Preclinical and Clinical Antioxidant Effects of Natural Compounds against Oxidative Stress-Induced Epigenetic Instability in Tumor Cells
Abstract
:1. Introduction
2. Oxidative Stress Signaling Pathways
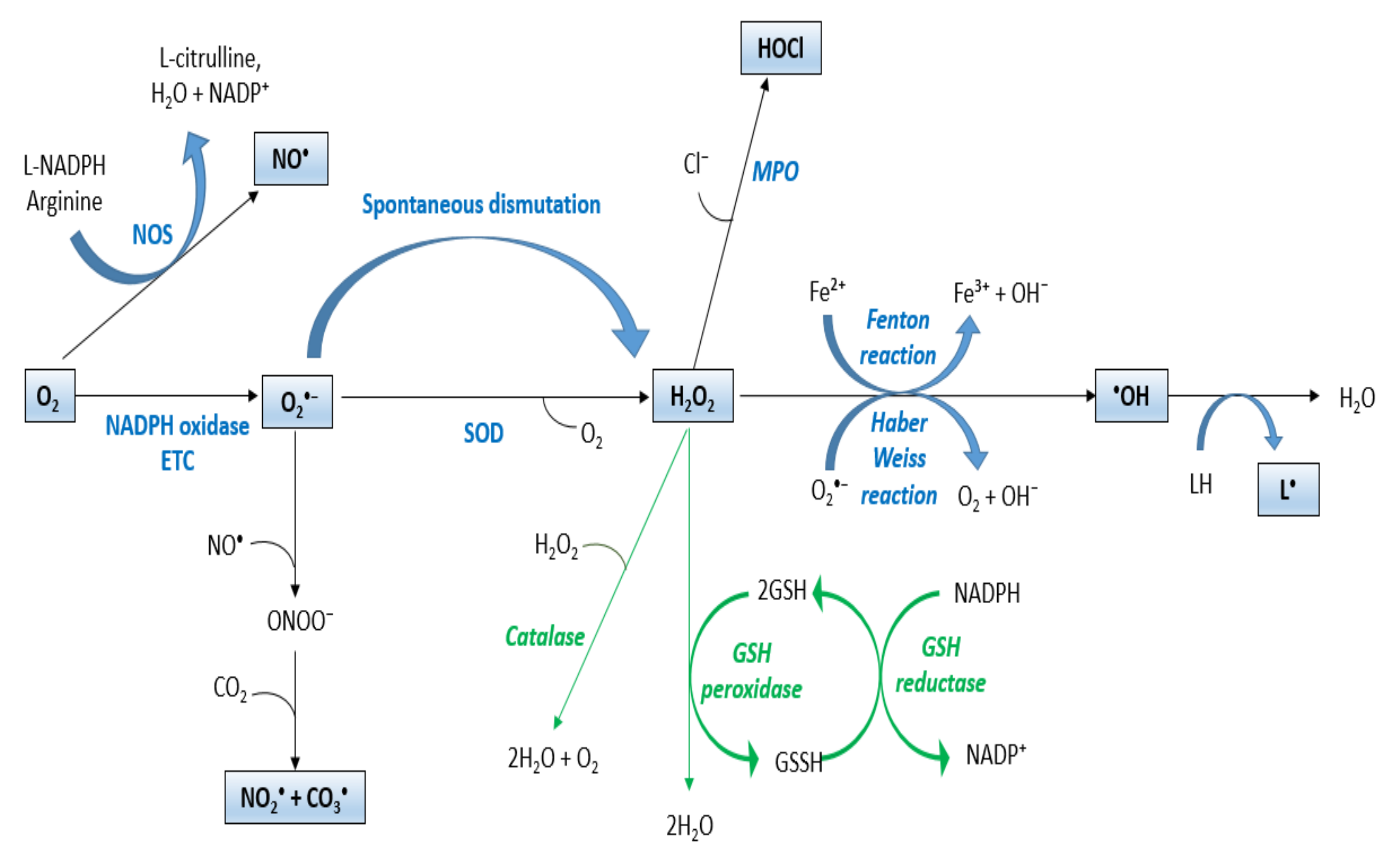
2.1. ROS
2.2. Redox Homeostasis
3. ROS Signaling Pathways in Cancer
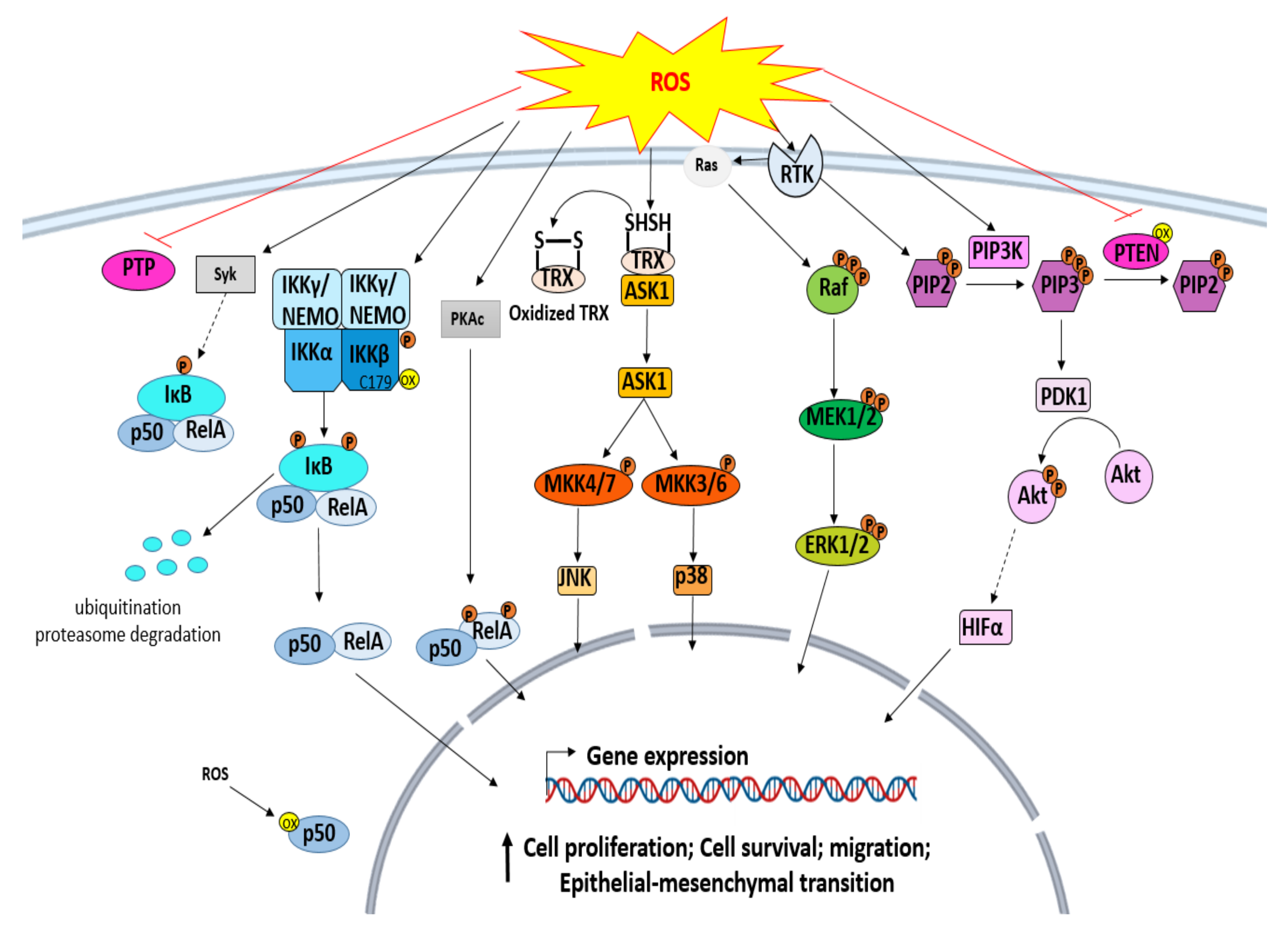
3.1. ROS and Protein Tyrosine Kinase (PTK)
3.2. Mitogen-Activated Protein Kinases (MAPKs) Signaling Pathway
3.3. ROS and IκB Kinase (IKK)/NF-κB Pathway
3.4. ROS and Phosphoinositide 3 Kinases (PI3K)/Akt Signaling Pathway
3.5. ROS and Hypoxia-Inducible Factor 1 (HIF-1) Signaling in Cancer
4. Oxidative-Stress-Induced Epigenetic Instability in Cancer
4.1. Oxidative Stress and DNA Methylation
4.2. Oxidative Stress and Histone Modification
4.3. Oxidative Stress and miRNAs
5. Antioxidant Protective Effects of Natural Compounds
5.1. In Vivo Preclinical Investigations
5.1.1. Flavonoids
5.1.2. Phenolic Acids
5.1.3. Terpenoids
5.2. Clinical Evidence
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Martinez-Cayuela, M. Oxygen free radicals and human disease. Biochimie 1995, 77, 147–161. [Google Scholar] [CrossRef]
- Szumiel, I. Ionizing radiation-induced oxidative stress, epigenetic changes and genomic instability: The pivotal role of mitochondria. Int. J. Radiat. Biol. 2015, 91, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Parohan, M.; Sadeghi, A.; Khatibi, S.R.; Nasiri, M.; Milajerdi, A.; Khodadost, M.; Sadeghi, O. Dietary Total antioxidant capacity and risk of cancer: A systematic review and meta-analysis on observational studies. Crit. Rev. Oncol. Hematol. 2019, 138, 70–86. [Google Scholar] [CrossRef] [PubMed]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative stress: Harms and benefits for human health. Oxid. Med. Cell. Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef]
- De Jager, T.L.; Cockrell, A.E.; Du Plessis, S.S. Ultraviolet light induced generation of reactive oxygen species. In Ultraviolet Light in Human Health, Diseases and Environment. Advances in Experimental Medicine and Biology; Springer: Cham, Switzerland, 2017; Volume 996, pp. 15–23. [Google Scholar]
- Kohen, R.; Nyska, A. Invited Review: Oxidation of biological systems: Oxidative stress phenomena, antioxidants, redox reactions, and methods for their quantification. Toxicol. Pathol. 2002, 30, 620–650. [Google Scholar] [CrossRef] [Green Version]
- Magnani, F.; Mattevi, A. Structure and mechanisms of ROS generation by NADPH oxidases. Curr. Opin. Struct. Biol. 2019, 59, 91–97. [Google Scholar] [CrossRef]
- Zhao, R.-Z.; Jiang, S.; Zhang, L.; Yu, Z.-B. Mitochondrial electron transport chain, ROS generation and uncoupling. Int. J. Mol. Med. 2019, 44, 3–15. [Google Scholar] [CrossRef] [Green Version]
- Cho, K.-J.; Seo, J.-M.; Kim, J.-H. Bioactive lipoxygenase metabolites stimulation of NADPH oxidases and reactive oxygen species. Mol. Cells 2011, 32, 1–5. [Google Scholar] [CrossRef]
- Battelli, M.G.; Polito, L.; Bortolotti, M.; Bolognesi, A. Xanthine Oxidoreductase-derived reactive species: Physiological and pathological effects. Oxid. Med. Cell. Longev. 2016, 2016, 3527579. [Google Scholar] [CrossRef] [Green Version]
- Veith, A.; Moorthy, B. Role of Cytochrome P450s in the generation and metabolism of reactive oxygen species. Curr. Opin. Toxicol. 2018, 7, 44–51. [Google Scholar] [CrossRef]
- Ali, E.M.; Soha, H.M.; Mohamed, T.M. Nitric oxide synthase and oxidative stress: Regulation of nitric oxide synthase. In Oxidative Stress: Molecular Mechanisms and Biological Effects; InTech: Rijeka, Croatia, 2012; pp. 61–72. [Google Scholar]
- Schröder, K. NADPH Oxidases: Current aspects and tools. Redox Biol. 2020, 34, 101512. [Google Scholar] [CrossRef]
- Wang, Y.; Branicky, R.; Noë, A.; Hekimi, S. Superoxide dismutases: Dual roles in controlling ROS damage and regulating ROS signaling. J. Cell Biol. 2018, 217, 1915–1928. [Google Scholar] [CrossRef]
- Stuehr, D.J. Enzymes of the L-arginine to nitric oxide pathway. J. Nutr. 2004, 134, 2748S–2751S. [Google Scholar] [CrossRef]
- Aratani, Y. Myeloperoxidase: Its Role for host defense, inflammation, and neutrophil function. Arch. Biochem. Biophys. 2018, 640, 47–52. [Google Scholar] [CrossRef]
- Kanti Das, T.; Wati, M.R.; Fatima-Shad, K. Oxidative stress gated by Fenton and Haber Weiss reactions and its association with Alzheimer’s Disease. Arch. Neurosci. 2015, 2, e60038. [Google Scholar] [CrossRef] [Green Version]
- Sies, H. Strategies of antioxidant defense. In EJB Reviews; Springer: Berlin/Heidelber, Germany, 1993; pp. 101–107. [Google Scholar] [CrossRef]
- Gutteridge, J.M. Lipid peroxidation initiated by superoxide-dependent hydroxyl radicals using complexed iron and hydrogen peroxide. FEBS Lett. 1984, 172, 245–249. [Google Scholar] [CrossRef] [Green Version]
- Redza-Dutordoir, M.; Averill-Bates, D.A. Activation of apoptosis signalling pathways by reactive oxygen species. Biochim. Biophys. Acta BBA Mol. Cell Res. 2016, 1863, 2977–2992. [Google Scholar] [CrossRef]
- Wang, L.; Kuang, Z.; Zhang, D.; Gao, Y.; Ying, M.; Wang, T. Reactive oxygen species in immune cells: A new antitumor target. Biomed. Pharmacother. 2021, 133, 110978. [Google Scholar] [CrossRef]
- Ji, A.-R.; Ku, S.-Y.; Cho, M.S.; Kim, Y.Y.; Kim, Y.J.; Oh, S.K.; Kim, S.H.; Moon, S.Y.; Choi, Y.M. Reactive oxygen species enhance differentiation of human embryonic stem cells into mesendodermal lineage. Exp. Mol. Med. 2010, 42, 175–186. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Wang, X.; Vikash, V.; Ye, Q.; Wu, D.; Liu, Y.; Dong, W. ROS and ROS-mediated cellular signaling. Oxid. Med. Cell. Longev. 2016, 2016, 4350965. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, G.; Almasry, M.; Dhillon, A.S.; Abuayyash, M.M.; Kothandaraman, N.; Cakar, Z. Overview and sources of reactive oxygen species (ROS) in the reproductive system. In Oxidative Stress in Human Reproduction; Springer: Cham, Schwitzerland, 2017; pp. 1–16. [Google Scholar]
