Hu, D.; Hanthorn, J.J.; Pratt, D.A. Unexpected acid catalysis in reactions of peroxyl radicals with phenols. Angew. Chem. Int. Ed. 2009, 48, 8348–8351. [Google Scholar] [CrossRef] [PubMed]
- Amorati, R.; Valgimigli, L.; Pedulli, G.F.; Grabovskiy, S.A.; Kabal´nova, N.N.; Chatgilialoglu, C. Base-promoted reaction of 5-hydroxyuracil derivatives with peroxyl radicals. Org. Lett. 2010, 12, 4130–4133. [Google Scholar] [CrossRef]
- Amorati, R.; Valgimigli, L.; Baschieri, A.; Guo, Y.; Mollica, F.; Menichetti, S.; Lupi, M.; Viglianisi, C. SET and HAT/PCET acid-mediated oxidation processes in helical shaped fused bis-phenothiazines. ChemPhysChem 2021, 22, 1446–1454. [Google Scholar] [CrossRef]
- Ingold, K.U.; Pratt, D.A. Advances in radical-trapping antioxidant chemistry in the 21st century: A kinetics and mechanisms perspective. Chem. Rev. 2014, 114, 9022–9046. [Google Scholar] [CrossRef] [Green Version]
- Mochizuki, M.; Yamazaki, S.-i.; Kano, K.; Ikeda, T. Kinetic analysis and mechanistic aspects of autoxidation of catechins. Biochim. Biophys. Acta Gen. Subj. 2002, 1569, 35–44. [Google Scholar] [CrossRef]
- Guernelli, S.; Cariola, A.; Baschieri, A.; Amorati, R.; Lo Meo, P. Nanosponges for the protection and release of the natural phenolic antioxidants quercetin, curcumin and phenethyl caffeate. Mater. Adv. 2020, 1, 2501–2508. [Google Scholar] [CrossRef]
- Sawyer, D.T.; Valentine, J.S. How super is superoxide? Acc. Chem. Res. 1981, 14, 393–400. [Google Scholar] [CrossRef]
- Agarwal, R.G.; Kim, H.J.; Mayer, J.M. Nanoparticle O-H Bond Dissociation Free Energies from Equilibrium Measurements of Cerium Oxide Colloids. J. Am. Chem. Soc. 2021, 143, 2896–2907. [Google Scholar] [CrossRef] [PubMed]
- Dunn, R.O. Oxidative stability of soybean oil fatty acid methyl esters by oil stability index (OSI). J. Am. Oil Chem. Soc. 2005, 82, 381–387. [Google Scholar] [CrossRef]
- Amorati, R.; Baschieri, A.; Valgimigli, L. Measuring Antioxidant Activity in Bioorganic Samples by the Differential Oxygen Uptake Apparatus: Recent Advances. J. Chem. 2017, 2017, 1–12. [Google Scholar] [CrossRef]