- Simon, H.-U.; Haj-Yehia, A.; Levi-Schaffer, F. Role of reactive oxygen species (ROS) in apoptosis induction. Apoptosis 2000, 5, 415–418. [Google Scholar] [CrossRef]
- Chaudière, J.; Ferrari-Iliou, R. Intracellular antioxidants: From chemical to biochemical mechanisms. Food Chem. Toxicol. 1999, 37, 949–962. [Google Scholar] [CrossRef]
- McCord, J.M.; Fridovich, I. Superoxide dismutase: An enzymic function for erythrocuprein (hemocuprein). J. Biol. Chem. 1969, 244, 6049–6055. [Google Scholar] [CrossRef]
- Nandi, A.; Yan, L.-J.; Jana, C.K.; Das, N. Role of catalase in oxidative stress-and age-associated degenerative diseases. Oxid. Med. Cell. Longev. 2019, 2019, 9613090. [Google Scholar] [CrossRef] [Green Version]
- Sarıkaya, E.; Doğan, S. Glutathione Peroxidase in health and diseases. Glutathione System and Oxidative Stress in Health and Disease; Intech: Rijeka, Croatia, 2020. [Google Scholar]
- Carlberg, I.; Mannervik, B. Glutathione reductase. Methods Enzymol. 1985, 113, 484–490. [Google Scholar]
- Freinbichler, W.; Colivicchi, M.A.; Stefanini, C.; Bianchi, L.; Ballini, C.; Misini, B.; Weinberger, P.; Linert, W.; Varešlija, D.; Tipton, K.F. Highly reactive oxygen species: Detection, formation, and possible functions. Cell. Mol. Life Sci. 2011, 68, 2067–2079. [Google Scholar] [CrossRef]
- Klaunig, J.E.; Wang, Z. Oxidative stress in carcinogenesis. Curr. Opin. Toxicol. 2018, 7, 116–121. [Google Scholar] [CrossRef]
- Nakashima, I.; Kawamoto, Y.; Takeda, K.; Kato, M. Control of genetically prescribed protein tyrosine kinase activities by environment-linked redox reactions. Enzyme Res. 2011, 2011, 896567. [Google Scholar] [CrossRef] [Green Version]
- Son, Y.; Cheong, Y.-K.; Kim, N.-H.; Chung, H.-T.; Kang, D.G.; Pae, H.-O. Mitogen-activated protein kinases and reactive oxygen species: How can ROS activate MAPK pathways? J. Signal Transduct. 2011, 2011, 792639. [Google Scholar] [CrossRef]
- Cheng, C.W.; Kuo, C.Y.; Fan, C.C.; Fang, W.C.; Jiang, S.S.; Lo, Y.K.; Wang, T.Y.; Kao, M.C.; Lee, A.Y. Overexpression of Lon contributes to survival and aggressive phenotype of cancer cells through mitochondrial complex I-mediated generation of reactive oxygen species. Cell Death Dis. 2013, 4, e681. [Google Scholar] [CrossRef]
- Shih, V.F.-S.; Tsui, R.; Caldwell, A.; Hoffmann, A. A Single NFκB System for both canonical and non-canonical signaling. Cell Res. 2011, 21, 86–102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morgan, M.J.; Liu, Z. Crosstalk of reactive oxygen species and NF-ΚB signaling. Cell Res. 2011, 21, 103–115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takada, Y.; Mukhopadhyay, A.; Kundu, G.C.; Mahabeleshwar, G.H.; Singh, S.; Aggarwal, B.B. Hydrogen peroxide activates NF-ΚB through tyrosine phosphorylation of IκBα and serine phosphorylation of P65: Evidence for the involvement of IκBα kinase and Syk protein-tyrosine kinase. J. Biol. Chem. 2003, 278, 24233–24241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, J.; Nie, J.; Ma, X.; Wei, Y.; Peng, Y.; Wei, X. Targeting PI3K in cancer: Mechanisms and advances in clinical trials. Mol. Cancer 2019, 18, 1–28. [Google Scholar] [CrossRef] [Green Version]
- Leslie, N.R.; Bennett, D.; Lindsay, Y.E.; Stewart, H.; Gray, A.; Downes, C.P. Redox regulation of PI 3-kinase signalling via inactivation of PTEN. EMBO J. 2003, 22, 5501–5510. [Google Scholar] [CrossRef]
- Kitagishi, Y.; Matsuda, S. Redox regulation of tumor suppressor PTEN in cancer and aging. Int. J. Mol. Med. 2013, 31, 511–515. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.-R.; Yang, K.-S.; Kwon, J.; Lee, C.; Jeong, W.; Rhee, S.G. Reversible inactivation of the tumor suppressor PTEN by H2O2. J. Biol. Chem. 2002, 277, 20336–20342. [Google Scholar] [CrossRef] [Green Version]
- Franke, T.F.; Wang, X.; McCullough, K.D.; Holbrook, N.J. Epidermal growth factor receptor-dependent akt activation by oxidative stress enhances cell survival. J. Biol. Chem. 2000, 275, 14624–14631. [Google Scholar]
- Galanis, A.; Pappa, A.; Giannakakis, A.; Lanitis, E.; Dangaj, D.; Sandaltzopoulos, R. Reactive oxygen species and HIF-1 signalling in cancer. Cancer Lett. 2008, 266, 12–20. [Google Scholar] [CrossRef]
- Liu, L.-Z.; Hu, X.-W.; Xia, C.; He, J.; Zhou, Q.; Shi, X.; Fang, J.; Jiang, B.-H. Reactive oxygen species regulate epidermal growth factor-induced vascular endothelial growth factor and hypoxia-inducible factor-1α expression through activation of AKT and P70S6K1 in human ovarian cancer cells. Free Radic. Biol. Med. 2006, 41, 1521–1533. [Google Scholar] [CrossRef]
- Lutz, W.K. Endogenous genotoxic agents and processes as a basis of spontaneous carcinogenesis. Mutat. Res. Genet. Toxicol. 1990, 238, 287–295. [Google Scholar] [CrossRef] [Green Version]
- Jena, N.R. DNA Damage by reactive species: Mechanisms, Mutation and Repair. J. Biosci. 2012, 37, 503–517. [Google Scholar] [CrossRef]
- Van Elsland, D.; Neefjes, J. Bacterial infections and cancer. EMBO Rep. 2018, 19, e46632. [Google Scholar] [CrossRef]
- Sharma, S.; Kelly, T.K.; Jones, P.A. Epigenetics in cancer. Carcinogenesis 2010, 31, 27–36. [Google Scholar] [CrossRef]
- Donkena, K.V.; Young, C.Y.; Tindall, D.J. Oxidative stress and DNA methylation in prostate cancer. Obstet. Gynecol. Int. 2010, 2010, 302051. [Google Scholar] [CrossRef] [Green Version]
- Bird, A. DNA Methylation patterns and epigenetic memory. Genes Dev. 2002, 16, 6–21. [Google Scholar] [CrossRef] [Green Version]
- Nan, X.; Ng, H.-H.; Johnson, C.A.; Laherty, C.D.; Turner, B.M.; Eisenman, R.N.; Bird, A. Transcriptional repression by the methyl-CpG-binding protein MeCP2 involves a histone deacetylase complex. Nature 1998, 393, 386–389. [Google Scholar] [CrossRef]
- Reyngold, M.; Chan, T.A. DNA methylation. In Molecular Oncology: Causes of Cancer and Targets for Treatment; Cambridge University Press: Cambridge, UK, 2018. [Google Scholar]
- Ehrlich, M. DNA Hypomethylation in Cancer Cells. Epigenomics 2009, 1, 239–259. [Google Scholar] [CrossRef] [Green Version]
- Valinluck, V.; Tsai, H.-H.; Rogstad, D.K.; Burdzy, A.; Bird, A.; Sowers, L.C. Oxidative Damage to methyl-CpG Sequences inhibits the binding of the methyl-CpG binding domain (MBD) of methyl-CpG binding protein 2 (MeCP2). Nucleic Acids Res. 2004, 32, 4100–4108. [Google Scholar] [CrossRef] [Green Version]
- Grollman, A.P.; Moriya, M. Mutagenesis by 8-Oxoguanine: An Enemy Within. Trends Genet. 1993, 9, 246–249. [Google Scholar] [CrossRef]
- Gening, L.V.; Volodin, A.A.; Kazachenko, K.Y.; Makarova, I.V.; Tarantul, V.Z. Estimation of the Mutagenic Potential of 8-Oxog in Nuclear Extracts of Mouse Cells Using the “Framed Mirror” Method. Methods Protoc. 2020, 3, 3. [Google Scholar] [CrossRef] [Green Version]
- Franco, R.; Schoneveld, O.; Georgakilas, A.G.; Panayiotidis, M.I. Oxidative stress, DNA methylation and carcinogenesis. Cancer Lett. 2008, 266, 6–11. [Google Scholar] [CrossRef]
- Lim, S.-O.; Gu, J.-M.; Kim, M.S.; Kim, H.-S.; Park, Y.N.; Park, C.K.; Cho, J.W.; Park, Y.M.; Jung, G. Epigenetic changes induced by reactive oxygen species in hepatocellular carcinoma: Methylation of the E-cadherin promoter. Gastroenterology 2008, 135, 2128–2140. [Google Scholar] [CrossRef]
- Zhang, R.; Kang, K.A.; Kim, K.C.; Na, S.-Y.; Chang, W.Y.; Kim, G.Y.; Kim, H.S.; Hyun, J.W. Oxidative stress causes epigenetic alteration of CDX1 expression in colorectal cancer cells. Gene 2013, 524, 214–219. [Google Scholar] [CrossRef]
- Romanenko, A.; Morell-Quadreny, L.; Lopez-Guerrero, J.A.; Pellin, A.; Nepomnyaschy, V.; Vozianov, A.; Llombart-Bosch, A. P16INK4A and P15INK4B gene alteration associated with oxidative stress in renal cell carcinomas after the chernobyl accident (Pilot Study). Diagn. Mol. Pathol. 2002, 11, 163–169. [Google Scholar] [CrossRef]
- Castelli, G.; Pelosi, E.; Testa, U. Targeting histone methyltransferase and demethylase in acute myeloid leukemia therapy. OncoTargets Ther. 2018, 11, 131. [Google Scholar] [CrossRef] [Green Version]
- Kouzarides, T. Chromatin Modifications and Their Function. Cell 2007, 128, 693–705. [Google Scholar] [CrossRef] [Green Version]
- Luan, Y.; Ngo, L.; Han, Z.; Wang, X.; Qu, M.; Zheng, Y.G. Histone acetyltransferases: Enzymes, assays, and inhibitors. In Epigenetic Technological Applications; Elsevier: Amsterdam, The Netherlands, 2015; pp. 291–317. [Google Scholar]
- O’Hagan, H.M.; Wang, W.; Sen, S.; Shields, C.D.; Lee, S.S.; Zhang, Y.W.; Clements, E.G.; Cai, Y.; Van Neste, L.; Easwaran, H. Oxidative damage targets complexes containing DNA methyltransferases, SIRT1, and polycomb members to promoter CpG Islands. Cancer Cell 2011, 20, 606–619. [Google Scholar] [CrossRef] [Green Version]
- Place, R.F.; Li, L.-C.; Pookot, D.; Noonan, E.J.; Dahiya, R. MicroRNA-373 induces expression of genes with complementary promoter sequences. Proc. Natl. Acad. Sci. USA 2008, 105, 1608–1613. [Google Scholar] [CrossRef] [Green Version]
- Piletič, K.; Kunej, T. MicroRNA epigenetic signatures in human disease. Arch. Toxicol. 2016, 90, 2405–2419. [Google Scholar] [CrossRef]
- Calin, G.A.; Sevignani, C.; Dumitru, C.D.; Hyslop, T.; Noch, E.; Yendamuri, S.; Shimizu, M.; Rattan, S.; Bullrich, F.; Negrini, M. Human MicroRNA genes are frequently located at fragile sites and genomic regions involved in cancers. Proc. Natl. Acad. Sci. USA 2004, 101, 2999–3004. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Humphries, B.; Wang, Z.; Yang, C. MicroRNA Regulation of Epigenetic Modifiers in Breast Cancer. Cancers 2019, 11, 897. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, J.; Jiang, B.-H. Interplay between reactive oxygen species and MicroRNAs in cancer. Curr. Pharmacol. Rep. 2016, 2, 82–90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, J.; Xu, Q.; Jing, Y.; Agani, F.; Qian, X.; Carpenter, R.; Li, Q.; Wang, X.-R.; Peiper, S.S.; Lu, Z. Reactive oxygen species regulate ERBB2 and ERBB3 expression via MiR-199a/125b and DNA methylation. EMBO Rep. 2012, 13, 1116–1122. [Google Scholar] [CrossRef] [Green Version]
- Tu, H.; Sun, H.; Lin, Y.; Ding, J.; Nan, K.; Li, Z.; Shen, Q.; Wei, Y. Oxidative stress upregulates PDCD4 expression in patients with gastric cancer via MiR-21. Curr. Pharm. Des. 2014, 20, 1917–1923. [Google Scholar] [CrossRef]
- Degli Esposti, D.; Aushev, V.N.; Lee, E.; Cros, M.-P.; Zhu, J.; Herceg, Z.; Chen, J.; Hernandez-Vargas, H. MiR-500a-5p regulates oxidative stress response genes in breast cancer and predicts cancer survival. Sci. Rep. 2017, 7, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Wang, P.; Zhu, C.; Ma, M.; Chen, G.; Song, M.; Zeng, Z.; Lu, W.; Yang, J.; Wen, S.; Chiao, P.J. Micro-RNA-155 Is Induced by K-Ras oncogenic signal and promotes ROS stress in pancreatic cancer. Oncotarget 2015, 6, 21148. [Google Scholar] [CrossRef] [Green Version]
- Demir, F.; Uzun, F.G.; Durak, D.; Kalender, Y. Subacute chlorpyrifos-induced oxidative stress in rat erythrocytes and the protective effects of catechin and quercetin. Pestic. Biochem. Physiol. 2011, 99, 77–81. [Google Scholar] [CrossRef]
- Ganesan, D.; Holkar, A.; Albert, A.; Paul, E.; Mariakuttikan, J.; Sadasivam Selvam, G. Combination of ramipril and rutin alleviate alloxan induced diabetic nephropathy targeting multiple stress pathways in vivo. Biomed. Pharmacother. 2018, 108, 1338–1346. [Google Scholar] [CrossRef]