- Poon, J.F.; Zilka, O.; Pratt, D.A. Potent Ferroptosis Inhibitors Can Catalyze the Cross-Dismutation of Phospholipid-Derived Peroxyl Radicals and Hydroperoxyl Radicals. J. Am. Chem. Soc. 2020, 142, 14331–14342. [Google Scholar] [CrossRef] [PubMed]
- Repetto, M.G.; Ferrarotti, N.F.; Boveris, A. The involvement of transition metal ions on iron-dependent lipid peroxidation. Arch. Toxicol. 2010, 84, 255–262. [Google Scholar] [CrossRef]
- Zhou, B.; Wu, L.M.; Yang, L.; Liu, Z.L. Evidence for alpha-tocopherol regeneration reaction of green tea polyphenols in SDS micelles. Free Radic. Biol. Med. 2005, 38, 78–84. [Google Scholar] [CrossRef]
- Kusio, J.; Sitkowska, K.; Konopko, A.; Litwinienko, G. Hydroxycinnamyl Derived BODIPY as a Lipophilic Fluorescence Probe for Peroxyl Radicals. Antioxidants 2020, 9, 88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Konopko, A.; Kusio, J.; Litwinienko, G. Antioxidant Activity of Metal Nanoparticles Coated with Tocopherol-Like Residues-The Importance of Studies in Homo- and Heterogeneous Systems. Antioxidants 2019, 9, 5. [Google Scholar] [CrossRef] [Green Version]
- Howard, J.A.; Ingold, K.U. Absolute rate constants for hydrocarbon autoxidation. VI. Alkyl aromatic and olefinic hydrocarbons. Can. J. Chem. 1967, 45, 793–802. [Google Scholar] [CrossRef]
- Amorati, R.; Fumo, M.G.; Menichetti, S.; Mugnaini, V.; Pedulli, G.F. Electronic and hydrogen bonding effects on the chain-breaking activity of sulfur-containing phenolic antioxidants. J. Org. Chem. 2006, 71, 6325–6332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baschieri, A.; Pizzol, R.; Guo, Y.; Amorati, R.; Valgimigli, L. Calibration of Squalene, p-Cymene, and Sunflower Oil as Standard Oxidizable Substrates for Quantitative Antioxidant Testing. J. Agric. Food Chem. 2019, 67, 6902–6910. [Google Scholar] [CrossRef]
- Howard, H.J.; Ingold, K.U. Absolute rate constants for hydrocarbon autoxidation. V. The hydroperoxy radical in chain propagation and termination. Can. J. Chem. 1967, 45, 785–792. [Google Scholar] [CrossRef]
- Barclay, L.R.C.; Baskin, K.A.; Dakin, K.A.; Locke, S.J.; Vinqvist, M.R. The antioxidant activities of phenolic antioxidants in free radical peroxidation of phospholipid membranes. Can. J. Chem. 1990, 68, 2258–2269. [Google Scholar] [CrossRef] [Green Version]