- Gautam, R.; Singh, M.; Gautam, S.; Rawat, J.K.; Saraf, S.A.; Kaithwas, G. Rutin attenuates intestinal toxicity induced by methotrexate linked with anti-oxidative and anti-inflammatory effects. BMC Complement. Altern. Med. 2016, 16, 99. [Google Scholar] [CrossRef] [Green Version]
- Han, J.-Y.; Ahn, S.-Y.; Kim, C.-S.; Yoo, S.-K.; Kim, S.-K.; Kim, H.-C.; Hong, J.T.; Oh, K.-W. Protection of apigenin against kainate-induced excitotoxicity by anti-oxidative effects. Biol. Pharm. Bull. 2012, 35, 1440–1446. [Google Scholar] [CrossRef] [Green Version]
- La Casa, C.; Villegas, I.; Alarcón de la Lastra, C.; Motilva, V.; Martín Calero, M.J. Evidence for protective and antioxidant properties of rutin, a natural flavone, against ethanol induced gastric lesions. J. Ethnopharmacol. 2000, 71, 45–53. [Google Scholar] [CrossRef]
- Liu, H.; Guo, X.; Chu, Y.; Lu, S. Heart protective effects and mechanism of quercetin preconditioning on anti-myocardial ischemia reperfusion (IR) injuries in rats. Gene 2014, 545, 149–155. [Google Scholar] [CrossRef]
- Liu, H.-J.; Fan, Y.-L.; Liao, H.-H.; Liu, Y.; Chen, S.; Ma, Z.-G.; Zhang, N.; Yang, Z.; Deng, W.; Tang, Q.-Z. Apigenin alleviates STZ-induced diabetic cardiomyopathy. Mol. Cell. Biochem. 2017, 428, 9–21. [Google Scholar] [CrossRef]
- Mao, X.-Y.; Yu, J.; Liu, Z.-Q.; Zhou, H.-H. Apigenin attenuates diabetes-associated cognitive decline in rats via suppressing oxidative stress and nitric oxide synthase pathway. Int. J. Clin. Exp. Med. 2015, 8, 15506–15513. [Google Scholar]
- Pan, X.; Shao, Y.; Wang, F.; Cai, Z.; Liu, S.; Xi, J.; He, R.; Zhao, Y.; Zhuang, R. Protective effect of apigenin magnesium complex on H2O2-induced oxidative stress and inflammatory responses in rat hepatic stellate cells. Pharm. Biol. 2020, 58, 553–560. [Google Scholar] [CrossRef]
- Rizvi, S.I.; Zaid, M.A.; Anis, R.; Mishra, N. Protective role of tea catechins against oxidation-induced damage of type 2 diabetic erythrocytes. Clin. Exp. Pharmacol. Physiol. 2005, 32, 70–75. [Google Scholar] [CrossRef]
- Roslan, J.; Giribabu, N.; Karim, K.; Salleh, N. Quercetin ameliorates oxidative stress, inflammation and apoptosis in the heart of streptozotocin-nicotinamide-induced adult male diabetic rats. Biomed. Pharmacother. 2017, 86, 570–582. [Google Scholar] [CrossRef]
- Samie, A.; Sedaghat, R.; Baluchnejadmojarad, T.; Roghani, M. Hesperetin, a citrus flavonoid, attenuates testicular damage in diabetic rats via inhibition of oxidative stress, inflammation, and apoptosis. Life Sci. 2018, 210, 132–139. [Google Scholar] [CrossRef]
- Somade, O.; Akinloye, O.; Adeyeye, M.; Fabunmi, G.; Idowu, O.; Badmus, F.; Salaudeen, B. Quercetin, a natural phytochemical and antioxidant protects against Sodium azide-induced hepatic and splenic oxidative stress in rats. J. Investig. Biochem. 2015, 4, 69. [Google Scholar] [CrossRef]
- Wan, J.; Kuang, G.; Zhang, L.; Jiang, R.; Chen, Y.; He, Z.; Ye, D. Hesperetin Attenuated acetaminophen-induced hepatotoxicity by inhibiting hepatocyte necrosis and apoptosis, oxidative stress and inflammatory response via upregulation of heme oxygenase-1 expression. Int. Immunopharmacol. 2020, 83, 106435. [Google Scholar] [CrossRef]
- Yang, X.; Yang, J.; Hu, J.; Li, X.; Zhang, X.; Li, Z. Apigenin attenuates myocardial ischemia/reperfusion injury via the inactivation of P38 mitogen-activated protein kinase. Mol. Med. Rep. 2015, 12, 6873–6878. [Google Scholar] [CrossRef] [Green Version]
- Yelumalai, S.; Giribabu, N.; Karim, K.; Omar, S.Z.; Salleh, N.B. In vivo administration of quercetin ameliorates sperm oxidative stress, inflammation, preserves sperm morphology and functions in streptozotocin-nicotinamide induced adult male diabetic rats. Arch. Med. Sci. 2019, 15, 240–249. [Google Scholar] [CrossRef]
- Yue, S.; Xue, N.; Li, H.; Huang, B.; Chen, Z.; Wang, X. Hepatoprotective effect of apigenin against liver injury via the non-canonical NF-ΚB pathway in vivo and in vitro. Inflammation 2020, 43, 1634–1648. [Google Scholar] [CrossRef]
- Chen, W.-M.; Shaw, L.-H.; Chang, P.-J.; Tung, S.-Y.; Chang, T.-S.; Shen, C.-H.; Hsieh, Y.-Y.; Wei, K.-L. Hepatoprotective effect of resveratrol against ethanol-induced oxidative stress through induction of superoxide dismutase in vivo and in vitro. Exp. Ther. Med. 2016, 11, 1231–1238. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.-L.; Gao, J.-P.; Han, Y.-L.; Xu, X.; Wu, R.; Gao, Y.; Cui, X.-H. Comparative studies of polydatin and resveratrol on mutual transformation and antioxidative effect in vivo. Phytomedicine 2015, 22, 553–559. [Google Scholar] [CrossRef]
- Prakash, R. Protective effect of resveratrol and celecoxib on lipopolysaccharide induced oxidative stress. Ramakrishnan Prakash J. Pharm. Sci. Res. 2019, 11, 1. [Google Scholar]
- Wang, X.; Meng, L.; Zhao, L.; Wang, Z.; Liu, H.; Liu, G.; Guan, G. Resveratrol ameliorates hyperglycemia-induced renal tubular oxidative stress damage via modulating the SIRT1/FOXO3a pathway. Diabetes Res. Clin. Pract. 2017, 126, 172–181. [Google Scholar] [CrossRef]
- Zhang, Y.-Q.; Liu, Y.-J.; Mao, Y.-F.; Dong, W.-W.; Zhu, X.-Y.; Jiang, L. Resveratrol ameliorates lipopolysaccharide-induced epithelial mesenchymal transition and pulmonary fibrosis through suppression of oxidative stress and transforming growth factor-Β1 signaling. Clin. Nutr. 2015, 34, 752–760. [Google Scholar] [CrossRef]
- Baranauskaite, J.; Sadauskiene, I.; Liekis, A.; Kasauskas, A.; Lazauskas, R.; Zlabiene, U.; Masteikova, R.; Kopustinskiene, D.M.; Bernatoniene, J. Natural compounds rosmarinic acid and carvacrol counteract aluminium-induced oxidative stress. Molecules 2020, 25, 1807. [Google Scholar] [CrossRef] [Green Version]
- Carrasco-Legleu, C.E. A single dose of caffeic acid phenethyl ester prevents initiation in a medium-term rat hepatocarcinogenesis model. World J. Gastroenterol. 2006, 12, 6779. [Google Scholar] [CrossRef] [PubMed]
- Dianat, M.; Hamzavi, G.R.; Badavi, M.; Samarbafzadeh, A. Effects of losartan and vanillic acid co-administration on ischemia-reperfusion-induced oxidative stress in isolated rat heart. Iran. Red Crescent Med. J. 2014, 16, e16664. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gerin, F.; Erman, H.; Erboga, M.; Sener, U.; Yilmaz, A.; Seyhan, H.; Gurel, A. The Effects of ferulic acid against oxidative stress and inflammation in formaldehyde-induced hepatotoxicity. Inflammation 2016, 39, 1377–1386. [Google Scholar] [CrossRef] [PubMed]
- Govindaraj, J.; Sorimuthu Pillai, S. Rosmarinic acid modulates the antioxidant status and protects pancreatic tissues from glucolipotoxicity mediated oxidative stress in high-fat diet: Streptozotocin-induced diabetic rats. Mol. Cell. Biochem. 2015, 404, 143–159. [Google Scholar] [CrossRef]
- Akdemir, F.N.E.; Gozeler, M.S.; Yildirim, S.; Askin, S.; Dortbudak, M.B.; Kiziltunc, A. The effect of ferulic acid against cisplatin-induced ototoxicit. Med. Sci. Int. Med. J. 2018, 7, 528–531. [Google Scholar] [CrossRef]
- Nabavi, S.F.; Habtemariam, S.; Di Lorenzo, A.; Sureda, A.; Khanjani, S.; Nabavi, S.M.; Daglia, M. Post-stroke depression modulation and in vivo antioxidant activity of gallic acid and its synthetic derivatives in a murine model system. Nutrients 2016, 8, 248. [Google Scholar] [CrossRef] [Green Version]
- Shen, Y.; Song, X.; Li, L.; Sun, J.; Jaiswal, Y.; Huang, J.; Liu, C.; Yang, W.; Williams, L.; Zhang, H.; et al. Protective effects of p-coumaric acid against oxidant and hyperlipidemia-an in vitro and in vivo evaluation. Biomed. Pharmacother. 2019, 111, 579–587. [Google Scholar] [CrossRef]
- Sun, J.; Li, Y.; Ding, Y.; Wang, J.; Geng, J.; Yang, H.; Ren, J.; Tang, J.; Gao, J. Neuroprotective effects of gallic acid against hypoxia/reoxygenation-induced mitochondrial dysfunctions in vitro and cerebral ischemia/reperfusion injury in vivo. Brain Res. 2014, 1589, 126–139. [Google Scholar] [CrossRef]
- Thingore, C.; Kshirsagar, V.; Juvekar, A. Amelioration of oxidative stress and neuroinflammation in lipopolysaccharide-induced memory impairment using rosmarinic acid in mice. Metab. Brain Dis. 2021, 36, 299–313. [Google Scholar] [CrossRef]
- Tolba, M.F.; El-Serafi, A.T.; Omar, H.A. Caffeic acid phenethyl ester protects against glucocorticoid-induced osteoporosis in vivo: Impact on oxidative stress and RANKL/OPG signals. Toxicol. Appl. Pharmacol. 2017, 324, 26–35. [Google Scholar] [CrossRef]
- Yuan, J.; Ge, K.; Mu, J.; Rong, J.; Zhang, L.; Wang, B.; Wan, J.; Xia, G. Ferulic acid attenuated acetaminophen-induced hepatotoxicity though down-regulating the cytochrome P 2E1 and inhibiting toll-like receptor 4 signaling-mediated inflammation in mice. Am. J. Transl. Res. 2016, 8, 4205–4214. [Google Scholar]
- Agarwal, S.; Tripathi, R.; Mohammed, A.; Rizvi, S.I.; Mishra, N. Effects of thymol supplementation against type 2 diabetes in streptozotocin-induced rat model. Plant Arch. 2020, 20, 7. [Google Scholar]
- Bagheri, S.; Sarabi, M.M.; Gholami, M.; Assadollahi, V.; Khorramabadi, R.M.; Moradi, F.H.; Ahmadvand, H. D-Limonene in diabetic rats. J. Ren. Inj. Prev. 2021, 10, 8. [Google Scholar]
- El-Emam, S.Z.; Soubh, A.A.; Al-Mokaddem, A.K.; Abo El-Ella, D.M. Geraniol activates Nrf-2/HO-1 signaling pathway mediating protection against oxidative stress-induced apoptosis in hepatic ischemia-reperfusion injury. Naunyn. Schmiedebergs Arch. Pharmacol. 2020, 393, 1849–1858. [Google Scholar] [CrossRef]
- Jamshidi, H.R.; Zeinabady, Z.; Zamani, E.; Shokrzadeh, M.; Shaki, F. Attenuation of diabetic nephropathy by carvacrol through anti-oxidative effects in alloxan-induced diabetic Rats. Res. J. Pharmacogn. 2018, 5, 57–64. [Google Scholar] [CrossRef]
- Jiang, Z.-S.; Pu, Z.-C.; Hao, Z.-H. Carvacrol protects against spinal cord injury in rats via suppressing oxidative stress and the endothelial nitric oxide synthase pathway. Mol. Med. Rep. 2015, 12, 5349–5354. [Google Scholar] [CrossRef]
- Mishra, C.; Khalid, M.; Fatima, N.; Singh, B.; Tripathi, D.; Waseem, M.; Mahdi, A.A. Effects of citral on oxidative stress and hepatic key enzymes of glucose metabolism in streptozotocin/high-fat-diet induced diabetic dyslipidemic Rats. Iran. J. Basic Med. Sci. 2018, 22, 49–57. [Google Scholar] [CrossRef]
- Park, H.; Seol, G.H.; Ryu, S.; Choi, I.-Y. Neuroprotective effects of (-)-Linalool against oxygen-glucose deprivation-induced neuronal injury. Arch. Pharm. Res. 2016, 39, 555–564. [Google Scholar] [CrossRef]
- Rajan, B.; Ravikumar, R.; Premkumar, T.; Devaki, T. Carvacrol attenuates N-nitrosodiethylamine induced liver injury in experimental Wistar rats. Food Sci. Hum. Wellness 2015, 4, 66–74. [Google Scholar] [CrossRef] [Green Version]
- Samarghandian, S.; Farkhondeh, T.; Samini, F.; Borji, A. Protective effects of carvacrol against oxidative stress induced by chronic stress in rat’s brain, liver, and kidney. Biochem. Res. Int. 2016, 2016, e2645237. [Google Scholar] [CrossRef] [Green Version]
- Shata, F.Y.H.; Eldebaky, H.A.A. Effects of camphor on hepatic enzymes, steroids and antioxidant capacity of male rats intoxicated with atrazine. Middle-East J. Sci. Res. 2014, 22, 9. [Google Scholar]
- Shoorei, H.; Khaki, A.; Khaki, A.A.; Hemmati, A.A.; Moghimian, M.; Shokoohi, M. The ameliorative effect of carvacrol on oxidative stress and germ cell apoptosis in testicular tissue of adult diabetic rats. Biomed. Pharmacother. 2019, 111, 568–578. [Google Scholar] [CrossRef]