- Barclay, L.R.C.; Locke, S.J.; Macneil, J.M.; Vankessel, J. Quantitative Studies of the Autoxidation of Linoleate Monomers Sequestered in Phosphatidylcholine Bilayers—Absolute Rate Constants in Bilayers. Can. J. Chem. 1985, 63, 2633–2638. [Google Scholar] [CrossRef]
- Amorati, R.; Baschieri, A.; Morroni, G.; Gambino, R.; Valgimigli, L. Peroxyl Radical Reactions in Water Solution: A Gym for Proton-Coupled Electron-Transfer Theories. Chem.-Eur. J. 2016, 22, 7924–7934. [Google Scholar] [CrossRef] [PubMed]
- Haidasz, E.A.; Van Kessel, A.T.; Pratt, D.A. A Continuous Visible Light Spectrophotometric Approach To Accurately Determine the Reactivity of Radical-Trapping Antioxidants. J. Org. Chem. 2016, 81, 737–744. [Google Scholar] [CrossRef]
- Uluata, S.; Durmaz, G.; Julian McClements, D.; Decker, E.A. Comparing DPPP fluorescence and UV based methods to assess oxidation degree of krill oil-in-water emulsions. Food Chem. 2021, 339, 127898. [Google Scholar] [CrossRef]
- Daoud, S.; Bou-Maroun, E.; Waschatko, G.; Cayot, P. Lipid oxidation in oil-in-water emulsions: Iron complexation by buffer ions and transfer on the interface as a possible mechanism. Food Chem. 2021, 342, 128273. [Google Scholar] [CrossRef]
- Amorati, R.; Valgimigli, L.; Diner, P.; Bakhtiari, K.; Saeedi, M.; Engman, L. Multi-faceted reactivity of alkyltellurophenols towards peroxyl radicals: Catalytic antioxidant versus thiol-depletion effect. Chem.-Eur. J. 2013, 19, 7510–7522. [Google Scholar] [CrossRef]
- Roschek, B., Jr.; Tallman, K.A.; Rector, C.L.; Gillmore, J.G.; Pratt, D.A.; Punta, C.; Porter, N.A. Peroxyl radical clocks. J. Org. Chem. 2006, 71, 3527–3532. [Google Scholar] [CrossRef]
- Zielinski, Z.A.; Pratt, D.A. Lipid Peroxidation: Kinetics, Mechanisms, and Products. J. Org. Chem. 2017, 82, 2817–2825. [Google Scholar] [CrossRef]
- Ghani, M.A.; Barril, C.; Bedgood, D.R., Jr.; Prenzler, P.D. Measurement of antioxidant activity with the thiobarbituric acid reactive substances assay. Food Chem. 2017, 230, 195–207. [Google Scholar] [CrossRef]