- Wan, L.; Meng, D.; Wang, H.; Wan, S.; Jiang, S.; Huang, S.; Wei, L.; Yu, P. Preventive and therapeutic effects of thymol in a lipopolysaccharide-induced acute lung injury mice model. Inflammation 2018, 41, 183–192. [Google Scholar] [CrossRef]
- Wang, M.; Sun, J.; Jiang, Z.; Xie, W.; Zhang, X. Hepatoprotective effect of kaempferol against alcoholic liver injury in mice. Am. J. Chin. Med. 2015, 43, 241–254. [Google Scholar] [CrossRef] [Green Version]
- Wei, H.-K.; Xue, H.-X.; Zhou, Z.X.; Peng, J. A carvacrol–thymol blend decreased intestinal oxidative stress and influenced selected microbes without changing the messenger RNA levels of tight junction proteins in Jejunal mucosa of weaning piglets. Animal 2017, 11, 193–201. [Google Scholar] [CrossRef] [Green Version]
- Xu, P.; Wang, K.; Lu, C.; Dong, L.; Gao, L.; Yan, M.; Aibai, S.; Yang, Y.; Liu, X. Protective effects of linalool against amyloid beta-induced cognitive deficits and damages in mice. Life Sci. 2017, 174, 21–27. [Google Scholar] [CrossRef]
- Yao, L.; Hou, G.; Wang, L.; Zuo, X.; Liu, Z. Protective effects of thymol on lps-induced acute lung injury in mice. Microb. Pathog. 2018, 116, 8–12. [Google Scholar] [CrossRef] [PubMed]
- Younis, N.S.; Abduldaium, M.S.; Mohamed, M.E. Protective effect of geraniol on oxidative, inflammatory and apoptotic alterations in isoproterenol-induced cardiotoxicity: Role of the Keap1/Nrf2/HO-1 and PI3K/Akt/MTOR pathways. Antioxidants 2020, 9, 977. [Google Scholar] [CrossRef]
- Yu, W.; Liu, Q.; Zhu, S. Carvacrol protects against acute myocardial infarction of rats via anti-oxidative and anti-apoptotic pathways. Biol. Pharm. Bull. 2013, 36, 579–584. [Google Scholar] [CrossRef] [Green Version]
- Agarwal, A.; Prasad, R.; Jain, A. Effect of green tea extract (catechins) in reducing oxidative stress seen in patients of pulmonary tuberculosis on DOTS Cat I regimen. Phytomedicine 2010, 17, 23–27. [Google Scholar] [CrossRef]
- Alavinezhad, A.; Hedayati, M.; Boskabady, M.H. The effect of Zataria multiflora and carvacrol on wheezing, FEV1 and plasma levels of nitrite in asthmatic patients. Avicenna J. Phytomed. 2017, 7, 531–541. [Google Scholar] [PubMed]
- Boots, A.W.; Drent, M.; de Boer, V.C.J.; Bast, A.; Haenen, G.R.M.M. Quercetin reduces markers of oxidative stress and inflammation in sarcoidosis. Clin. Nutr. 2011, 30, 506–512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bumrungpert, A.; Lilitchan, S.; Tuntipopipat, S.; Tirawanchai, N.; Komindr, S. Ferulic acid supplementation improves lipid profiles, oxidative stress, and inflammatory status in hyperlipidemic subjects: A randomized, double-blind, placebo-controlled clinical trial. Nutrients 2018, 10, 713. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Groote, D.; Van Belleghem, K.; Devière, J.; Van Brussel, W.; Mukaneza, A.; Amininejad, L. Effect of the Intake of resveratrol, resveratrol phosphate, and catechin-rich grape seed extract on markers of oxidative stress and gene expression in adult obese subjects. Ann. Nutr. Metab. 2012, 61, 15–24. [Google Scholar] [CrossRef]
- Duranti, G.; Ceci, R.; Patrizio, F.; Sgrò, P.; Di Luigi, L.; Sabatini, S.; Felici, F.; Bazzucchi, I. Chronic consumption of quercetin reduces erythrocytes oxidative damage: Evaluation at resting and after eccentric exercise in humans. Nutr. Res. 2018, 50, 73–81. [Google Scholar] [CrossRef]
- Ferk, F.; Kundi, M.; Brath, H.; Szekeres, T.; Al-Serori, H.; Mišík, M.; Saiko, P.; Marculescu, R.; Wagner, K.-H.; Knasmueller, S. Gallic Acid improves health-associated biochemical parameters and prevents oxidative damage of DNA in type 2 diabetes patients: Results of a placebo-controlled pilot study. Mol. Nutr. Food Res. 2018, 62, 1700482. [Google Scholar] [CrossRef]
- Quindry, J.C.; McAnulty, S.R.; Hudson, M.B.; Hosick, P.; Dumke, C.; McAnulty, L.S.; Henson, D.; Morrow, J.D.; Nieman, D. Oral quercetin supplementation and blood oxidative capacity in response to ultramarathon competition. Int. J. Sport Nutr. Exerc. Metab. 2008, 18, 601–616. [Google Scholar] [CrossRef]
- Takahashi, M.; Miyashita, M.; Suzuki, K.; Bae, S.-R.; Kim, H.-K.; Wakisaka, T.; Matsui, Y.; Takeshita, M.; Yasunaga, K. Acute ingestion of catechin-rich green tea improves postprandial glucose status and increases serum thioredoxin concentrations in postmenopausal women. Br. J. Nutr. 2014, 112, 1542–1550. [Google Scholar] [CrossRef] [Green Version]
| Compounds | Experimental Approach | Key Results | Refs |
|---|---|---|---|
| Apigenin | CCl4-induced hepatotoxicity in mice | SOD, CAT, GSH-Px, and GSH levels increased. | [91] |
| MDA level was decreased. | |||
| Apigenin | Kainic acid (KA)-induced excitotoxicity | GSH levels were increased. | [78] |
| Apigenin | STZ-induced diabetic cardiomyopathy | SOD and GPx activity were increased. | [81] |
| Decreased GSH levels. | |||
| Apigenin | H2O2-induced rat hepatic stellate cells | SOD and GSH levels were enhanced. | [83] |
| ROS, MDA, and NO levels were inhibited. | |||
| Apigenin | Diabetes-associated cognitive decline a diabetic rat model | Decreased the MDA content. | [82] |
| Increased SOD activity and GSH level. | |||
| Inhibited the activities of cNOS and iNOS. | |||
| Apigenin | Myocardial ischemia/reperfusion injury in mice | Significantly decreased MDA. | |
| Elevated SOD activity. | |||
| Catechin | Subacute chlorpyrifos-induced oxidative stress | Reduced MDA content. | [75] |
| SOD, CAT, and GPx activities were increased. | |||
| Catechin | Type 2 diabetic erythrocytes | Decreased MDA. | [84] |
| Increased GSH. | |||
| Hesperetin | Acetaminophen-induced hepatotoxicity | Increased levels of glutathione. Increased SOD and CAT activities. | [88] |
| Reduced MDA levels. | |||
| Hesperetin | Streptozotocin-induced diabetic in rat | Increased GSH. | [86] |
| Improved CAT, SOD, and GPx. | |||