- Mollica, F.; Lucarini, M.; Passerini, C.; Carati, C.; Pavoni, S.; Bonoldi, L.; Amorati, R. Effect of Antioxidants on High-Temperature Stability of Renewable Bio-Oils Revealed by an Innovative Method for the Determination of Kinetic Parameters of Oxidative Reactions. Antioxidants 2020, 9, 399. [Google Scholar] [CrossRef]
- Grebowski, J.; Konopko, A.; Krokosz, A.; DiLabio, G.A.; Litwinienko, G. Antioxidant activity of highly hydroxylated fullerene C60 and its interactions with the analogue of alpha-tocopherol. Free Radic. Biol. Med. 2020, 160, 734–744. [Google Scholar] [CrossRef]
- Haidasz, E.A.; Meng, D.; Amorati, R.; Baschieri, A.; Ingold, K.U.; Valgimigli, L.; Pratt, D.A. Acid Is Key to the Radical-Trapping Antioxidant Activity of Nitroxides. J. Am. Chem. Soc. 2016, 138, 5290–5298. [Google Scholar] [CrossRef] [Green Version]
- Datta, A.; Mishra, S.; Manna, K.; Saha, K.D.; Mukherjee, S.; Roy, S. Pro-Oxidant Therapeutic Activities of Cerium Oxide Nanoparticles in Colorectal Carcinoma Cells. ACS Omega. 2020, 5, 9714–9723. [Google Scholar] [CrossRef] [Green Version]
- Baschieri, A.; Del Secco, B.; Zaccheroni, N.; Valgimigli, L.; Amorati, R. The Role of Onium Salts in the Pro-Oxidant Effect of Gold Nanoparticles in Lipophilic Environments. Chem.-Eur. J. 2018, 24, 9113–9119. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://site.unibo.it/free-radical-antiox-chem/en/downloads (accessed on 27 September 2021).
- Klaper, R.D. The Known and Unknown about the Environmental Safety of Nanomaterials in Commerce. Small 2020, 16, e2000690. [Google Scholar] [CrossRef]
- Miller, M.R.; Poland, C.A. Nanotoxicology: The Need for a Human Touch? Small 2020, 16, e2001516. [Google Scholar] [CrossRef] [PubMed]


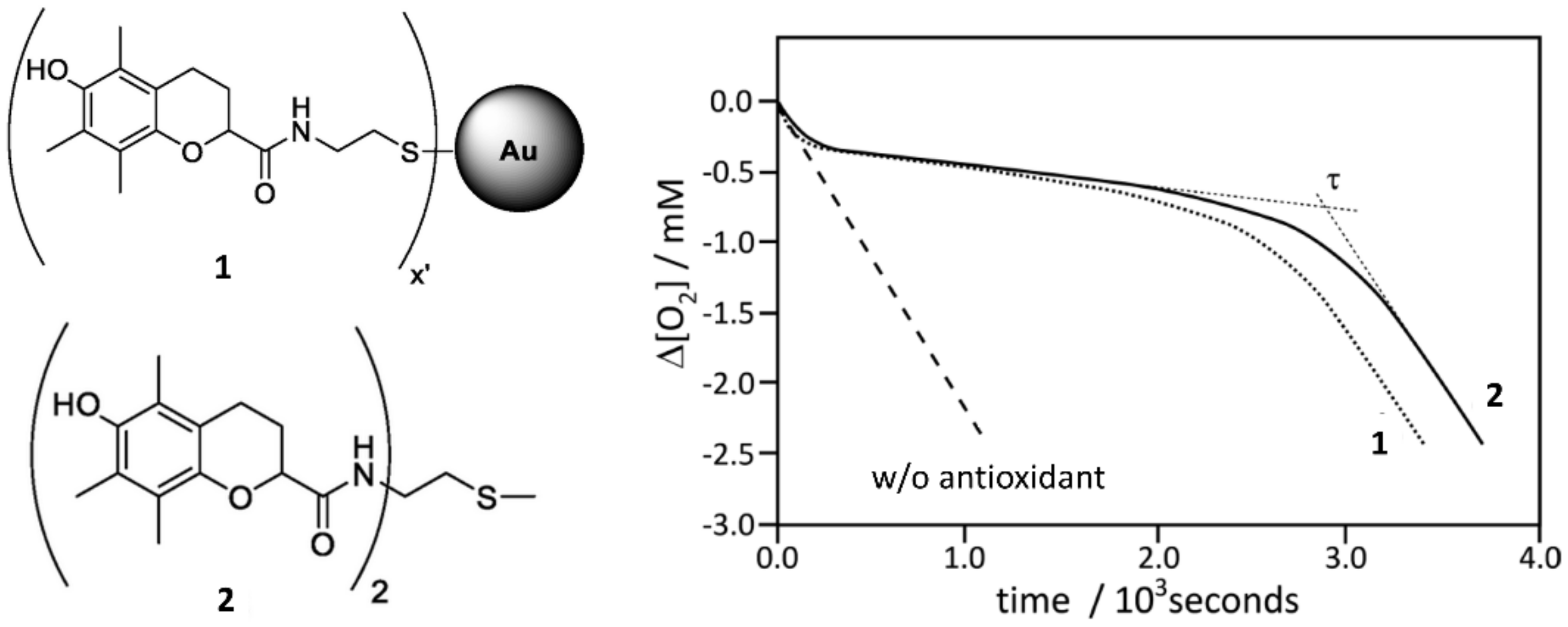
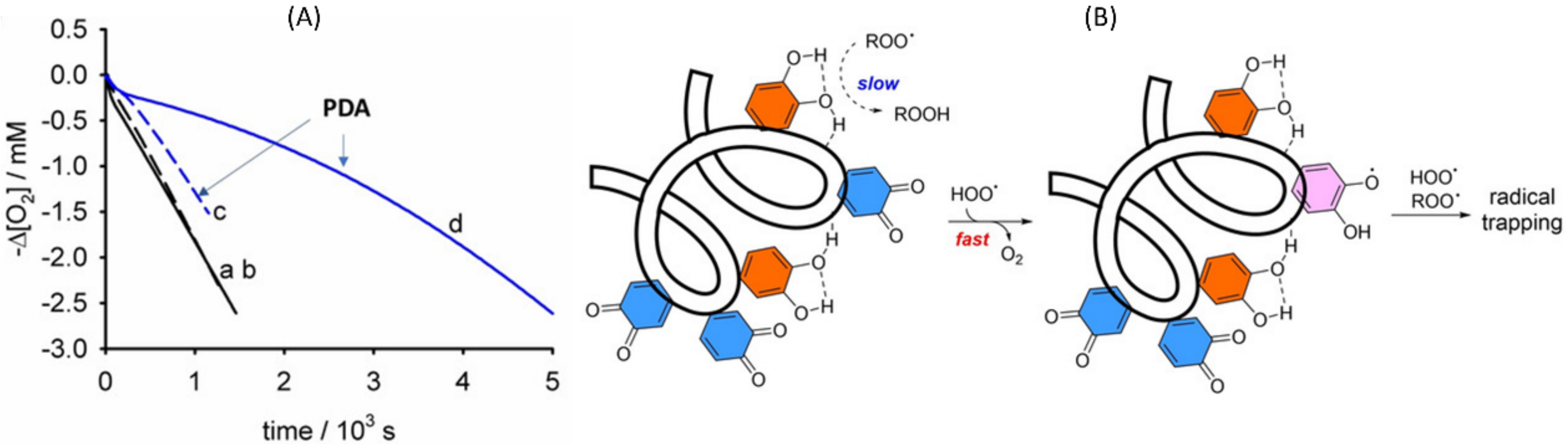
| Radical | Trapped by RTA | Reason | |
|---|---|---|---|
| HO• | Hydroxyl | No | Reaction with organic substrates is too fast |
| RO• | Alkoxyl | No | Reaction with organic substrates is too fast |
| R• | Alkyl | No | Reaction with O2 is diffusion-controlled and [O2] ≈ 10−4–10−3 M |
| ROO• | Alkyl peroxyl | Yes | Reaction with organic substrate is slow |