| Decreased levels of MDA. | |||
| Reduced protein carbonyl. | |||
| Quercetin | Subacute-chlorpyrifos-induced oxidative stress | Decreased malondialdehyde levels. | [75] |
| Enhanced SOD, CAT, and GPx. | |||
| Quercetin | Streptozotocin-nicotinamide-induced diabetic rats | Improved SOD, CAT, GPx. | [90] |
| Increased mRNA expression levels. | |||
| Ameliorated MDA levels. | |||
| Quercetin | Streptozotocin-nicotinamide-induced diabetic rats | Improved cardiac SOD-1, CAT, and GPx-1. | [85] |
| Quercetin | Myocardial ischemia reperfusion (IR) injuries | Reduced MDA content. | [80] |
| Increased the activities of GSH, SOD, CAT, GSH-Px, GR. | |||
| Quercetin | Sodium-azide-induced hepatic and splenic oxidative stress in vivo | SOD and GPx activities were significantly increased. | [87] |
| Considerably reduced MDA concentrations. | |||
| Rutin | Intestinal toxicity induced by methotrexate | Decreased TBARS and protein carbonyl. | [77] |
| Increased SOD, catalase, and GSH. | |||
| Rutin | Alloxan-induced diabetic nephropathy | Increased SOD and catalase. | [76] |
| Reduced lipid peroxidation. | |||
| Downregulated endoplasmic reticulum stress markers GRP78 and CHOP. | |||
| Rutin | Gastric lesions induced by 50% ethanol | Significantly increased GSH-Px activity. | [79] |
| Decreased the levels of thiobarbituric acid. | |||
| Resveratrol | Ethanol-induced oxidative stress in vivo | Increased SOD activity. | [92] |
| Increased catalase. | |||
| Increased glutathione peroxidase. | |||
| Resveratrol | Oxidative stress cardiomyopathy induced by doxorubicin | Reduced MDA content. | [93] |
| Promoted SOD, CAT, and GPx activities. | |||
| Increased GSH. | |||
| Resveratrol | Lipopolysaccharide-induced oxidative stress | Significantly reduced the level of TBARS. | [94] |
| Significantly increased glutathione level and the superoxide dismutase. | |||
| Resveratrol | Hyperglycemia-induced renal tubular oxidative stress damage | Prevented the SOD activity downregulation and MDA upregulation. | [95] |
| Significantly increased CAT levels. | |||
| Modulates the SIRT1/FOXO3a pathway. | |||
| Resveratrol | Murine model of lipopolysaccharide (LPS)-induced pulmonary fibrosis | Decreased MDA levels. | [96] |
| Increased total antioxidant activity, superoxide dismutase, and catalase activities. |
| Compounds | Experimental Approach | Key Results | Refs |
|---|---|---|---|
| Caffeic acid | Glucocorticoid-induced osteoporosis in vivo | Elevated glutathione peroxidase content and superoxide dismutase. | [107] |
| Significantly decreased malondialdehyde levels. | |||
| Caffeic acid | Medium-term rat hepatocarcinogenesis model | Decreased lipid peroxidation. | [98] |
| p-Coumaric acid | High-fat diet (HFD) mice model | Elevated CAT, total antioxidant capacity, and GSH-Px levels. | [104] |
| Ferulic acid | Cisplatin-induced ototoxicity | Increased SOD and GPx activities. | [100,102,108] |
| Reduced MDA levels. | |||
| Ferulic acid | Acetaminophen-induced hepatotoxicity | Enhanced superoxide dismutase and catalase activities. | [108] |
| Increased GSH-Px levels. | |||
| Ferulic acid | Formaldehyde-induced hepatotoxicity | Increased CAT, GPx content, and SOD activities. | [100] |
| Decreased malondialdehyde content. | |||
| Gallic acid | Balb/c mice with post-stroke depression | Increased SOD and CAT activities. | [103] |
| Elevated glutathione peroxidase content. | |||
| Decreased TBARS levels. | |||
| Gallic acid | Cerebral ischemia/reperfusion-induced by middle cerebral artery occlusion | Reduced MDA levels. | [105] |
| Rosmarinic acid | Aluminum-induced oxidative stress | Increased GSH concentration. | [97] |
| Decreased MDA concentration. | |||
| Increased CAT and SOD activities. | |||
| Rosmarinic acid | High-fat diet and streptozotocin-induced diabetic rats. | Elevated the levels of vitamin C, vitamin E, and GSH. | [101] |
| Elevated SOD, CAT, and GPx activities. | |||
| Decreased lipid peroxide, AOPP, and protein carbonyl levels. | |||
| Rosmarinic acid | Lipopolysaccharide-induced memory impairment | SOD activity increased. | [106] |
| GSH levels reduced. | |||
| Decreased lipid peroxidation in the brain. | |||
| Vanillic acid | Ischemia-reperfusion-induced oxidative stress in isolated rat heart | Decreased MDA. | [99] |
| Elevated SOD, CAT, and GPx activities. |
| Compounds | Experimental Approach | Key Results | Refs |
|---|---|---|---|
| Camphor | Atrazine-induced toxicity | Increased SOD activity. | [118] |
| Reduced MDA levels. | |||
| Carvacrol | N-nitrosodiethylamine-induced liver injury in mice | Decreased the levels of lipid peroxides. | [116] |
| Elevated superoxide dismutase and catalase activities. | |||
| Significantly increased the activities of GPx, GR, GSH, G6PD, vitamin (Vit. A), Vit. C and Vit. E. | |||
| Carvacrol | Acute myocardial infarction | Decreased MDA content. | [126] |
| in mice | Increased SOD, GSH, and GSH-Px activities. | ||
| Carvacrol | Restraint-stress-induced oxidative stress damage in the brain, liver, and kidney | Reduced MDA content. | [117] |
| Elevated GSH, SOD, GPx, GR, and CAT activities. | |||
| Carvacrol | Alloxan-induced diabetic rats | Reduced malondialdehyde. | [112] |
| Increased significantly glutathione levels. | |||
| Carvacrol | STZ-induced diabetic rats | Reduced levels of tissue malondialdehyde. | [119] |
| Increased antioxidant enzymes (SOD and GPx,). | |||
| Carvacrol | Weaning-induced intestinal dysfunction in piglets | Significantly elevated superoxide dismutase and glutathione peroxidase activities. | [122] |
| Decreased TBARS levels. | |||
| Carvacrol | Aluminium-induced oxidative stress | Increased GSH concentration. | [97] |
| Decreased MDA concentration. | |||
| Increased CAT and SOD activities. | |||