| HOO• | Hydroperoxyl | Yes | Reaction with organic substrate is slow |
| O2•− | Superoxide | No (Yes) 1 | No reaction with organic substrates nor with most RTA |
| Substrate | kp | 2kt | Solvent | Reference |
|---|---|---|---|---|
| Styrene | 41 | 4.2 × 107 | PhCl | [99] |
| Cumene | 0.32 | 4.6 × 104 | PhCl | [100] |
| Squalene | 0.68 | 7.4 × 106 | PhCl | [101] |
| 1,4-Cyclohexadiene | 1400 | 1.3 × 109 a | PhCl | [102] |
| p-Cymene | 0.83 | 2.9 × 106 | PhCl | [101] |
| Stripped sunflower oil | 66.9 | 3.5 × 106 | PhCl | [101] |
| Methyl linoleate | 62 | 8.8 × 106 | PhCl | [99] |
| 36 | 3.5 × 105 | Triton X-100 micelles | [103] | |
| 41 | 0.6 × 106 | DMPC b liposomes | [104] | |
| Tetrahydrofuran | 4.8 | 6.6 × 107 | H2O | [105] |
| Nanoantioxidant | kinh | kinh’ | Solvent | Reference |
|---|---|---|---|---|
| Trolox-HNT | (1.1 ± 0.1) × 106 | (9.8 ± 0.5) × 105 | Cumene/PhCl | [52] |
| TroloxS-AuNPs | (6.9 ± 0.4) × 105 | (6.9 ± 0.3) × 105 | Styrene/PhCl | [98] |
| CoNPs-Trolox | (5.6 ± 1.5) × 106 | (6.4 ± 1.0) × 105 | Styrene/PhCN | [60] |
| Ascorbic Acid/HNT | (1.4 ± 0.3) × 106 | (1.7 ± 0.2) × 106 | THF/Water | [3] |
| Nitroxides NPs | (1.5 ± 0.4) × 105 | (5.1 ± 1.5) × 106 | THF/Water | [56] |
| Curcumin-HNT | (1.7 ± 0.1) × 104 | (1.8 ± 0.2) × 104 | Cumene/PhCl | [49] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baschieri, A.; Amorati, R. Methods to Determine Chain-Breaking Antioxidant Activity of Nanomaterials beyond DPPH•. A Review. Antioxidants 2021, 10, 1551. https://doi.org/10.3390/antiox10101551
Baschieri A, Amorati R. Methods to Determine Chain-Breaking Antioxidant Activity of Nanomaterials beyond DPPH•. A Review. Antioxidants. 2021; 10(10):1551. https://doi.org/10.3390/antiox10101551
Chicago/Turabian StyleBaschieri, Andrea, and Riccardo Amorati. 2021. "Methods to Determine Chain-Breaking Antioxidant Activity of Nanomaterials beyond DPPH•. A Review" Antioxidants 10, no. 10: 1551. https://doi.org/10.3390/antiox10101551
APA StyleBaschieri, A., & Amorati, R. (2021). Methods to Determine Chain-Breaking Antioxidant Activity of Nanomaterials beyond DPPH•. A Review. Antioxidants, 10(10), 1551. https://doi.org/10.3390/antiox10101551