| Citral | Streptozotocin/high-fat-diet-induced diabetic dyslipidemic rats | Significant reduction in the level of MDA. | [114] |
| Attenuated protein carbonyl content. | |||
| Significantly improved the activity of SOD. | |||
| Significantly restored the activity of catalase. | |||
| Significant increase in Gpx activity. | |||
| D-limonene | Alloxan-induced diabetic rats | Reduced malondialdehyde and NO. | [110] |
| Elevated GSH levels. | |||
| Increased GPx, CAT, and SOD activities. | |||
| Significant elevation in mRNA levels of superoxide dismutase, catalase, and glutathione peroxidase activities. | |||
| Geraniol | Hepatic ischemia reperfusion injury | Increased GSH levels. | [111] |
| Normalized malondialdehyde. | |||
| Decreased Keap1 expression. | |||
| Elevated the nuclear accumulation of Nrf2. | |||
| Elevated expression levels of HO-1. | |||
| Geraniol | Isoproterenol-induced cardiotoxicity | Increased GSH levels. | [125] |
| Elevated GPx, CAT, and SOD activities. | |||
| Activated Nrf2. | |||
| Upregulation of HO-1 expression. | |||
| Kaempferol | Alcohol-induced liver injury in mice | Increased antioxidant enzymes (superoxide dismutase and glutathione). | [121] |
| Decreased malondialdehyde. | |||
| Attenuating the activity and expression of CYP2E1. | |||
| (-)-linalool | Oxygen–glucose-deprivation-induced neuronal injury | Significantly increased SOD. | [115] |
| Linalool | Amyloid-beta-induced cognitive deficits and damages in mice | Elevated dismutase and glutathione peroxidase activities. | [123] |
| Reduced the malondialdehyde content. | |||
| Reduced the AChE level. | |||
| Activated the Nrf2/HO-1 signaling. | |||
| Thymol | Lipopolysaccharide-induced acute lung injury mice | Decreased malondialdehyde and MPO levels. | [120] |
| Model | Increased superoxide dismutase activity. | ||
| Thymol | Type 2 diabetes in a streptozotocin-induced rat model | Significantly improved FRAP value. | [109] |
| Decreased the levels of AOPP value. | |||
| Significantly decreased MDA level. | |||
| Elevated erythrocyte GSH levels. | |||
| Elevated % DPPH. | |||
| Thymol | LPS-induced acute lung injury in mice | Significantly reduced the MPO activity. | [124] |
| Significantly reduced MDA content. |
| Molecules | Human Subjects | Effects | Refs. |
|---|---|---|---|
| Catechins | Patients of AFB-positive pulmonary tuberculosis. | Decreased MDA concentration. | [127] |
| Decreased the level of NO production. | |||
| Significantly increased SOD. | |||
| Significantly decreased catalase and GPx level. | |||
| Significantly increased GSH. | |||
| Significant decrease in SH level. | |||
| Catechin | Adult obese subjects | Increased the gene expression, and also SOD and GPX activity. | [131] |
| Reduced the levels of GSH. | |||
| Increased TAP and GSSG. | |||
| Decreased lipid peroxides. | |||
| Altered the expression of genes involved in redox. | |||
| Catechins | Healthy postmenopausal women | No impact on serum d-ROM concentrations and plasma H2O2. | [135] |
| Elevated postprandial plasma TRX concentrations. | |||
| Quercetin | Athletes | Significantly increased plasma FRAP. | [134] |
| Unaffected TEAC Plasma F2-isoprostane values and protein carbonyls. | |||
| Quercetin | Non-smoking patients with symptomatic sarcoidosis | Increased total plasma antioxidant capacity. | [129] |
| Decreased MDA concentration. | |||
| Quercetin | Healthy young | Decrease in GSSG levels. | [132] |
| Improved GSH/GSSG ratio. | |||
| Significantly decreased TBARs levels. | |||
| No effect in erythrocytes CAT, GPx, SOD activities, and SOD/GPx ratio. | |||
| Ferulic acid | Subjects with hyperlipidemia | Decreased the oxidative stress biomarker, MDA. | [130] |
| Gallic acid | Type 2 diabetes patients | No impact on plasma MDA and FRAP. | [133] |
| Carvacrol | Asthmatic patients | Significantly decreased plasma level of NO2-. | [128] |
| Resveratrol | Adult obese subjects | Increased the gene expression, and also SOD and GPX activity. | [131] |
| Reduced the levels of GSH and GSSG. | |||
| Increased TAP. | |||
| Decreased lipid peroxides. | |||
| Altered the expression of genes involved in redox. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bouyahya, A.; El Menyiy, N.; Oumeslakht, L.; El Allam, A.; Balahbib, A.; Rauf, A.; Muhammad, N.; Kuznetsova, E.; Derkho, M.; Thiruvengadam, M.; et al. Preclinical and Clinical Antioxidant Effects of Natural Compounds against Oxidative Stress-Induced Epigenetic Instability in Tumor Cells. Antioxidants 2021, 10, 1553. https://doi.org/10.3390/antiox10101553
Bouyahya A, El Menyiy N, Oumeslakht L, El Allam A, Balahbib A, Rauf A, Muhammad N, Kuznetsova E, Derkho M, Thiruvengadam M, et al. Preclinical and Clinical Antioxidant Effects of Natural Compounds against Oxidative Stress-Induced Epigenetic Instability in Tumor Cells. Antioxidants. 2021; 10(10):1553. https://doi.org/10.3390/antiox10101553
Chicago/Turabian StyleBouyahya, Abdelhakim, Naoual El Menyiy, Loubna Oumeslakht, Aicha El Allam, Abdelaali Balahbib, Abdur Rauf, Naveed Muhammad, Elena Kuznetsova, Marina Derkho, Muthu Thiruvengadam, and et al. 2021. "Preclinical and Clinical Antioxidant Effects of Natural Compounds against Oxidative Stress-Induced Epigenetic Instability in Tumor Cells" Antioxidants 10, no. 10: 1553. https://doi.org/10.3390/antiox10101553
APA StyleBouyahya, A., El Menyiy, N., Oumeslakht, L., El Allam, A., Balahbib, A., Rauf, A., Muhammad, N., Kuznetsova, E., Derkho, M., Thiruvengadam, M., Shariati, M. A., & El Omari, N. (2021). Preclinical and Clinical Antioxidant Effects of Natural Compounds against Oxidative Stress-Induced Epigenetic Instability in Tumor Cells. Antioxidants, 10(10), 1553. https://doi.org/10.3390/antiox10101553










